Safety and Efficacy of 177Lutetium-PSMA-617 Radioligand Therapy Shortly after Failing 223Radium-Dichloride
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. 177Lutetium-PSMA-617 Radioligand Therapy
2.3. Response Assessment
2.4. Toxicity Assessment
2.5. Data Analysis
3. Results
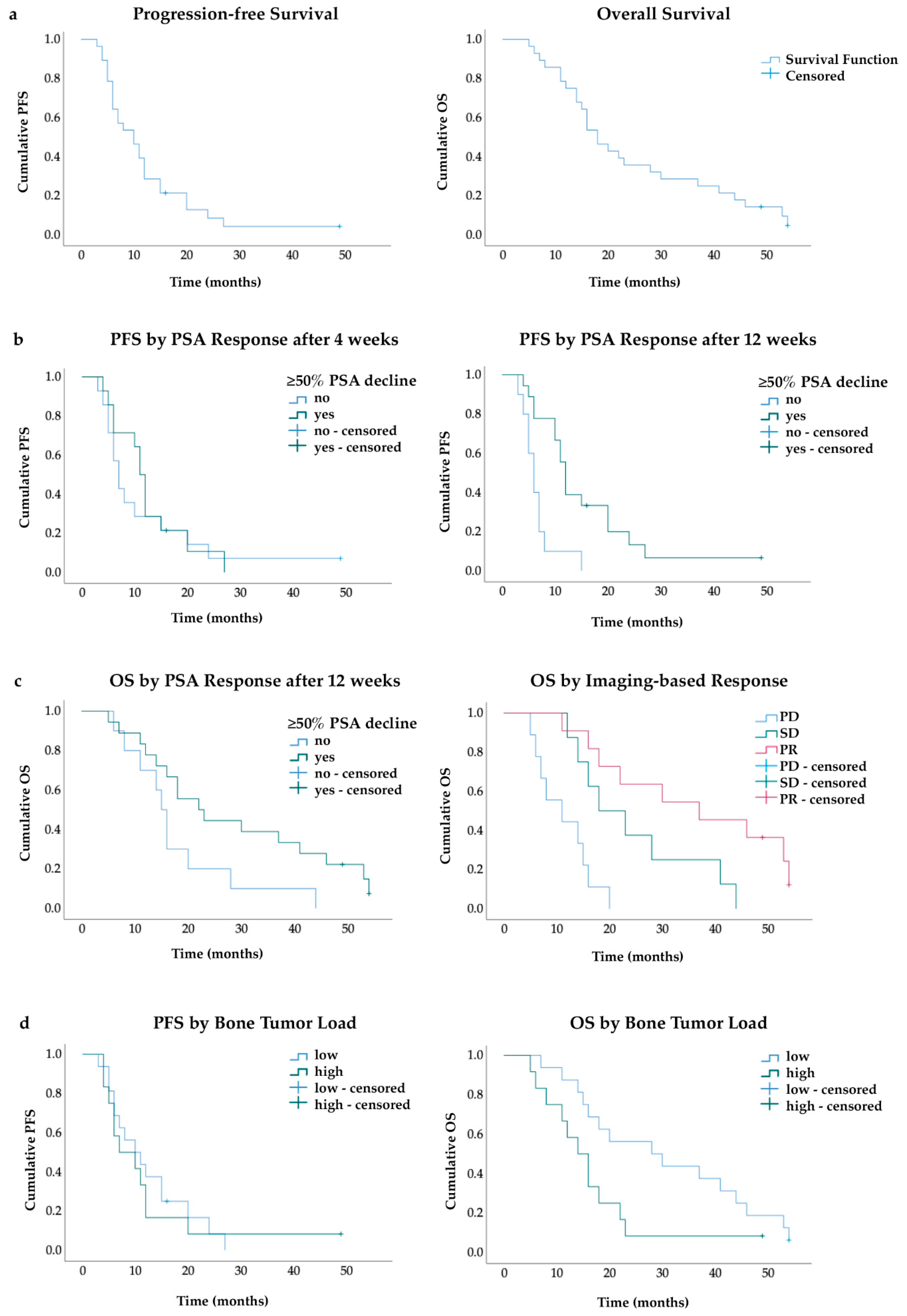
3.1. Response and Survival
3.2. Safety
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Harris, W.P.; Mostaghel, E.A.; Nelson, P.S.; Montgomery, B. Androgen deprivation therapy: Progress in understanding mechanisms of resistance and optimizing androgen depletion. Nat. Clin. Pract. Urol. 2009, 6, 76–85. [Google Scholar] [CrossRef]
- Bubendorf, L.; Schöpfer, A.; Wagner, U.; Sauter, G.; Moch, H.; Willi, N.; Gasser, T.C.; Mihatsch, M.J. Metastatic patterns of prostate cancer: An autopsy study of 1589 patients. Hum. Pathol. 2000, 31, 578–583. [Google Scholar] [CrossRef] [PubMed]
- Saad, F. Impact of bone metastases on patient’s quality of life and importance of treatment. Eur. Urol. Suppl. 2006, 5, 547–550. [Google Scholar] [CrossRef]
- Beer, T.M.; Armstrong, A.J.; Rathkopf, D.E.; Loriot, Y.; Sternberg, C.N.; Higano, C.S.; Iversen, P.; Bhattacharya, S.; Carles, J.; Chowdhury, S.; et al. Enzalutamide in metastatic prostate cancer before chemotherapy. N. Engl. J. Med. 2014, 371, 424–433. [Google Scholar] [CrossRef] [Green Version]
- Tannock, I.F.; de Wit, R.; Berry, W.R.; Horti, J.; Pluzanska, A.; Chi, K.N.; Oudard, S.; Théodore, C.; James, N.D.; Turesson, I.; et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N. Engl. J. Med. 2004, 351, 1502–1512. [Google Scholar] [CrossRef] [Green Version]
- Gartrell, B.A.; Saad, F. Managing bone metastases and reducing skeletal related events in prostate cancer. Nat. Rev. Clin. Oncol. 2014, 11, 335–345. [Google Scholar] [CrossRef]
- Fizazi, K.; Scher, H.I.; Molina, A.; Logothetis, C.J.; Chi, K.N.; Jones, R.J.; Staffurth, J.N.; North, S.; Vogelzang, N.J.; Saad, F.; et al. Abiraterone acetate for treatment of metastatic castration-resistant prostate cancer: Final overall survival analysis of the COU-AA-301 randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol. 2012, 13, 983–992. [Google Scholar] [CrossRef]
