Particle Therapy in Adult Patients with Pelvic Ewing Sarcoma—Tumor and Treatment Characteristics and Early Clinical Outcomes
Abstract
Simple Summary
Abstract
1. Introduction
2. Material and Methods
2.1. Patients and Treatment Concepts
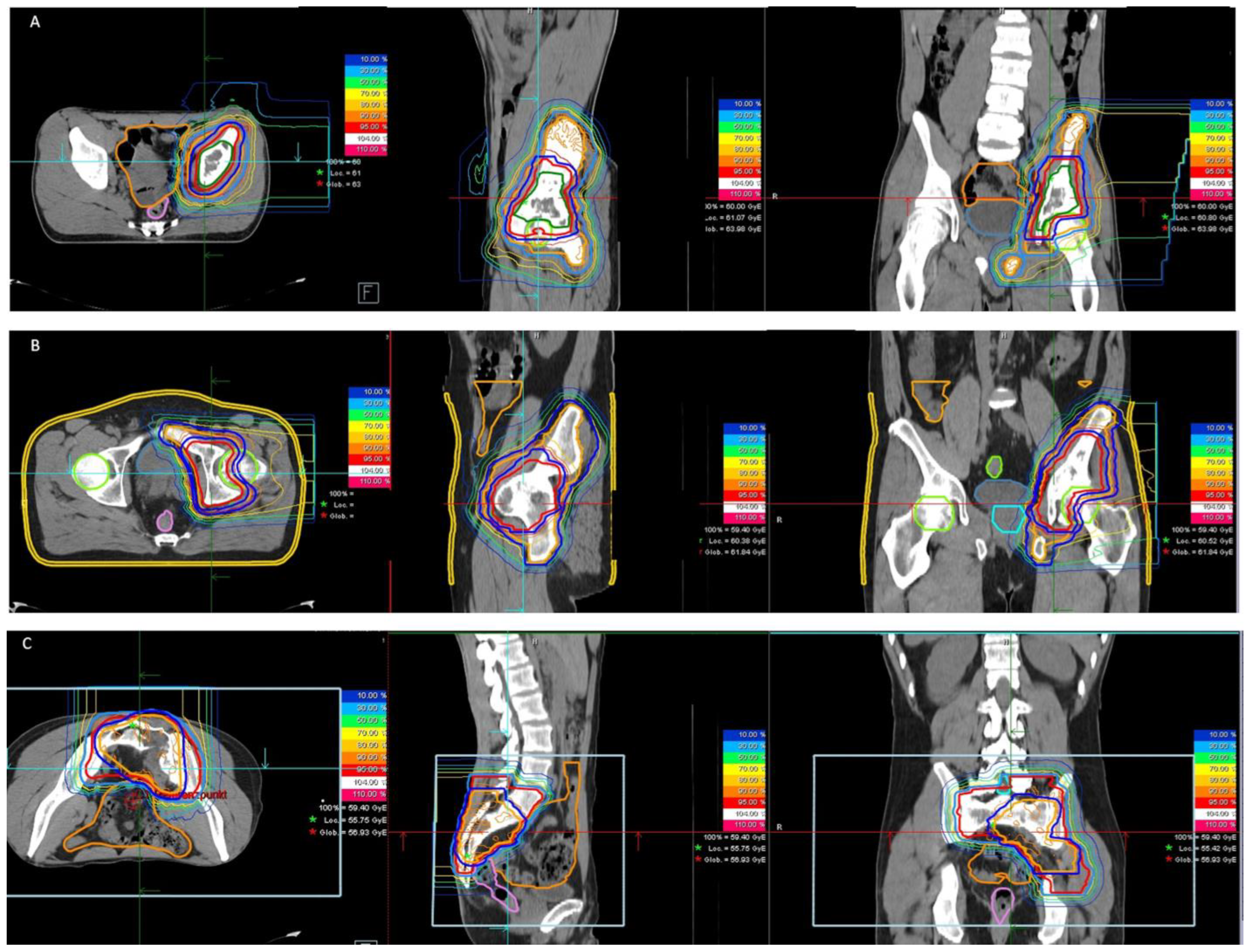
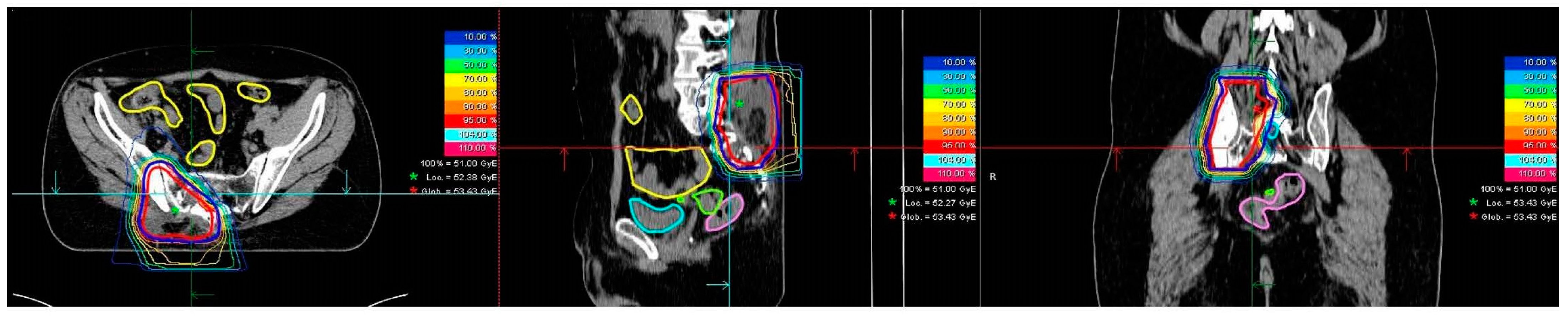
2.2. Radiotherapy
2.3. Data Collection and Study Endpoints
3. Results
3.1. Patient Cohort
3.2. Treatment Characteristics
3.3. Clinical Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bernstein, M.; Kovar, H.; Paulussen, M.; Randall, R.L.; Schuck, A.; Teot, L.A.; Juergens, H. Ewing’s sarcoma family of tumors: Current management. Oncologist 2006, 11, 503–519. [Google Scholar] [CrossRef] [PubMed]
- Scobioala, S.; Eich, H.T. Risk stratification of pulmonary toxicities in the combination of whole lung irradiation and high-dose chemotherapy for Ewing sarcoma patients with lung metastases: A review. Strahlenther. Onkol. 2020, 196, 495–504. [Google Scholar] [CrossRef] [PubMed]
- König, L.; Bougatf, N.; Hörner-Rieber, J.; Chaudhri, N.; Mielke, T.; Klüter, S.; Haefner, M.F.; Rieken, S.; Haberer, T.; Debus, J.; et al. Considative mediastinal irradiation of malignant lymphoma using active scanning proton beams: Clinical outcome and dosimetric comparison. Strahlenther. Onkol. 2019, 195, 677–687. [Google Scholar] [CrossRef] [PubMed]
- Baumann, B.C.; Mitra, N.; Harton, J.; Xiao, Y.; Wojcieszynski, A.P.; Gabriel, P.E.; Zhong, H.; Geng, H.; Doucette, A.; Wei, J.; et al. Comparative Effectiveness of Proton vs Photon Therapy as Part of Concurrent Chemoradiotherapy for Locally Advanced Cancer. JAMA Oncol. 2020, 6, 237–246. [Google Scholar] [CrossRef]
- Weber, U.; Kraft, G. Comparison of Carbon Ions Versus Protons. Cancer J. 2009, 15, 325–332. [Google Scholar] [CrossRef]
- Uhl, M.; Herfarth, K.; Debus, J. Comparing the Use of Protons and Carbon Ions for Treatment. Cancer J. 2014, 20, 433–439. [Google Scholar] [CrossRef]
- Haberer, T.; Becher, W.; Schardt, D.; Kraft, G. Magnetic scanning system for heavy ion therapy. NIM A 1993, 330, 296–305. [Google Scholar] [CrossRef]
- Hesla, A.C.; Tsagozis, P.; Jebsen, N.; Zaikova, O.; Bauer, H.; Brosjo, O. Improved prognosis for patients with Ewing sarcoma in the sacrum compared with the innominate bones: The Scandinavian Sarcoma Group experience. J. Bone Joint. Surg. Am. 2016, 98, 199–210. [Google Scholar] [CrossRef]
