Postoperative Radiotherapy and the Role of Regional Lymph Node Irradiation in Localized Merkel Cell Carcinoma: A Single-Center Retrospective Analysis
Abstract
Simple Summary
Abstract
1. Introduction
2. Patients and Methods
2.1. Patients and Treatment Characteristics
2.2. Objectives
2.3. Statistical Analysis
2.4. Ethics
3. Results
3.1. Patient Characteristics
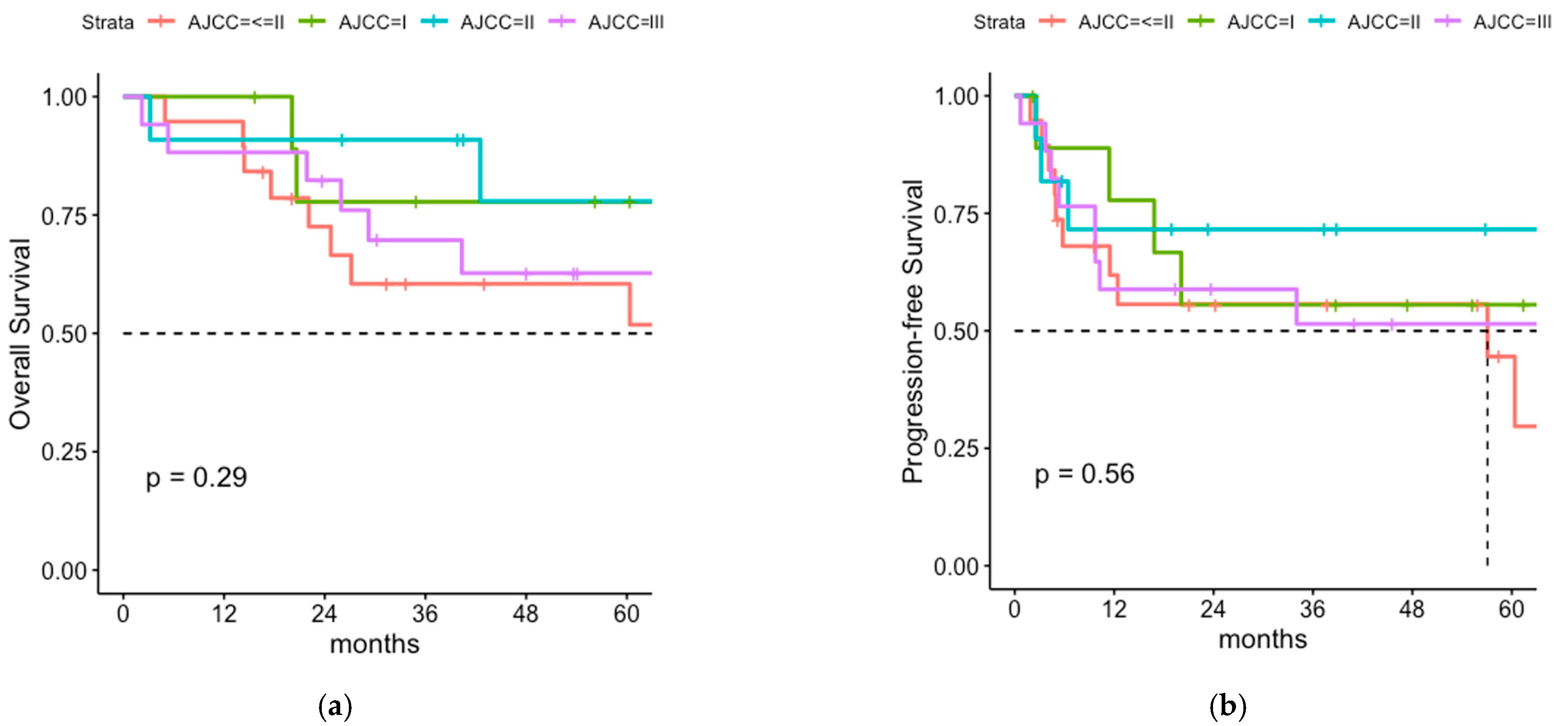
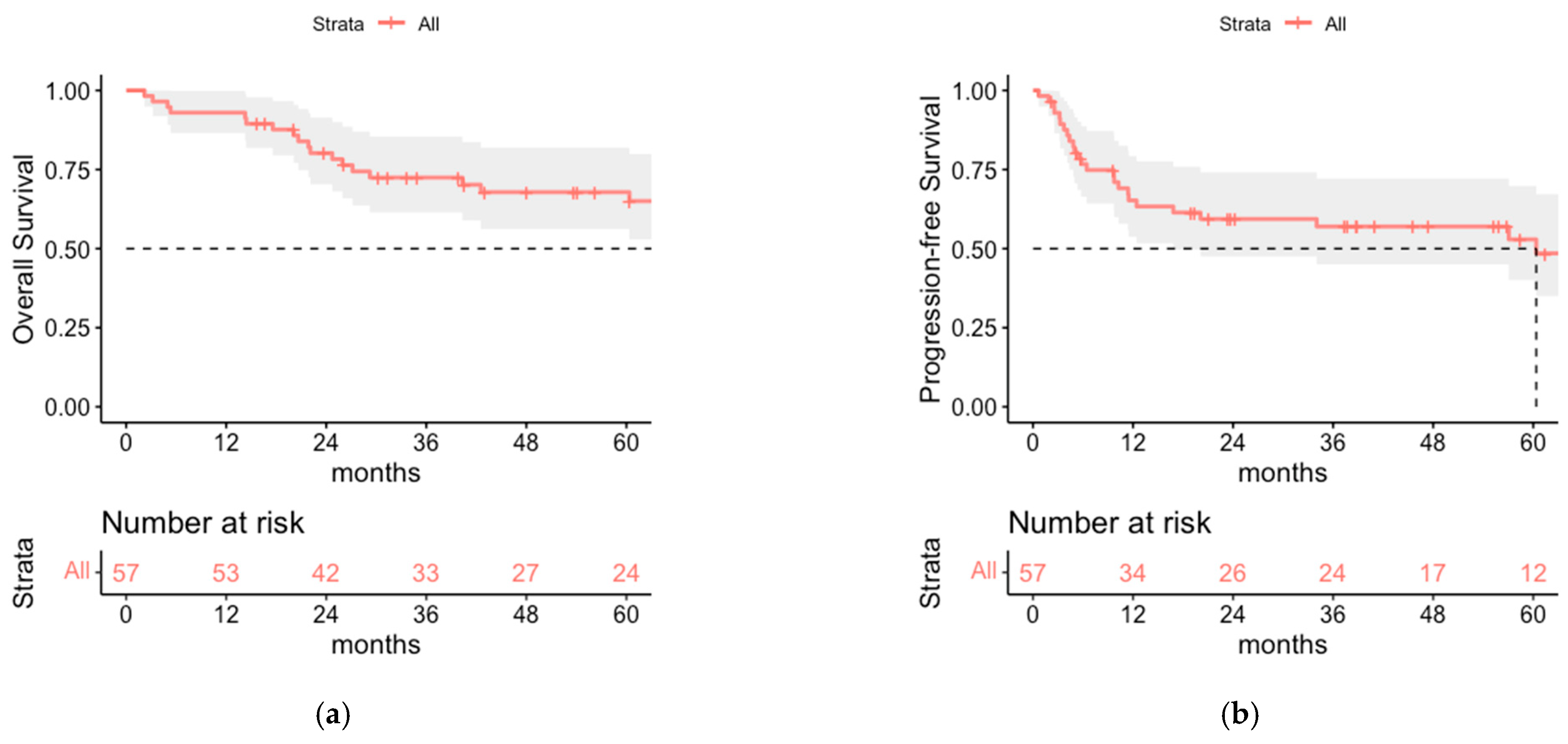
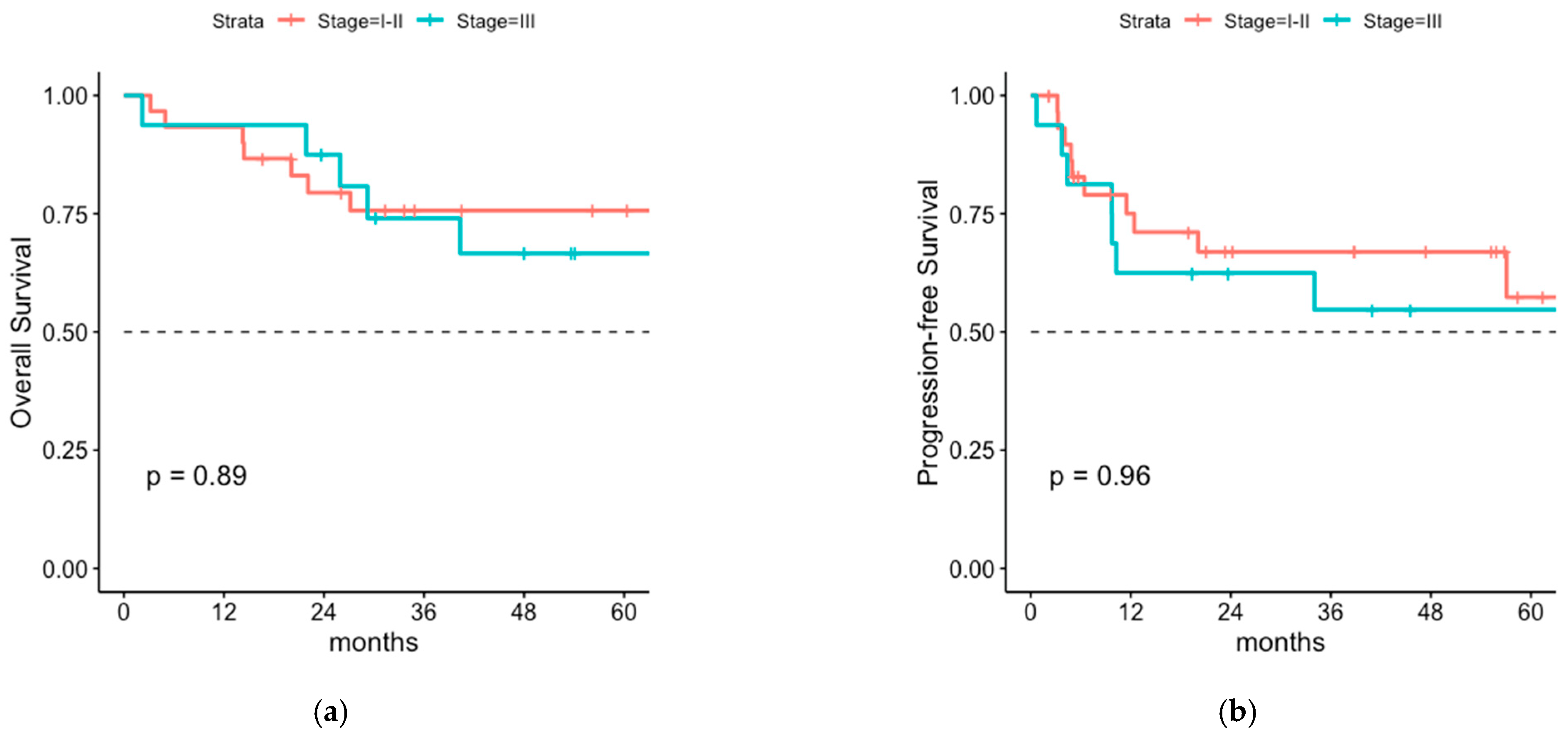
3.2. Oncologic Outcome and Patterns of Relapse of the Total Cohort
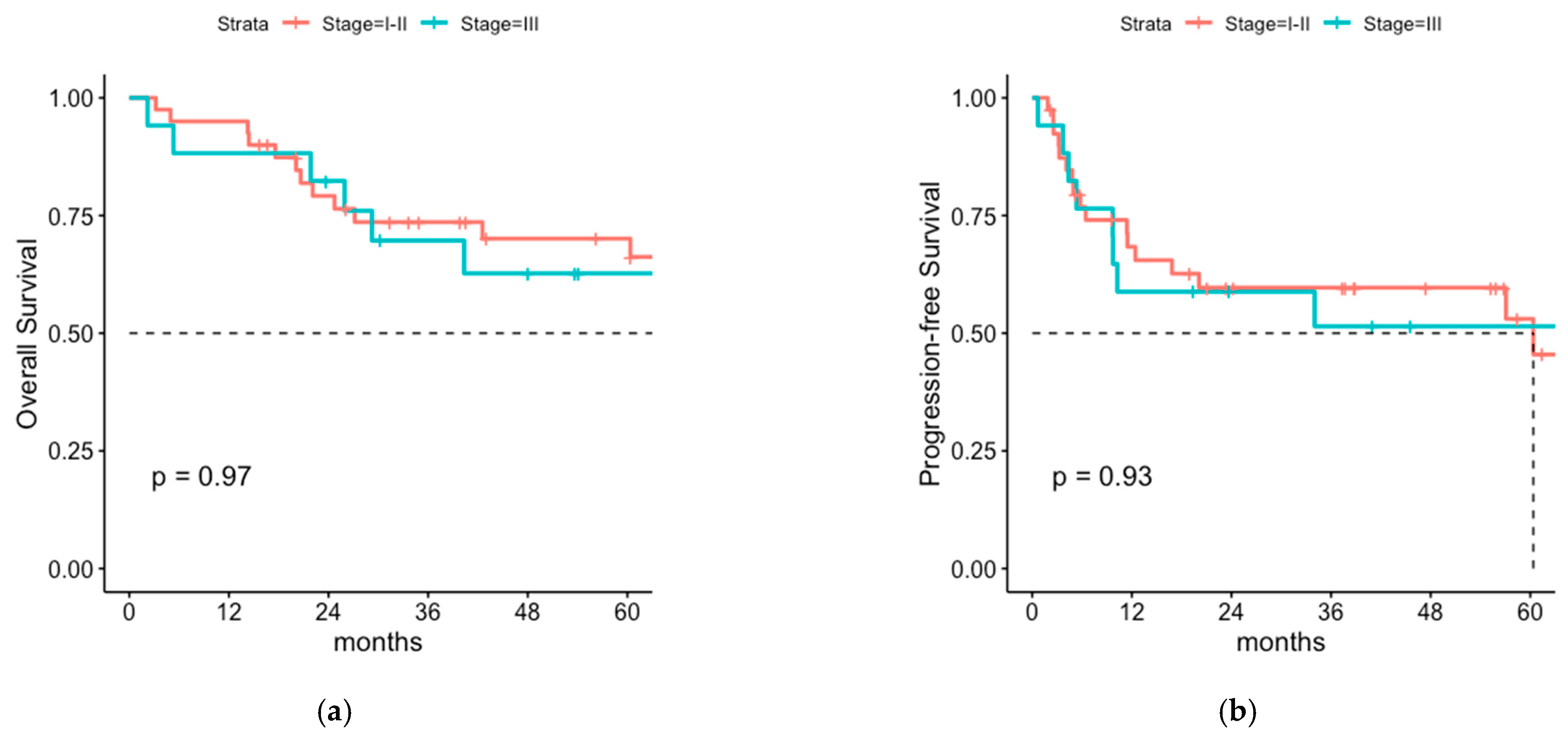
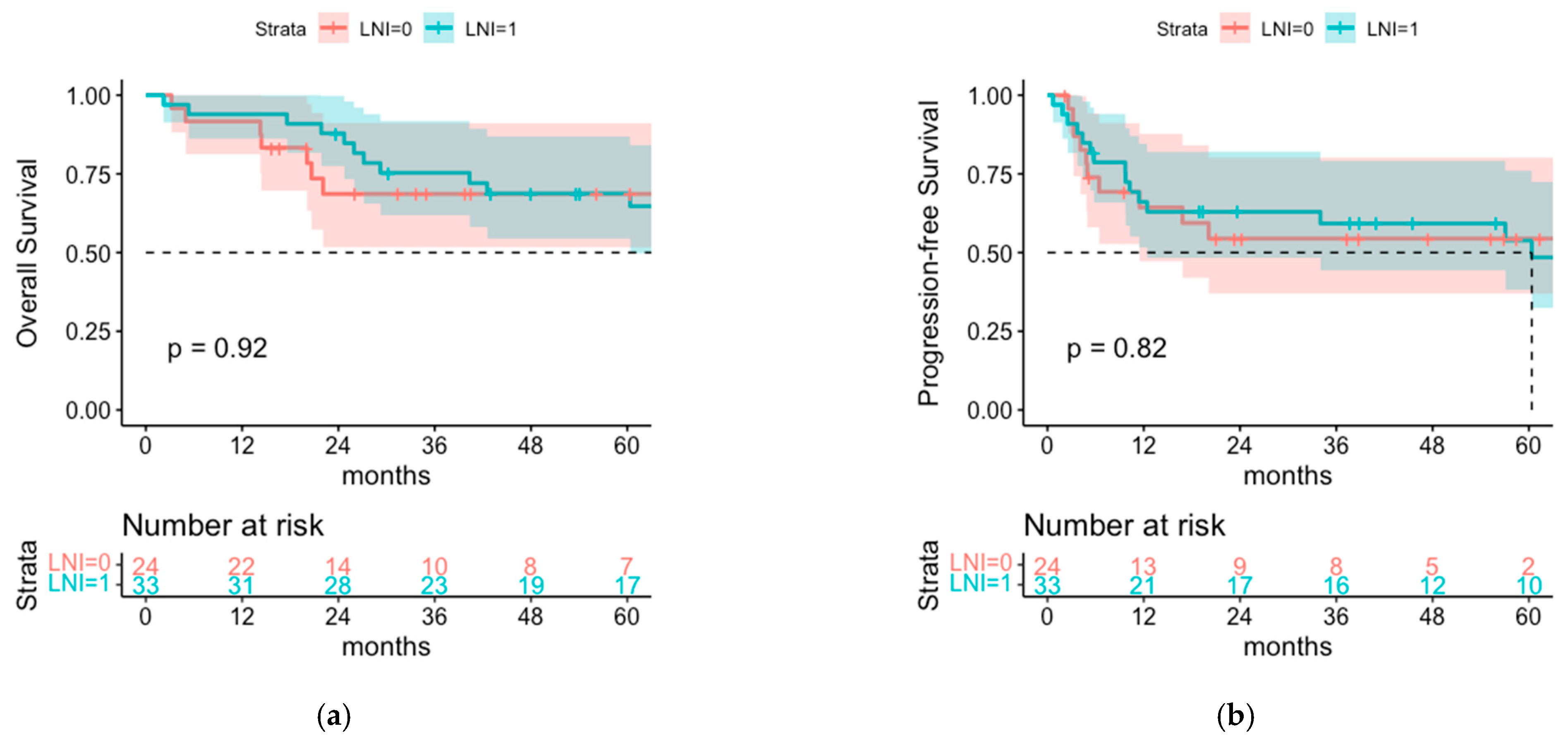
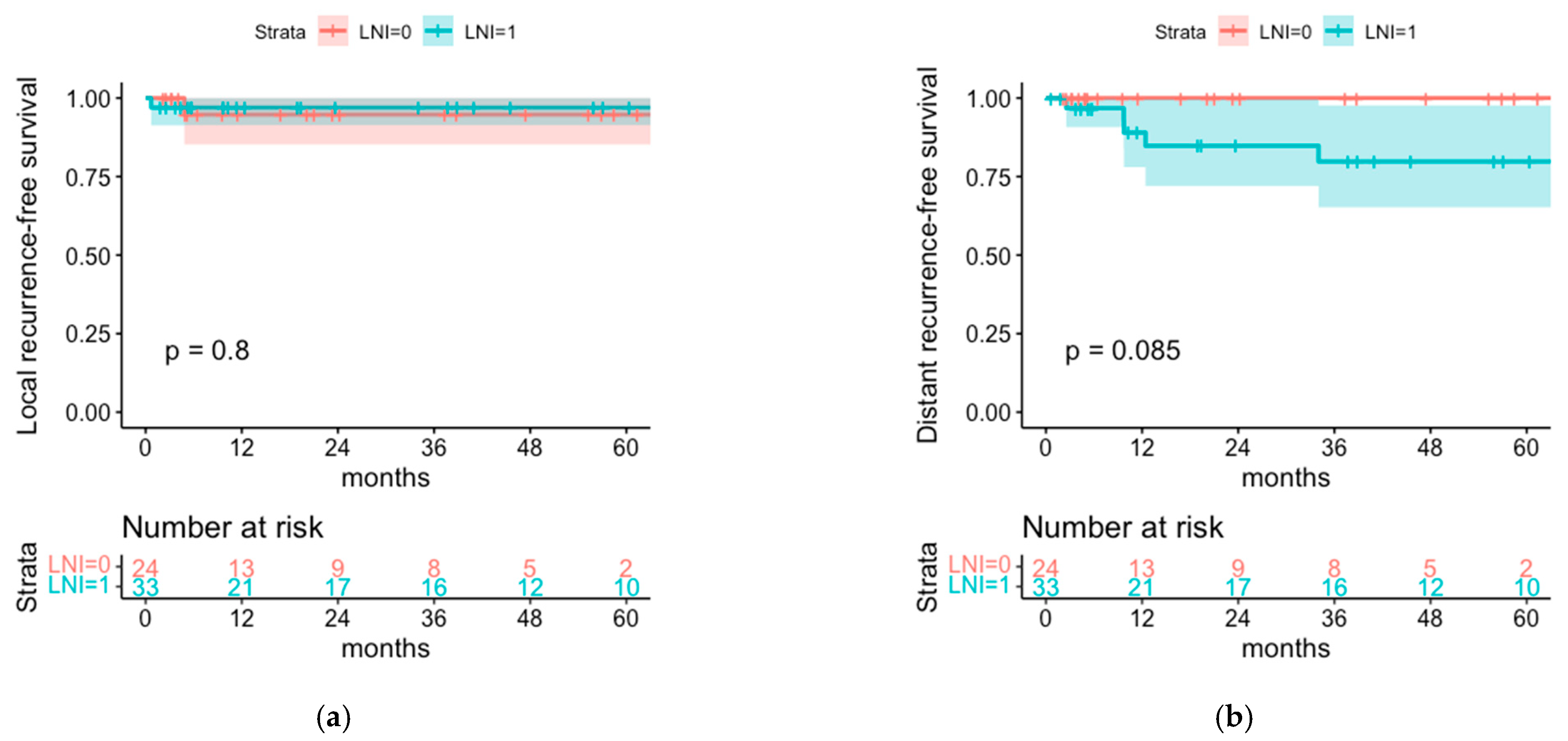
3.3. Comparison of Postoperative Radiotherapy with or without Regional Lymph Node Irradiation (LNI)
3.4. Cox Proportional Hazard Model for OS, PFS and Regional Recurrence-Free Survival
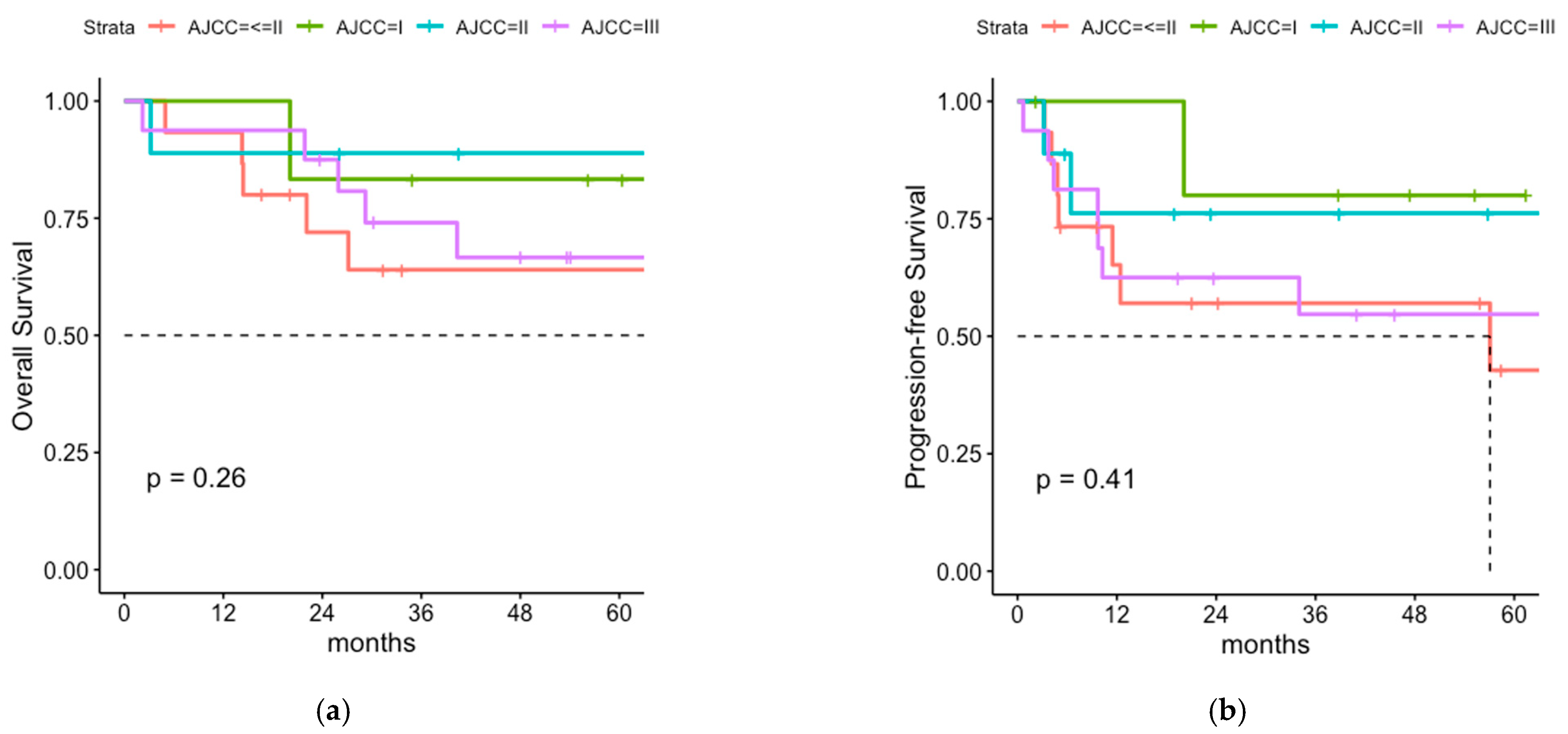
3.5. Postoperative Radiotherapy with or without Regional LNI for Patients with Negative SLNB
3.6. Lymph Node Irradiation of Sentinel Node Positive Patients without Previous LAD
4. Discussion
4.1. Overall Survival and Progression-Free Survival of Patients with or without LNI
4.2. Patterns of Relapse
4.3. Sentinel Node-Negative Patients
4.4. Radiation Monotherapy for Microscopical Lymph Node Involvement
4.5. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
References