- Parker, C.; Nilsson, S.; Heinrich, D.; Helle, S.I.; O’Sullivan, J.M.; Fosså, S.D.; Chodacki, A.; Wiechno, P.; Logue, J.; Seke, M.; et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N. Engl. J. Med. 2013, 369, 213–223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sartor, O.; de Bono, J.; Chi, K.N.; Fizazi, K.; Herrmann, K.; Rahbar, K.; Tagawa, S.T.; Nordquist, L.T.; Vaishampayan, N.; El-Haddad, G.; et al. Lutetium-177–PSMA-617 for metastatic castration-resistant prostate cancer. N. Engl. J. Med. 2021, 385, 1091–1103. [Google Scholar] [CrossRef]
- Hofman, M.S.; Violet, J.; Hicks, R.J.; Ferdinandus, J.; Thang, S.P.; Akhurst, T.; Iravani, A.; Kong, G.; Kumar, A.R.; Murphy, D.G.; et al. [177Lu]-PSMA-617 radionuclide treatment in patients with metastatic castration-resistant prostate cancer (LuPSMA Trial): A single-centre, single-arm, phase 2 study. Lancet Oncol. 2018, 19, 825–833. [Google Scholar] [CrossRef]
- Sartor, A.O.; la Fougère, C.; Essler, M.; Ezziddin, S.; Kramer, G.; Elllinger, J.; Nordquist, L.; Sylvester, J.; Paganelli, G.; Peer, A.; et al. Lutetium-177–prostate-specific membrane antigen ligand following radium-223 treatment in men with bone-metastatic castration-resistant prostate cancer: Real-world clinical experience. J. Nucl. Med. 2021, jnumed.121.262240. [Google Scholar] [CrossRef]
- Ahmadzadehfar, H.; Zimbelmann, S.; Yordanova, A.; Fimmers, R.; Kürpig, S.; Eppard, E.; Gaertner, F.C.; Wei, X.; Hauser, S.; Essler, M. Radioligand therapy of metastatic prostate cancer using 177 Lu-PSMA-617 after radiation exposure to 223 Ra-dichloride. Oncotarget 2015, 5, 55567–55574. [Google Scholar] [CrossRef]
- Leibowitz, R.; Davidson, T.; Gadot, M.; Aharon, M.; Malki, A.; Levartovsky, M.; Oedegaard, C.; Saad, A.; Sandler, I.; Ben-Haim, S.; et al. A retrospective analysis of the safety and activity of lutetium-177-prostate-specific membrane antigen radionuclide treatment in older patients with metastatic castration-resistant prostate cancer. Oncologist 2020, 25, 787–792. [Google Scholar] [CrossRef] [PubMed]
- Kratochwil, C.; Giesel, F.L.; Stefanova, M.; Benešová, M.; Bronzel, M.; Afshar-Oromieh, A.; Mier, W.; Eder, M.; Kopka, K.; Haberkorn, U. PSMA-targeted radionuclide therapy of metastatic castration-resistant prostate cancer with 177Lu-labeled PSMA-617. J. Nucl. Med. 2016, 57, 1170–1176. [Google Scholar] [CrossRef] [Green Version]
