Preoperative Cancer Inflammation Prognostic Index as a Superior Predictor of Short- and Long-Term Outcomes in Patients with Stage I–III Colorectal Cancer after Curative Surgery
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Collection and Study Design
2.2. Cancer-Inflammation Prognostic Index (CIPI)
2.3. Measurement Outcomes
2.4. Statistics
3. Results
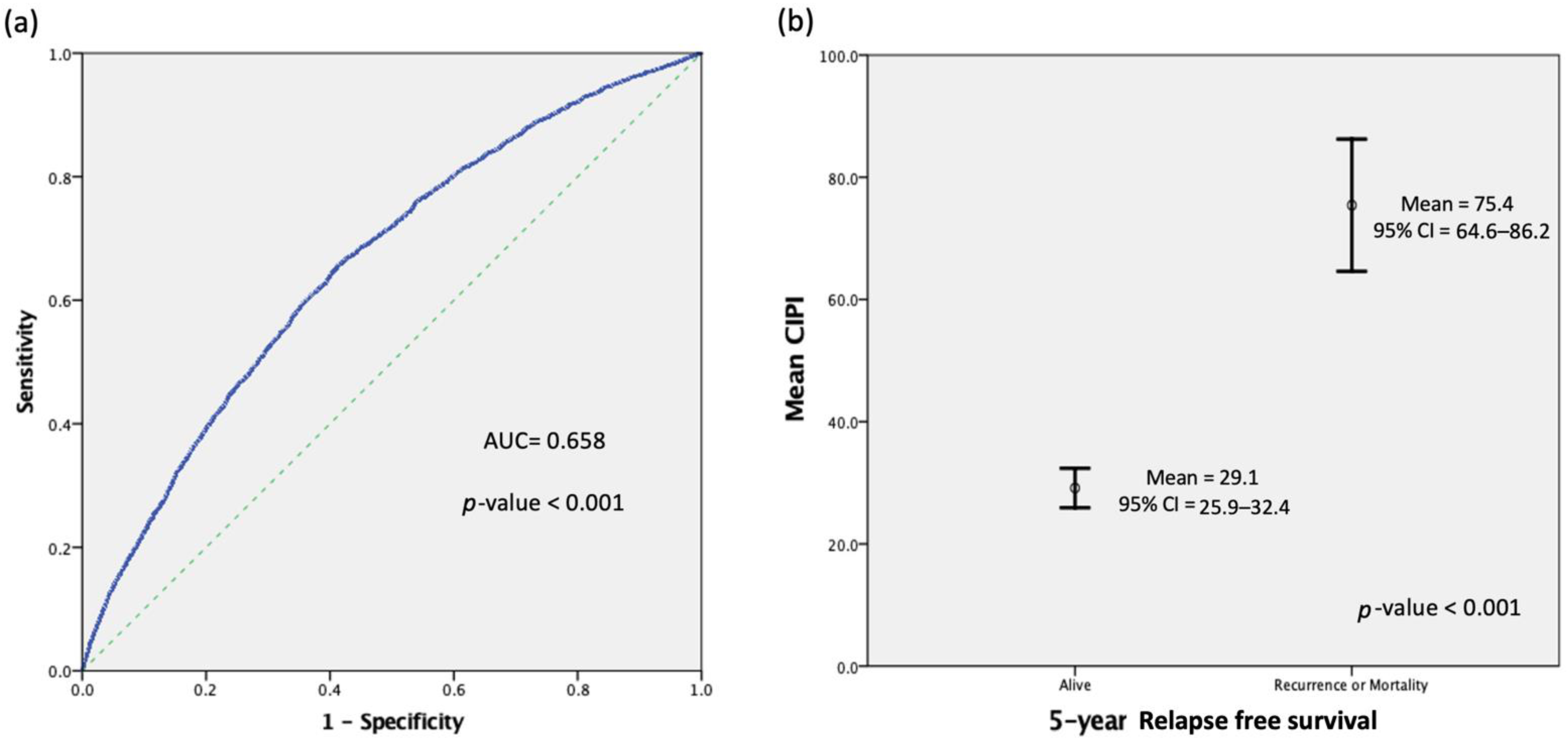
3.1. The CIPI Cutoff Level
3.2. CIPI and Clinicopathological Features
3.3. CIPI and Short- and Long-Term Outcomes
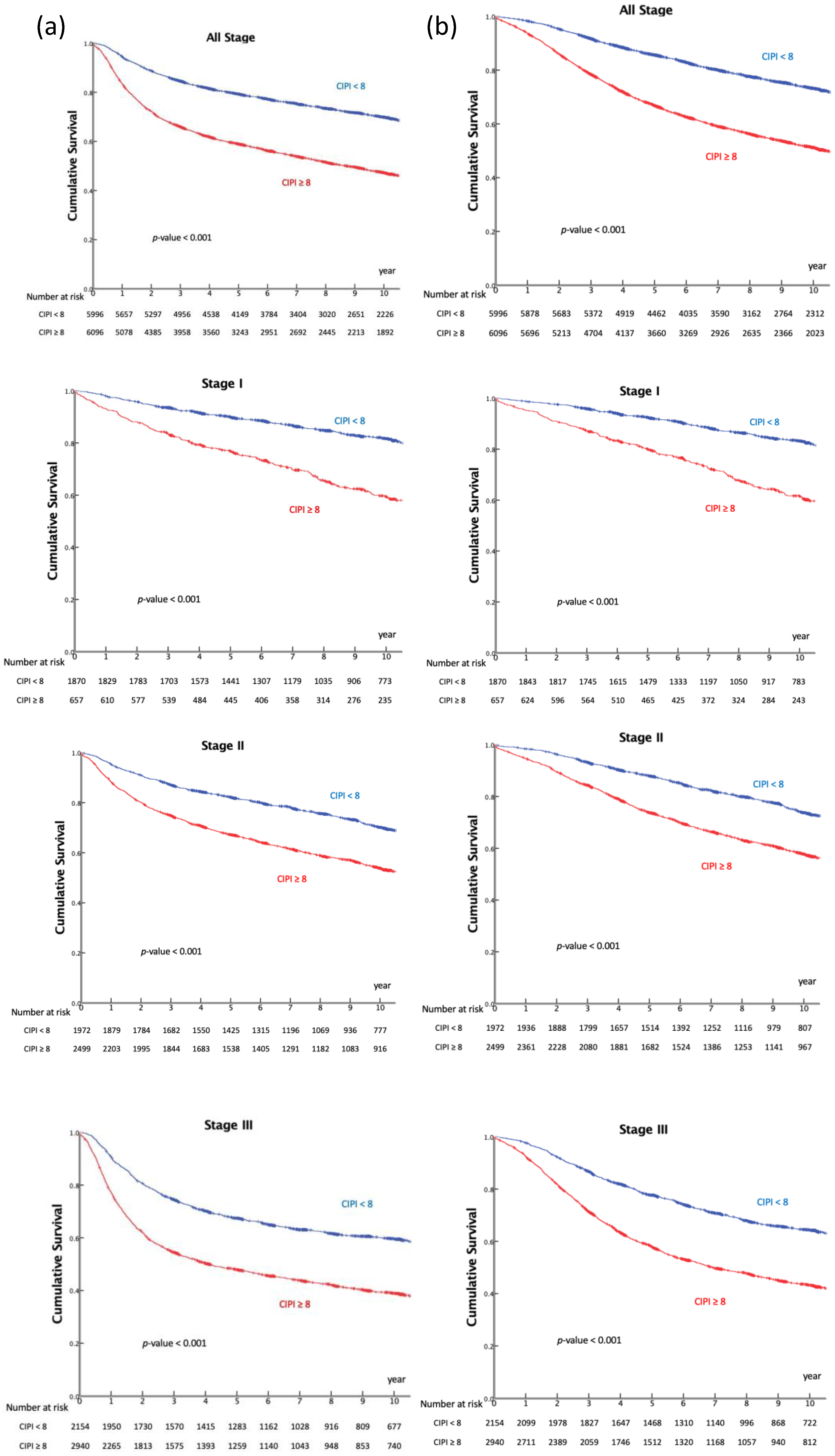
3.4. CIPI and Survival Analyses According to Tumor Stage
3.5. Univariate and Multivariate Analysis of RFS and OS
3.6. Subgroup Analyses of Survival Based on CIPI
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Iyengar, N.M.; Gucalp, A.; Dannenberg, A.J.; Hudis, C.A. Obesity and Cancer Mechanisms: Tumor Microenvironment and Inflammation. J. Clin. Oncol. 2016, 34, 4270–4276. [Google Scholar] [CrossRef] [PubMed]
- Balkwill, F.; Charles, K.A.; Mantovani, A. Smoldering and polarized inflammation in the initiation and promotion of malignant disease. Cancer Cell 2005, 7, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Murata, M. Inflammation and cancer. Environ. Health Prev Med. 2018, 23, 50. [Google Scholar] [CrossRef]
- Singh, N.; Baby, D.; Rajguru, J.P.; Patil, P.B.; Thakkannavar, S.S.; Pujari, V.B. Inflammation and cancer. Ann. Afr. Med. 2019, 18, 121–126. [Google Scholar] [CrossRef]
- Lucas, C.; Barnich, N.; Nguyen, H.T.T. Microbiota, Inflammation and Colorectal Cancer. Int. J. Mol. Sci. 2017, 18, 1310. [Google Scholar] [CrossRef] [PubMed]
- Tuomisto, A.E.; Mäkinen, M.J.; Väyrynen, J.P. Systemic inflammation in colorectal cancer: Underlying factors, effects, and prognostic significance. World J. Gastroenterol. 2019, 25, 4383–4404. [Google Scholar] [CrossRef] [PubMed]
- Lichtenstern, C.R.; Ngu, R.K.; Shalapour, S.; Karin, M. Immunotherapy, Inflammation and Colorectal Cancer. Cells 2020, 9, 618. [Google Scholar] [CrossRef]
- Yamamoto, T.; Kawada, K.; Obama, K. Inflammation-Related Biomarkers for the Prediction of Prognosis in Colorectal Cancer Patients. Int. J. Mol. Sci. 2021, 22, 8002. [Google Scholar] [CrossRef]
- Moutachakkir, M.; Hanchi, A.L.; Baraou, A.; Boukhira, A.; Chellak, S. Immunoanalytical characteristics of C-reactive protein and high sensitivity C-reactive protein. Ann. Biol. Clin. 2017, 75, 225–229. [Google Scholar] [CrossRef]
- Sproston, N.R.; Ashworth, J.J. Role of C-Reactive Protein at Sites of Inflammation and Infection. Front. Immunol. 2018, 9, 754. [Google Scholar] [CrossRef]
- Pathak, S.; Nunes, Q.; Daniels, I.; Smart, N.J. Is C-reactive protein useful in prognostication for colorectal cancer? A systematic review. Colorectal Dis. 2014, 16, 769–776. [Google Scholar] [CrossRef] [PubMed]