- Becker, J.C.; Eigentler, T.; Frerich, B.; Gambichler, T.; Grabbe, S.; Höller, U.; Klumpp, B.; Loquai, C.; Krause-Bergmann, A.; Müller-Richter, U.; et al. S2k-Guideline Merkel Cell Carcinoma (MCC, Neuroendocrine Carcinoma of the Skin)—Update 2018. JDDG 2019. [Google Scholar] [CrossRef]
- Becker, J.C.; Stang, A.; DeCaprio, J.A.; Cerroni, L.; Lebbé, C.; Veness, M.; Nghiem, P. Merkel cell carcinoma. Nat. Rev. Dis. Primers 2017, 3, 17077. [Google Scholar] [CrossRef] [PubMed]
- Fondain, M.; Dereure, O.; Uhry, Z.; Guizard, A.V.; Woronoff, A.S.; Colonna, M.; Molinie, F.; Bara, S.; Velten, M.; Marrer, E.; et al. Merkel cell carcinoma in France: A registries-based, comprehensive epidemiological survey. J. Eur. Acad. Dermatol. Venereol. 2018, 32, 1292–1296. [Google Scholar] [CrossRef] [PubMed]
- Sridharan, V.; Muralidhar, V.; Margalit, D.N.; Tishler, R.B.; DeCaprio, J.A.; Thakuria, M.; Rabinowits, G.; Schoenfeld, J.D. Merkel cell carcinoma: A populationanalysis on survival. J. Natl. Compr. Cancer Netw. 2016, 14, 1247–1257. [Google Scholar] [CrossRef] [PubMed]
- Strom, T.; Carr, M.; Zager, J.S.; Naghavi, A.; Smith, F.O.; Cruse, C.W.; Messina, J.L.; Russell, J.; Rao, N.G.; Fulp, W.; et al. Radiation therapy is associated with improved outcomes in Merkel cell carcinoma. Ann. Surg. Oncol. 2016, 23, 3572–3578. [Google Scholar] [CrossRef] [PubMed]