- Fanti, S.; Goffin, K.; Hadaschik, B.A.; Herrmann, K.; Maurer, T.; MacLennan, S.; Oprea-Lager, D.E.; Oyen, W.J.; Rouvière, O.; Mottet, N.; et al. Consensus statements on PSMA PET/CT response assessment criteria in prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 469–476. [Google Scholar] [CrossRef]
- Scher, H.I.; Morris, M.J.; Stadler, W.M.; Higano, C.; Basch, E.; Fizazi, K.; Antonarakis, E.S.; Beer, T.M.; Carducci, M.A.; Chi, K.N.; et al. Trial design and objectives for castration-resistant prostate cancer: Updated recommendations from the prostate cancer clinical trials working group 3. J. Clin. Oncol. 2016, 34, 1402–1418. [Google Scholar] [CrossRef] [Green Version]
- Eiber, M.; Herrmann, K.; Calais, J.; Hadaschik, B.; Giesel, F.L.; Hartenbach, M.; Hope, T.; Reiter, R.; Maurer, T.; Weber, W.A.; et al. Prostate cancer molecular imaging standardized evaluation (PROMISE): Proposed MiTNM classification for the interpretation of PSMA-ligand PET/CT. J. Nucl. Med. 2018, 59, 469–478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yordanova, A.; Linden, P.; Hauser, S.; Meisenheimer, M.; Kürpig, S.; Feldmann, G.; Gaertner, F.C.; Essler, M.; Ahmadzadehfar, H. Outcome and safety of rechallenge [177Lu]Lu-PSMA-617 in patients with metastatic prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 1073–1080. [Google Scholar] [CrossRef] [PubMed]
- Groener, D.; Nguyen, C.T.; Baumgarten, J.; Bockisch, B.; Davis, K.; Happel, C.; Mader, N.; Ngoc, C.N.; Wichert, J.; Banek, S.; et al. Hematologic safety of 177Lu-PSMA-617 radioligand therapy in patients with metastatic castration-resistant prostate cancer. EJNMMI Res. 2021, 11, 61. [Google Scholar] [CrossRef]
- Seifert, R.; Kessel, K.; Schlack, K.; Weckesser, M.; Bögemann, M.; Rahbar, K. Radioligand therapy using [177Lu]Lu-PSMA-617 in MCRPC: A pre-VISION single-center analysis. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 2106–2112. [Google Scholar] [CrossRef] [Green Version]
- Ahmadzadehfar, H.; Rahbar, K.; Baum, R.P.; Seifert, R.; Kessel, K.; Bögemann, M.; Kulkarni, H.R.; Zhang, J.; Gerke, C.; Fimmers, R.; et al. Prior therapies as prognostic factors of overall survival in metastatic castration-resistant prostate cancer patients treated with [177Lu]Lu-PSMA-617. A WARMTH multicenter study (the 617 Trial). Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 113–122. [Google Scholar] [CrossRef]
