Exact Primary Tumor Location in mCRC: Prognostic Value and Predictive Impact on Anti-EGFR mAb Efficacy
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Trials
2.2. Patients
2.3. Primary Tumor Location
2.4. Treatment
2.5. Definition of Overall Survival and Objective Response Rate
2.6. Statistical Analysis
3. Results
3.1. Patient and Tumor Characteristics
3.2. Objective Response Rates According to Primary Tumor Location
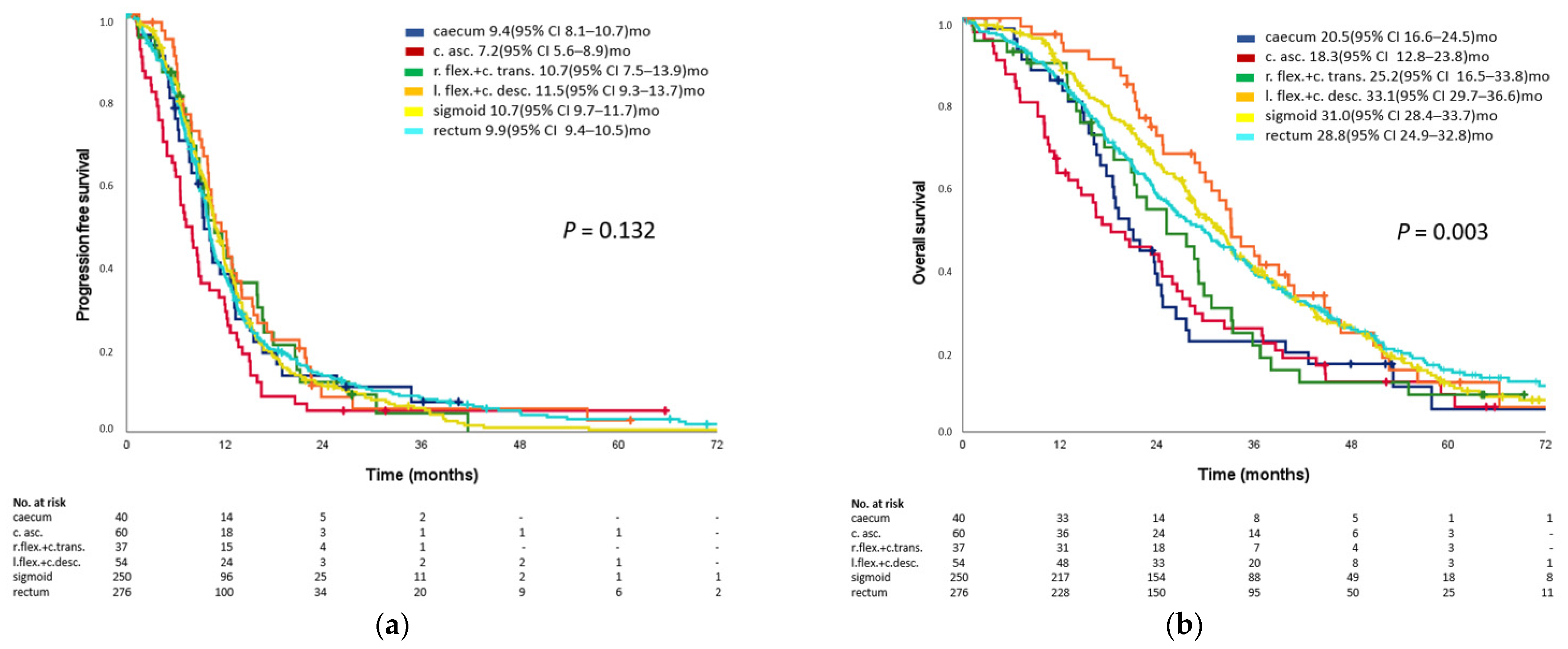
3.3. Prognostic Impact of Primary Tumor Location
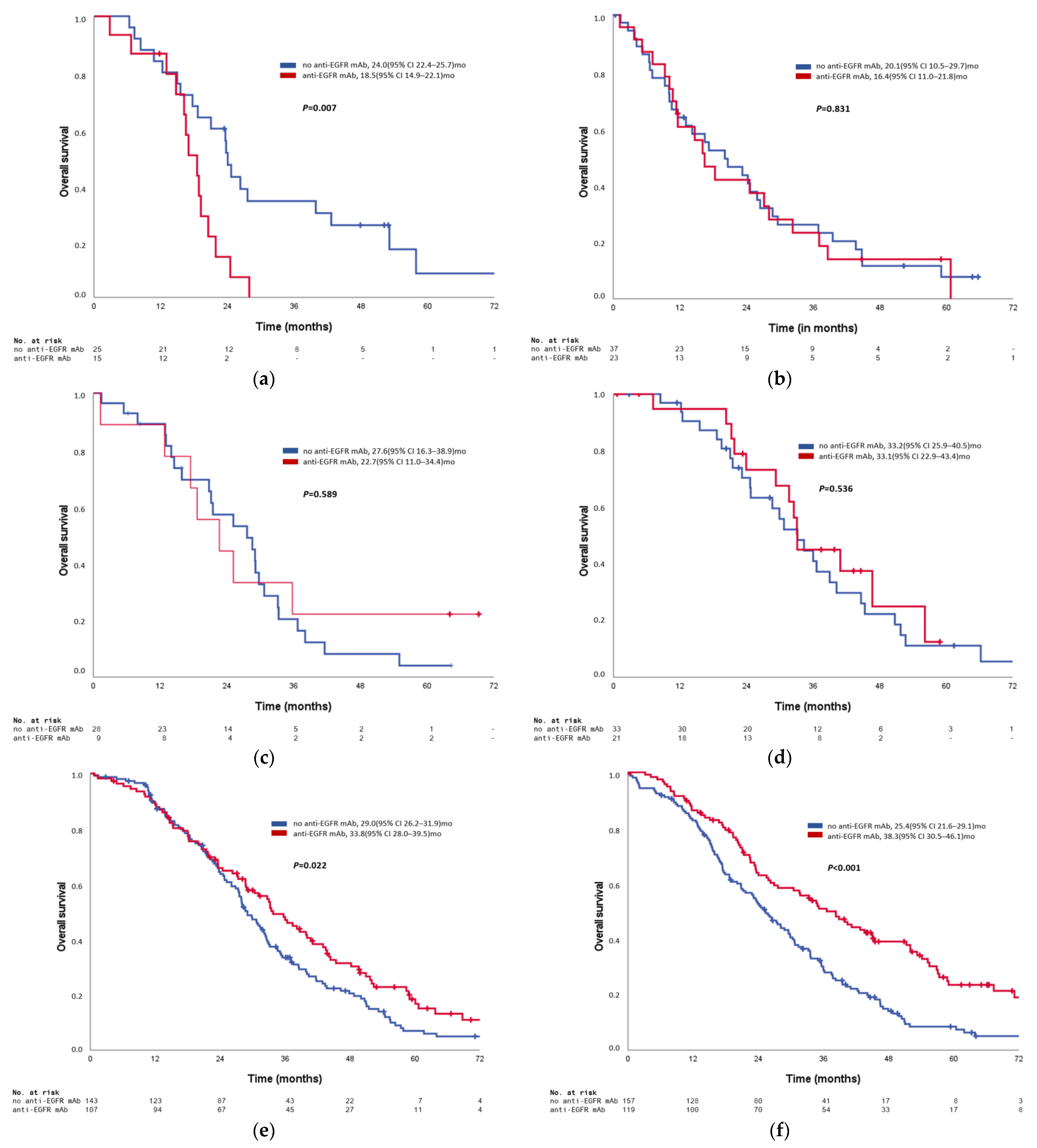
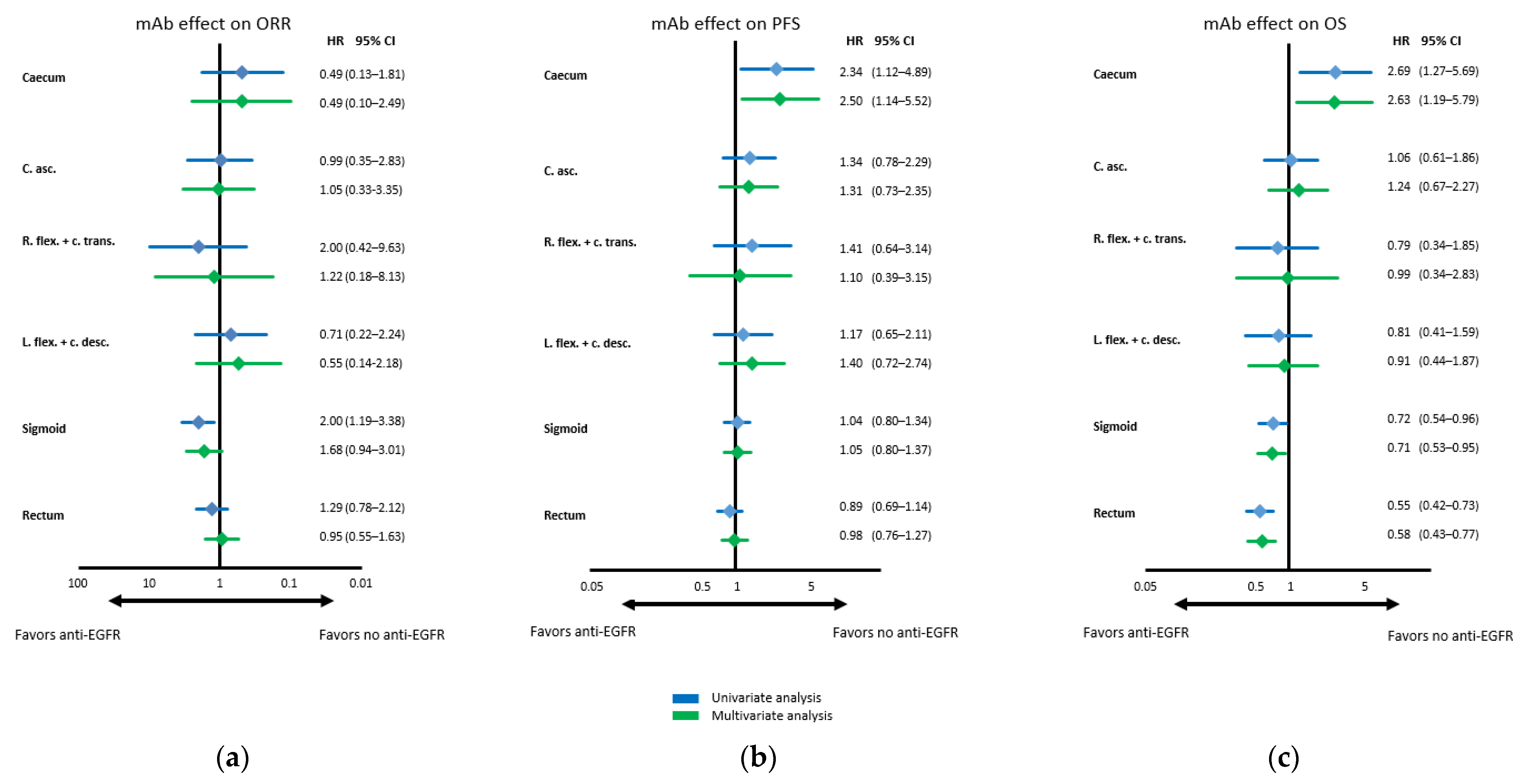
3.4. Predictive Impact on Anti-EGFR Antibody Efficacy of Primary Tumor Location
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Holch, J.W.; Ricard, I.; Stintzing, S.; Modest, D.P.; Heinemann, V. The relevance of primary tumour location in patients with metastatic colorectal cancer: A meta-analysis of first-line clinical trials. Eur. J. Cancer 2017, 70, 87–98. [Google Scholar] [CrossRef]
- Arnold, D.; Lueza, B.; Douillard, J.Y.; Peeters, M.; Lenz, H.J.; Venook, A.; Heinemann, V.; Van Cutsem, E.; Pignon, J.P.; Tabernero, J.; et al. Prognostic and predictive value of primary tumour side in patients with RAS wild-type metastatic colorectal cancer treated with chemotherapy and EGFR directed antibodies in six randomized trials. Ann. Oncol. 2017, 28, 1713–1729. [Google Scholar] [CrossRef]
- Modest, D.P.; Schulz, C.; von Weikersthal, L.F.; Quietzsch, D.; von Einem, J.C.; Schalhorn, A.; Vehling-Kaiser, U.; Laubender, R.P.; Giessen, C.; Stintzing, S.; et al. Outcome of patients with metastatic colorectal cancer depends on the primary tumor site (midgut vs. hindgut): Analysis of the FIRE1-trial (FuFIRI or mIROX as first-line treatment). Anti-Cancer Drug 2014, 25, 212–218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tejpar, S.; Stintzing, S.; Ciardiello, F. Prognostic and Predictive Relevance of Primary Tumor Location in Patients with RAS Wild-Type Metastatic Colorectal Cancer: Retrospective Analyses of the CRYSTAL and FIRE-3 Trials. JAMA Oncol. 2017, 3, 1742. [Google Scholar] [CrossRef] [PubMed]
- Pietrantonio, F.; Petrelli, F.; Coinu, A.; Di Bartolomeo, M.; Borgonovo, K.; Maggi, C.; Cabiddu, M.; Iacovelli, R.; Bossi, I.; Lonati, V.; et al. Predictive role of BRAF mutations in patients with advanced colorectal cancer receiving cetuximab and panitumumab: A meta-analysis. Eur. J. Cancer 2015, 51, 587–594. [Google Scholar] [CrossRef]
- Fontana, E.; Nyamundanda, G.; Cunningham, D.; Tu, D.S.; Cheang, M.C.U.; Jonker, D.J.; Siu, L.L.; Sclafani, F.; Eason, K.; Ragulan, C.; et al. Intratumoral Transcriptome Heterogeneity Is Associated with Patient Prognosis and Sidedness in Patients With Colorectal Cancer Treated With Anti-EGFR Therapy From the CO.20 Trial. JCO Precis. Oncol. 2020, 4, 1152–1162. [Google Scholar] [CrossRef] [PubMed]
- Lenz, H.J.; Ou, F.S.; Venook, A.P.; Hochster, H.S.; Niedzwiecki, D.; Goldberg, R.M.; Mayer, R.J.; Bertagnolli, M.M.; Blanke, C.D.; Zemla, T.; et al. Impact of Consensus Molecular Subtype on Survival in Patients with Metastatic Colorectal Cancer: Results From CALGB/SWOG 80405 (Alliance). J. Clin. Oncol. 2019, 37, 1876–1885. [Google Scholar] [CrossRef]