- Strom, T.; Naghavi, A.O.; Messina, J.L.; Kim, S.; Torres–Roca, J.F.; Russell, J.; Sondak, V.K.; Padhya, T.A.; Trotti, A.M.; Caudell, J.J.; et al. Improved local and regional control with radiotherapy for Merkel cell carcinoma of the head and neck. Head Neck 2016, 39, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Haymerle, G.; Fochtmann, A.; Kunstfeld, R.; Pammer, J.; Erovic, B.M. Merkel cell carcinoma: Overall survival after open biopsy versus wide local excision. Head Neck 2015, 38, E1014–E1018. [Google Scholar] [CrossRef]
- Wright, G.P.; Holtzman, M.P. Surgical resection improves median overall survival with marginal improvement in long-term survival when compared with definitive radiotherapy in Merkel cell carcinoma: A propensity score matched analysis of the National Cancer Database. Am. J. Surg. 2018, 215, 384–387. [Google Scholar] [CrossRef]
- Schwartz, J.L.; Bichakjian, C.K.; Lowe, L.; Griffith, K.A.; Frohm, M.L.; Fullen, D.R.; Hayman, J.A.; Lao, C.D.; Shah, K.S.; McLean, S.A.; et al. Clinicopathologic features of primary merkel cell carcinoma: A detailed descriptive analysis of a large contemporary cohort. Dermatol. Surg. 2013, 39, 1009–1016. [Google Scholar] [CrossRef]
- Lim, C.S.; Whalley, D.; Haydu, L.E.; Murali, R.; Tippett, J.; Thompson, J.F.; Hruby, G.; Scolyer, R.A. Increasing tumor thickness is associated with recurrence and poorer survival in patients with Merkel cell carcinoma. Ann. Surg. Oncol. 2012, 19, 3325–3334. [Google Scholar] [CrossRef]
