Extensive Phenotypic Characterization of T Cells Infiltrating Liver Metastasis from Colorectal Cancer: A Potential Role in Precision Medicine
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants and Sample Collection
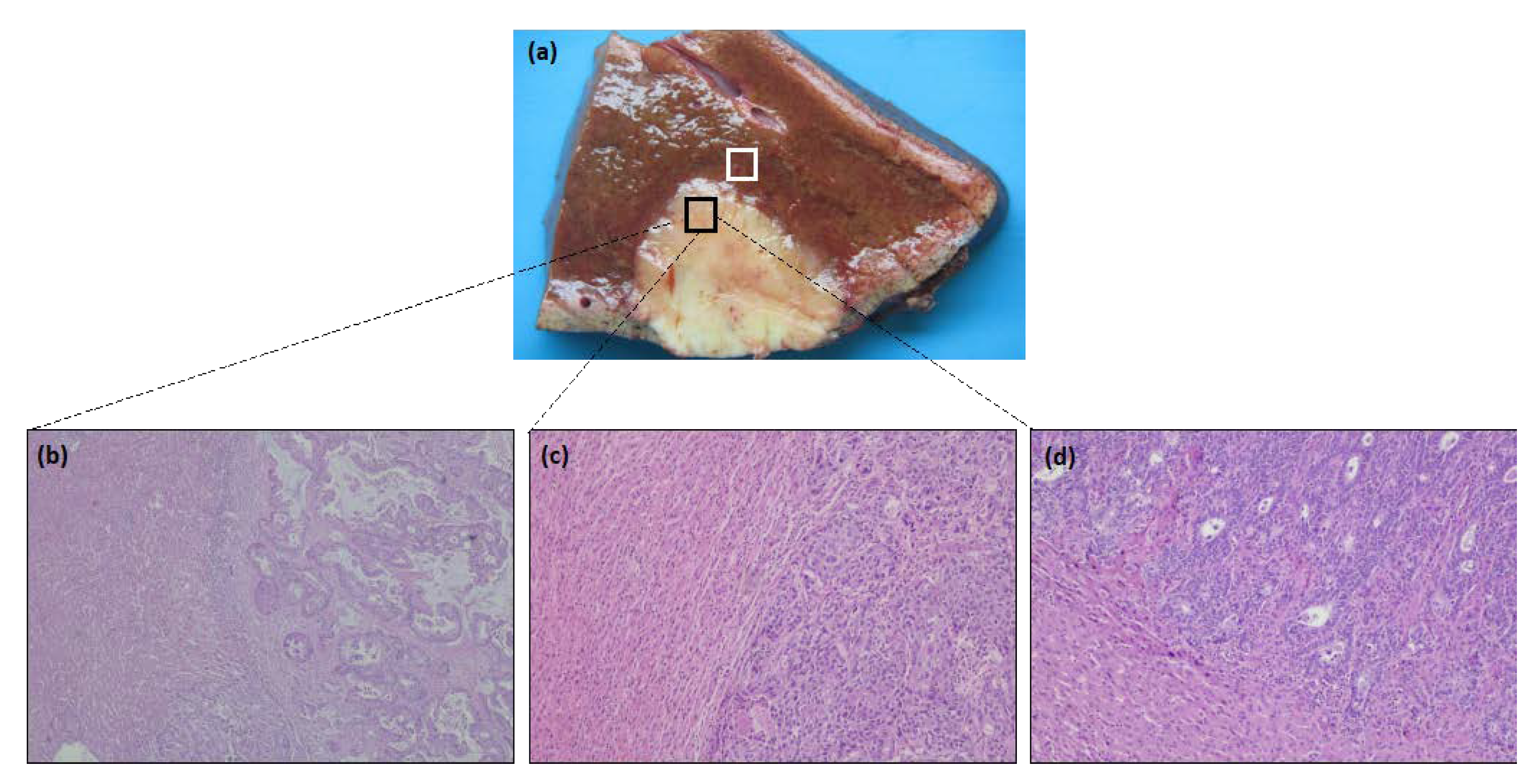
2.2. Histological Analysis
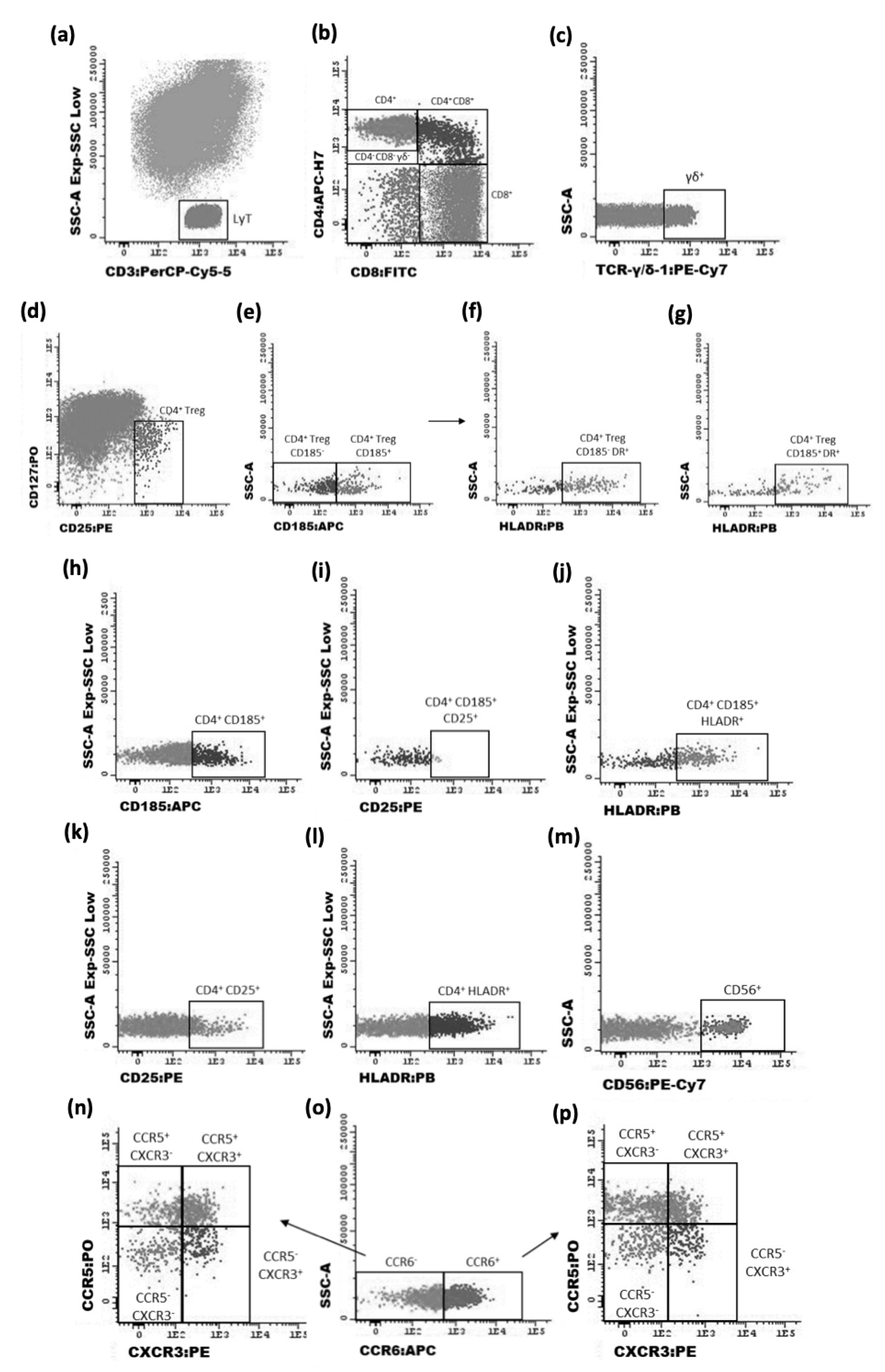
2.3. Flow Cytometry Characterization of T Cells
2.3.1. Staining Protocol
2.3.2. Data Acquisition and Analysis
2.4. Statistical Analysis
3. Results
3.1. The Liver Niche Decreases the Migration of Pro-Inflammatory Cells to the CRC Metastasis Location
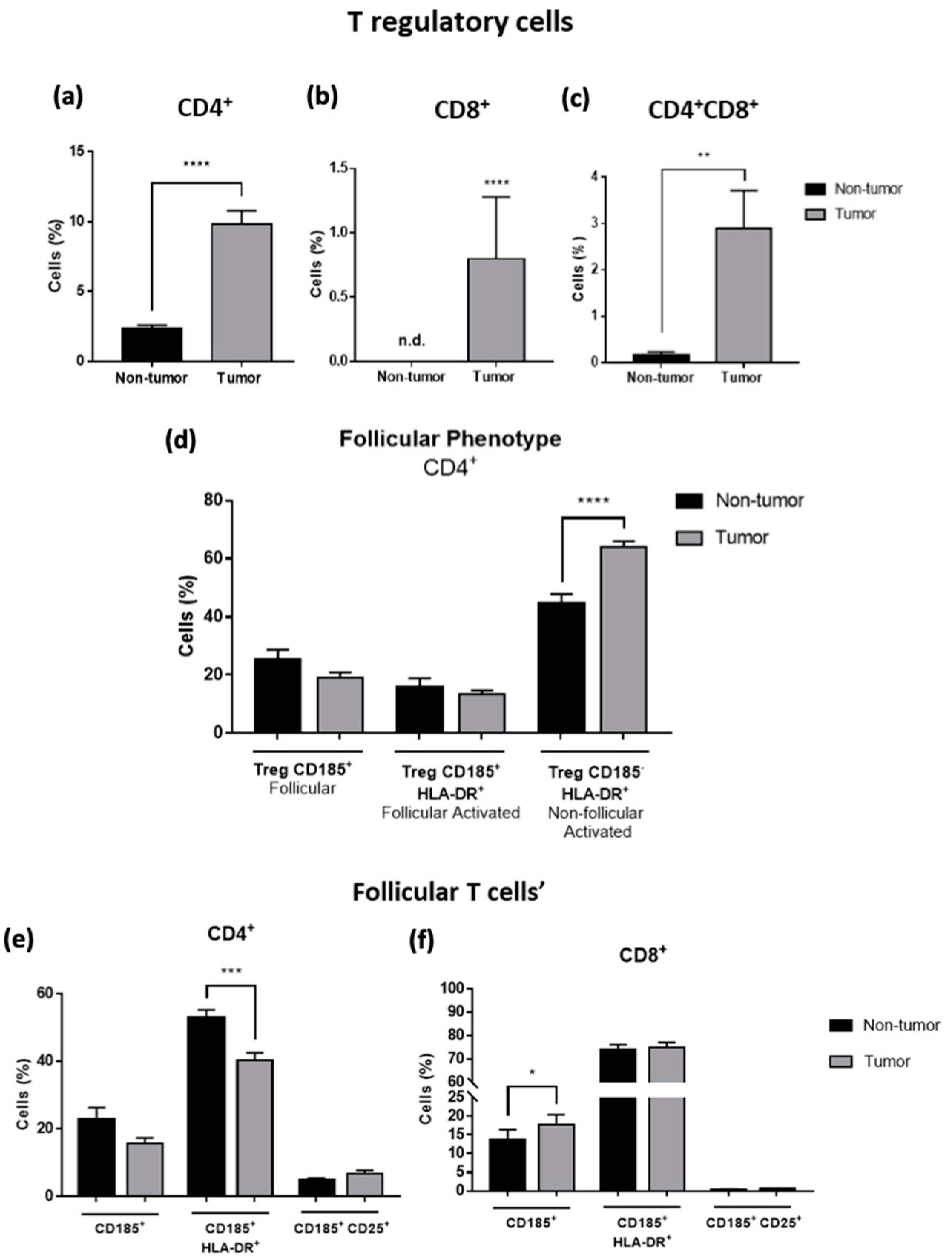
3.2. T Regulatory and Follicular-like Cells Are Increased in Tumor Samples of CRC Liver Metastasis
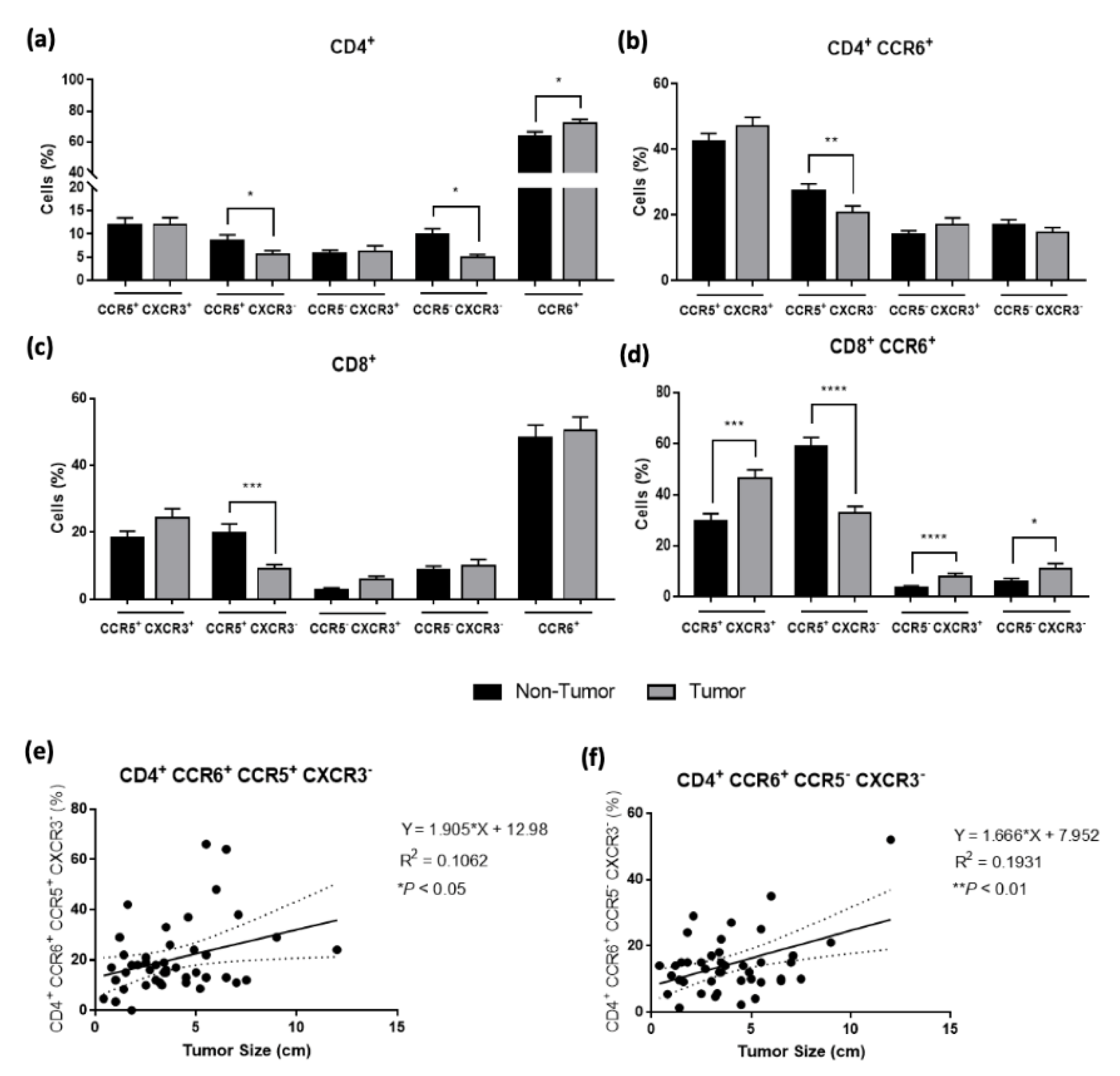
3.3. Th17 Cells Are Attracted to the Tumor Microenvironment According to the Tumor Size
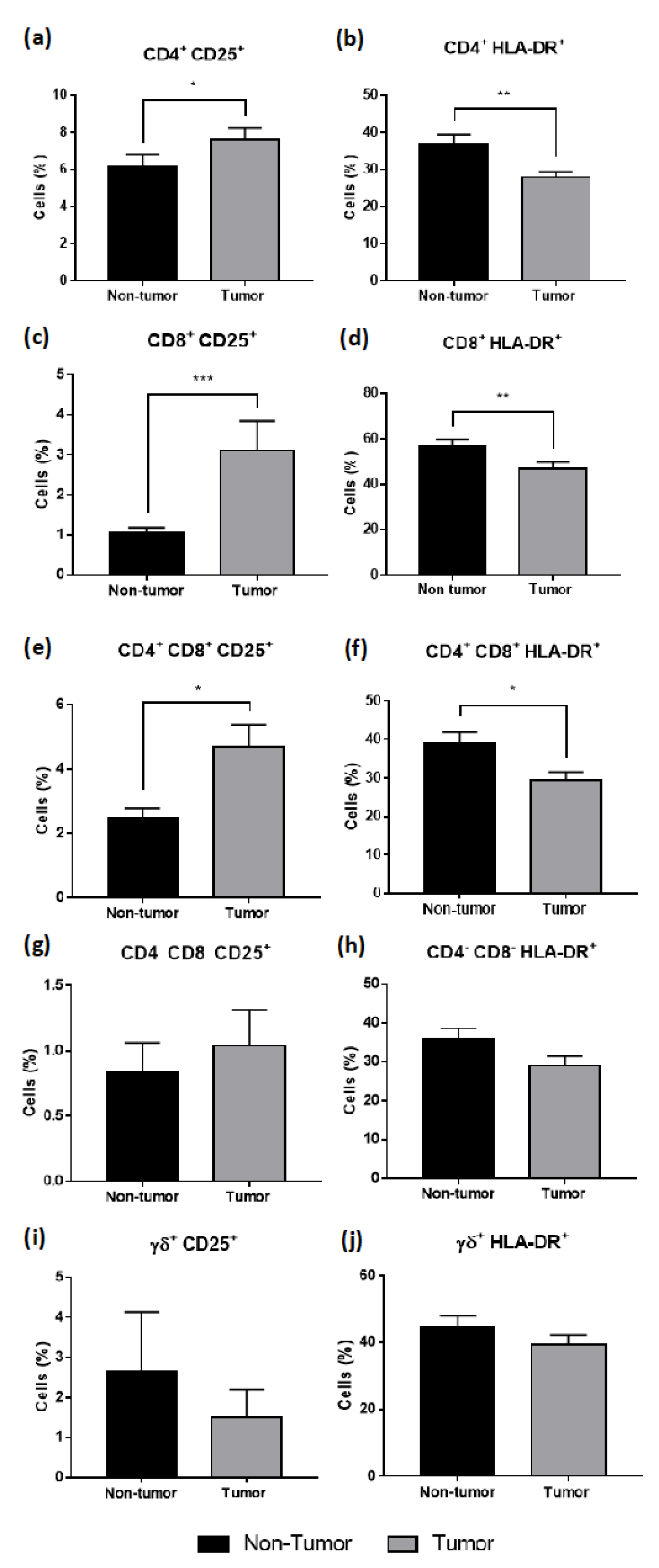
3.4. T Cells Infiltrating CRC Liver Metastasis Present an Intermediate Activation Phenotype
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Tsilimigras, D.I.; Brodt, P.; Clavien, P.A.; Muschel, R.J.; D’Angelica, M.I.; Endo, I.; Parks, R.W.; Doyle, M.; de Santibañes, E.; Pawlik, T.M. Liver metastases. Nat. Rev. Dis Primers 2021, 7, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Chandra, R.; Karalis, J.D.; Liu, C.; Murimwa, G.Z.; Park, J.V.; Heid, C.A.; Reznik, S.I.; Huang, E.; Minna, J.D.; Brekken, R.A. The colorectal cancer tumor microenvironment and its impact on liver and lung metastasis. Cancers 2021, 13, 6206. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Ward, S.E.; Zhou, J.; Cheng, A.S.L. Liver Immune Microenvironment and Metastasis from Colorectal Cancer-Pathogenesis and Therapeutic Perspectives. Cancers 2021, 13, 2418. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Zhang, W.; Xu, Y.; Li, Y.-H.; Xing, B.-C. A Prognostic Scoring System to Predict Survival Outcome of Resectable Colorectal Liver Metastases in this Modern Era. Ann. Surg. Oncol. 2021, 28, 7709–7718. [Google Scholar] [CrossRef]
- Höppener, D.J.; Nierop, P.M.; Hof, J.; Sideras, K.; Zhou, G.; Visser, L.; Gouw, A.S.; de Jong, K.P.; Sprengers, D.; Kwekkeboom, J.; et al. Enrichment of the tumour immune microenvironment in patients with desmoplastic colorectal liver metastasis. Br. J. Cancer 2020, 123, 196–206. [Google Scholar] [CrossRef]
- Garcia-vicién, G.; Mezheyeuski, A.; Bañuls, M.; Ruiz-roig, N.; Molleví, D.G. The tumor microenvironment in liver metastases from colorectal carcinoma in the context of the histologic growth patterns. Int. J. Mol. Sci. 2021, 22, 1544. [Google Scholar] [CrossRef]
