Deploying Indocyanine Green Fluorescence-Guided Navigation System in Precise Laparoscopic Resection of Pediatric Hepatoblastoma
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
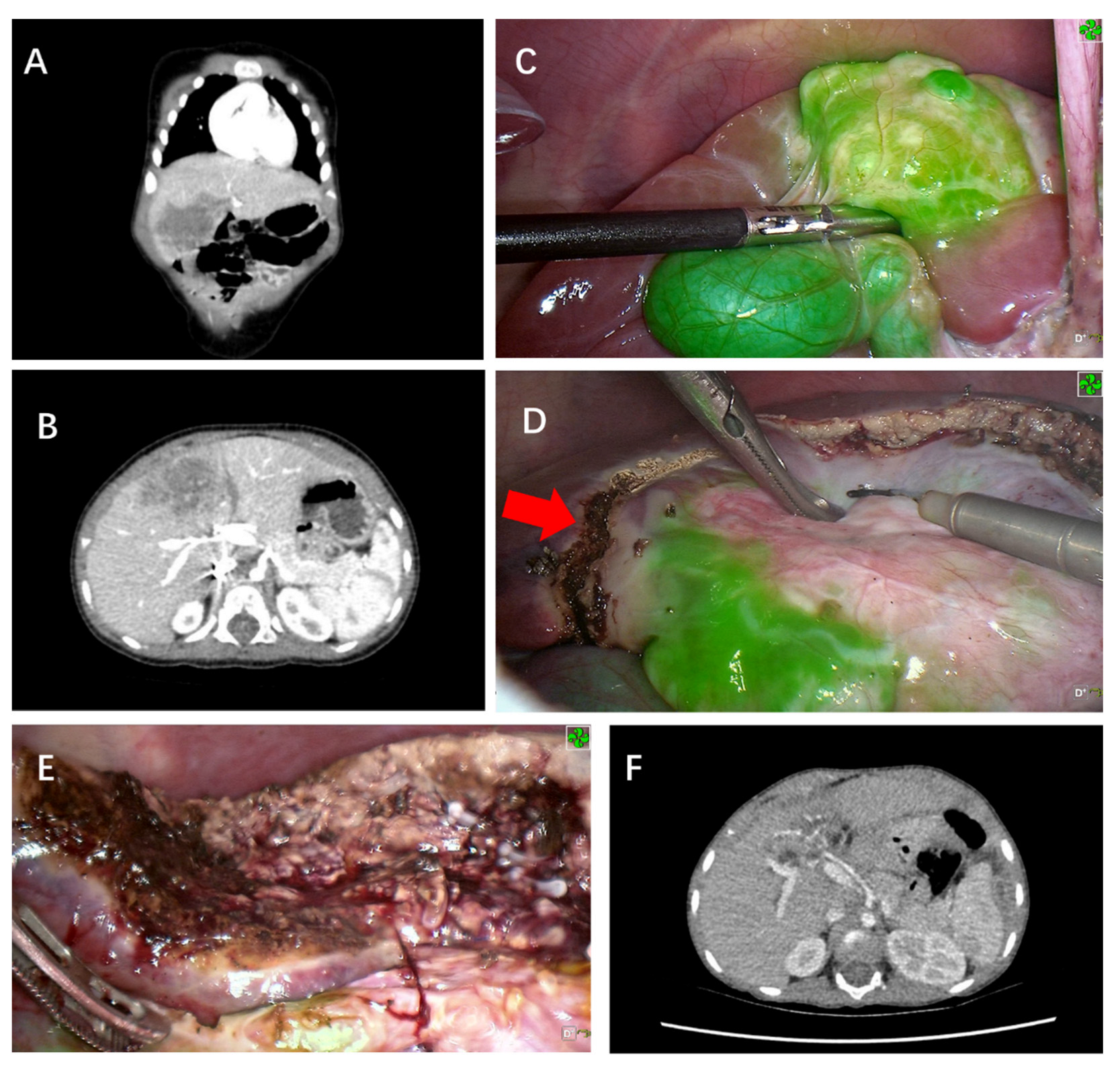
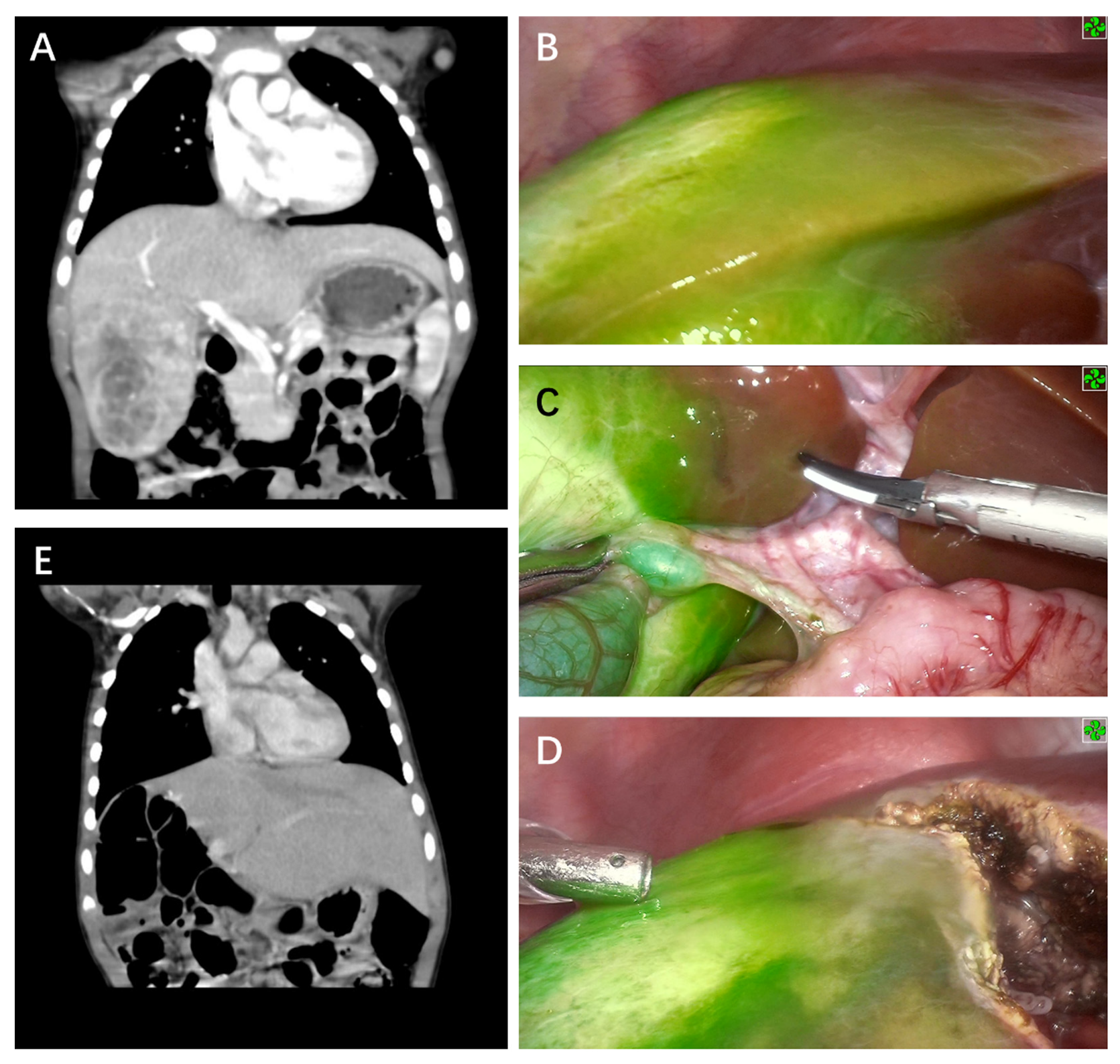
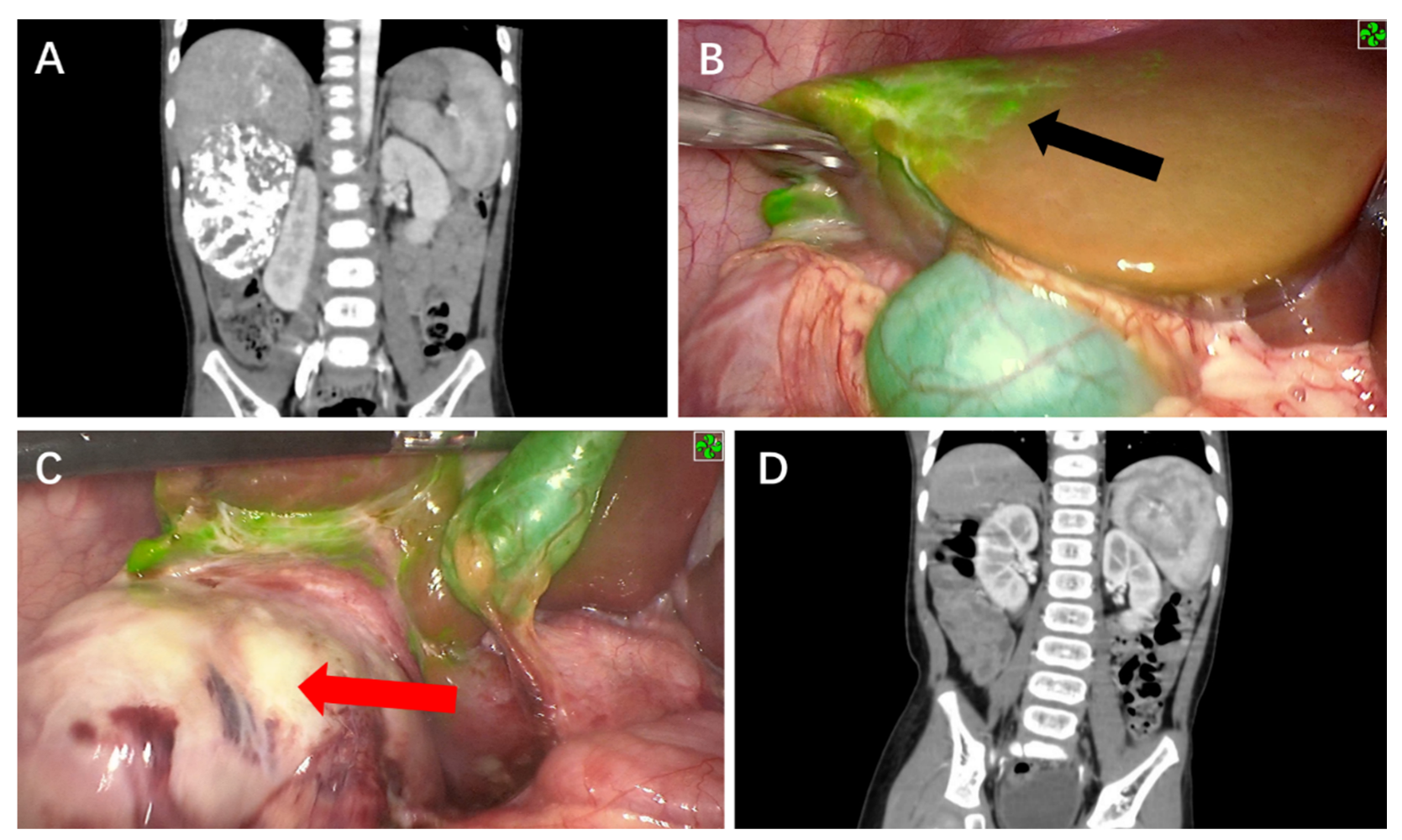
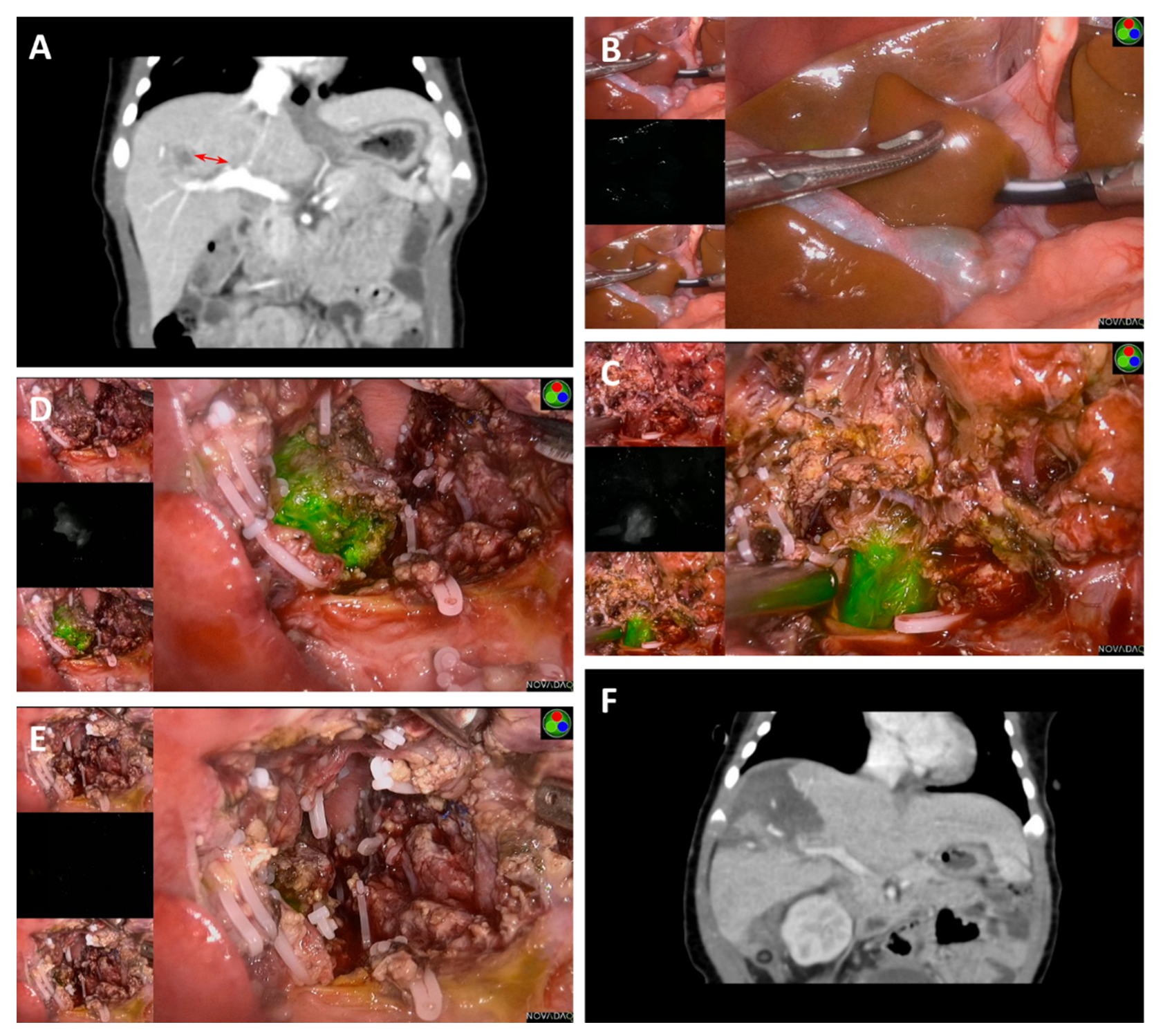
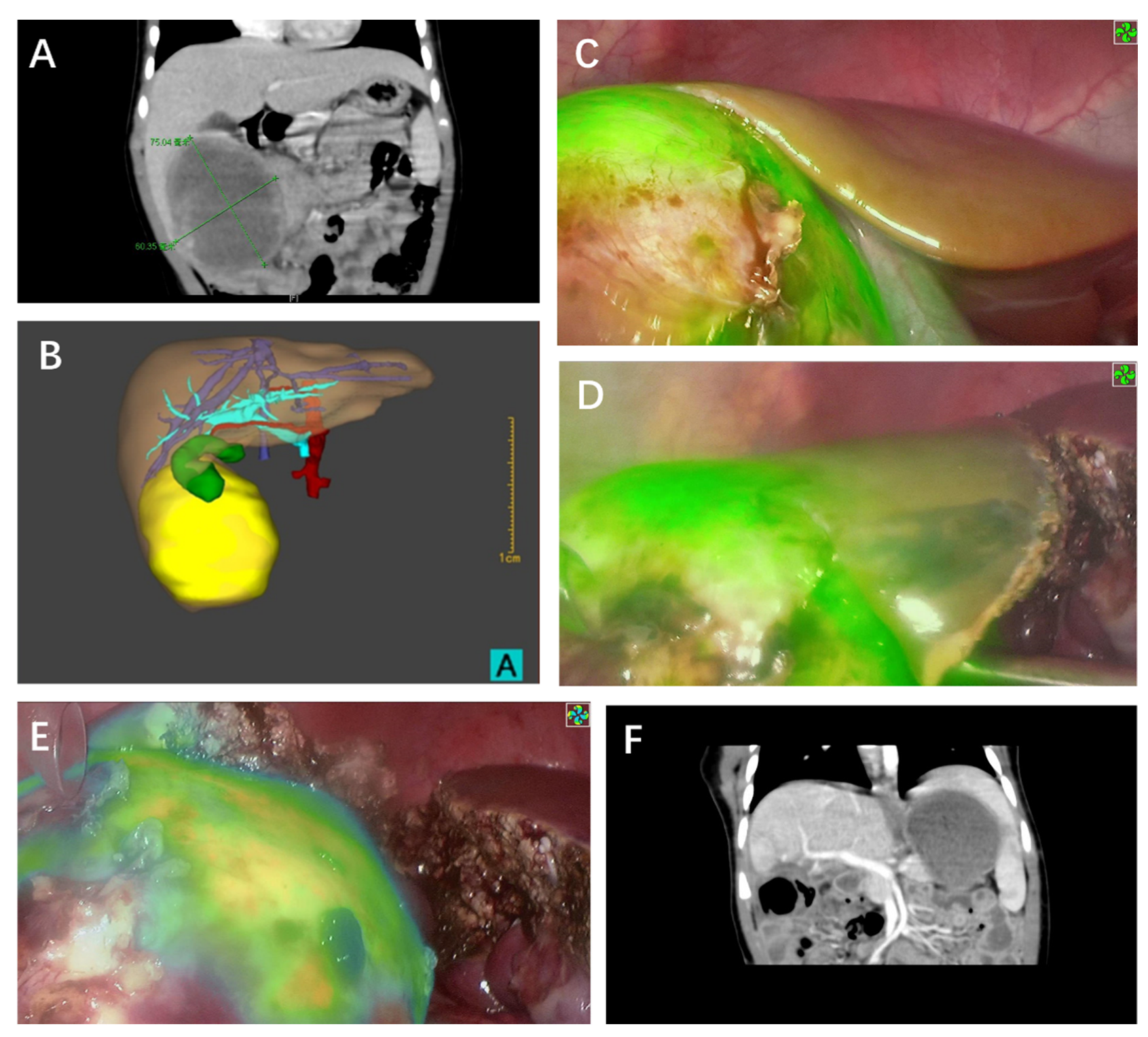
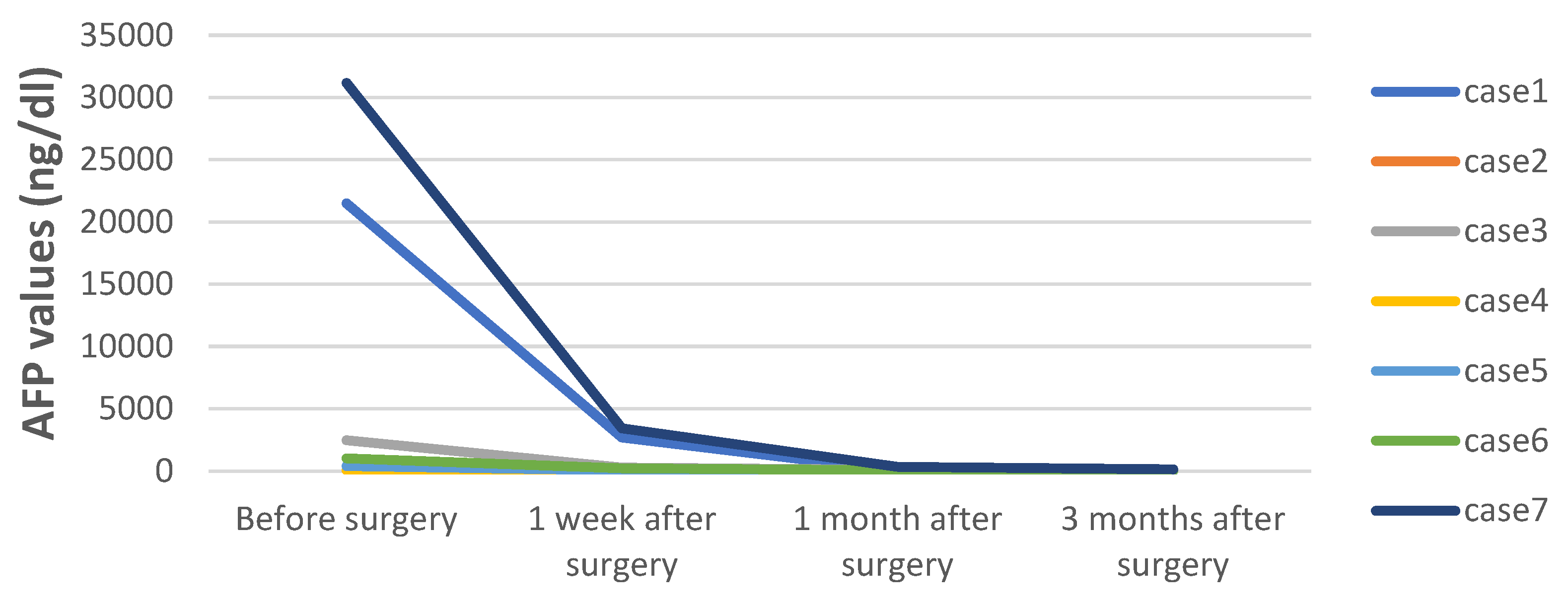
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Meyers, R.; Hiyama, E.; Czauderna, P.; Tiao, G.M. Liver tumors in pediatric patients. Surg. Oncol. Clin. N. Am. 2021, 30, 253–274. [Google Scholar] [CrossRef]
- Busweiler, L.A.; Wijnen, M.H.; Wilde, J.C.; Sieders, E.; Terwisscha, V.S.S.; van Heurn, L.W.; Ziros, J.; Bakx, R.; Heij, H.A. Surgical treatment of childhood hepatoblastoma in the netherlands (1990–2013). Pediatr. Surg. Int. 2017, 33, 23–31. [Google Scholar] [CrossRef]
- Shi, Y.; Commander, S.J.; Masand, P.M.; Heczey, A.; Goss, J.A.; Vasudevan, S.A. Vascular invasion is a prognostic indicator in hepatoblastoma. J. Pediatr. Surg. 2017, 52, 956–961. [Google Scholar] [CrossRef]
- Narasaki, H.; Noji, T.; Wada, H.; Ebihara, Y.; Tsuchikawa, T.; Okamura, K.; Tanaka, E.; Shichinohe, T.; Hirano, S. Intraoperative real-time assessment of liver function with near-infrared fluorescence imaging. Eur. Surg. Res. 2017, 58, 235–245. [Google Scholar] [CrossRef]