- Frohm, M.L.; Griffith, K.A.; Harms, K.L.; Hayman, J.A.; Fullen, D.R.; Nelson, C.C.; Wong, S.L.; Schwartz, J.L.; Bichakjian, C.K. Recurrence and survival in patients with Merkel cell carcinoma undergoing surgery without adjuvant radiation therapy to the primary site. JAMA Dermatol. 2016, 152, 1001–1007. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, S.; Storer, B.E.; Iyer, J.G.; Moshiri, A.; Parvathaneni, U.; Byrd, D.; Sober, A.J.; Sondak, V.K.; Gershenwald, J.E.; Nghiem, P. Adjuvant radiation therapy and chemotherapy in Merkel cell carcinoma: Survival analyses of 6908 cases from the National Cancer Data Base. J. Natl. Cancer Inst. 2016, 108, djw042. [Google Scholar] [CrossRef] [PubMed]
- Harrington, C.; Kwan, W. Radiotherapy and Conservative surgery in the locoregional management of Merkel cell carcinoma: The British Columbia Cancer Agency experience. Ann. Surg. Oncol. 2016, 23, 573–578. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.M.; Roman, S.A.; Sosa, J.A.; Judson, B.L. The role of adjuvant therapy in the management of head and neck Merkel cell carcinoma. JAMA Otolaryngol. Head Neck Surg. 2015, 141, 137–141. [Google Scholar] [CrossRef] [PubMed]
- Sexton, K.W.; Poteet, S.P.; Hill, J.B.; Schmidt, A.; Patel, A.; Del Corral, G.A.; Axt, J.; Kelley, M.C.; Thayer, W.P.; Shack, R.B. Adjuvant radiation therapy increases disease-free survival in stage Ib Merkel cell carcinoma. Ann. Plast. Surg. 2014, 73, 531–534. [Google Scholar] [CrossRef]