- Liang, J.Y.; Xi, S.Y.; Shao, Q.; Yuan, Y.F.; Li, B.K.; Zheng, Y.; Wang, D.S.; Wu, X.J.; Ding, P.R.; Chen, G.; et al. Histopathological growth patterns correlate with the immunoscore in colorectal cancer liver metastasis patients after hepatectomy. Cancer Immunol. Immunother. 2020, 69, 2623–2634. [Google Scholar] [CrossRef]
- Garcia-Vicién, G.; Mezheyeuski, A.; Micke, P.; Ruiz, N.; Ruffinelli, J.C.; Mils, K.; Bañuls, M.; Molina, N.; Losa, F.; Lladó, L.; et al. Spatial Immunology in Liver Metastases from Colorectal Carcinoma according to the Histologic Growth Pattern. Cancers 2022, 14, 689. [Google Scholar] [CrossRef]
- Zhou, H.; Liu, Z.; Wang, Y.; Wen, X.; Amador, E.H.; Yuan, L.; Ran, X.; Xiong, L.; Ran, Y.; Chen, W.; et al. Colorectal liver metastasis: Molecular mechanism and interventional therapy. Signal Transduct. Target. Ther. 2022, 7. [Google Scholar] [CrossRef]
- Itatani, Y.; Kawada, K.; Inamoto, S.; Yamamoto, T.; Ogawa, R.; Taketo, M.M.; Sakai, Y. The role of chemokines in promoting colorectal cancer invasion/metastasis. Int. J. Mol. Sci. 2016, 17, 643. [Google Scholar] [CrossRef] [PubMed]
- Kuwahara, T.; Hazama, S.; Suzuki, N.; Yoshida, S.; Tomochika, S.; Nakagami, Y.; Matsui, H.; Shindo, Y.; Kanekiyo, S.; Tokumitsu, Y.; et al. Intratumoural-infiltrating CD4 + and FOXP3 + T cells as strong positive predictive markers for the prognosis of resectable colorectal cancer. Br. J. Cancer 2019, 121, 659–665. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Wang, H.; An, F.; Wu, F.; Tao, Q.; Li, Y.; Ruan, Y.; Zhai, Z. CD4+CD25+CD127low regulatory T cells associated with the effect of CD19 CAR-T therapy for relapsed/refractory B-cell acute lymphoblastic leukemia. Int. Immunopharmacol. 2021, 96, 107742. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Feng, M.; Yu, T.; Liu, X.; Zhang, P. Intratumoral regulatory T cells are associated with suppression of colorectal carcinoma metastasis after resection through overcoming IL-17 producing T cells. Cell Immunol. 2014, 287, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Jiang, P.; Wei, S.; Xu, X.; Wang, J. Regulatory T cells in tumor microenvironment: New mechanisms, potential therapeutic strategies and future prospects. Mol. Cancer 2020, 19, 116. [Google Scholar] [CrossRef] [PubMed]