- Indelicato, D.J.; Keole, S.R.; Shahlaee, A.H.; Shi, W.; Morris, C.G.; Gibbs, C.P., Jr.; Scarborough, M.T.; Marcus, R.B., Jr. Impact of local man-agement on long-term outcomes in Ewing tumors of the pelvis and sacral bones: The University of Florida experience. Int. J. Radiat. Oncol. Biol. Phys. 2008, 72, 41–48. [Google Scholar] [CrossRef]
- Andreou, D.; Ranft, A.; Gosheger, G.; Timmermann, B.; Ladenstein, R.; Hartmann, W.; Bauer, S.; Baumhoer, D.; van den Berg, H.; Dijkstra, P.D.S.; et al. Which Factors Are Associated with Local Control and Survival of Patients with Lo-calized Pelvic Ewing’s Sarcoma? A Retrospective Analysis of Data from the Euro-EWING99 Trial. Clin. Orthop. Relat. Res. 2020, 478, 290–302. [Google Scholar] [CrossRef]
- Uezono, H.; Indelicato, D.J.; Rotondo, R.L.; Vega, R.B.M.; Bradfield, S.M.; Morris, C.G.; Bradley, J.A. Treatment Outcomes After Proton Therapy for Ewing Sarcoma of the Pelvis. Int. J. Radiat. Oncol. 2020, 107, 974–981. [Google Scholar] [CrossRef] [PubMed]
- Bosma, S.E.; Ayu, O.; Fiocco, M.; Gelderblom, H.; Dijkstra, P.D.S. Prognostic factors for survival in Ewing sarcoma: A systematic review. Surg. Oncol. 2018, 27, 603–610. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.K.; Robinson, S.I.; Arndt, C.A.S.; Petersen, I.A.; Haddock, M.G.; Rose, P.S.; Laack, N.N.I. Pelvis Ewing sarcoma: Local control and survival in the modern era. Pediatr. Blood Cancer 2017, 64, 26540. [Google Scholar] [CrossRef] [PubMed]
- Mounessi, F.S.; Lehrich, P.; Haverkamp, U.; Willich, N.; Bölling, T.; Eich, H.T. Pelvic Ewing sarcomas. Three-dimensional conformal vs. intensity-modulated radiotherapy. Strahlenther. Onkol. 2013, 189, 308–314. [Google Scholar] [CrossRef] [PubMed]
- Doyen, J.; Falk, A.T.; Floquet, V.; Herault, J.; Hannoun-Levi, J.M. Proton beams in cancer treatments: Clinical outcomes and dosi-metric comparisons with photon therapy. Cancer Treat. Rev. 2016, 43, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Guerrero Urbano, T.; Khoo, V.; Staffurth, J.; Norman, A.; Buffa, F.; Jackson, A.; Adams, E.; Hansen, V.; Clark, C.; Miles, E.; et al. Intensity-modulated radiotherapy allows escalation of the radiation dose to the pelvic lymph nodes in patients with locally advanced prostate cancer: Preliminary results of a phase I dose escalation study. Clin. Oncol. 2010, 22, 236–244. [Google Scholar] [CrossRef]
- Chopra, S.; Dora, T.; Chinnachamy, A.; Thomas, B.; Kannan, S.; Engineer, R.; Mahantshetty, U.; Phurailatpam, R.; Paul, S.N.; Shrivastava, S.K. Predictors of Grade 3 or Higher Late Bowel Toxicity in Patients Undergoing Pelvic Radiation for Cervical Cancer: Results From a Prospective Study. Int. J. Radiat. Oncol. 2014, 88, 630–635. [Google Scholar] [CrossRef]