- Jouary, T.; Leyral, C.; Dreno, B.; Doussau, A.; Sassolas, B.; Beylot-Barry, M.; Renaud-Vilmer, C.; Guillot, B.; Bernard, P.; Lok, C.; et al. Adjuvant prophylactic regional radiotherapy versus observation in stage I Merkel cell carcinoma: A multicentric prospective randomized study. Ann. Oncol. 2012, 23, 1074–1080. [Google Scholar] [CrossRef]
- Mojica, P.; Smith, D.; Ellenhorn, J.D.I. Adjuvant radiation therapy is associated with improved survival in Merkel cell carcinoma of the skin. J. Clin. Oncol. 2007, 25, 1043–1047. [Google Scholar] [CrossRef]
- Kang, S.H.; Haydu, L.E.; Goh, R.Y.H.; Fogarty, G.B. Radiotherapy is associated with significant improvement in local and regional control in Merkel cell carcinoma. Radiat. Oncol. 2012, 7, 171. [Google Scholar] [CrossRef]
- Hoeller, U.; Mueller, T.; Schubert, T.; Budach, V.; Ghadjar, P.; Brenner, W.; Kiecker, F.; Schicke, B.; Haase, O. Regional nodal relapse in surgically staged Merkel cell carcinoma. Strahlenther. Und Onkol. 2015, 191, 51–58. [Google Scholar] [CrossRef]
- McAfee, W.J.; Morris, C.G.; Mendenhall, C.M.; Werning, J.W.; Mendenhall, N.P.; Mendenhall, W.M. Merkel cell carcinoma. Cancer 2005, 104, 1761–1764. [Google Scholar] [CrossRef]
- Allen, P.J.; Bowne, W.B.; Jaques, D.P.; Brennan, M.F.; Busam, K.; Coit, D.G. Merkel Cell Carcinoma: Prognosis and Treatment of Patients From a Single Institution. J. Clin. Oncol. 2005, 23, 2300–2309. [Google Scholar] [CrossRef] [PubMed]