- Stahler, A.; Stintzing, S.; von Einem, J.C.; Westphalen, C.B.; Heinrich, K.; Kramer, N.; Michl, M.; Modest, D.P.; von Weikersthal, L.F.; Decker, T.; et al. Single-nucleotide variants, tumour mutational burden and microsatellite instability in patients with metastatic colorectal cancer: Next-generation sequencing results of the FIRE-3 trial. Eur. J. Cancer 2020, 137, 250–259. [Google Scholar] [CrossRef]
- Stintzing, S.; Wirapati, P.; Lenz, H.J.; Neureiter, D.; von Weikersthal, L.F.; Decker, T.; Kiani, A.; Kaiser, F.; Al-Batran, S.; Heintges, T.; et al. Consensus molecular subgroups (CMS) of colorectal cancer (CRC) and first-line efficacy of FOLFIRI plus cetuximab or bevacizumab in the FIRE3 (AIO KRK-0306) trial. Ann. Oncol. 2019, 30, 1796–1803. [Google Scholar] [CrossRef] [Green Version]
- Yamauchi, M.; Morikawa, T.; Kuchiba, A.; Imamura, Y.; Qian, Z.R.; Nishihara, R.; Liao, X.Y.; Waldron, L.; Hoshida, Y.; Huttenhower, C.; et al. Assessment of colorectal cancer molecular features along bowel subsites challenges the conception of distinct dichotomy of proximal versus distal colorectum. Gut 2012, 61, 847–854. [Google Scholar] [CrossRef]
- Loree, J.M.; Pereira, A.A.L.; Lam, M.; Willauer, A.N.; Raghav, K.; Dasari, A.; Morris, V.K.; Advani, S.; Menter, D.G.; Eng, C.; et al. Classifying Colorectal Cancer by Tumor Location Rather than Sidedness Highlights a Continuum in Mutation Profiles and Consensus Molecular Subtypes. Clin. Cancer Res. 2018, 24, 1062–1072. [Google Scholar] [CrossRef] [Green Version]
- Von Weikersthal, L.F.; Schalhorn, A.; Stauch, M.; Quietzsch, D.; Maubach, P.A.; Lambertz, H.; Oruzio, D.; Schlag, R.; Weigang-Kohler, K.; Vehling-Kaiser, U.; et al. Phase III trial of irinotecan plus infusional 5-fluorouracil/folinic acid versus irinotecan plus oxaliplatin as first-line treatment of advanced colorectal cancer. Eur. J. Cancer 2011, 47, 206–214. [Google Scholar] [CrossRef]
- Heinemann, V.; von Weikersthal, L.F.; Decker, T.; Kiani, A.; Kaiser, F.; Al-Batran, S.E.; Heintges, T.; Lerchenmuller, C.; Kahl, C.; Seipelt, G.; et al. FOLFIRI plus cetuximab or bevacizumab for advanced colorectal cancer: Final survival and per-protocol analysis of FIRE-3, a randomised clinical trial. Br. J. Cancer 2021, 124, 587–594. [Google Scholar] [CrossRef]
- Heinemann, V.; von Weikersthal, L.F.; Decker, T.; Kiani, A.; Vehling-Kaiser, U.; Al-Batran, S.E.; Heintges, T.; Lerchenmuller, C.; Kahl, C.; Seipelt, G.; et al. FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab as first-line treatment for patients with metastatic colorectal cancer (FIRE-3): A randomised, open-label, phase 3 trial. Lancet Oncol. 2014, 15, 1065–1075. [Google Scholar] [CrossRef]
- Modest, D.P.; von Weikersthal, L.F.; Decker, T.; Vehling-Kaiser, U.; Uhlig, J.; Schenk, M.; Freiberg-Richter, J.; Peuser, B.; Denzlinger, C.; Reddemann, C.P.G.; et al. Sequential Versus Combination Therapy of Metastatic Colorectal Cancer Using Fluoropyrimidines, Irinotecan, and Bevacizumab: A Randomized, Controlled StudyXELAVIRI (AIO KRK0110). J. Clin. Oncol. 2019, 37, 22–32. [Google Scholar] [CrossRef] [Green Version]
- Modest, D.P.; Martens, U.M.; Riera-Knorrenschild, J.; Greeve, J.; Florschutz, A.; Wessendorf, S.; Ettrich, T.; Kanzler, S.; Norenberg, D.; Ricke, J.; et al. FOLFOXIRI Plus Panitumumab as First-Line Treatment of RAS Wild-Type Metastatic Colorectal Cancer: The Randomized, Open-Label, Phase II VOLFI Study (AIO KRK0109). J. Clin. Oncol. 2019, 37, 3401–3411. [Google Scholar] [CrossRef] [PubMed]
