A Phase II Study to Compare the Safety and Efficacy of Direct Oral Anticoagulants versus Subcutaneous Dalteparin for Cancer-Associated Venous Thromboembolism in Patients with Advanced Upper Gastrointestinal, Hepatobiliary and Pancreatic Cancer: PRIORITY
Abstract
:Simple Summary
Abstract
1. Introduction
2. Material and Methods
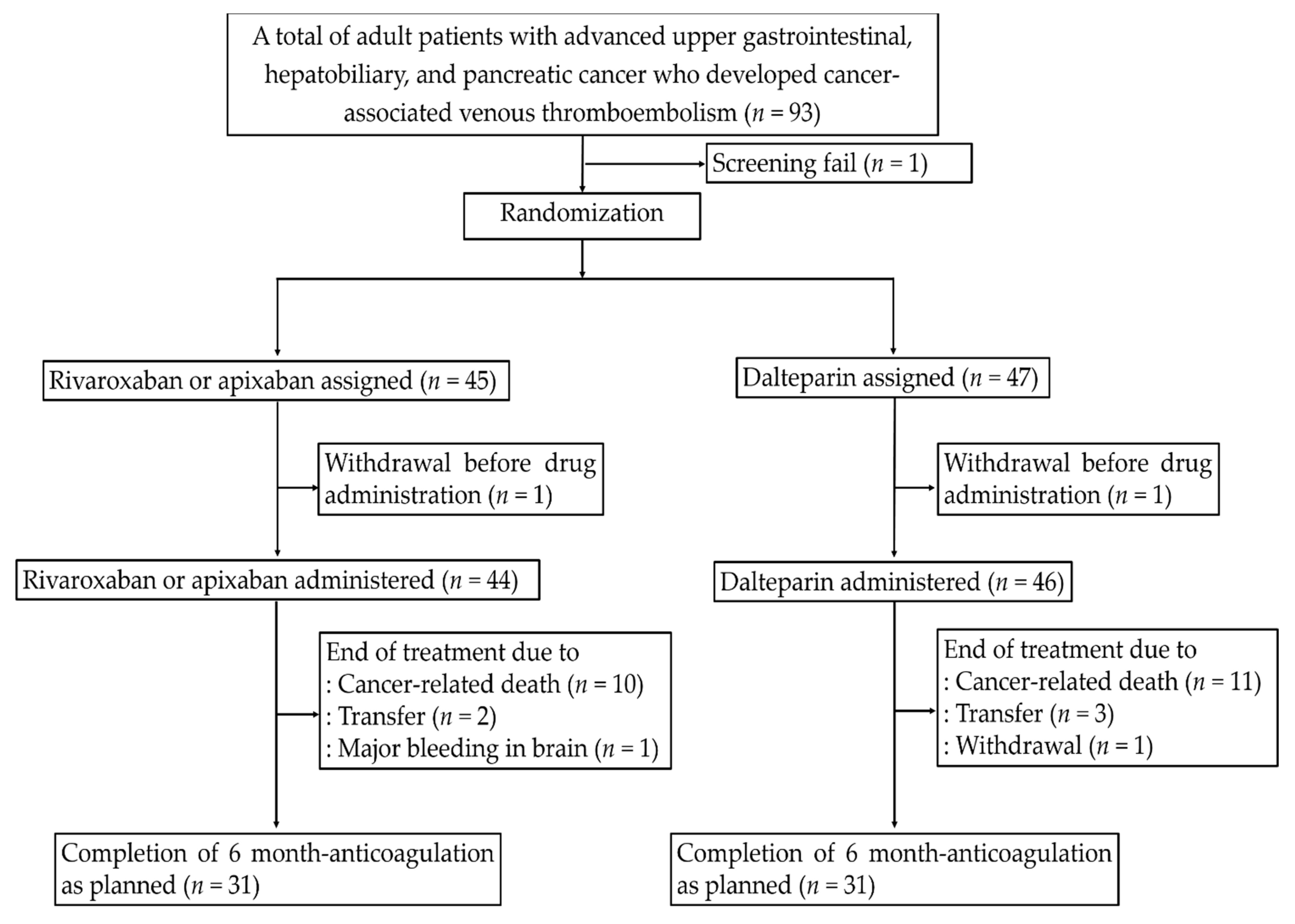
2.1. Study Design and Participants
2.2. Randomization and Trial Intervention
2.3. Outcomes
2.4. Surveillance and Follow-Up
2.5. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Primary Safety Outcomes
3.3. Secondary Safety and Efficacy Outcomes
3.4. Subgroup Analysis Based on the Drug Administered during Bleeding Events
4. Discussion
5. Conclusion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Baron, J.A.; Gridley, G.; Weiderpass, E.; Nyrén, O.; Linet, M. Venous thromboembolism and cancer. Lancet 1998, 351, 1077–1080. [Google Scholar] [CrossRef]
- Prandoni, P.; Lensing, A.W.; Piccioli, A.; Bernardi, E.; Simioni, P.; Girolami, B.; Marchiori, A.; Sabbion, P.; Prins, M.H.; Noventa, F.; et al. Recurrent venous thromboembolism and bleeding complications during anticoagulant treatment in patients with cancer and venous thrombosis. Blood 2002, 100, 3484–3488. [Google Scholar] [CrossRef] [Green Version]
- Hutten, B.A.; Prins, M.H.; Gent, M.; Ginsberg, J.; Tijssen, J.G.; Büller, H.R. Incidence of recurrent thromboembolic and bleeding complications among patients with venous thromboembolism in relation to both malignancy and achieved international normalized ratio: A retrospective analysis. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2000, 18, 3078–3083. [Google Scholar] [CrossRef]
- Khorana, A.A.; Kuderer, N.M.; Culakova, E.; Lyman, G.H.; Francis, C.W. Development and validation of a predictive model for chemotherapy-associated thrombosis. Blood 2008, 111, 4902–4907. [Google Scholar] [CrossRef] [Green Version]
- Patell, R.; Gutierrez, A.; Rybicki, L.; Khorana, A.A. Identifying predictors for bleeding in hospitalized cancer patients: A cohort study. Thromb. Res. 2017, 158, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Pabinger, I.; van Es, N.; Heinze, G.; Posch, F.; Riedl, J.; Reitter, E.M.; Di Nisio, M.; Cesarman-Maus, G.; Kraaijpoel, N.; Zielinski, C.C.; et al. A clinical prediction model for cancer-associated venous thromboembolism: A development and validation study in two independent prospective cohorts. Lancet Haematol. 2018, 5, e289–e298. [Google Scholar] [CrossRef]