- Reichgelt, B.A.; Visser, O. Epidemiology and survival of Merkel cell carcinoma in the Netherlands. A population-based study of 808 cases in 1993-2007. Eur. J. Cancer 2011, 47, 579–585. [Google Scholar] [CrossRef] [PubMed]
- Harms, K.L.; Healy, M.A.; Nghiem, P.; Sober, A.J.; Johnson, T.M.; Bichakjian, C.K.; Wong, S.L. Analysis of prognostic factors from 9387 Merkel cell carcinoma cases forms the basis for the New 8th Edition AJCC staging system. Ann. Surg. Oncol. 2016, 23, 3564–3571. [Google Scholar] [CrossRef] [PubMed]
- Becker, J.C.; Ugurel, S.; Leiter-Stoppke, U.; Meier, F.; Gutzmer, R.; Haferkamp, S.; Zimmer, L.; Livingstone, E.; Eigentler, T.; Hauschild, A.; et al. Adjuvant immunotherapy with nivolumab (NIVO) versus observation in completely resected Merkel cell carcinoma (MCC): Disease-free survival (DFS) results from ADMEC-O, a randomized, open-label phase II trial. Ann. Oncol. 2022, 33, S903. [Google Scholar] [CrossRef]
- Fang, L.C.; Lemos, B.; Douglas, J.; Iyer, J.; Nghiem, P. Radiation monotherapy as regional treatment for lymph node-positive Merkel cell carcinoma. Cancer 2010, 116, 1783–1790. [Google Scholar] [CrossRef]


| Total Cohort (n = 57) | with LNI (n = 33) | without LNI (n = 24) | p-Value | ||
|---|---|---|---|---|---|
| median age (range) in years | 73 (50–89) | 73 (50–88) | 72.5 (52–89) | 0.517 | |
| sex | male | 33 (57.9%) | 20 (60.6%) | 13 (54.2%) | 0.629 |
| female | 24 (42.1%) | 13 (39.4%) | 11 (45.8%) | ||