- Kroemer, M.; Turco, C.; Spehner, L.; Viot, J.; Idirène, I.; Bouard, A.; Renaude, E.; Deschamps, M.; Godet, Y.; Adotévi, O.; et al. Investigation of the prognostic value of CD4 T cell subsets expanded from tumor-infiltrating lymphocytes of colorectal cancer liver metastases. J. Immunother. Cancer 2020, 8, e001478. [Google Scholar] [CrossRef]
- Wu, D.; Wu, P.; Huang, Q.; Liu, Y.; Ye, J.; Huang, J. Interleukin-17: A promoter in colorectal cancer progression. Clin. Dev. Immunol. 2013, 2013. [Google Scholar] [CrossRef]
- Ling, A.; Lundberg, I.V.; Eklöf, V.; Wikberg, M.L.; Öberg, Å.; Edin, S.; Palmqvist, R. The infiltration, and prognostic importance, of Th1 lymphocytes vary in molecular subgroups of colorectal cancer. J. Pathol. Clin. Res. 2016, 2, 21–31. [Google Scholar] [CrossRef]
- Toor, S.M.; Sasidharan Nair, V.; Saleh, R.; Taha, R.Z.; Murshed, K.; Al-Dhaheri, M.; Khawar, M.; Ahmed, A.A.; Kurer, M.A.; Abu Nada, M.; et al. Transcriptome of Tumor-Infiltrating T Cells in Colorectal Cancer Patients Uncovered a Unique Gene Signature in CD4+ T Cells Associated with Poor Disease-Specific Survival. Vaccines 2021, 9, 334. [Google Scholar] [CrossRef]
- Lückel, C.; Picard, F.S.R.; Huber, M. Tc17 biology and function: Novel concepts. Eur. J. Immunol. 2020, 50, 1257–1267. [Google Scholar] [CrossRef]
- Qi, H. T follicular helper cells in space-time. Nat. Rev. Immunol. 2016, 16, 612–625. [Google Scholar] [CrossRef] [PubMed]
- Hetta, H.F.; Elkady, A.; Yahia, R.; Meshall, A.K.; Saad, M.M.; Mekky, M.A.; Al-Kadmy, I.M.S. T follicular helper and T follicular regulatory cells in colorectal cancer: A complex interplay. J. Immunol. Methods 2020, 480, 112753. [Google Scholar] [CrossRef] [PubMed]
- Koutsakos, M.; Nguyen, T.H.O.; Kedzierska, K. With a Little Help from T Follicular Helper Friends: Humoral Immunity to Influenza Vaccination. J. Immunol. 2019, 202, 360–367. [Google Scholar] [CrossRef] [PubMed]
- van den Eynden, G.G.; Bird, N.C.; Majeed, A.W.; van Laere, S.; Dirix, L.Y.; Vermeulen, P.B. The histological growth pattern of colorectal cancer liver metastases has prognostic value. Clin. Exp. Metastasis 2012, 29, 541–549. [Google Scholar] [CrossRef] [PubMed]
- Vermeulen, P.B.; Colpaert, C.; Salgado, R.; Royers, R.; Hellemans, H.; van den Heuvel, E.; Goovaerts, G.; Dirix, L.Y.; van Marck, E. Liver metastases from colorectal adenocarcinomas grow in three patterns with different angiogenesis and desmoplasia. J. Pathol. 2001, 195, 336–342. [Google Scholar] [CrossRef]