- Casadei Gardini, A.; Carloni, S.; Scarpi, E.; Maltoni, P.; Dorizzi, R.M.; Passardi, A.; Frassineti, G.L.; Cortesi, P.; Giannini, M.B.; Marisi, G.; et al. Prognostic role of serum concentrations of high-sensitivity C-reactive protein in patients with metastatic colorectal cancer: Results from the ITACa trial. Oncotarget 2016, 7, 10193–10202. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Xiang, S.; Dai, Z.; Zou, C.; Wang, X.; Gao, Z. Prognostic significance of C-reactive protein to albumin ratio in colorectal cancer patients: A meta-analysis. Int. J. Colorectal Dis. 2019, 34, 1105–1111. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.-K.; Yu, Y.-L.; Lin, Y.-C.; Hsu, Y.-J.; Chern, Y.-J.; Chiang, J.-M.; You, J.-F. Prognostic value of the C-reactive protein to albumin ratio in colorectal cancer: An updated systematic review and meta-analysis. World J. Surg. Oncol. 2021, 19, 139. [Google Scholar] [CrossRef]
- Pan, Y.; Lou, Y.; Wang, L. Prognostic value of C-reactive protein to albumin ratio in metastatic colorectal cancer: A systematic review and meta-analysis. Medicine 2021, 100, e27783. [Google Scholar] [CrossRef]
- Mik, M.; Dziki, L.; Berut, M.; Trzcinski, R.; Dziki, A. Neutrophil to Lymphocyte Ratio and C-Reactive Protein as Two Predictive Tools of Anastomotic Leak in Colorectal Cancer Open Surgery. Dig. Surg. 2017, 35, 77–84. [Google Scholar] [CrossRef]
- Duffy, M.; Lamerz, R.; Haglund, C.; Nicolini, A.; Kalousova, M.; Holubec, L.; Sturgeon, C. Tumor markers in colorectal cancer, gastric cancer and gastrointestinal stromal cancers: European group on tumor markers 2014 guidelines update. Int. J. Cancer 2013, 134, 2513–2522. [Google Scholar] [CrossRef]
- Das, V.; Kalita, J.; Pal, M. Predictive and prognostic biomarkers in colorectal cancer: A systematic review of recent advances and challenges. Biomed. Pharmacother. 2017, 87, 8–19. [Google Scholar] [CrossRef]
- Campos-Da-Paz, M.; Dórea, J.G.; Galdino, A.S.; Lacava, Z.G.M.; Santos, M.D.F.M.A. Carcinoembryonic Antigen (CEA) and Hepatic Metastasis in Colorectal Cancer: Update on Biomarker for Clinical and Biotechnological Approaches. Recent Patents Biotechnol. 2018, 12, 269–279. [Google Scholar] [CrossRef]
- Kim, J.Y.; Kim, N.K.; Sohn, S.K.; Kim, Y.W.; Kim, K.J.S.; Hur, H.; Min, B.S.; Cho, C.H. Prognostic Value of Postoperative CEA Clearance in Rectal Cancer Patients with High Preoperative CEA Levels. Ann. Surg. Oncol. 2009, 16, 2771–2778. [Google Scholar] [CrossRef]
- Treska, V.; Topolcan, O.; Stanislav, K.; Liska, V.; Holubec, L. Preoperative tumor markers as prognostic factors of colorectal liver metastases. Hepatogastroenterology 2009, 56, 317–320. [Google Scholar]
- Nesteruk, D.; Rutkowski, A.; Fabisiewicz, S.; Pawlak, J.; Siedlecki, J.A.; Fabisiewicz, A. Evaluation of Prognostic Significance of Circulating Tumor Cells Detection in Rectal Cancer Patients Treated with Preoperative Radiotherapy: Prospectively Collected Material Data. BioMed Res. Int. 2014, 2014, 712827. [Google Scholar] [CrossRef] [PubMed]
- Stillwell, A.P.; Ho, Y.-H.; Veitch\ud, C. Systematic Review of Prognostic Factors Related to Overall Survival in Patients with Stage IV Colorectal Cancer and Unresectable Metastases. World J. Surg. 2010, 35, 684–692. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.-Y.; Chen, J.-H.; Chien, C.-R.; Chen, W.T.-L.; Tsai, S.-C.; Lin, W.-Y.; Kao, C.-H. Use of FDG-PET or PET/CT to detect recurrent colorectal cancer in patients with elevated CEA: A systematic review and meta-analysis. Int. J. Colorectal Dis. 2013, 28, 1039–1047. [Google Scholar] [CrossRef] [PubMed]