| AJCC-stage | I | 13 (22.8%) | 5 (15.2%) | 8 (33.3%) | <0.001 |
| ≤II | 19 (33.3%) | 8 (24.2%) | 11 (45.8%) | ||
| II | 8 (14.0%) | 3 (9.1%) | 5 (20.8%) | ||
| III | 17 (29.8%) | 17 (51.5%) | 0 (0%) | ||
| SLNB | done | 42 (73.7%) | 22 (66.7%) | 20 (83.3%) | 0.162 |
| not done | 15 (26.3%) | 11 (33.3%) | 4 (16.7%) | ||
| SLNB result | positive | 12 (21.1%) | 12 (54.6%) | 0 (0%) | <0.001 |
| negative | 30 (52.6%) | 10 (45.5%) | 20 (100%) | ||
| lymph node dissection | done | 9 (15.8%) | 8 (24.2%) | 1 (4.2%) | 0.064 |
| not done | 47 (82.4%) | 24 (72.7%) | 23 (95.8%) | ||
| unknown | 1 (1.8%) | 1 (3.0%) | 0 (0%) | ||
| resection status (R-status) | 0 | 52 (91.2%) | 29 (87.9%) | 23 (95.8%) | 0.073 |
| 1 | 4 (7.0%) | 4 (12.1%) | 0 (0%) | ||
| X | 1 (1.8%) | 0 (0%) | 1 (4.2%) | ||
| primary tumor localization | craniofacial | 19 (33.3%) | 14 (42.4%) | 5 (20.9%) | 0.185 |
| body trunk | 6 (10.5%) | 4 (12.1%) | 2 (8.3%) | ||
| extremities | 32 (56.2%) | 15 (45.5%) | 17 (70.8%) | ||
| Site of Relapse | with LNI (n = 33) | without LNI (n = 24) | Total (n = 57) |
|---|---|---|---|
| Local | 1 (3.0%) | 1 (4.2%) | 2 (3.5%) |
| Regional | 3 (9.1%) | 6 (25.0%) | 9 (15.8%) |
| Distant | 5 (15.2%) | 0 (0%) | 5 (8.8%) |
| Site of Relapse | Patients with Negative SLNB Only | ||
|---|---|---|---|
| with LNI (n = 10) | without LNI (n = 20) | Total (n = 30) | |
| Local | 0 (0%) | 1 (5%) | 1 (3.3%) |
| Regional | 1 (10%) | 4 (20%) | 5 (16.7%) |