- Lee, A.Y.; Levine, M.N.; Baker, R.I.; Bowden, C.; Kakkar, A.K.; Prins, M.; Rickles, F.R.; Julian, J.A.; Haley, S.; Kovacs, M.J.; et al. Low-molecular-weight heparin versus a coumarin for the prevention of recurrent venous thromboembolism in patients with cancer. N. Engl. J. Med. 2003, 349, 146–153. [Google Scholar] [CrossRef] [Green Version]
- Meyer, G.; Marjanovic, Z.; Valcke, J.; Lorcerie, B.; Gruel, Y.; Solal-Celigny, P.; Le Maignan, C.; Extra, J.M.; Cottu, P.; Farge, D. Comparison of low-molecular-weight heparin and warfarin for the secondary prevention of venous thromboembolism in patients with cancer: A randomized controlled study. Arch. Intern. Med. 2002, 162, 1729–1735. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, A.Y.Y.; Kamphuisen, P.W.; Meyer, G.; Bauersachs, R.; Janas, M.S.; Jarner, M.F.; Khorana, A.A.; CATCH Investigators. Tinzaparin vs warfarin for treatment of acute venous thromboembolism in patients with active cancer: A randomized clinical trial. JAMA 2015, 314, 677–686. [Google Scholar] [CrossRef] [PubMed]
- Raskob, G.E.; van Es, N.; Verhamme, P.; Carrier, M.; Di Nisio, M.; Garcia, D.; Grosso, M.A.; Kakkar, A.K.; Kovacs, M.J.; Mercuri, M.F.; et al. Edoxaban for the treatment of cancer-associated venous thromboembolism. N. Engl. J. Med. 2018, 378, 615–624. [Google Scholar] [CrossRef] [PubMed]
- Young, A.M.; Marshall, A.; Thirlwall, J.; Chapman, O.; Lokare, A.; Hill, C.; Hale, D.; Dunn, J.A.; Lyman, G.H.; Hutchinson, C.; et al. Comparison of an oral factor Xa inhibitor with low molecular weight heparin in patients with cancer with venous thromboembolism: Results of a randomized trial (SELECT-D). J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2018, 36, 2017–2023. [Google Scholar] [CrossRef]
- Agnelli, G.; Becattini, C.; Meyer, G.; Munoz, A.; Huisman, M.V.; Connors, J.M.; Cohen, A.; Bauersachs, R.; Brenner, B.; Torbicki, A.; et al. Apixaban for the treatment of venous thromboembolism associated with cancer. N. Engl. J. Med. 2020, 382, 1599–1607. [Google Scholar] [CrossRef] [PubMed]
- McBane, R.D.; Wysokinski, W.E.; Le-Rademacher, J.G.; Zemla, T.; Ashrani, A.; Tafur, A.; Perepu, U.; Anderson, D.; Gundabolu, K.; Kuzma, C.; et al. Apixaban and dalteparin in active malignancy-associated venous thromboembolism: The ADAM VTE trial. J. Thromb. Haemost. 2020, 18, 411–421. [Google Scholar] [CrossRef]