- Van Dongen, J.J.M.; Lhermitte, L.; Böttcher, S.; Almeida, J.; Van Der Velden, V.H.J.; Flores-Montero, J.; Rawstron, A.; Asnafi, V.; Lecrevisse, Q.; Lucio, P.; et al. EuroFlow antibody panels for standardized n-dimensional flow cytometric immunophenotyping of normal, reactive and malignant leukocytes. Leukemia 2012, 26, 1908–1975. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Q.; Xing, B.; Luo, N.; Gao, R.; Yu, K.; Hu, X.; Bu, Z.; Peng, J.; Ren, X.; et al. Immune phenotypic linkage between colorectal cancer and liver metastasis. Cancer Cell 2022, 40, 424–437. [Google Scholar] [CrossRef]
- Zhang, L.; Yu, X.; Zheng, L.; Zhang, Y.; Li, Y.; Fang, Q.; Gao, R.; Kang, B.; Zhang, Q.; Huang, J.Y.; et al. Lineage tracking reveals dynamic relationships of T cells in colorectal cancer. Nature 2018, 564, 268–272. [Google Scholar] [CrossRef]
- Hu, W.; Sun, R.; Chen, L.; Zheng, X.; Jiang, J. Prognostic significance of resident CD103+CD8+T cells in human colorectal cancer tissues. Acta Histochem. 2019, 121, 657–663. [Google Scholar] [CrossRef]
- Yu, X.; Zhu, L.; Liu, J.; Xie, M.; Chen, J.; Li, J. Emerging role of immunotherapy for colorectal cancer with liver metastasis. Onco Targets Ther. 2020, 13, 11645–11658. [Google Scholar] [CrossRef]
- De Simone, M.; Arrigoni, A.; Rossetti, G.; Gruarin, P.; Ranzani, V.; Politano, C.; Bonnal, R.J.; Provasi, E.; Sarnicola, M.L.; Panzeri, I.; et al. Transcriptional Landscape of Human Tissue Lymphocytes Unveils Uniqueness of Tumor-Infiltrating T Regulatory Cells. Immunity 2016, 45, 1135–1147. [Google Scholar] [CrossRef] [PubMed]
- Salama, P.; Phillips, M.; Grieu, F.; Morris, M.; Zeps, N.; Joseph, D.; Platell, C.; Iacopetta, B. Tumor-infiltrating FOXP3+ T regulatory cells show strong prognostic significance in colorectal cancer. J. Clin. Oncol. 2009, 27, 186–192. [Google Scholar] [CrossRef] [PubMed]
- Timperi, E.; Pacella, I.; Schinzari, V.; Focaccetti, C.; Sacco, L.; Farelli, F.; Caronna, R.; Del Bene, G.; Longo, F.; Ciardi, A.; et al. Regulatory T cells with multiple suppressive and potentially pro-tumor activities accumulate in human colorectal cancer. Oncoimmunology 2016, 5, e1175800. [Google Scholar] [CrossRef] [PubMed]
- Kuca-Warnawin, E.; Janicka, I.; Szczęsny, P.; Olesińska, M.; Bonek, K.; Głuszko, P.; Kontny, E. Modulation of T-Cell Activation Markers Expression by the Adipose Tissue–Derived Mesenchymal Stem Cells of Patients with Rheumatic Diseases. Cell Transplant. 2020, 29, 096368972094568. [Google Scholar] [CrossRef] [PubMed]