- Smet, S.; Pötter, R.; Haie-Meder, C.; Lindegaard, J.C.; Schulz-Juergenliemk, I.; Mahantshetty, U.; Segedin, B.; Bruheim, K.; Hoskin, P.; Rai, B.; et al. Fatigue, insomnia and hot flashes after definitive radiochemotherapy and image-guided adaptive brachytherapy for locally advanced cervical cancer: An analysis from the EMBRACE study. Radiother. Oncol. 2018, 127, 440–448. [Google Scholar] [CrossRef]
- Smet, S.; Spampinato, S.; Pötter, R.; Jürgenliemk-Schulz, I.M.; Nout, R.A.; Chargari, C.; Mahantshetty, U.; Sturdza, A.; Segedin, B.; Bruheim, K.; et al. Risk factors for persistent late fatigue after radiochemotherapy in cervical cancer (EM-BRACE study). Int. J. Radiat. Oncol. Biol. Phys. 2022, 112, 1177–1189. [Google Scholar] [CrossRef]
- Paulino, A.C.; Nguyen, T.X.; Mai, W.Y.; Teh, B.S.; Wen, B.-C. Dose response and local control using radiotherapy in non-metastatic Ewing sarcoma. Pediatr. Blood Cancer 2007, 49, 145–148. [Google Scholar] [CrossRef]
- Laskar, S.; Sinha, S.; Chatterjee, A.; Khanna, N.; Manjali, J.J.; Puri, A.; Gulia, A.; Nayak, P.; Vora, T.; Chinnaswamy, G.; et al. Radiation Therapy Dose Escalation in Unresectable Ewing Sarcoma: Final Results of a Phase 3 Randomized Controlled Trial. Int. J. Radiat. Oncol. 2022, 113, 996–1002. [Google Scholar] [CrossRef] [PubMed]
- Yock, T.I.; Krailo, M.; Fryer, C.J.; Donaldson, S.S.; Miser, J.S.; Chen, Z.; Bernstein, M.; Laurie, F.; Gebhardt, M.C.; Grier, H.E.; et al. Local control in pelvic Ewing sarcoma: Analysis from INT-0091—A report from the Children’s On-cology Group. J. Clin. Oncol. 2006, 24, 3838–3843. [Google Scholar] [CrossRef] [PubMed]
| Parameter | (n) | |
|---|---|---|
| Total number of patients (n) | 21 | |
| Median age at diagnosis in years (range) | 23 (18–55) | |
| Gender (n) | ||
| male | 16 | |
| female | 5 | |
| Median Karnofsky Index at radiotherapy (range) | 90 (60–100) | |
| Local tumor status at diagnosis (n) | ||
| primary | 18 | |
| recurrent | 3 | |
| Location (n) | ||
| innominate bones | 8 | |
| sacrum | 6 | |
| both (sacrum + innominate) | 6 | |
| extraskeletal | 1 | |
| ESWR1 rearrangement (n) | ||
| yes | 12 | |
| no | 1 | |
| unknown | 8 | |
| Distant tumor status at diagnosis (n) | ||
| no metastasis | 19 | |
| bone metastasis | 1 | |
| bone + lung metastasis | 1 | |
| Chemotherapy regimen (n) | ||
| Euro-Ewing 2008 | 13 | |
| ISG/SSG-3 | 3 | |
| ISG/SSG-4 | 1 | |