- Key, N.S.; Khorana, A.A.; Kuderer, N.M.; Bohlke, K.; Lee, A.Y.Y.; Arcelus, J.I.; Wong, S.L.; Balaban, E.P.; Flowers, C.R.; Francis, C.W.; et al. Venous thromboembolism prophylaxis and treatment in patients with cancer: ASCO clinical practice guideline update. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2020, 38, 496–520. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Seo, S.; Kim, K.P.; Chang, H.M.; Ryoo, B.Y.; Yoo, C.; Jeong, J.H.; Lee, J.L.; Im, H.S.; Jeong, H.; et al. Rivaroxaban versus low-molecular-weight heparin for venous thromboembolism in advanced upper gastrointestinal tract and hepatopancreatobiliary cancer. In Vivo 2020, 34, 829–837. [Google Scholar] [CrossRef] [Green Version]
- Schulman, S.; Kearon, C.; Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J. Thromb. Haemost. 2005, 3, 692–694. [Google Scholar] [CrossRef]
- Gordon Lan, K.K.; DeMets, D.L. Discrete sequential boundaries for clinical trials. Biometrika 1983, 70, 659–663. [Google Scholar] [CrossRef]
- Kraaijpoel, N.; Di Nisio, M.; Mulder, F.I.; van Es, N.; Beyer-Westendorf, J.; Carrier, M.; Garcia, D.; Grosso, M.; Kakkar, A.K.; Mercuri, M.F.; et al. Clinical impact of bleeding in cancer-associated venous thromboembolism: Results from the Hokusai VTE cancer study. Thromb. Haemost. 2018, 118, 1439–1449. [Google Scholar] [CrossRef]
- Ay, C.; Beyer-Westendorf, J.; Pabinger, I. Treatment of cancer-associated venous thromboembolism in the age of direct oral anticoagulants. Ann. Oncol. 2019, 30, 897–907. [Google Scholar] [CrossRef]
- Houghton, D.E.; Vlazny, D.T.; Casanegra, A.I.; Brunton, N.; Froehling, D.A.; Meverden, R.A.; Hodge, D.O.; Peterson, L.G.; McBane, R.D.; Wysokinski, W.E. Bleeding in patients with gastrointestinal cancer compared with nongastrointestinal cancer treated with apixaban, rivaroxaban, or enoxaparin for acute venous thromboembolism. Mayo Clin. Proc. 2021, 96, 2793–2805. [Google Scholar] [CrossRef] [PubMed]
- White, R.H.; Keenan, C.R. Effects of race and ethnicity on the incidence of venous thromboembolism. Thromb. Res. 2009, 123, S11–S17. [Google Scholar] [CrossRef]
- Gervaso, L.; Dave, H.; Khorana, A.A. Venous and Arterial Thromboembolism in Patients With Cancer: JACC: CardioOncology State-of-the-Art Review. JACC CardioOncol. 2021, 3, 173–190. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M.; Yamada, N.; Asamura, T.; Shiosakai, K.; Uchino, K. Safety and Effectiveness of Edoxaban in Japanese Venous Thromboembolism Patients―Final Analysis of One-Year Follow-up Data From a Japanese Postmarketing Observational Study (ETNA-VTE-Japan). Circ. Rep. 2020, CR-19-0127. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| DOAC (n = 44) | Dalteparin (n = 46) | p | |