- Cristaudo, A.T.; Jennings, S.B.; Morris, D.L. Comparison of Proportion of Elevated Carcinoembryonic Antigen Levels in Patients With Appendiceal and Colorectal Adenocarcinoma: A Systematic Review and Meta-analysis. Anticancer Res. 2022, 42, 4217–4235. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.-L.; Tsai, K.-L.; Chiu, T.-J.; Lin, Y.-M.; Lee, K.-C.; Lu, C.-C.; Chen, H.-H.; Wu, C.-C.; Hsu, H.-C. Development and Validation of a Novel Serum Prognostic Marker for Patients with Metastatic Colorectal Cancer on Regorafenib Treatment. Cancers 2021, 13, 5080. [Google Scholar] [CrossRef]
- Horino, T.; Tokunaga, R.; Miyamoto, Y.; Hiyoshi, Y.; Akiyama, T.; Daitoku, N.; Sakamoto, Y.; Yoshida, N.; Baba, H. The advanced lung cancer inflammation index is a novel independent prognosticator in colorectal cancer patients after curative resection. Ann. Gastroenterol. Surg. 2021, 6, 83–91. [Google Scholar] [CrossRef]
- Thompson, J.A.; Grunert, F.; Zimmermann, W. Carcinoembryonic antigen gene family: Molecular biology and clinical perspectives. J. Clin. Lab. Anal. 1991, 5, 344–366. [Google Scholar] [CrossRef] [PubMed]
- Dallas, M.R.; Liu, G.; Chen, W.; Thomas, S.N.; Wirtz, D.; Huso, D.L.; Konstantopoulos, K. Divergent roles of CD44 and carcinoembryonic antigen in colon cancer metastasis. FASEB J. 2012, 26, 2648–2656. [Google Scholar] [CrossRef]
- Alexiou, D.; Karayiannakis, A.; Syrigos, K.; Zbar, A.; Kremmyda, A.; Bramis, I.; Tsigris, C. Serum levels of E-selectin, ICAM-1 and VCAM-1 in colorectal cancer patients: Correlations with clinicopathological features, patient survival and tumour surgery. Eur. J. Cancer 2001, 37, 2392–2397. [Google Scholar] [CrossRef]
- Becerra, A.Z.; Probst, C.P.; Tejani, M.A.; Aquina, C.; González, M.G.; Hensley, B.J.; Noyes, K.; Monson, J.R.; Fleming, F. Evaluating the Prognostic Role of Elevated Preoperative Carcinoembryonic Antigen Levels in Colon Cancer Patients: Results from the National Cancer Database. Ann. Surg. Oncol. 2016, 23, 1554–1561. [Google Scholar] [CrossRef]
- Thirunavukarasu, P.; Talati, C.; Munjal, S.; Attwood, K.; Edge, S.B.; Francescutti, V. Effect of Incorporation of Pretreatment Serum Carcinoembryonic Antigen Levels Into AJCC Staging for Colon Cancer on 5-Year Survival. JAMA Surg. 2015, 150, 747–755. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.H.; Tsai, W.-S.; You, J.-F.; Hung, H.-Y.; Yeh, C.-Y.; Hsieh, P.-S.; Chiang, S.-F.; Lai, C.-C.; Chiang, J.-M.; Tang, R.; et al. Preoperative Carcinoembryonic Antigen as a Poor Prognostic Factor in Stage I–III Colorectal Cancer After Curative-Intent Resection: A Propensity Score Matching Analysis. Ann. Surg. Oncol. 2019, 26, 1685–1694. [Google Scholar] [CrossRef] [PubMed]
- Takagawa, R.; Fujii, S.; Ohta, M.; Nagano, Y.; Kunisaki, C.; Yamagishi, S.; Osada, S.; Ichikawa, Y.; Shimada, H. Preoperative Serum Carcinoembryonic Antigen Level as a Predictive Factor of Recurrence After Curative Resection of Colorectal Cancer. Ann. Surg. Oncol. 2008, 15, 3433–3439. [Google Scholar] [CrossRef]