- Ishizawa, T.; Fukushima, N.; Shibahara, J.; Masuda, K.; Tamura, S.; Aoki, T.; Hasegawa, K.; Beck, Y.; Fukayama, M.; Kokudo, N. Real-time identification of liver cancers by using indocyanine green fluorescent imaging. Cancer-Am. Cancer Soc. 2009, 115, 2491–2504. [Google Scholar] [CrossRef]
- Liberale, G.; Bourgeois, P.; Larsimont, D.; Moreau, M.; Donckier, V.; Ishizawa, T. Indocyanine green fluorescence-guided surgery after iv injection in metastatic colorectal cancer: A systematic review. Eur. J. Surg. Oncol. 2017, 43, 1656–1667. [Google Scholar] [CrossRef]
- Yamamichi, T.; Oue, T.; Yonekura, T.; Owari, M.; Nakahata, K.; Umeda, S.; Nara, K.; Ueno, T.; Uehara, S.; Usui, N. Clinical application of indocyanine green (icg) fluorescent imaging of hepatoblastoma. J. Pediatr. Surg. 2015, 50, 833–836. [Google Scholar] [CrossRef]
- Lake, C.M.; Bondoc, A.J.; Dasgupta, R.; Jenkins, T.M.; Towbin, A.J.; Smith, E.A.; Alonso, M.H.; Geller, J.I.; Tiao, G.M. Indocyanine green is a sensitive adjunct in the identification and surgical management of local and metastatic hepatoblastoma. Cancer Med. 2021, 10, 4322–4343. [Google Scholar] [CrossRef]