- Corneau, A.; Cosma, A.; Even, S.; Katlama, C.; le Grand, R.; Frachet, V.; Blanc, C.; Autran, B. Comprehensive Mass Cytometry Analysis of Cell Cycle, Activation, and Coinhibitory Receptors Expression in CD4 T Cells from Healthy and HIV-Infected Individuals. Cytom. B Clin. Cytom. 2017, 92, 21–32. [Google Scholar] [CrossRef]
- Olguín, J.E.; Medina-Andrade, I.; Rodríguez, T.; Rodríguez-Sosa, M.; Terrazas, L.I. Relevance of Regulatory T Cells during Colorectal Cancer Development. Cancers 2020, 12, 1888. [Google Scholar] [CrossRef]
- Valentine, K.M.; Hoyer, K.K. CXCR5+ CD8 T cells: Protective or pathogenic? Front. Immunol. 2019, 10, 01322. [Google Scholar] [CrossRef]
- Niogret, J.; Berger, H.; Rebe, C.; Mary, R.; Ballot, E.; Truntzer, C.; Thibaudin, M.; Derangère, V.; Hibos, C.; Hampe, L.; et al. Follicular helper-T cells restore CD8 + -dependent antitumor immunity and anti-PD-L1/PD-1 efficacy. J. Immunother. Cancer 2021, 9, e002157. [Google Scholar] [CrossRef]
- Xing, J.; Li, X.; Wang, J.E.C.; Wang, H. Inverse relationship between CD40L expression and cytolytic molecule expression by CD8+CXCR5+ T follicular cytotoxic cells in colorectal cancer. Exp. Cell Res. 2020, 389, 4–11. [Google Scholar] [CrossRef]
- Flynn, M.J.; Hartley, J.A. The emerging role of anti-CD25 directed therapies as both immune modulators and targeted agents in cancer. Br. J. Haematol. 2017, 179, 20–35. [Google Scholar] [CrossRef]
- Naghizadeh, M.; Hatamzade, N.; Larsen, F.T.; Kjaerup, R.B.; Wattrang, E.; Dalgaard, T.S. Kinetics of activation marker expression after in vitro polyclonal stimulation of chicken peripheral T cell. Cytom. Part A 2022, 101, 45–56. [Google Scholar] [CrossRef]
- Xing, J.; Zhang, C.; Yang, X.; Wang, S.; Wang, Z.; Li, X.; Yu, E. CXCR5+CD8+ T cells infiltrate the colorectal tumors and nearby lymph nodes, and are associated with enhanced IgG response in B cells. Exp. Cell Res. 2017, 356, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Chu, F.; Li, H.S.; Liu, X.; Cao, J.; Ma, W.; Ma, Y.; Weng, J.; Zhu, Z.; Cheng, X.; Wang, Z.; et al. CXCR5+CD8+ T cells are a distinct functional subset with an antitumor activity. Leukemia 2019, 33, 2640–2653. [Google Scholar] [CrossRef] [PubMed]
- Bai, M.; Zheng, Y.; Liu, H.; Su, B.; Zhan, Y.; He, H. CXCR5+ CD8+ T cells potently infiltrate pancreatic tumors and present high functionality. Exp. Cell Res. 2017, 361, 39–45. [Google Scholar] [CrossRef]