- Kim, C.W.; Yoon, Y.S.; Park, I.J.; Lim, S.-B.; Yu, C.S.; Kim, J.C. Elevation of Preoperative s-CEA Concentration in Stage IIA Colorectal Cancer Can Also Be a High Risk Factor for Stage II Patients. Ann. Surg. Oncol. 2013, 20, 2914–2920. [Google Scholar] [CrossRef]
- Moreno García, V.; Cejas, P.; Blanco Codesido, M.; Feliu Batlle, J.; de Castro Carpeño, J.; Belda-Iniesta, C.; Barriuso, J.; Sánchez, J.J.; Larrauri, J.; González-Barón, M.; et al. Prognostic value of carcinoembryonic antigen level in rectal cancer treated with neoadjuvant chemoradiotherapy. Int. J. Colorectal. Dis. 2009, 24, 741–748. [Google Scholar] [CrossRef] [PubMed]
- Ruibal Morell, A. CEA serum levels in non-neoplastic disease. Int. J. Biol. Markers 1992, 7, 160–166. [Google Scholar] [CrossRef]
- Yang, Y.; Xu, M.; Huang, H.; Jiang, X.; Gong, K.; Liu, Y.; Kuang, X.; Yang, X. Serum carcinoembryonic antigen elevation in benign lung diseases. Sci. Rep. 2021, 11, 19044. [Google Scholar] [CrossRef]
- Bulut, I.; Arbak, P.; Coskun, A.; Balbay, O.; Annakkaya, A.; Yavuz, O.; Gulcan, E. Comparison of Serum CA 19.9, CA 125 and CEA Levels with Severity of Chronic Obstructive Pulmonary Disease. Med Princ. Pract. 2009, 18, 289–293. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, S.; Takeuchi, Y.; Arai, K.; Oishi, C.; Norose, T.; Yamochi-Onizuka, T.; Kushima, M.; Ota, H.; Imawari, M. Elevation of carcinoembryonic antigen coinciding with disease activity of ulcerative colitis. Clin. J. Gastroenterol. 2012, 5, 150–154. [Google Scholar] [CrossRef]
- Kang, H.Y.; Choe, E.K.; Park, K.J.; Lee, Y. Factors Requiring Adjustment in the Interpretation of Serum Carcinoembryonic Antigen: A Cross-Sectional Study of 18,131 Healthy Nonsmokers. Gastroenterol. Res. Pract. 2017, 2017, 9858931. [Google Scholar] [CrossRef] [PubMed]
- Dupré, A.; Malik, H.Z. Inflammation and cancer: What a surgical oncologist should know. Eur. J. Surg. Oncol. 2018, 44, 566–570. [Google Scholar] [CrossRef] [PubMed]
- Korniluk, A.; Koper, O.; Kemona, H.; Dymicka-Piekarska, V. From inflammation to cancer. Ir. J. Med. Sci. 2017, 186, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Waldman, A.D.; Fritz, J.M.; Lenardo, M.J. A guide to cancer immunotherapy: From T cell basic science to clinical practice. Nat. Rev. Immunol. 2020, 20, 651–668. [Google Scholar] [CrossRef] [PubMed]
- Herrero-Cervera, A.; Soehnlein, O.; Kenne, E. Neutrophils in chronic inflammatory diseases. Cell. Mol. Immunol. 2022, 19, 177–191. [Google Scholar] [CrossRef] [PubMed]
- Golder, A.M.; McMillan, D.C.; Park, J.H.; Mansouri, D.; Horgan, P.G.; Roxburgh, C.S. The prognostic value of combined measures of the systemic inflammatory response in patients with colon cancer: An analysis of 1700 patients. Br. J. Cancer 2021, 124, 1828–1835. [Google Scholar] [CrossRef]
- Hamid, H.K.; Emile, S.H.; Davis, G.N. Prognostic Significance of Lymphocyte-to-Monocyte and Platelet-to-Lymphocyte Ratio in Rectal Cancer: A Systematic Review, Meta-analysis, and Meta-regression. Dis. Colon Rectum 2021, 65, 178–187. [Google Scholar] [CrossRef]