- Khreish, F.; Ghazal, Z.; Marlowe, R.J.; Rosar, F.; Sabet, A.; Maus, S.; Linxweiler, J.; Bartholomä, M.; Ezziddin, S. 177 Lu-PSMA-617 radioligand therapy of metastatic castration-resistant prostate cancer: Initial 254-patient results from a prospective registry (REALITY study). Eur. J. Nucl. Med. Mol. Imaging 2021, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Heck, M.M.; Tauber, R.; Schwaiger, S.; Retz, M.; D’Alessandria, C.; Maurer, T.; Gafita, A.; Wester, H.-J.; Gschwend, J.E.; Weber, W.A.; et al. Treatment outcome, toxicity, and predictive factors for radioligand therapy with 177Lu-PSMA-I&T in metastatic castration-resistant prostate cancer. Eur. Urol. 2018, 75, 920–926. [Google Scholar] [CrossRef] [PubMed]
- Hofman, M.S.; Emmett, L.; Sandhu, S.; Iravani, A.; Joshua, A.M.; Goh, J.C.; Pattison, D.A.; Tan, T.H.; Kirkwood, I.D.; Ng, S.; et al. [177Lu]Lu-PSMA-617 versus cabazitaxel in patients with metastatic castration-resistant prostate cancer (TheraP): A randomised, open-label, phase 2 trial. Lancet 2021, 397, 797–804. [Google Scholar] [CrossRef]
- Groener, D.; Baumgarten, J.; Haefele, S.; Happel, C.; Klimek, K.; Mader, N.; Ngoc, C.N.; Tselis, N.; Chun, F.K.H.; Grünwald, F.; et al. Salvage radioligand therapy with repeated cycles of 177Lu-PSMA-617 in metastatic castration-resistant prostate cancer with diffuse bone marrow involvement. Cancers 2021, 13, 4017. [Google Scholar] [CrossRef] [PubMed]
- Mader, N.; Groener, D.; Tselis, N.; Banek, S.; Nagarajah, J.; Grünwald, F.; Sabet, A. Outcome of 177Lu-PSMA-617 radioligand therapy in chemo-refractory patients with metastatic castration-resistant early-onset prostate cancer. Cancers 2021, 13, 4193. [Google Scholar] [CrossRef]
- Bruland, Ø.S.; Nilsson, S.; Fisher, D.R.; Larsen, R.H. High-linear energy transfer irradiation targeted to skeletal metastases by the α-Emitter 223Ra: Adjuvant or alternative to conventional modalities? Clin. Cancer Res. 2006, 12, 6250s–6257s. [Google Scholar] [CrossRef] [Green Version]
- Rahbar, K.; Ahmadzadehfar, H.; Kratochwil, C.; Haberkorn, U.; Schäfers, M.; Essler, M.; Baum, R.P.; Kulkarni, H.R.; Schmidt, M.; Drzezga, A.; et al. German multicenter study investigating 177Lu-PSMA-617 radioligand therapy in advanced prostate cancer patients. J. Nucl. Med. 2017, 58, 85–90. [Google Scholar] [CrossRef] [Green Version]