|---|---|---|---|
| Median age, years (range) | 64 (3977) | 63 (4278) | 0.864 |
| Sex | 0.662 | ||
| Male | 25 (56.8) | 23 (50.0) | |
| Female | 19 (43.2) | 23 (50.0) | |
| BMI | 22.5 ± 3.3 | 22.4 ± 3.1 | 0.916 |
| CA-VTE | 0.250 | ||
| Deep vein thromboembolism | 4 (9.1) | 10 (21.7) | |
| Pulmonary thromboembolism | 35 (79.5) | 32 (69.6) | |
| Both | 5 (11.4) | 4 (8.7) | |
| Tumor types | 0.453 | ||
| Esophageal cancer | 8 (18.2) | 5 (10.9) | |
| Gastric cancer | 19 (43.2) | 18 (39.1) | |
| Ampulla of vater cancer | 1 (2.3) | 1 (2.2) | |
| Duodenal cancer | 0 (0) | 1 (2.2) | |
| Hepatocellular carcinoma | 2 (4.5) | 0 (0) | |
| Biliary cancer | 8 (18.2) | 9 (19.6) | |
| Pancreatic cancer | 6 (13.6) | 12 (26.1) | |
| ECOG PS | 0.662 | ||
| 0–1 | 37 (84.1) | 36 (78.3) | |
| ≥2 | 7 (15.9) | 10 (21.7) | |
| Metastatic disease | 38 (86.4) | 33 (71.7) | 0.122 |
| Chemotherapy during anticoagulation | 41 (93.2) | 40 (87.0) | 0.486 |
| Lines of chemotherapy during anticoagulation * | 0.447 | ||
| First-line | 24 (58.5) | 28 (70.0) | |
| Second-line | 12 (29.3) | 7 (17.5) | |
| Third or later line | 5 (12.2) | 5 (12.5) | |
| Radiotherapy during anticoagulation | 2 (4.5) | 1 (2.2) | 0.969 |
| DOAC (n = 44, %) | Dalteparin (n = 46, %) | p * | HR (95% CI) | p ** | Adjusted HR *** (95% CI) | p ** | |
|---|---|---|---|---|---|---|---|
| Recurrent CA-VTE | 1 (2.3) | 1 (2.2) | 1.000 | 1.06 (0.07–16.98) | 0.966 | 0.97 (0.05–19.23) | 0.985 |
| Category of bleeding events | |||||||
| Major bleeding | 8 (18.2) | 2 (4.3) | 0.047 | 4.32 (0.92–20.36) | 0.064 | 4.05 (0.86–19.11) | 0.077 |
| Clinically relevant nonmajor bleeding | 8 (18.2) | 4 (8.7) | 0.186 | 2.11 (0.64–7.02) | 0.222 | 1.70 (0.49–5.88) | 0.404 |
| Clinically relevant bleeding | 15 (34.1) | 6 (13.0) | 0.017 | 2.83 (1.10–7.30) | 0.031 | 2.83 (1.09–7.29) | 0.031 |
| Total bleeding | 26 (59.1) | 23 (50.0) | 0.387 | 1.19 (0.68–2.09) | 0.545 | 1.12 (0.61–2.04) | 0.719 |
| All (n = 90, %) | DOAC (n = 44, %) | Dalteparin (n = 46, %) | p * | |
|---|---|---|---|---|
| Major bleeding | 10/90 (11.1) | 8/44 (18.2) | 2/46 (4.3) | 0.047 |
| 0.732 | ||||
| GI tract | 8/10 (80.0) | 6/8 (75.0) | 2/2 (100.0) | |