- Huang, X.; Huan, Y.; Liu, L.; Ye, Q.; Guo, J.; Yan, B. Preoperative low absolute lymphocyte count to fibrinogen ratio correlated with poor survival in nonmetastatic colorectal cancer. World J. Surg. Oncol. 2022, 20, 309. [Google Scholar] [CrossRef]
- Liu, X.-C.; Dai, Y.-L.; Huang, F.; Zhong, Z.-J. Diagnostic Value of Carcinoembryonic Antigen Combined with Multi-Inflammatory Cell Ratios in Colorectal Cancer. Dis. Markers 2022, 2022, 4889616. [Google Scholar] [CrossRef]
- Cheng, K.-C.; Lin, Y.-M.; Liu, C.-C.; Wu, K.-L.; Lee, K.-C. High Red Cell Distribution Width Is Associated with Worse Prognosis in Early Colorectal Cancer after Curative Resection: A Propensity-Matched Analysis. Cancers 2022, 14, 945. [Google Scholar] [CrossRef]
- Yin, X.; Wu, L.; Yang, H.; Yang, H. Prognostic significance of neutrophil-lymphocyte ratio (NLR) in patients with ovarian cancer: A systematic review and meta-analysis. Medicine 2019, 98, e17475. [Google Scholar] [CrossRef] [PubMed]
- Cupp, M.A.; Cariolou, M.; Tzoulaki, I.; Aune, D.; Evangelou, E.; Berlanga-Taylor, A.J. Neutrophil to lymphocyte ratio and cancer prognosis: An umbrella review of systematic reviews and meta-analyses of observational studies. BMC Med. 2020, 18, 360. [Google Scholar] [CrossRef] [PubMed]
- Jakubowska, K.; Koda, M.; Kisielewski, W.; Kańczuga-Koda, L.; Grudzińska, M.; Famulski, W. Pre- and postoperative neutrophil and lymphocyte count and neutrophil-to-lymphocyte ratio in patients with colorectal cancer. Mol. Clin. Oncol. 2020, 13, 56. [Google Scholar] [CrossRef]
- Mazaki, J.; Katsumata, K.; Kasahara, K.; Tago, T.; Wada, T.; Kuwabara, H.; Enomoto, M.; Ishizaki, T.; Nagakawa, Y.; Tsuchida, A. Neutrophil-to-lymphocyte ratio is a prognostic factor for colon cancer: A propensity score analysis. BMC Cancer 2020, 20, 922. [Google Scholar] [CrossRef]
- Dell’Aquila, E.; Cremolini, C.; Zeppola, T.; Lonardi, S.; Bergamo, F.; Masi, G.; Stellato, M.; Marmorino, F.; Schirripa, M.; Urbano, F.; et al. Prognostic and predictive role of neutrophil/lymphocytes ratio in metastatic colorectal cancer: A retrospective analysis of the TRIBE study by GONO. Ann. Oncol. 2018, 29, 924–930. [Google Scholar] [CrossRef] [PubMed]
- Hung, H.-Y.; Chen, J.-S.; Yeh, C.Y.; Changchien, C.-R.; Tang, R.; Hsieh, P.-S.; Tasi, W.-S.; You, J.-F.; You, Y.-T.; Fan, C.-W.; et al. Effect of preoperative neutrophil–lymphocyte ratio on the surgical outcomes of stage II colon cancer patients who do not receive adjuvant chemotherapy. Int. J. Colorectal Dis. 2011, 26, 1059–1065. [Google Scholar] [CrossRef] [PubMed]
- Chiang, S.F.; Hung, H.Y.; Tang, R.; Changchien, C.R.; Chen, J.S.; You, Y.T.; Chiang, J.M.; Lin, J.R. Can neutrophil-to-lymphocyte ratio predict the survival of colorectal cancer patients who have received curative surgery electively? Int. J. Colorectal Dis. 2012, 27, 1347–1357. [Google Scholar] [CrossRef] [PubMed]

| Variables | CIPI (n = 12,092) | p Values | |
|---|---|---|---|
| Low < 8 (n = 5996) | High ≥ 8 (n = 6096) | ||
| Age, mean ± SD | 62.10 ± 12.67 | 64.94 ± 13.12 | <0.001 |
| Age (years), n (%) | |||
| <65 | 3331 (55.6) | 2798 (45.9) | <0.001 |
| ≥65 | 2665 (44.4) | 3298 (54.1) | |
| Gender, n (%) | |||