| temodal + irinotecan/topotecan | 3 | |
| VAC + IE | 1 | |
| Radiotherapy setting (n) | ||
| definitive | 16 | |
| adjuvant | 4 | |
| additive | 1 | |
| Type of radiotherapy (n) | ||
| proton | 18 | |
| carbon ion | 3 |
| Parameter | |
|---|---|
| Median GTV-init in cm3 (range) | 307 (15–2599) |
| Median GTV at radiotherapy in cm3 (range) * | 161 (13–2095) |
| Median tumor regression after Cht induction in % | 55 (15–95) |
| Median CTV-init in cm3 (range) | 1215 (207–3385) |
| Median CTV-boost in cm3 (range) * | 458 (121–2110) |
| Median PTV-init in cm3 (range) | 1630 (337–4798) |
| Median PTV-boost in cm3 (range) * | 655 (212–2630) |
| Proton | VMAT | p-Value | ||
|---|---|---|---|---|
| Primary tumor treated in definitive setting (protons) | n = 15 | |||
| median total prescribed dose (range) in Gy (RBE) | 59.4 (54–60) | |||
| median single prescribed dose (range) in Gy (RBE) | 1.8 (1.5 *–2) | |||
| Primary tumor treated in adjuvant setting (protons) | n = 3 | |||
| median total prescribed dose (range) in Gy (RBE) | 45 (45–54) | |||
| median single prescribed dose (range) in Gy (RBE) | 1.8 (1.8) | |||
| Recurrent tumor treated in definitive/additive/ adjuvant setting (carbon ions) | n = 3 | |||
| median total prescribed dose (range) in Gy (RBE) | 51 | |||
| median single prescribed dose (range) in Gy (RBE) | 3 | |||
| n = 18 ** | ||||
| PTV (median (range) in Gy (RBE)) *** | ||||
| D2% | 57 (46–62) | 58(46–62) | 0.920 | |
| Dmean | 56 (44–60) | 53 (45–59) | 0.130 | |
| D98% | 51 (37–58) | 45(40–51) | 0.002 | |
| Bladder (median (range) in Gy (RBE)) | ||||
| Dmean | 0.6 (0–47) | 17 (0–54) | 0.034 | |
| Dmax | 31 (0–62) | 44 (0–62) | 0.288 | |
| D2cc | 11 (0–61) | 38 (0–61) | 0.327 | |
| V40 Gy in % | 0 (0–80) | 1 (0–100) | 0.560 | |
| Rectum (median (range) in Gy (RBE)) | ||||
| Dmean | 9 (0–23) | 29 (1–40) | 0.000 | |
| Dmax | 43 (0–60) | 53 (2–60) | 0.112 | |
| D2cc | 32 (0–56) | 47 (1–58) | 0.063 | |
| V40 Gy in % | 0.5 (0–18) | 17 (0–59) | 0.005 | |
| Bowel (median (range) in Gy (RBE)) | ||||
| Dmax | 50 (27–53) | 50 (42–57) | 0.058 | |
| D1% | 41 (11–50) | 46(38–52) | 0.014 | |
| V40 Gy in cm3 | 15 (0–199) | 59 (2–198) | 0.015 | |
| V30 Gy in cm3 | 42 (0–260) | 149 (35–394) | 0.001 | |
| V15 Gy in cm3 | 82 (2–347) | 502 (170–1174) | 0.000 | |
| Femoral head in proximity to target volume in Gy (RBE) | ||||
| Dmean | 6 (0–58) | 24 (0–59) | 0.428 | |
| Dmax | 43 (0–62) | 45 (0–62) | 0.640 | |
| Cauda in Gy (RBE) | ||||
| Dmax | 52 (0–59) | 55 (3–62) | 0.340 | |
| Uterus in Gy (RBE) | ||||