- Yamada, Y.; Hoshino, K.; Mori, T.; Kawaida, M.; Abe, K.; Takahashi, N.; Fujimura, T.; Kameyama, K.; Kuroda, T. Metastasectomy of hepatoblastoma utilizing a novel overlay fluorescence imaging system. J. Laparoendosc. Adv. Surg. Tech. 2018, 28, 1152–1155. [Google Scholar] [CrossRef]
- Souzaki, R.; Kawakubo, N.; Matsuura, T.; Yoshimaru, K.; Koga, Y.; Takemoto, J.; Shibui, Y.; Kohashi, K.; Hayashida, M.; Oda, Y.; et al. Navigation surgery using indocyanine green fluorescent imaging for hepatoblastoma patients. Pediatr. Surg. Int. 2019, 35, 551–557. [Google Scholar] [CrossRef]
- Cho, Y.J.; Namgoong, J.; Kwon, H.H.; Kwon, Y.J.; Kim, D.Y.; Kim, S.C. The advantages of indocyanine green fluorescence imaging in detecting and treating pediatric hepatoblastoma: A preliminary experience. Front. Pediatr. 2021, 9, 635394. [Google Scholar] [CrossRef]
- Misa, Y.; Mio, T.; Norihiko, K.; Kumiko, N.; Masato, S.; Hiroaki, G.; Yukichi, T. Clinicopathological study of surgery for pulmonary metastases of hepatoblastoma with indocyanine green fluorescent imaging. Pediatr. Blood Cancer. 2022, 69, e29488. [Google Scholar]
- Yuanchao, S.; Manna, Z.; Jiahao, L.; Tianbao, T.; Jiliang, Y.; Jing, P.; Chao, H.; Yan, Z.; Tianyou, Y. Clinical Application of Indocyanine Green Fluorescence Imaging in the Resection of Hepatoblastoma: A Single Institution’s Experiences. Front. Surg. 2022, 9, 932721. [Google Scholar]