| Male | 3267 (54.5) | 3581 (58.7) | <0.001 |
| Female | 2729 (45.5) | 2515 (41.3) | |
| Body mass index, kg/m2, n (%) | |||
| <25 | 3509 (59.3) | 3970 (67.4) | <0.001 |
| ≥25 | 2407 (40.7) | 1916 (32.6) | |
| missing | 80 | 210 | |
| Underlying illness, n (%) | |||
| Hypertension | 1978 (33.0) | 2045 (33.5) | 0.493 |
| Cardiovascular disease | 511 (8.5) | 550 (9.0) | 0.376 |
| Diabetes mellitus | 843 (14.1) | 1153 (18.9) | <0.001 |
| Peptic ulcer disease | 447 (7.5) | 458 (7.5) | 0.993 |
| Hepatitis | 326 (5.4) | 263 (4.3) | 0.006 |
| Chronic renal disease | 282 (4.8) | 586 (9.8) | <0.001 |
| Tumor location, n (%) | |||
| Colon | 3440 (57.4) | 3411 (56.0) | 0.119 |
| Rectum | 2556 (42.6) | 2685 (44.0) | |
| Tumor stage, n (%) | |||
| Stage I | 1870 (31.2) | 657 (10.8) | <0.001 |
| Stage II | 1972 (32.9) | 2499 (41.0) | |
| Stage III | 2154 (35.9) | 2940 (48.2) | |
| pT status, n (%) | |||
| T1 | 1129 (18.8) | 276 (4.5) | <0.001 |
| T2 | 1094 (18.2) | 518 (8.5) | |
| T3 | 2842 (47.4) | 3097 (50.8) | |
| T4 | 931 (15.5) | 22.5 (36.2) | |
| pN status, n (%) | |||
| N0 | 3842 (64.1) | 3153 (51.7) | <0.001 |
| N1 | 1394 (23.2) | 1738 (28.5) | |
| N2 | 760 (12.7) | 1205 (19.8) | |
| Histologic type, n (%) | |||
| Adenocarcinoma | 5733 (95.6) | 5611 (92.0) | <0.001 |
| Mucinous adenocarcinoma | 238 (4.0) | 432 (7.1) | |
| Signet ring cell adenocarcinoma | 25 (0.4) | 53 (0.9) | |
| Histologic grade, n (%) | |||
| Well differentiated | 1063 (17.7) | 767 (12.6) | <0.001 |
| Moderate differentiated | 4515 (75.3) | 4814 (79.0) | |
| Poor differentiated | 362 (6.0) | 498 (8.2) | |
| Unclassified | 56 (0.9) | 17 (0.3) | |
| Operative finding | |||
| Obstruction | 312 (5.2) | 814 (13.4) | <0.001 |
| Perforation | 70 (1.2) | 314 (5.2) | <0.001 |
| Preoperative laboratory values, n (%) | |||
| Hemoglobin (g/dL) | |||
| <10 | 743 (12.4) | 1429 (23.5) | <0.001 |
| ≥10 | 5247 (87.6) | 4663 (76.5) | |
| missing | 6 | 4 | |
| Albumin (g/dL) | |||
| <3.5 | 303 (5.1) | 1065 (17.9) | <0.001 |
| ≥3.5 | 5630 (94.9) | 4891 (82.1) | |
| missing | 63 | 140 | |
| Variables | CIPI (n = 12,092) | p Values | |
|---|---|---|---|
| Low < 8 (n = 5996) | High ≥ 8 (n = 6096) | ||
| Short-term postoperative outcome, n (%) | |||
| 30-day morbidity | 660 (11.0) | 1032 (16.9) | <0.001 |
| 30-day mortality | 14 (0.2) | 73 (1.2) | <0.001 |
| Long-term outcome, n (survival%) | |||
| Recurrence | 961 (16.0) | 1865 (30.6) | <0.001 |
| three-year RFS | 4956 (81.5) | 3958 (61.8) | <0.001 |
| three-year OS | 5372 (88.4) | 4704 (72.0) | <0.001 |
| five-year RFS | 4149 (77.3) | 3243 (56.2) | <0.001 |
| five-year OS | 4462 (82.9) | 3660 (62.7) | <0.001 |
| 10-year RFS | 2226 (68.2) | 1892 (45.5) | <0.001 |
| 10-year OS | 2312 (71.5) | 2023 (49.1) | <0.001 |
| Characteristics | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p Values | HR | 95% CI | p Values | |
| Age | ||||||
| <65 | 1 | 1 | ||||
| ≥65 | 1.814 | 1.711–1.923 | <0.001 | 1.59 | 1.49–1.696 | <0.001 |
| Gender | ||||||
| Female | 1 | 1 | ||||
| Male | 1.251 | 1.180–1.326 | <0.001 | 1.252 | 1.176–1.333 | <0.001 |
| Body mass index | ||||||
| <25 | 1 | 1 | ||||