- Modest, D.P.; Jung, A.; Moosmann, N.; Laubender, R.P.; Giessen, C.; Schulz, C.; Haas, M.; Neumann, J.; Boeck, S.; Kirchner, T.; et al. The influence of KRAS and BRAF mutations on the efficacy of cetuximab-based first-line therapy of metastatic colorectal cancer: An analysis of the AIO KRK-0104-trial. Int. J. Cancer 2012, 131, 980–986. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moosmann, N.; von Weikersthal, L.F.; Stauch, M.; Hass, H.G.; Dietzfelbinger, H.; Oruzio, D.; Klein, S.; Zellmann, K.; Decker, T.; Schulze, M.; et al. Cetuximab Plus Capecitabine and Irinotecan Compared with Cetuximab Plus Capecitabine and Oxaliplatin As First-Line Treatment for Patients With Metastatic Colorectal Cancer: AIO KRK-0104-A Randomized Trial of the German AIO CRC Study Group. J. Clin. Oncol. 2011, 29, 1050–1058. [Google Scholar] [CrossRef]
- Stahler, A.; Heinemann, V.; Giessen-Jung, C.; Crispin, A.; Schalhorn, A.; Stintzing, S.; von Weikersthal, L.F.; Vehling-Kaiser, U.; Stauch, M.; Quietzsch, D.; et al. Influence of mRNA expression of epiregulin and amphiregulin on outcome of patients with metastatic colorectal cancer treated with 5-FU/LV plus irinotecan or irinotecan plus oxaliplatin as first-line treatment (FIRE 1-trial). Int. J. Cancer 2016, 138, 739–746. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stintzing, S.; von Weikersthal, L.F.; Decker, T.; Vehling-Kaiser, U.; Jager, E.; Heintges, T.; Stoll, C.; Giessen, C.; Modest, D.P.; Neumann, J.; et al. FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab as first-line treatment for patients with metastatic colorectal cancer-subgroup analysis of patients with KRAS: Mutated tumours in the randomised German AIO study KRK-0306. Ann. Oncol. 2012, 23, 1693–1699. [Google Scholar] [CrossRef]
- Stintzing, S.; Modest, D.P.; Rossius, L. FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab for metastatic colorectal cancer (FIRE-3): A post-hoc analysis of tumour dynamics in the final RAS wild-type subgroup of this randomised open-label phase 3 trial. Lancet Oncol. 2016, 17, E479. [Google Scholar] [CrossRef]
- Stintzing, S.; Miller-Phillips, L.; Modest, D.P.; von Weikersthal, L.F.; Decker, T.; Kiani, A.; Vehling-Kaiser, U.; Al-Batran, S.E.; Heintges, T.; Kahl, C.; et al. Impact of BRAF and RAS mutations on first-line efficacy of FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab: Analysis of the FIRE-3 (AIO KRK-0306) study. Eur. J. Cancer 2017, 79, 50–60. [Google Scholar] [CrossRef]
- Lee, K.H.; Chen, W.S.; Jiang, J.K.; Yang, S.H.; Wang, H.S.; Chang, S.C.; Lan, Y.T.; Lin, C.C.; Lin, H.H.; Huang, S.C.; et al. The efficacy of anti-EGFR therapy in treating metastatic colorectal cancer differs between the middle/low rectum and the left-sided colon. Br. J. Cancer 2021. [Google Scholar] [CrossRef]
- Cremolini, C.; Benelli, M.; Fontana, E.; Pagani, F.; Rossini, D.; Fuca, G.; Busico, A.; Conca, E.; Di Donato, S.; Loupakis, F.; et al. Benefit from anti-EGFRs in RAS and BRAF wild-type metastatic transverse colon cancer: A clinical and molecular proof of concept study. ESMO Open 2019, 4, e000489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khattak, M.A.; Martin, H.; Davidson, A.; Phillips, M. Role of First-Line Anti-Epidermal Growth Factor Receptor Therapy Compared with Anti-Vascular Endothelial Growth Factor Therapy in Advanced Colorectal Cancer: A Meta-Analysis of Randomized Clinical Trials. Clin. Colorectal Cancer 2015, 14, 81–90. [Google Scholar] [CrossRef]