| Dmean | 0.2 (0–0.5) | 10 (1–23) | 0.022 | |
| Dmax | 8 (0–15) | 27 (2–45) | ||
| Testicles in Gy (RBE) | ||||
| Dmax | 0 (0–4) | 1 (0–23) | 0.049 |
| G1 | G2 | Overall | ||
|---|---|---|---|---|
| n | n | n (%) | ||
| Start of radiotherapy (n = 21) | ||||
| Skin | 0 | 0 | 0 (0%) | |
| Fatigue | 1 | 0 | 1 (5%) | |
| Pain | 7 | 2 | 9 (43%) | |
| Gastrointestinal | 3 | 0 | 3 (14%) | |
| Urinary | 1 | 0 | 1 (5%) | |
| Sensory | 7 | 0 | 7 (33%) | |
| Motor function | 1 | 4 | 5 (24%) | |
| End of radiotherapy (n = 21) | ||||
| Skin | 10 | 6 | 16 (76%) | |
| Fatigue | 8 | 1 | 9 (43%) | |
| Pain | 5 | 1 | 6 (29%) | |
| Gastrointestinal | 4 | 0 | 4 (19%) | |
| Urinary | 3 | 1 | 4 (19%) | |
| Sensory | 5 | 0 | 5 (24%) | |
| Motor function | 2 | 3 | 5 (24%) | |
| Follow-up (n = 11) | ||||
| Skin | 4 | 0 | 4 (36%) | |
| Fatigue | 1 | 0 | 1 (9%) | |
| Pain | 2 | 0 | 2 (18%) | |
| Gastrointestinal | 0 | 0 | 0 (0%) | |
| Urinary | 1 | 0 | 1 (9%) | |
| Sensory | 0 | 1 | 1 (9%) | |
| Motor function | 0 | 1 | 1 (9%) | |
| Soft tissue | 3 | 0 | 3 (27%) | |
| Bone | 2 | 0 | 2 (18%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schmid, M.P.; Harrabi, S.; Herfarth, K.; Bruland, Ø.S.; Welzel, T.; Haberer, T.; Ellerbrock, M.; Debus, J.; Uhl, M.; Seidensaal, K. Particle Therapy in Adult Patients with Pelvic Ewing Sarcoma—Tumor and Treatment Characteristics and Early Clinical Outcomes. Cancers 2022, 14, 6045. https://doi.org/10.3390/cancers14246045
Schmid MP, Harrabi S, Herfarth K, Bruland ØS, Welzel T, Haberer T, Ellerbrock M, Debus J, Uhl M, Seidensaal K. Particle Therapy in Adult Patients with Pelvic Ewing Sarcoma—Tumor and Treatment Characteristics and Early Clinical Outcomes. Cancers. 2022; 14(24):6045. https://doi.org/10.3390/cancers14246045
Chicago/Turabian StyleSchmid, Maximilian P., Semi Harrabi, Klaus Herfarth, Øyvind S. Bruland, Thomas Welzel, Thomas Haberer, Malte Ellerbrock, Jürgen Debus, Matthias Uhl, and Katharina Seidensaal. 2022. "Particle Therapy in Adult Patients with Pelvic Ewing Sarcoma—Tumor and Treatment Characteristics and Early Clinical Outcomes" Cancers 14, no. 24: 6045. https://doi.org/10.3390/cancers14246045
APA StyleSchmid, M. P., Harrabi, S., Herfarth, K., Bruland, Ø. S., Welzel, T., Haberer, T., Ellerbrock, M., Debus, J., Uhl, M., & Seidensaal, K. (2022). Particle Therapy in Adult Patients with Pelvic Ewing Sarcoma—Tumor and Treatment Characteristics and Early Clinical Outcomes. Cancers, 14(24), 6045. https://doi.org/10.3390/cancers14246045