- Barber, T.W.; Singh, A.; Kulkarni, H.R.; Niepsch, K.; Billah, B.; Baum, R.P. Clinical outcomes of 177Lu-PSMA radioligand therapy in earlier and later phases of metastatic castration-resistant prostate cancer grouped by previous taxane chemotherapy. J. Nucl. Med. 2019, 60, 955–962. [Google Scholar] [CrossRef] [Green Version]
- Ahmadzadehfar, H.; Matern, R.; Baum, R.P.; Seifert, R.; Kessel, K.; Bögemann, M.; Kratochwil, C.; Rathke, H.; Ilhan, H.; Svirydenka, H.; et al. The impact of the extent of the bone involvement on overall survival and toxicity in MCRPC patients receiving [177Lu]Lu-PSMA-617: A WARMTH multicentre study. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 4067–4076. [Google Scholar] [CrossRef] [PubMed]
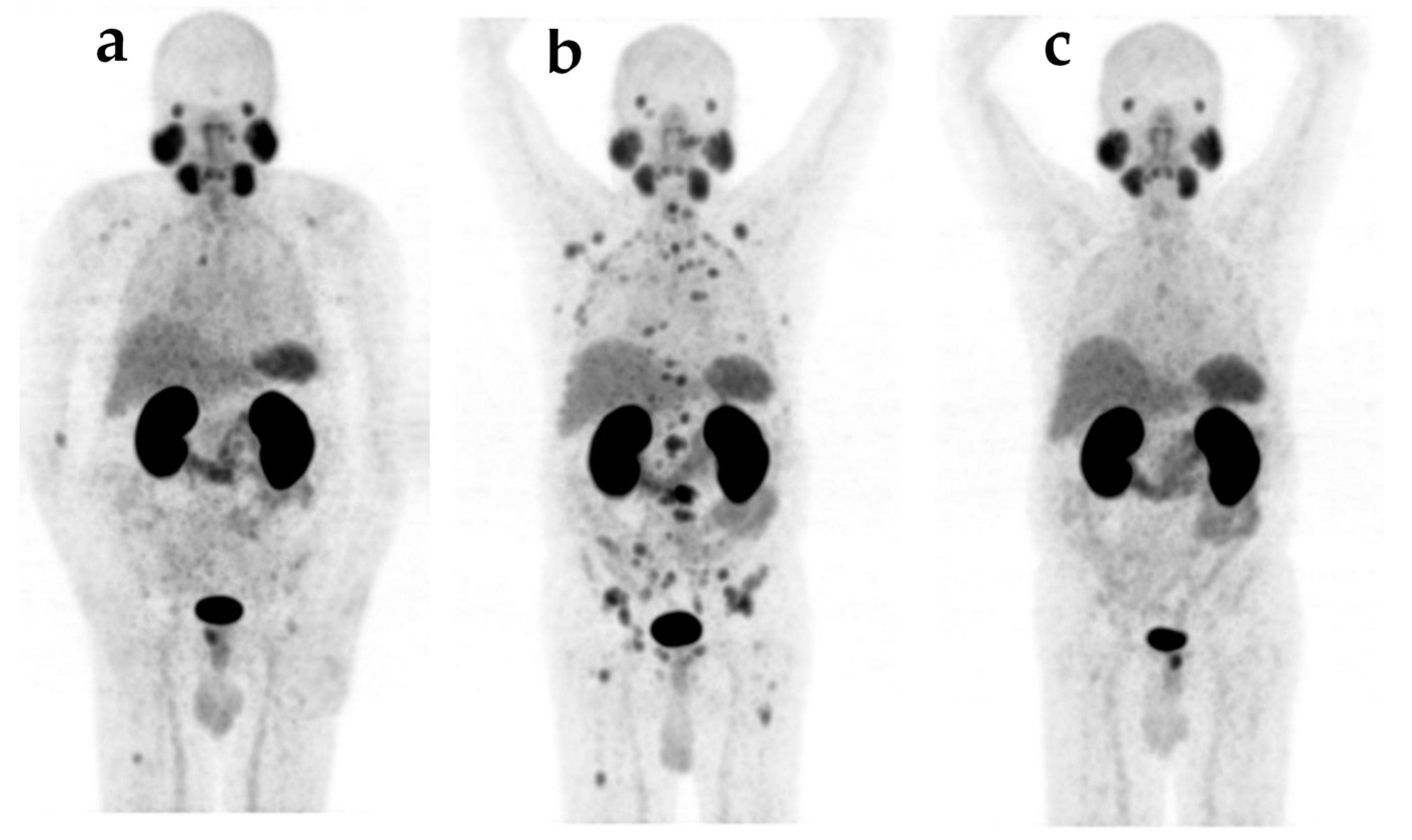
| Treatment | Prior to 223Ra | After 177Lu-PSMA-617 |
|---|---|---|
| Abiraterone | 16 (57) | 4 (14) |
| Enzalutamide | 10 (36) | 5 (18) |
| Docetaxel | 9 (32) | 8 (29) |
| Cabazitaxel | 0 (0) | 1 (3) |
| Re-treatment 177Lu-PSMA-617 | - | 7 (25) |
| Variable | Before 223Ra | Before RLT |
|---|---|---|
| PSA (ng/mL) | 35.2 (15.9–147) | 161 (76–336) |
| Hemoglobin (g/L) | 13.6 (12.6–14.3) | 12.1 (11.2–13.3) |
| White blood cells (109/L) | 5.5 (4.6–7.4) | 5.2 (4.0–7.0) |
| Platelets (109/L) | 192 (224–276) | 189 (169–240) |
| Sites of metastases | ||
| Bone | ||
| -oligofocal/multifocal | 18 (64) | 16 (57) |
| -disseminiated/diffuse | 10 (36) | 12 (43) |
| Local recurrence | 3 (11) | 8 (29) |
| Lymph nodes | 16 (57) | 20 (71) |
| Visceral | 0 (0) | 3 (11) |
| Toxicity | Prior to 223Ra (Grade) | Prior to RLT (Grade) | Post RLT (Grade) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 1 | 2 | 3 | 4 | 1 | 2 | 3 | 4 | |
| Anemia | 13 (46) | 1 (4) | 0 (0) | 0 (0) | 23 (82) | 1 (3) | 0 (0) | 0 (0) | 15 (54) | 8 (29) | 5 (18) | 0 (0) |
| Leukopenia | 1 (4) | 0 (0) | 0 (0) | 0 (0) | 5 (18) | 2 (7) | 0 (0) | 0 (0) | 6 (21) | 4 (18) | 3 (11) | 1 (4) |
| Thrombocytopenia | 0 (0) | 1 (4) | 0 (0) | 0 (0) | 4 (14) | 0 (0) | 0 (0) | 0 (0) | 12 (43) | 0 (0) | 2 (7) | 4 (14) |
| Blood Parameter | Prior to 223Ra | Prior to RLT | Lowest Post RLT | Follow-Up |
|---|---|---|---|---|
| Hb (g/L) | 13.6 ± 1.4 | 12.1 ± 1.4 | 10.1 ± 2.1 | 10.8 ± 2.1 |