- Yoshida, N.; Kinugasa, T.; Miyoshi, H.; Sato, K.; Yuge, K.; Ohchi, T.; Fujino, S.; Shiraiwa, S.; Katagiri, M.; Akagi, Y.; et al. A High RORγT/CD3 Ratio is a Strong Prognostic Factor for Postoperative Survival in Advanced Colorectal Cancer: Analysis of Helper T Cell Lymphocytes (Th1, Th2, Th17 and Regulatory T Cells). Ann. Surg. Oncol. 2016, 23, 919–927. [Google Scholar] [CrossRef]
- Watanabe, S.; Yamada, Y.; Murakami, H. Expression of Th1/Th2 cell–related chemokine receptors on CD4+ lymphocytes under physiological conditions. Int. J. Lab. Hematol. 2020, 42, 68–76. [Google Scholar] [CrossRef]
- Razi, S.; Noveiry, B.B.; Keshavarz-Fathi, M.; Rezaei, N. IL-17 and colorectal cancer: From carcinogenesis to treatment. Cytokine 2019, 116, 7–12. [Google Scholar] [CrossRef]
- Chen, J.; Chen, Z. The effect of immune microenvironment on the progression and prognosis of colorectal cancer. Med. Oncol. 2014, 31, 82. [Google Scholar] [CrossRef]
- Rosshirt, N.; Trauth, R.; Platzer, H.; Tripel, E.; Nees, T.A.; Lorenz, H.M.; Tretter, T.; Moradi, B. Proinflammatory T cell polarization is already present in patients with early knee osteoarthritis. Arthritis. Res. Ther. 2021, 23, 1–12. [Google Scholar] [CrossRef]
- Liu, J.; Chang, H.W.; Huang, Z.M.; Nakamura, M.; Sekhon, S.; Ahn, R.; Munoz-Sandoval, P.; Bhattarai, S.; Beck, K.M.; Sanchez, I.M.; et al. Single-cell RNA sequencing of psoriatic skin identifies pathogenic Tc17 cell subsets and reveals distinctions between CD8+ T cells in autoimmunity and cancer. J. Allergy Clin. Immunol. 2021, 147, 2370–2380. [Google Scholar] [CrossRef]
- Shi, Y.; Lin, H.; Cui, J.; Qi, H.; Florholmen, J.; Liu, Z.; Cui, G. The role of interleukin-17A in colorectal tumorigenesis. Cancer Biother. Radiopharm. 2013, 28, 429–432. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Cai, C.; Samir, J.; Palgen, J.L.; Keoshkerian, E.; Li, H.; Bull, R.A.; Luciani, F.; An, H.; Lloyd, A.R. Human CD8 T-stem cell memory subsets phenotypic and functional characterization are defined by expression of CD122 or CXCR3. Eur J. Immunol. 2021, 51, 1732–1747. [Google Scholar] [CrossRef]
- Duckworth, B.C.; Lafouresse, F.; Wimmer, V.C.; Broomfield, B.J.; Dalit, L.; Alexandre, Y.O.; Sheikh, A.A.; Qin, R.Z.; Alvarado, C.; Mielke, L.A.; et al. Effector and stem-like memory cell fates are imprinted in distinct lymph node niches directed by CXCR3 ligands. Nat. Immunol. 2021, 22, 434–448. [Google Scholar] [CrossRef]
- Groom, J.R.; Luster, A.D. CXCR3 in T cell function. Exp. Cell Res. 2011, 317, 620–631. [Google Scholar] [CrossRef] [PubMed]