- Giessen, C.; Laubender, R.P.; Ankerst, D.P.; Stintzing, S.; Modest, D.P.; Mansmann, U.; Heinemann, V. Progression-Free Survival as a Surrogate Endpoint for Median Overall Survival in Metastatic Colorectal Cancer: Literature-Based Analysis from 50 Randomized First-Line Trials. Clin. Cancer Res. 2013, 19, 225–235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franko, J.; Shi, Q.; Meyers, J.P.; Maughan, T.S.; Adams, R.A.; Seymour, M.T.; Saltz, L.; Punt, C.J.A.; Koopman, M.; Tournigand, C.; et al. Prognosis of patients with peritoneal metastatic colorectal cancer given systemic therapy: An analysis of individual patient data from prospective randomised trials from the Analysis and Research in Cancers of the Digestive System (ARCAD) database. Lancet Oncol. 2016, 17, 1709–1719. [Google Scholar] [CrossRef]
- Piessevaux, H.; Buyse, M.; Schlichting, M.; Van Cutsem, E.; Bokemeyer, C.; Heeger, S.; Tejpar, S. Use of Early Tumor Shrinkage to Predict Long-Term Outcome in Metastatic Colorectal Cancer Treated with Cetuximab. J. Clin. Oncol. 2013, 31, 3764–3775. [Google Scholar] [CrossRef]
- Douillard, J.Y.; Siena, S.; Peeters, M.; Koukakis, R.; Terwey, J.H.; Tabernero, J. Impact of early tumour shrinkage and resection on outcomes in patients with wild-type RAS metastatic colorectal cancer. Eur. J. Cancer 2015, 51, 1231–1242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stahler, A.; Stintzing, S.; Modest, D.P.; Ricard, I.; Giessen-Jung, C.; Kapaun, C.; Ivanova, B.; Kaiser, F.; von Weikersthal, L.F.; Moosmann, N.; et al. Amphiregulin Expression Is a Predictive Biomarker for EGFR Inhibition in Metastatic Colorectal Cancer: Combined Analysis of Three Randomized Trials. Clin. Cancer Res. 2020, 26, 6559–6567. [Google Scholar] [CrossRef] [PubMed]
- Seligmann, J.F.; Hatch, A.J.; Richman, S.D.; Elliott, F.; Jacobs, B.; Brown, S.; Hurwitz, H.; Barrett, J.H.; Quirk, P.; Nixon, A.B.; et al. Association of Tumor HER3 Messenger RNA Expression with Panitumumab Efficacy in Advanced Colorectal Cancer. JAMA Oncol. 2018, 4, 564–568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwartzberg, L.S.; Rivera, F.; Karthaus, M.; Fasola, G.; Canon, J.L.; Hecht, J.R.; Yu, H.; Oliner, K.S.; Go, W.Y. PEAK: A Randomized, Multicenter Phase II Study of Panitumumab Plus Modified Fluorouracil, Leucovorin, and Oxaliplatin (mFOLFOX6) or Bevacizumab Plus mFOLFOX6 in Patients With Previously Untreated, Unresectable, Wild-Type KRAS Exon 2 Metastatic Colorectal Cancer. J. Clin. Oncol. 2014, 32, 2240–2247. [Google Scholar] [CrossRef] [PubMed]
- Laurent-Puig, P.; Grisoni, M.L.; Heinemann, V.; Liebaert, F.; Neureiter, D.; Jung, A.; Montestruc, F.; Gaston-Mathe, Y.; Thiebaut, R.; Stintzing, S. Validation of miR-31-3p Expression to Predict Cetuximab Efficacy When Used as First-Line Treatment in RAS Wild-Type Metastatic Colorectal Cancer. Clin. Cancer Res. 2019, 25, 134–141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seligmann, J.F.; Elliott, F.; Richman, S.D.; Jacobs, B.; Hemmings, G.; Brown, S.; Barrett, J.H.; Tejpar, S.; Quirke, P.; Seymour, M.T. Combined Epiregulin and Amphiregulin Expression Levels as a Predictive Biomarker for Panitumumab Therapy Benefit or Lack of Benefit in Patients with RAS Wild-Type Advanced Colorectal Cancer. JAMA Oncol. 2016, 2, 633–642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| FIRE-1 | CIOX | FIRE-3 | XELAVIRI | VOLFI | |