| Distant | 2 (20%) | 0 (0%) | 2 (6.7%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dinges, L.-A.; Eichkorn, T.; Regnery, S.; Hörner-Rieber, J.; Debus, J.; Hassel, J.C.; Lang, K. Postoperative Radiotherapy and the Role of Regional Lymph Node Irradiation in Localized Merkel Cell Carcinoma: A Single-Center Retrospective Analysis. Cancers 2022, 14, 6140. https://doi.org/10.3390/cancers14246140
Dinges L-A, Eichkorn T, Regnery S, Hörner-Rieber J, Debus J, Hassel JC, Lang K. Postoperative Radiotherapy and the Role of Regional Lymph Node Irradiation in Localized Merkel Cell Carcinoma: A Single-Center Retrospective Analysis. Cancers. 2022; 14(24):6140. https://doi.org/10.3390/cancers14246140
Chicago/Turabian StyleDinges, Lisa-Antonia, Tanja Eichkorn, Sebastian Regnery, Juliane Hörner-Rieber, Jürgen Debus, Jessica C. Hassel, and Kristin Lang. 2022. "Postoperative Radiotherapy and the Role of Regional Lymph Node Irradiation in Localized Merkel Cell Carcinoma: A Single-Center Retrospective Analysis" Cancers 14, no. 24: 6140. https://doi.org/10.3390/cancers14246140
APA StyleDinges, L.-A., Eichkorn, T., Regnery, S., Hörner-Rieber, J., Debus, J., Hassel, J. C., & Lang, K. (2022). Postoperative Radiotherapy and the Role of Regional Lymph Node Irradiation in Localized Merkel Cell Carcinoma: A Single-Center Retrospective Analysis. Cancers, 14(24), 6140. https://doi.org/10.3390/cancers14246140