- Koffron, A.J.; Auffenberg, G.; Kung, R.; Abecassis, M. Evaluation of 300 minimally invasive liver resections at a single institution: Less is more. Ann. Surg. 2007, 246, 385–394. [Google Scholar] [CrossRef]
- Reddy, S.K.; Tsung, A.; Geller, D.A. Laparoscopic liver resection. World J. Surg. 2011, 35, 1478–1486. [Google Scholar] [CrossRef]
- Kwon, H.; Lee, J.Y.; Cho, Y.J.; Kim, D.Y.; Kim, S.C.; Namgoong, J. How to safely perform laparoscopic liver resection for children: A case series of 19 patients. J. Pediatr. Surg. 2019, 54, 2579–2584. [Google Scholar] [CrossRef]
- Urade, T.; Sawa, H.; Iwatani, Y.; Abe, T.; Fujinaka, R.; Murata, K.; Mii, Y.; Man-i, M.; Oka, S.; Kuroda, D. Laparoscopic anatomical liver resection using indocyanine green fluorescence imaging. Asian J. Surg. 2020, 43, 362–368. [Google Scholar] [CrossRef]
- Yada, K.; Ishibashi, H.; Mori, H.; Shimada, M. Laparoscopic resection of hepatoblastoma: Report of a case. Asian J. Endosc. Surg. 2014, 7, 267–270. [Google Scholar] [CrossRef]
- Yuan, X.J.; Wang, H.M.; Jiang, H.; Tang, M.J.; Li, Z.L.; Zou, X.; Fang, Y.J.; Pan, C.; Tou, J.F.; Zhang, K.R.; et al. Multidisciplinary effort in treating children with hepatoblastoma in china. Cancer Lett. 2016, 375, 39–46. [Google Scholar] [CrossRef]