|---|---|---|---|---|---|
| Phase of study | III | II | III | III | II |
| Country | Germany | Germany | Germany, Austria | Germany | Germany |
| No. of centers | 48 | 35 | 110, 6 | 82 | 21 |
| Recruiting period | 07/2000–10/2004 | 09/2004–12/2006 | 01/2007–09/2012 | 12/2010–04/2016 | 06/2011–01/2016 |
| Primary endpoint | PFS | ORR | ORR | TFS | ORR |
| OS censored in database | 12.9% | 19.8% | 12.7% | 15.2% | 34.4% * |
| Treatment arms | |||||
| Arm A | FUFIRI | CAPIRI + Cet | FOLFIRI + Cet | FP + Bev –> PD –> FP + Iri + Bev | mFOLFOXIRI + Pani |
| Arm B | mIROX | CAPOX + Cet | FOLFIRI + Bev | FP + Iri + Bev | FOLFOXIRI |
| Previous adj. chemotherapy allowed | yes (no TOP1 inh., no platinum) | yes (no TOP1 inh.) | yes | yes | yes |
| Required time between adj. chemotherapy and relapse | 6 months | 6 months | 6 months | 6 months | 6 months |
| RECIST version | - (WHO) | 1.0 | 1.0 | 1.1 | 1.1 |
| Trial finder registration | - | NCT00254137 | NCT004 33927 | NCT012 49638 | NCT013 28171 |
| Eligibility criteria | |||||
| Age (in years) | 18–75 | 18–75 | 18–75 | ≥18 | ≥18 |
| ECOG | - | - | ≤2 | ≤1 | ≤1 |
| Karnowsky | ≥70% | ≥70% | - | - | - |
| Induction | Escalation | ||
|---|---|---|---|
| XELAVIRI | Sequential therapy arm | CAP + Bev (q3w) CAP 1250 mg/m2 1-0-1 p.o.; days 1–14 + Bev 7.5 mg/kg/bw i.v.; day 1 | CAPIRI + Bev (q3w) CAP 800 mg/m2 1-0-1 p.o.; days 1–14 + Iri 200 mg/m2 i.v.; day 1 + Bev 7.5 mg/kg/bw i.v.; day 1 |
| FUFA + Bev (q2w) FA 400 mg/m2 i.v.; day 1 + 5-FU bolus 400 mg/m2 i.v.; day 1 + 5-FU 2400 mg/m2 over 46 h i.v.; starting day 1 + Bev 5 mg/kg/bw i.v.; day 1 | FOLFIRI + Bev (q2w) Iri 180 mg/m2 i.v.; day 1 + FA 400 mg/m2 i.v.; day 1 + 5-FU bolus 400 mg/m2 i.v.; day 1 + 5-FU 2400 mg/m2 over 46h i.v.; starting day 1 + Bev 5 mg/kg/bw i.v.; day 1 | ||
| Combination therapy arm | Induction | Intermittent de-escalation (in case of at least stable disease for more than six months) | |
| CAPIRI + Bev (q3w) or FOLFIRI + Bev (q2w) | CAP + Bev (q3w) or FUFA + Bev (q2w) | ||
| FIRE-1 | Arm A | FUFIRI (q7w) Iri 80 mg/m2 i.v.; day 1, 8, 15, 22, 29, 36, 43 + FA 500 mg/m2 i.v.; day 1, 8, 15, 22, 29, 36, 43 + 5-FU 2000 mg/m2 over 24 h i.v.; starting day 1, 8, 15, 22, 29, 36, 43 | |
| Arm B | mIROX (q7w) Iri 80 mg/m2 i.v.; day 1, 8, 15, 22, 29, 36, 43 + Ox 85 mg/m2 i.v.; days 1, 15, 29 | ||
| CIOX | Arm A | CAPIRI + Cet (q3w) CAPIRI + Cet 400 mg/m2 i.v. on day 1 of c1 or Cet 250 mg/m2 i.v.; day 8, 15; starting c2 day 1 | |
| Arm B | CAPOX + Cet (q3w) CAP 1000 mg/m2 1-0-1 p.o.; days 1–14 + Ox 130 mg/m2 i.v.; day 1 + Cet 400 mg/m2 i.v. on day 1 of c1 or Cet 250 mg/m2 i.v.; day 8, 15; starting c2 day 1 | ||
| FIRE-3 | Arm A | FOLFIRI + Cet (q2w) FOLFIRI + Cet 400 mg/m2 i.v. on day 1 of c1 or Cet 250 mg/m2 i.v.; day 8; starting c2 additionally on day 1 | |
| Arm B | FOLFIRI + Bev (q2w) | ||
| VOLFI | Arm A | mFOLFOXIRI + Pani (q2w) Pani 6 mg/kg/bw i.v.; day 1 + Iri 150 mg/m2 i.v.; day 1 + Ox 85 mg/m2 i.v.; day 1 + FA 200 mg/m2 i.v.; day 1 + 5-FU 3000 mg/m2 over 48h i.v.; starting day 1 | |