| ≥25 | 0.877 | 0.825–0.933 | <0.001 | 0.937 | 0.877–1.001 | 0.053 |
| Underlying illness | ||||||
| Hypertension | 1.229 | 1.163–1.299 | <0.001 | 1.131 | 1.060–1.206 | <0.001 |
| Cardiovascular | 1.381 | 1.282–1.488 | <0.001 | 1.26 | 1.150–1.381 | <0.001 |
| Diabetes mellitus | 1.33 | 1.238–1.428 | <0.001 | 1.147 | 1.060–1.242 | 0.001 |
| Peptic ulcer | 1.053 | 0.958–1.158 | 0.282 | |||
| Hepatitis | 0.802 | 0.697–0.924 | 0.002 | 0.945 | 0.821–1.087 | 0.427 |
| Renal disease | 2.006 | 1.835–2.193 | <0.001 | 1.447 | 1.308–1.599 | <0.001 |
| Tumor location | ||||||
| Colon | 1 | 1 | ||||
| Rectum | 1.31 | 1.237–1.386 | <0.001 | 1.493 | 1.401–1.591 | <0.001 |
| Tumor stage | ||||||
| Stage I | 1 | |||||
| Stage II | 1.821 | 1.653–2.006 | <0.001 | |||
| Stage III | 3.008 | 2.743–3.300 | <0.001 | |||
| pT status | ||||||
| T1 | 1 | 1 | ||||
| T2 | 1.818 | 1.550–2.132 | <0.001 | 1.45 | 1.221–1.721 | <0.001 |
| T3 | 2.823 | 2.463–3.236 | <0.001 | 1.841 | 1.577–2.148 | <0.001 |
| T4 | 4.418 | 3.846–5.076 | <0.001 | 2.542 | 2.165–2.986 | <0.001 |
| pN status | ||||||
| N0 | 1 | 1 | ||||
| N1 | 1.602 | 1.498–1.714 | <0.001 | 1.46 | 1.357–1.572 | <0.001 |
| N2 | 2.777 | 2.587–2.980 | <0.001 | 2.421 | 2.236–2.621 | <0.001 |
| Histologic type | ||||||
| Adenocarcinoma | 1 | 1 | ||||
| Mucinous | 1.208 | 1.075–1.357 | 0.001 | 0.978 | 0.856–1.117 | 0.740 |
| Signet ring cell | 2.621 | 2.004–3.428 | <0.001 | 1.452 | 1.071–1.968 | 0.016 |
| Histologic grade | ||||||
| Well | 1 | 1 | ||||
| Moderate | 1.37 | 1.256–1.494 | <0.001 | 0.966 | 0.875–1.066 | 0.488 |
| Poor | 1.743 | 1.535–1.980 | <0.001 | 1.014 | 0.876–1.175 | 0.848 |
| Operative finding | ||||||
| Obstruction | 1.778 | 1.632–1.936 | <0.001 | 1.276 | 1.159–1.404 | <0.001 |
| Perforation | 1.769 | 1.540–2.033 | <0.001 | 1.145 | 0.980–1.337 | 0.087 |
| Preoperative values | ||||||
| Hemoglobin | ||||||
| <10 | 1 | 1 | ||||
| ≥10 | 0.657 | 0.614–0.703 | <0.001 | 0.873 | 0.807–0.945 | <0.001 |
| Albumin | ||||||
| <3.5 | 1 | 1 | ||||
| ≥3.5 | 0.471 | 0.438–0.508 | <0.001 | 0.658 | 0.601–0.720 | <0.001 |
| CIPI | ||||||
| <8 | 1 | 1 | ||||
| ≥8 | 2.178 | 2.052–2.312 | <0.001 | 1.478 | 1.383–1.579 | <0.001 |
| Characteristics | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p Values | HR | 95% CI | p Values | |
| Age | ||||||
| <65 | 1 | 1 | ||||
| ≥65 | 2.253 | 2.114–2.401 | <0.001 | 1.958 | 1.825–2.097 | <0.001 |
| Gender | ||||||
| Female | 1 | 1 | ||||
| Male | 1.282 | 1.205–1.364 | <0.001 | 1.286 | 1.204–1.375 | <0.001 |
| Body mass index | ||||||
| <25 | 1 | 1 | ||||
| ≥25 | 0.834 | 0.781–0.890 | <0.001 | 0.889 | 0.829–0.953 | 0.001 |
| Underlying illness | ||||||
| Hypertension | 1.353 | 1.271–1.439 | <0.001 | 1.189 | 1.107–1.277 | <0.001 |
| Cardiovascular | 1.648 | 1.502–1.807 | <0.001 | 1.345 | 1.219–1.485 | <0.001 |
| Diabetes mellitus | 1.434 | 1.330–1.546 | <0.001 | 1.185 | 1.092–1.285 | <0.001 |
| Peptic ulcer | 1.114 | 0.998–1.244 | 0.053 | |||
| Hepatitis | 0.775 | 0.662–0.906 | 0.001 | 0.959 | 0.813–1.130 | 0.616 |
| Renal disease | 2.359 | 2.153–2.585 | <0.001 | 1.587 | 1.435–1.755 | <0.001 |
| Tumor location | ||||||