- Lisowska, K.A.; Pindel, M.; Pietruczuk, K.; Kuźmiuk-Glembin, I.; Storoniak, H.; Dębska-Ślizień, A.; Witkowski, J.M. The influence of a single hemodialysis procedure on human T lymphocytes. Sci. Rep. 2019, 9, 5041. [Google Scholar] [CrossRef] [PubMed]
- Nowicka, A.; Rogala, E.; Bednarek, W.; Barczyński, B.; Wertel, I.; Piekarczyk, W.; Kotarski, J. Lymphocyte activation markers in patients with ovarian cancer. Ginekol. Pol. 2012, 83, 737–743. [Google Scholar] [PubMed]
- Dunne, M.R.; Phelan, J.J.; Michielsen, A.J.; Maguire, A.A.; Dunne, C.; Martin, P.; Noonan, S.; Tosetto, M.; Geraghty, R.; Fennelly, D.; et al. Characterising the prognostic potential of HLA-DR during colorectal cancer development. Cancer Immunol. Immunother. 2020, 69, 1577–1588. [Google Scholar] [CrossRef]

| Variable | (n = 47) |
|---|---|
| Age at surgical resection | |
| Mean ± SD; range | 59.1 ± 12.8; 36–90 |
| Variable | Number (%) |
| Gender | |
| Male | 31 (66) |
| Female | 16 (34) |
| Presentation | |
| Synchronous | 26 (55) |
| Metachronous | 21 (45) |
| Primary tumor location | |
| Sigmoid | 16 (34) |
| Ascending | 10 (21) |
| Descending | 5 (11) |
| Splenic Flexure | 4 (9) |
| Hepatic Flexure | 1 (2) |
| Transverse | 2 (4) |
| Rectal | 9 (19) |
| T stage of the primary colorectal tumor | |
| 3 | 31 (66) |
| 4a | 12 (26) |
| 4b | 2 (4) |
| First approach | |
| Liver First | 4 (9) |
| Colectomy | 34 (72) |
| Synchronous resection | 7 (15) |
| Preoperative systemic chemotherapy | 27 (57) |
| Variable | (n = 47) |
| Number of colorectal liver metastases | |
| Mean ± SD; range | 2.3 ± 1.8; 1–10 |
| Size of the largest colorectal liver metastases (cm) | |
| Mean ± SD; range | 3.8 ± 2.4; 0.4–12 |
| Variable | Number (%) |
| Histologic growth pattern | |
| Desmoplastic | 17 (36) |
| Non-desmoplastic | 30 (64) |
| Status | |
| Dead | 3 (6) |
| Alive | 44 (94) |
| Fluorochromes | ||||||||
|---|---|---|---|---|---|---|---|---|
| Tube | PB | PO | FITC | PE | PerCP-Cy5.5 | PE-Cy7 | APC | APC-H7 |
| 1 | HLA-DR | CD127 | CD8 | CD25 | CD3 | TCR-γ/δ-1 | CD185 | CD4 |
| BD (L243) | BD Horizon (HIL-7R-M21) | BD (SK1) | BD (2A3) | BD (SK7) | BD (11F2) | R&D Systems (51505) | BD (SK3) | |
| 2 μL | 5 μL | 10 μL | 10 μL | 10 μL | 1 μL | 5 μL | 5 μL | |
| 2 | CD4 | CCR5 | TCR-γ/δ-1 | CXCR3 | CD3 | CD56 | CCR6 | CD8 |
| BD Horizon (RPA-T4) | BD OptiBuild (2D7/CCR5) | BD (11F2) | BD Pharmingen 1C6/CXCR3 | BD (SK7) | Beckman Coulter N901 (NKH-1) | BD Pharmingen (11A9) | BD (SK1) | |