| Arm B | FOLFOXIRI (q2w) Iri 165 mg/m2 i.v.; day 1 + Ox 85 mg/m2 i.v.; day 1 + FA 200 mg/m2 i.v.; day 1 + 5-FU 3200 mg/m2 over 48 h i.v.; starting day 1 | ||
| A. Tumor characteristics: RAS/BRAF wild-type tumors, n = 717. | ||||||
| Characteristics | Caecum (n = 40) | C. asc. (n = 60) | R. flex. + c. trans. (n = 37) | L. flex. + c. desc. (n = 54) | Sigmoid (n = 250) | Rectum (n = 276) |
| Study | ||||||
| FIRE-1 | 6 (15.0%) | 7 (11.7%) | 2 (5.4%) | 11 (20.4%) | 31 (12.4%) | 30 (10.9%) |
| CIOX | 6 (15.0%) | 6 (10.0%) | 4 (10.8%) | 3 (5.6%) | 29 (11.6%) | 31 (11.2%) |
| FIRE-3 | 16 (40.0%) | 32 (53.3%) | 17 (50.0%) | 30 (55.6%) | 125 (50.0%) | 130 (47.1%) |
| XELAVIRI | 7 (17.5%) | 15 (25.0%) | 13 (35.1%) | 8 (14.8%) | 39 (12.4%) | 60 (21.7%) |
| VOLFI | 5 (12.5%) | 0 (0.0%) | 1 (2.7%) | 2 (3.7%) | 26 (10.4%) | 25 (9.1%) |
| Antibody | ||||||
| none | 8 (20.0%) | 7 (11.7%) | 3 (8.1%) | 11 (20.4%) | 41 (16.4%) | 35 (12.7%) |
| Anti-EGFR | 15 (37.5%) | 23 (38.3%) | 9 (24.3%) | 21 (38.9%) | 107 (42.8%) | 119 (43.1%) |
| Anti-VEGF | 17 (42.5%) | 30 (50.0%) | 25 (67.6%) | 22 (40.7%) | 102 (40.8%) | 122 (44.2%) |
| Sex | ||||||
| Male | 25 (62.5%) | 42 (70.0%) | 23 (62.2%) | 35 (64.8%) | 182 (72.8%) | 210 (76.1%) |
| Female | 15 (37.5%) | 18 (30.0%) | 14 (37.8%) | 19 (35.2%) | 68 (27.2%) | 66 (23.9%) |
| Age (in years) | ||||||
| ≤60 | 11 (31.4%) | 20 (33.3%) | 5 (13.9%) | 15 (28.8%) | 76 (33.9%) | 89 (35.5%) |
| >60–≤70 | 10 (28.6%) | 20 (33.3%) | 18 (50.0%) | 22 (42.3%) | 91 (40.6%) | 101 (40.2%) |
| >70 | 14 (40.0%) | 20 (33.3%) | 13 (36.1%) | 15 (28.8%) | 57 (25.4%) | 61 (24.3%) |
| ECOG | ||||||
| 0 | 23 (57.5%) | 28 (46.7%) | 20 (54.1%) | 35 (64.8%) | 156(62.9%) | 180 (65.2%) |
| ≥1 | 17 (42.5%) | 32 (53.3%) | 17 (45.9%) | 19 (35.2%) | 92 (36.8%) | 95 (34.4%) |
| Unknown | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1 (0.4%) |
| Metastatic spread | ||||||
| Liver | 33 (82.5%) | 50 (83.3%) | 36 (97.3%) | 50 (92.6%) | 225 (90.0%) | 216 (78.3%) |
| Liver-limited | 12 (30.0%) | 20 (33.3%) | 15 (40.5%) | 29 (53.7%) | 99 (39.6%) | 86 (31.2%) |
| Lung | 12 (30.0%) | 23 (38.3%) | 11 (29.7%) | 13 (24.1%) | 75 (30.0%) | 113 (40.9%) |
| Peritoneum | 8 (20.0%) | 4 (6.7%) | 4 (10.8%) | 2 (3.7%) | 17 (6.8%) | 6 (2.2%) |
| No. of metastatic sites | ||||||
| 1 | 16 (40.0%) | 26 (43.3%) | 15 (40.5%) | 31 (57.4%) | 106(42.4%) | 106 (38.4%) |
| ≥2 | 19 (47.5%) | 33 (55.0%) | 21 (56.8%) | 20 (37.0%) | 117 (46.8%) | 143 (51.8%) |
| Unknown | 5 (12.5%) | 1 (1.7%) | 1 (2.7%) | 3 (5.6%) | 27 (10.8%) | 27 (9.8%) |
| Onset of metastases | ||||||
| Synchronous | 20 (50.0%) | 48 (80.0%) | 19 (51.4%) | 33 (61.1%) | 147 (58.8%) | 149 (54.0%) |
| Metachronous | 8 (20.0%) | 6 (10.0%) | 13 (35.1%) | 16 (29.6%) | 48 (19.2%) | 69 (25.0%) |
| Unknown | 12 (30.0%) | 6 (10.0%) | 5 (13.5%) | 5 (9.3%) | 55 (22.0%) | 58 (21.0%) |
| Previous chemotherapy | ||||||
| No | 32 (80.0%) | 57 (95.0%) | 31 (83.8%) | 47 (87.0%) | 214 (85.6%) | 188 (68.1%) |
| Yes | 8 (20.0%) | 3 (5.0%) | 6 (16.2%) | 7 (13.0%) | 36 (14.4%) | 87 (31.5%) |
| Unknown | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1(0.4%) |
| B. Tumor characteristics of patients with RAS/BRAF wild-type tumors treated with anti-EGFR targeted therapy, n = 294. | ||||||