| Brain | 1/10 (10.0) | 1/8 (12.5) | 0 (0) | |
| Vagina | 1/10 (10.0) | 1/8 (12.5) | 0 (0) | |
| Clinically relevant bleeding | 15/44 (34.1) | 6/46 (13.0) | 0.018 | |
| 0.528 | ||||
| GI tract | 11/21 (52.4) | 9/15 (60.0) | 2/6 (33.3) | |
| GU tract | 6/21 (28.6) | 3/15 (20.0) | 3/6 (50.0) | |
| Brain | 1/21 (4.8) | 1/15 (6.7) | 0/6 (0) | |
| Vagina | 2/21 (9.5) | 1/15 (6.7) | 1/6 (16.7) | |
| Epistaxis | 1/21 (4.8) | 1/15 (6.7) | 0/6 (0) |
| Major Bleeding | Clinically Relevant Bleeding | |||
|---|---|---|---|---|
| HR (95% CI) | p * | HR (95% CI) | p * | |
| Male vs. Female | 2.01 (0.52–7.79) | 0.311 | 1.80 (0.73–4.47) | 0.203 |
| Age ≥65 years vs. <65 years | 0.28 (0.06–1.30) | 0.103 | 0.55 (0.22–1.36) | 0.197 |
| ECOG PS ≥2 vs. ECOG PS 0–1 | 0.56 (0.07–4.44) | 0.584 | 1.30 (0.44–3.89) | 0.638 |
| BMI (<18.5) vs. BMI (18.5) | 0.90 (0.11–7.20) | 0.921 | 1.33 (0.39–4.53) | 0.651 |
| Primary cancer type | 0.397 | 0.246 | ||
| Upper GI tract cancer | 1 | 1 | ||
| Hepatobiliary and pancreas cancer | 1.71 (0.49–5.94) | 1.66 (0.70–3.94) | ||
| Hemoglobin <9.0 g/dL vs. 9.0 g/dL | 2.21 (0.57–8.55) | 0.252 | 1.66 (0.61–4.55) | 0.322 |
| Platelets <100 × 106/µL vs. 100 × 106/µL | 2.37 (0.50–11.17) | 0.275 | 0.94 (0.22–4.02) | 0.928 |
| Cr clearance <60 mL/min vs. 60 mL/min | 1.10 (0.23–5.27) | 0.910 | 1.04 (0.35–3.12) | 0.945 |
| Albumin <3.5 g/dL vs. ≥3.5 g/dL | 1.87 (0.48–7.25) | 0.366 | 1.68 (0.68–4.19) | 0.262 |
| Activated partial thromboplastin time (aPTT) >35 s vs. ≤35 s | 1.14 (0.14–8.99) | 0.901 | 0.51 (0.07–3.83) | 0.515 |
| Anticancer systemic therapy during anticoagulation (yes vs. no) | 23.04 (0.0–798,399.18) | 0.556 | 22.96 (0.01–38,485.883) | 0.408 |
| Treatment lines of systemic therapy during anticoagulation | ||||
| First-line | 1 | 1 | ||
| Second line | 1.64 (0.39–6.86) | 0.499 | 2.13 (0.81–5.61) | 0.127 |
| Third or later line | 2.45 (0.47–12.68) | 0.286 | 2.58 (0.80–8.30) | 0.111 |
| Radiotherapy during anticoagulation (yes vs. no) | 4.07 (0.51–32.17) | 0.184 | 1.65 (0.22–12.35) | 0.624 |
| Cancer involvement at GI mucosa (yes vs. no) | 2.27 (0.64–8.06) | 0.204 | 2.57 (1.06–6.21) | 0.036 |
| Type of anticoagulant (DOAC vs. dalteparin) | 4.32 (0.92–20.36) | 0.064 | 2.83 (1.10–7.30) | 0.031 |
| DOAC (n = 46 †, %) | Dalteparin (n = 44 †, %) | p * | HR (95% CI) | p ** | Adjusted HR *** (95% CI) | p ** | |
|---|---|---|---|---|---|---|---|
| Recurrent CA-VTE | 1 (2.2) | 1 (2.2) | 1.000 | 0.91 (0.06–14.52) | 0.946 | 0.87 (0.05–16.34) | 0.924 |