| 2 μL | 5 μL | 7 μL | 15 μL | 10 μL | 2.5 μL | 5 μL | 2.5 μL | |
| Cell Types | Non-Tumor (%) ± SEM | Tumor (%) ± SEM | Tumor | |
|---|---|---|---|---|
| Non-Desmoplastic (%) ± SEM | Desmoplastic (%) ± SEM | |||
| T cells | 21.75 ± 2.29 | 6.78 ± 1.30 d | 5.40 ± 1.17 | 9.21 ± 2.90 |
| T cells (after removing the non-hematopoietic cells) | 30.21 ± 3.18 | 21.87 ± 4.18 b | 24.30 ± 3.35 | 34.83 ± 5.88 |
| CD4+ | 21.71 ± 1.46 | 40.54 ± 2.72 d | 40.12 ± 3.66 | 41.29 ± 3.96 |
| CD56+ | 8.14 ± 0.83 | 2.07 ± 0.27 d | 1.43 ± 0.18 | 3.09 ± 0.56 ** |
| CD8+ | 60.71 ± 2.10 | 39.35 ± 2.51 d | 35.16 ± 3.09 | 46.75 ± 3.78 * |
| CD56+ | 34.69 ± 2.22 | 16.04 ± 1.49 d | 14.91 ± 1.67 | 17.90 ± 2.84 |
| CD4+ CD8+ | 4.00 ± 0.39 | 2.21 ± 0.28 d | 1.93 ± 0.28 | 2.69 ± 0.60 |
| CD4− CD8− | 7.59 ± 1.45 | 13.16 ± 3.22 | 17.14 ± 4.54 | 6.13 ± 3.39 * |
| γδ + | 5.90 ± 0.68 | 2.76 ± 0.55 d | 2.52 ± 0.77 | 3.18 ± 0.73 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sampaio-Ribeiro, G.; Ruivo, A.; Silva, A.; Santos, A.L.; Oliveira, R.C.; Laranjeira, P.; Gama, J.; Cipriano, M.A.; Tralhão, J.G.; Paiva, A. Extensive Phenotypic Characterization of T Cells Infiltrating Liver Metastasis from Colorectal Cancer: A Potential Role in Precision Medicine. Cancers 2022, 14, 6069. https://doi.org/10.3390/cancers14246069
Sampaio-Ribeiro G, Ruivo A, Silva A, Santos AL, Oliveira RC, Laranjeira P, Gama J, Cipriano MA, Tralhão JG, Paiva A. Extensive Phenotypic Characterization of T Cells Infiltrating Liver Metastasis from Colorectal Cancer: A Potential Role in Precision Medicine. Cancers. 2022; 14(24):6069. https://doi.org/10.3390/cancers14246069
Chicago/Turabian StyleSampaio-Ribeiro, Gabriela, Ana Ruivo, Ana Silva, Ana Lúcia Santos, Rui Caetano Oliveira, Paula Laranjeira, João Gama, Maria Augusta Cipriano, José Guilherme Tralhão, and Artur Paiva. 2022. "Extensive Phenotypic Characterization of T Cells Infiltrating Liver Metastasis from Colorectal Cancer: A Potential Role in Precision Medicine" Cancers 14, no. 24: 6069. https://doi.org/10.3390/cancers14246069
APA StyleSampaio-Ribeiro, G., Ruivo, A., Silva, A., Santos, A. L., Oliveira, R. C., Laranjeira, P., Gama, J., Cipriano, M. A., Tralhão, J. G., & Paiva, A. (2022). Extensive Phenotypic Characterization of T Cells Infiltrating Liver Metastasis from Colorectal Cancer: A Potential Role in Precision Medicine. Cancers, 14(24), 6069. https://doi.org/10.3390/cancers14246069