| Characteristics | Caecum (n = 15) | C. asc. (n = 23) | R. flex. + c. trans. (n = 9) | L. flex. + c. desc. (n = 21) | Sigmoid (n = 107) | Rectum (n = 119) |
| Study | ||||||
| CIOX | 6 (40.0%) | 6 (26.1%) | 4 (44.4%) | 3 (14.3%) | 29 (27.1%) | 31 (26.1%) |
| FIRE-3 | 6 (40.0%) | 17 (73.9%) | 5 (55.6%) | 16 (76.2%) | 62 (57.9%) | 68 (57.1%) |
| VOLFI | 3 (20.0%) | 0 (0%) | 0 (0%) | 2 (9.5%) | 16 (15.0%) | 20 (16.8%) |
| Sex | ||||||
| Male | 6 (40.0%) | 16 (69.6%) | 6 (66.7%) | 12 (57.1%) | 81 (75.7%) | 92 (77.3%) |
| Female | 9 (60.0%) | 7 (30.4%) | 3 (33.3%) | 9 (42.9%) | 26 (24.3%) | 27 (22.7%) |
| Age (in years) | ||||||
| ≤60 | 4 (26.7%) | 6 (26.1%) | 4 (44.4%) | 6 (28.6%) | 40 (37.4%) | 43 (36.1%) |
| >60-≤70 | 6 (40.0%) | 10 (43.5%) | 3 (33.3%) | 9 (42.9%) | 31 (29.0%) | 38 (31.9%) |
| >70 | 2 (13.3%) | 7 (30.4%) | 2 (22.2%) | 4 (19.0%) | 20 (18.7%) | 18 (15.1%) |
| unknown | 3 (20.0%) | 0 (0.0%) | 0 (0.0%) | 2 (9.5%) | 16 (15.0%) | 20 (16.8%) |
| ECOG | ||||||
| 0 | 8 (53.3%) | 9 (%) | 5 (55.6%) | 15 (71.4%) | 69 (64.5%) | 83 (69.7%) |
| ≥1 | 7 (46.7%) | 14 (%) | 4 (44.4%) | 6 (28.6%) | 38 (35.5%) | 36 (30.3%) |
| Unknown | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | (0.0%) |
| Metastatic spread | ||||||
| Liver-limited * | 6 (40.0%) | 8 (34.8%) | 4 (44.4%) | 14 (66.7%) | 40 (37.4%) | 40 (33.6%) |
| Liver * | 14 (93.3%) | 19 (82.6%) | 9 (100%) | 20 (95.2%) | 96 (89.7%) | 101(84.9%) |
| Lung * | 3 (20.0%) | 10 (43.5%) | 2 (22.2%) | 3 (14.3%) | 33 (30.8%) | 45 (37.8%) |
| Peritoneum * | 2 (13.3%) | 2 (8.7%) | 1 (11.1%) | 1 (4.8%) | 1 (1.0%) | 3 (2.5%) |
| No. of metastatic sites | ||||||
| 1 | 6 (40.0%) | 11 (47.8%) | 4(44.4%) | 14 (66.7%) | 42 (39.3%) | 43 (36.1%) |
| ≥2 | 6 (40.0%) | 12 (52.2%) | 5 (55.6%) | 4 (19.0%) | 48 (44.9%) | 56 (47.1%) |
| Unknown | 3 (20.0%) | 0 (0.0%) | 0 (0.0%) | 1 (4.8%) | 17 (15.9%) | 20 (16.8%) |
| Onset of metastases | ||||||
| Synchronous | 4 (26.7%) | 15 (65.2%) | 4 (44.4%) | 11 (52.4%) | 51 (47.7%) | 47 (39.5%) |
| Metachronous | 2 (13.3%) | 2 (8.7%) | 1 (11.1%) | 5 (23.8%) | 11 (10.3%) | 21 (17.6%) |
| Unknown | 9 (40.0%) | 6 (26.1%) | 4 (44.4%) | 5 (23.8%) | 45 (42.1%) | 49 (41.2%) |
| Previous chemotherapy | ||||||
| No | 13 (86.7%) | 21 (91.3%) | 8 (88.9%) | 18 (85.7%) | 93 (86.9%) | 89 (74.8%) |
| Yes | 2 (13.3%) | 2 (8.7%) | 1 (11.1%) | 3 (14.3%) | 14 (13.1%) | 30 (25.2%) |
| Unknown | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| C. Tumor characteristics of patients with RAS/BRAF wild-type tumors not treated with anti-EGFR targeted therapy, n = 423. | ||||||
| Characteristics | Caecum (n = 25) | C. asc. (n = 37) | R. flex. + c. trans. (n = 28) | L. flex. + c. desc. (n = 28) | Sigmoid (n = 143) | Rectum (n = 157) |
| Study | ||||||
| FIRE-1 | 6 (24.0%) | 7 (18.9%) | 2 (7.1%) | 11 (33.3%) | 31 (21.7%) | 30 (19.1%) |
| FIRE-3 | 10 (40.0%) | 15 (40.5%) | 12 (42.9%) | 14 (42.4%) | 63 (44.1%) | 62 (39.5%) |
| XELAVIRI | 7 (28.0%) | 15 (40.5%) | 13 (46.4%) | 8 (24.2%) | 39 (27.3%) | 60 (38.2%) |
| VOLFI | 2 (8.0%) | 0 (0.0%) | 1 (3.6%) | 0 (0.0%) | 10 (7.0%) | 5 (3.2%) |
| Sex | ||||||
| Male | 19 (76.0%) | 26 (70.3%) | 17 (60.7%) | 23 (69.7%) | 101 (70.6%) | 118 (75.2%) |
| Female | 6 (24.0%) | 11 (29.7%) | 11 (39.3%) | 10 (30.3%) | 42 (29.4%) | 39 (24.8%) |
| Age (in years) | ||||||
| ≤60 | 7 (28.0%) | 14 (37.8%) | 1 (3.6%) | 9 (27.3%) | 36 (25.2%) | 46 (29.3%) |