- Hiyama, E.; Hishiki, T.; Watanabe, K.; Ida, K.; Yano, M.; Oue, T.; Iehara, T.; Hoshino, K.; Koh, K.; Tanaka, Y.; et al. Resectability and tumor response after preoperative chemotherapy in hepatoblastoma treated by the Japanese study group for pediatric liver tumor (jplt)-2 protocol. J. Pediatr. Surg. 2016, 51, 2053–2057. [Google Scholar] [CrossRef]
- Wu, Y.; Zeng, L.; Qiu, R.; Zhang, J.; Su, J.; Liao, M.; Deng, X. Two-stage laparoscopic resection of giant hepatoblastoma in infants combined with liver partial partition and artery ligation. World J. Surg. Oncol. 2021, 19, 63. [Google Scholar] [CrossRef]
- Landsman, M.L.; Kwant, G.; Mook, G.A.; Zijlstra, W.G. Light-absorbing properties, stability, and spectral stabilization of indocyanine green. J. Appl. Physiol. 1976, 40, 575–583. [Google Scholar] [CrossRef]
- Nakaseko, Y.; Ishizawa, T.; Saiura, A. Fluorescence-guided surgery for liver tumors. J. Surg. Oncol. 2018, 118, 324–331. [Google Scholar] [CrossRef]
- Kitagawa, N.; Shinkai, M.; Mochizuki, K.; Usui, H.; Miyagi, H.; Nakamura, K.; Tanaka, M.; Tanaka, Y.; Kusano, M.; Ohtsubo, S. Navigation using indocyanine green fluorescence imaging for hepatoblastoma pulmonary metastases surgery. Pediatr. Surg. Int. 2015, 31, 407–411. [Google Scholar] [CrossRef]
- Yamada, Y.; Ohno, M.; Fujino, A.; Kanamori, Y.; Irie, R.; Yoshioka, T.; Miyazaki, O.; Uchida, H.; Fukuda, A.; Sakamoto, S.; et al. Fluorescence-Guided Surgery for Hepatoblastoma with Indocyanine Green. Cancers 2019, 11, 1215. [Google Scholar] [CrossRef]