| WBC (109/L) | 5.5 ± 1.8 | 5.2 ± 1.9 | 4.0 ± 1.6 | 4.6 ± 1.9 |
| Plt (109/L) | 224 ± 64 | 189 ± 51 | 125 ± 74 | 128 ± 88 |
| Patient | Previous Therapies after Castration Resistance | Toxicity (CTCmax) | Course of Treatment/Disease after RLT | ||
|---|---|---|---|---|---|
| Hb | WBC | Plt | |||
| 1 | ENZA, ABI, 223Ra | 3 | 4 | 4 | transfusion (RBC, BP), PD, death 15 weeks after last cycle |
| 2 | DOCE, ABI, 223Ra | 3 | 4 | 4 | transfusion (RBC, BP), PD, death 18 weeks after last cycle |
| 3 | ABI, 223Ra | 3 | 3 | 4 | transfusion (RBC, BP), PD, death 15 weeks after last cycle |
| 4 | 223Ra | 3 | 3 | 4 | transfusion (RBC, BP), PD, DOCE, death 60 weeks after last cycle |
| 5 | DOCE, ABI, ENZA, 223Ra | 2 | 3 | 3 | transfusion (RBC), PD, death 14 weeks after last cycle |
| 6 | ENZA, 223Ra | 3 | 3 | 2 | transfusion (RBC, BP), PD, Re-ENZA, ABI, death 48 weeks after last cycle |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baumgarten, J.; Groener, D.; Nguyen Ngoc, C.; Mader, N.; Chaurasia, M.; Davis, K.; Wichert, J.; Chun, F.K.H.; Tselis, N.; Happel, C.; et al. Safety and Efficacy of 177Lutetium-PSMA-617 Radioligand Therapy Shortly after Failing 223Radium-Dichloride. Cancers 2022, 14, 557. https://doi.org/10.3390/cancers14030557
Baumgarten J, Groener D, Nguyen Ngoc C, Mader N, Chaurasia M, Davis K, Wichert J, Chun FKH, Tselis N, Happel C, et al. Safety and Efficacy of 177Lutetium-PSMA-617 Radioligand Therapy Shortly after Failing 223Radium-Dichloride. Cancers. 2022; 14(3):557. https://doi.org/10.3390/cancers14030557
Chicago/Turabian StyleBaumgarten, Justus, Daniel Groener, Christina Nguyen Ngoc, Nicolai Mader, Maximilian Chaurasia, Karen Davis, Jennifer Wichert, Felix K. H. Chun, Nikolaos Tselis, Christian Happel, and et al. 2022. "Safety and Efficacy of 177Lutetium-PSMA-617 Radioligand Therapy Shortly after Failing 223Radium-Dichloride" Cancers 14, no. 3: 557. https://doi.org/10.3390/cancers14030557
APA StyleBaumgarten, J., Groener, D., Nguyen Ngoc, C., Mader, N., Chaurasia, M., Davis, K., Wichert, J., Chun, F. K. H., Tselis, N., Happel, C., Grünwald, F., & Sabet, A. (2022). Safety and Efficacy of 177Lutetium-PSMA-617 Radioligand Therapy Shortly after Failing 223Radium-Dichloride. Cancers, 14(3), 557. https://doi.org/10.3390/cancers14030557