| Category of bleeding events | |||||||
| Major bleeding | 9 (19.6) | 1 (2.3) | 0.015 | 8.89 (1.13–70.17) | 0.038 | 8.88 (1.12–70.17) | 0.038 |
| Clinically relevant nonmajor bleeding | 9 (19.6) | 3 (6.8) | 0.120 | 2.93 (0.80–10.85) | 0.106 | 2.42 (0.64–9.22) | 0.195 |
| Clinically relevant bleeding | 17 (37.0) | 4 (9.1) | 0.002 | 4.52 (1.52–13.44) | 0.007 | 4.51 (1.52–13.44) | 0.007 |
| Total bleeding | 28 (60.9) | 21 (47.7) | 0.211 | 1.31 (0.75–2.31) | 0.346 | 1.21 (0.67–2.19) | 0.525 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.H.; Yoo, C.; Seo, S.; Jeong, J.H.; Ryoo, B.-Y.; Kim, K.-p.; Lee, J.B.; Lee, K.-W.; Kim, J.-W.; Kim, I.-H.; et al. A Phase II Study to Compare the Safety and Efficacy of Direct Oral Anticoagulants versus Subcutaneous Dalteparin for Cancer-Associated Venous Thromboembolism in Patients with Advanced Upper Gastrointestinal, Hepatobiliary and Pancreatic Cancer: PRIORITY. Cancers 2022, 14, 559. https://doi.org/10.3390/cancers14030559
Kim JH, Yoo C, Seo S, Jeong JH, Ryoo B-Y, Kim K-p, Lee JB, Lee K-W, Kim J-W, Kim I-H, et al. A Phase II Study to Compare the Safety and Efficacy of Direct Oral Anticoagulants versus Subcutaneous Dalteparin for Cancer-Associated Venous Thromboembolism in Patients with Advanced Upper Gastrointestinal, Hepatobiliary and Pancreatic Cancer: PRIORITY. Cancers. 2022; 14(3):559. https://doi.org/10.3390/cancers14030559
Chicago/Turabian StyleKim, Jwa Hoon, Changhoon Yoo, Seyoung Seo, Jae Ho Jeong, Baek-Yeol Ryoo, Kyu-pyo Kim, Jung Bok Lee, Keun-Wook Lee, Ji-Won Kim, Il-Hwan Kim, and et al. 2022. "A Phase II Study to Compare the Safety and Efficacy of Direct Oral Anticoagulants versus Subcutaneous Dalteparin for Cancer-Associated Venous Thromboembolism in Patients with Advanced Upper Gastrointestinal, Hepatobiliary and Pancreatic Cancer: PRIORITY" Cancers 14, no. 3: 559. https://doi.org/10.3390/cancers14030559
APA StyleKim, J. H., Yoo, C., Seo, S., Jeong, J. H., Ryoo, B.-Y., Kim, K.-p., Lee, J. B., Lee, K.-W., Kim, J.-W., Kim, I.-H., Kang, M., Ryu, H., Cheon, J., & Park, S. R. (2022). A Phase II Study to Compare the Safety and Efficacy of Direct Oral Anticoagulants versus Subcutaneous Dalteparin for Cancer-Associated Venous Thromboembolism in Patients with Advanced Upper Gastrointestinal, Hepatobiliary and Pancreatic Cancer: PRIORITY. Cancers, 14(3), 559. https://doi.org/10.3390/cancers14030559