| Colon | 1 | 1 | ||||
| Rectum | 1.267 | 1.193–1.346 | <0.001 | 1.491 | 1.395–1.594 | <0.001 |
| Tumor stage | ||||||
| Stage I | 1 | |||||
| Stage II | 1.704 | 1.539–1.887 | <0.001 | |||
| Stage III | 2.717 | 2.466–2.994 | <0.001 | |||
| pT status | ||||||
| T1 | 1 | 1 | ||||
| T2 | 1.675 | 1.419–1.977 | <0.001 | 1.3 | 1.089–1.551 | 0.004 |
| T3 | 2.433 | 2.112–2.803 | <0.001 | 1.524 | 1.300–1.785 | <0.001 |
| T4 | 3.977 | 3.446–4.591 | <0.001 | 2.193 | 1.860–2.586 | <0.001 |
| pN status | ||||||
| N0 | 1 | 1 | ||||
| N1 | 1.504 | 1.399–1.617 | <0.001 | 1.392 | 1.288–1.504 | <0.001 |
| N2 | 2.612 | 2.424–2.815 | <0.001 | 2.381 | 2.192–2.587 | <0.001 |
| Histologic type | ||||||
| Adenocarcinoma | 1 | 1 | ||||
| Mucinous | 1.192 | 1.053–1.349 | 0.001 | 0.954 | 0.830–1.096 | 0.504 |
| Signet ring cell | 3.143 | 2.396–4.122 | <0.001 | 1.839 | 1.360–2.486 | <0.001 |
| Histologic grade | ||||||
| Well | 1 | 1 | ||||
| Moderate | 1.291 | 1.178–1.414 | <0.001 | 0.951 | 0.859–1.053 | 0.334 |
| Poor | 1.726 | 1.510–1.974 | <0.001 | 1.055 | 0.906–1.229 | 0.488 |
| Operative finding | ||||||
| Obstruction | 1.784 | 1.630–1.953 | <0.001 | 1.258 | 1.136–1.393 | <0.001 |
| Perforation | 1.811 | 1.567–2.093 | <0.001 | 1.131 | 0.962–1.331 | 0.136 |
| Preoperative values | ||||||
| Hemoglobin | ||||||
| <10 | 1 | 1 | ||||
| ≥10 | 0.617 | 0.575–0.663 | <0.001 | 0.855 | 0.788–0.929 | <0.001 |
| Albumin | ||||||
| <3.5 | 1 | 1 | ||||
| ≥3.5 | 0.406 | 0.376–0.439 | <0.001 | 0.589 | 0.538–0.646 | <0.001 |
| CIPI | ||||||
| <8 | 1 | 1 | ||||
| ≥8 | 2.262 | 2.121–2.411 | <0.001 | 1.503 | 1.400–1.613 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
You, J.-F.; Hsu, Y.-J.; Chern, Y.-J.; Cheng, C.-C.; Jong, B.-K.; Liao, C.-K.; Hsieh, P.-S.; Hsu, H.-C.; Tsai, W.-S. Preoperative Cancer Inflammation Prognostic Index as a Superior Predictor of Short- and Long-Term Outcomes in Patients with Stage I–III Colorectal Cancer after Curative Surgery. Cancers 2022, 14, 6232. https://doi.org/10.3390/cancers14246232
You J-F, Hsu Y-J, Chern Y-J, Cheng C-C, Jong B-K, Liao C-K, Hsieh P-S, Hsu H-C, Tsai W-S. Preoperative Cancer Inflammation Prognostic Index as a Superior Predictor of Short- and Long-Term Outcomes in Patients with Stage I–III Colorectal Cancer after Curative Surgery. Cancers. 2022; 14(24):6232. https://doi.org/10.3390/cancers14246232
Chicago/Turabian StyleYou, Jeng-Fu, Yu-Jen Hsu, Yih-Jong Chern, Ching-Chung Cheng, Bor-Kang Jong, Chun-Kai Liao, Pao-Shiu Hsieh, Hung-Chih Hsu, and Wen-Sy Tsai. 2022. "Preoperative Cancer Inflammation Prognostic Index as a Superior Predictor of Short- and Long-Term Outcomes in Patients with Stage I–III Colorectal Cancer after Curative Surgery" Cancers 14, no. 24: 6232. https://doi.org/10.3390/cancers14246232
APA StyleYou, J.-F., Hsu, Y.-J., Chern, Y.-J., Cheng, C.-C., Jong, B.-K., Liao, C.-K., Hsieh, P.-S., Hsu, H.-C., & Tsai, W.-S. (2022). Preoperative Cancer Inflammation Prognostic Index as a Superior Predictor of Short- and Long-Term Outcomes in Patients with Stage I–III Colorectal Cancer after Curative Surgery. Cancers, 14(24), 6232. https://doi.org/10.3390/cancers14246232