- de Graaf, W.; Bennink, R.J.; Vetelainen, R.; van Gulik, T.M. Nuclear imaging techniques for the assessment of hepatic function in liver surgery and transplantation. J. Nucl. Med. 2010, 51, 742–752. [Google Scholar] [CrossRef]
- Gotoh, K.; Yamada, T.; Ishikawa, O.; Takahashi, H.; Eguchi, H.; Yano, M.; Ohigashi, H.; Tomita, Y.; Miyamoto, Y.; Imaoka, S. A novel image-guided surgery of hepatocellular carcinoma by indocyanine green fluorescence imaging navigation. J. Surg. Oncol. 2009, 100, 75–79. [Google Scholar] [CrossRef]
- Lim, C.; Vibert, E.; Azoulay, D.; Salloum, C.; Ishizawa, T.; Yoshioka, R.; Mise, Y.; Sakamoto, Y.; Aoki, T.; Sugawara, Y.; et al. Indocyanine green fluorescence imaging in the surgical management of liver cancers: Current facts and future implications. J. Visc. Surg. 2014, 151, 117–124. [Google Scholar] [CrossRef]
- Hsu, C.W. Lymph node mapping and anastomosis evaluation by visera elite ii®, a novel surgical endoscope system, with infrared fluorescence imaging during laparoscopic rectal cancer surgery—A video vignette. Color. Dis. 2019, 21, 375–376. [Google Scholar] [CrossRef]
- Morita, Y.; Sakaguchi, T.; Unno, N.; Shibasaki, Y.; Suzuki, A.; Fukumoto, K.; Inaba, K.; Baba, S.; Takehara, Y.; Suzuki, S.; et al. Detection of hepatocellular carcinomas with near-infrared fluorescence imaging using indocyanine green: Its usefulness and limitation. Int. J. Clin. Oncol. 2013, 18, 232–241. [Google Scholar] [CrossRef]