| >60–≤70 | 4 (16.0%) | 10 (27.0%) | 15 (53.6%) | 13 (39.4%) | 60 (42.0%) | 63 (40.1%) |
| >70 | 12 (48.0%) | 13 (35.1%) | 11 (39.3%) | 11 (33.3%) | 37 (25.9%) | 43 (27.4%) |
| unknown | 2 (8.0%) | 0 (0.0%) | 1 (36%) | 0 (0.0%) | 10 (7.0%) | 5 (3.2%) |
| ECOG | ||||||
| 0 | 15 (60.0%) | 19 (51.4%) | 15 (53.6%) | 20 (60.6%) | 87 (60.8%) | 97 (61.8%) |
| ≥1 | 10 (40.0%) | 18 (48.6%) | 13 (46.4%) | 13 (39.4%) | 54 (37.8%) | 59 (37.6%) |
| Unknown | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 2 (1.4%) | 1 (0.6%) |
| Metastatic spread | ||||||
| Liver-limited * | 6 (24.0%) | 12 (32.4%) | 11 (39.3%) | 15 (45.5%) | 59 (41.3%) | 46 (29.3%) |
| Liver * | 19 (76.0%) | 31 (83.8%) | 27 (96.4%) | 30 (90.9%) | 129 (90.2%) | 115 (73.2%) |
| Lung * | 9 (36.0%) | 13 (35.1%) | 9 (32.1%) | 10 (30.3%) | 42 (29.4%) | 68 (43.3%) |
| Peritoneum * | 6 (24.0%) | 2 (5.4%) | 3 (10.7%) | 1 (3.0%) | 6 (4.2%) | 3 (1.9%) |
| No. of metastatic sites | ||||||
| 1 | 10 (40.0%) | 15 (40.5%) | 11 (39.3%) | 17 (51.5%) | 64 (44.8%) | 63 (40.1%) |
| ≥2 | 13 (52.0%) | 21 (56.8%) | 16 (57.1%) | 16 (48.5%) | 69 (48.3%) | 87 (55.4%) |
| Unknown | 2 (8.0%) | 1 (2.7%) | 1 (3.6%) | 0 (0.0%) | 10 (7.0%) | 7 (4.5%) |
| Onset of metastases | ||||||
| Synchronous | 16 (64.0%) | 33 (89.2%) | 23 (82.1%) | 29 (87.9%) | 121 (84.6%) | 99 (63.1%) |
| Metachronous | 6 (24.0%) | 4 (10.8%) | 5 (17.9%) | 4 (12.1%) | 22 (15.4%) | 57 (36.3%) |
| Unknown | 3 (12.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1 (0.6%) |
| Previous chemotherapy | ||||||
| No | 19 (76.0%) | 36 (97.3%) | 23 (82.1%) | 29 (87.9%) | 121 (84.6%) | 102 (65.0%) |
| Yes | 6 (24.0%) | 1 (2.7%) | 5 (17.9%) | 4 (12.1%) | 22 (15.4%) | 48 (30.6%) |
| Unknown | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 7 (4.5%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alig, A.H.S.; Heinemann, V.; Geissler, M.; Fischer von Weikersthal, L.; Decker, T.; Heinrich, K.; Held, S.; Weiss, L.; Fischer, L.E.; Moosmann, N.; et al. Exact Primary Tumor Location in mCRC: Prognostic Value and Predictive Impact on Anti-EGFR mAb Efficacy. Cancers 2022, 14, 526. https://doi.org/10.3390/cancers14030526
Alig AHS, Heinemann V, Geissler M, Fischer von Weikersthal L, Decker T, Heinrich K, Held S, Weiss L, Fischer LE, Moosmann N, et al. Exact Primary Tumor Location in mCRC: Prognostic Value and Predictive Impact on Anti-EGFR mAb Efficacy. Cancers. 2022; 14(3):526. https://doi.org/10.3390/cancers14030526
Chicago/Turabian StyleAlig, Annabel H. S., Volker Heinemann, Michael Geissler, Ludwig Fischer von Weikersthal, Thomas Decker, Kathrin Heinrich, Swantje Held, Lena Weiss, Laura E. Fischer, Nicolas Moosmann, and et al. 2022. "Exact Primary Tumor Location in mCRC: Prognostic Value and Predictive Impact on Anti-EGFR mAb Efficacy" Cancers 14, no. 3: 526. https://doi.org/10.3390/cancers14030526
APA StyleAlig, A. H. S., Heinemann, V., Geissler, M., Fischer von Weikersthal, L., Decker, T., Heinrich, K., Held, S., Weiss, L., Fischer, L. E., Moosmann, N., Stahler, A., Jelas, I., Kurreck, A., von Einem, J. C., Reinacher-Schick, A. C., Tannapfel, A., Giessen-Jung, C., Stintzing, S., & Modest, D. P. (2022). Exact Primary Tumor Location in mCRC: Prognostic Value and Predictive Impact on Anti-EGFR mAb Efficacy. Cancers, 14(3), 526. https://doi.org/10.3390/cancers14030526