| Case | Sex | Age at Surgery | PRETEXT | POST-TEXT | Chemotherapy | AFP at Surgery (ng/dL) | Tumor Diameter (mm) | Interval Time of ICG Injection/Surgery | ICG Dosage (mg/Kg) | Tumor Depth from Liver Surface | Surgical Procedure | Operation Time (min) | Pathology | Follow-up Days | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Male | 14 M | III | II | 4 C5VD | 21,492 | 75 | 48 h | 0.5 | 0mm | PH (S5,S6) | 250 | Fetal and embryonal | 1127 | Alive without disease |
| 2 | Male | 22 M | III | II | 3 C5VD | 60.37 | 70 | 48 h | 0.5 | 0mm | PH (S5,6,7) | 295 | Embryonal | 1085 | Alive without disease |
| 3 | Male | 9 M | III | II | 4 C5VD | 2468 | 65 | 48 h | 0.5 | 0mm | RH | 410 | Epithelial and mesenchymal | 1012 | Alive without disease |
| 4 | Male | 36 M | III | II | 3 CCD + 2 ICE | 82.5 | 87 | 48 h | 0.5 | 0mm | CBS | 570 | Fetal | 779 | Alive without disease |
| 5 | Male | 13 M | III | II | 4 C5VD | 398.6 | 47 | 48 h | 0.5 | 0mm | PH (S4a) | 310 | Fetal | 448 | Alive without disease |
| 6 | Male | 3 M | II | I | 2 C5VD | 1025 | 25 | 48 h | 0.5 | 11.5mm | PH (S8) | 430 | Epithelial and mesenchymal | 259 | Alive without disease |
| 7 | Female | 13 D | I | I | None | 31,178 | 30 | 48 h | 0.5 | 0mm | LLS | 250 | Fetal | 247 | Alive without disease |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiu, R.; Wu, Y.; Su, J.; Chen, L.; Liao, M.; Zhao, Z.; Lu, Z.; Xiong, X.; Jin, S.; Deng, X. Deploying Indocyanine Green Fluorescence-Guided Navigation System in Precise Laparoscopic Resection of Pediatric Hepatoblastoma. Cancers 2022, 14, 6057. https://doi.org/10.3390/cancers14246057
Qiu R, Wu Y, Su J, Chen L, Liao M, Zhao Z, Lu Z, Xiong X, Jin S, Deng X. Deploying Indocyanine Green Fluorescence-Guided Navigation System in Precise Laparoscopic Resection of Pediatric Hepatoblastoma. Cancers. 2022; 14(24):6057. https://doi.org/10.3390/cancers14246057
Chicago/Turabian StyleQiu, Ronglin, Yaohao Wu, Jianhang Su, Luping Chen, Minyi Liao, Zhuangjie Zhao, Zijie Lu, Xiangang Xiong, Shikai Jin, and Xiaogeng Deng. 2022. "Deploying Indocyanine Green Fluorescence-Guided Navigation System in Precise Laparoscopic Resection of Pediatric Hepatoblastoma" Cancers 14, no. 24: 6057. https://doi.org/10.3390/cancers14246057
APA StyleQiu, R., Wu, Y., Su, J., Chen, L., Liao, M., Zhao, Z., Lu, Z., Xiong, X., Jin, S., & Deng, X. (2022). Deploying Indocyanine Green Fluorescence-Guided Navigation System in Precise Laparoscopic Resection of Pediatric Hepatoblastoma. Cancers, 14(24), 6057. https://doi.org/10.3390/cancers14246057





