Resistin Promotes Nasopharyngeal Carcinoma Metastasis through TLR4-Mediated Activation of p38 MAPK/NF-κB Signaling Pathway
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Samples, Ethical Approval and Resistin Determination
2.2. Animal Study
2.3. Cell Culture and Regents
2.4. Cell Viability and Proliferation Assays
2.5. Wound-Healing Assay
2.6. Migration and Invasion Assays
2.7. Transient Transfection with Small Interfering RNA (siRNA)
2.8. RNA Extraction and qRT-PCR
2.9. Western Blot Analysis
2.10. Immunofluorescence Staining
2.11. Dual-Luciferase Reporter Assay
2.12. Immunohistochemistry Staining
2.13. Statistical Analysis
3. Results
3.1. Clinical Correlation of Serum Resistin Levels with the Risk of NPC
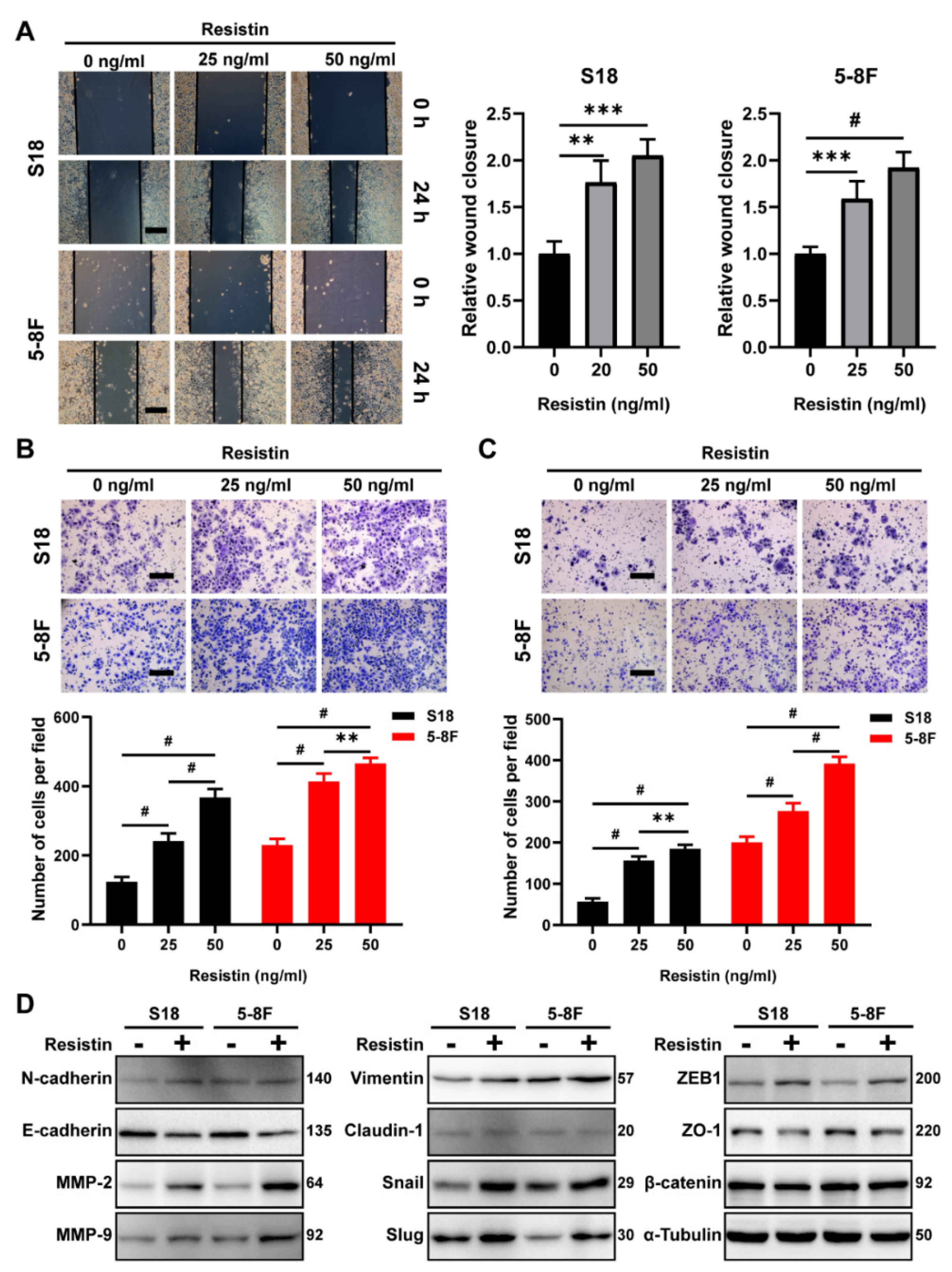
3.2. Resistin Does Not Affect the Proliferation but Promotes the Migration and Invasion in NPC Cells
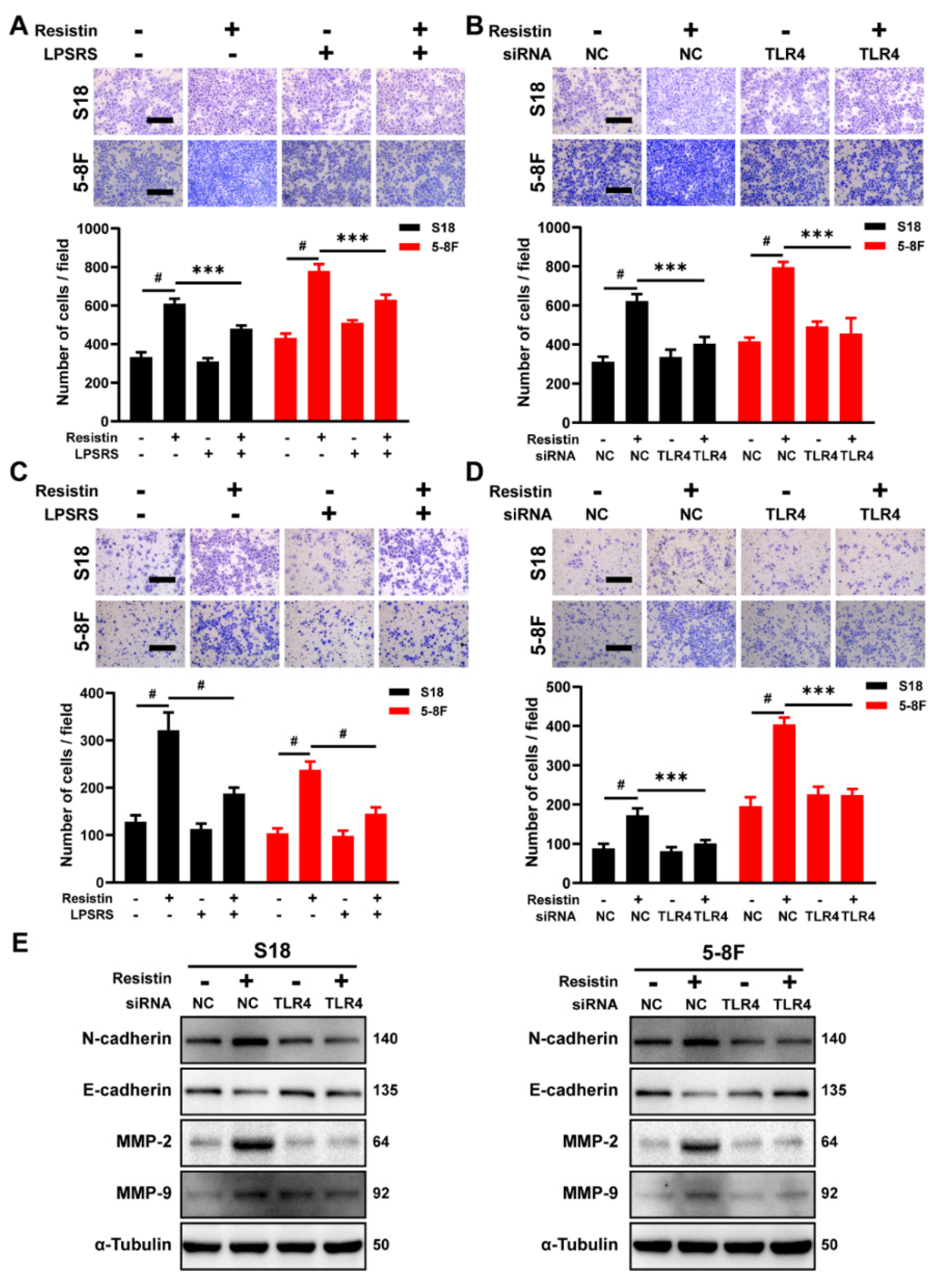
3.3. TLR4 Is Necessary for Resistin-Induced NPC Cell Migration
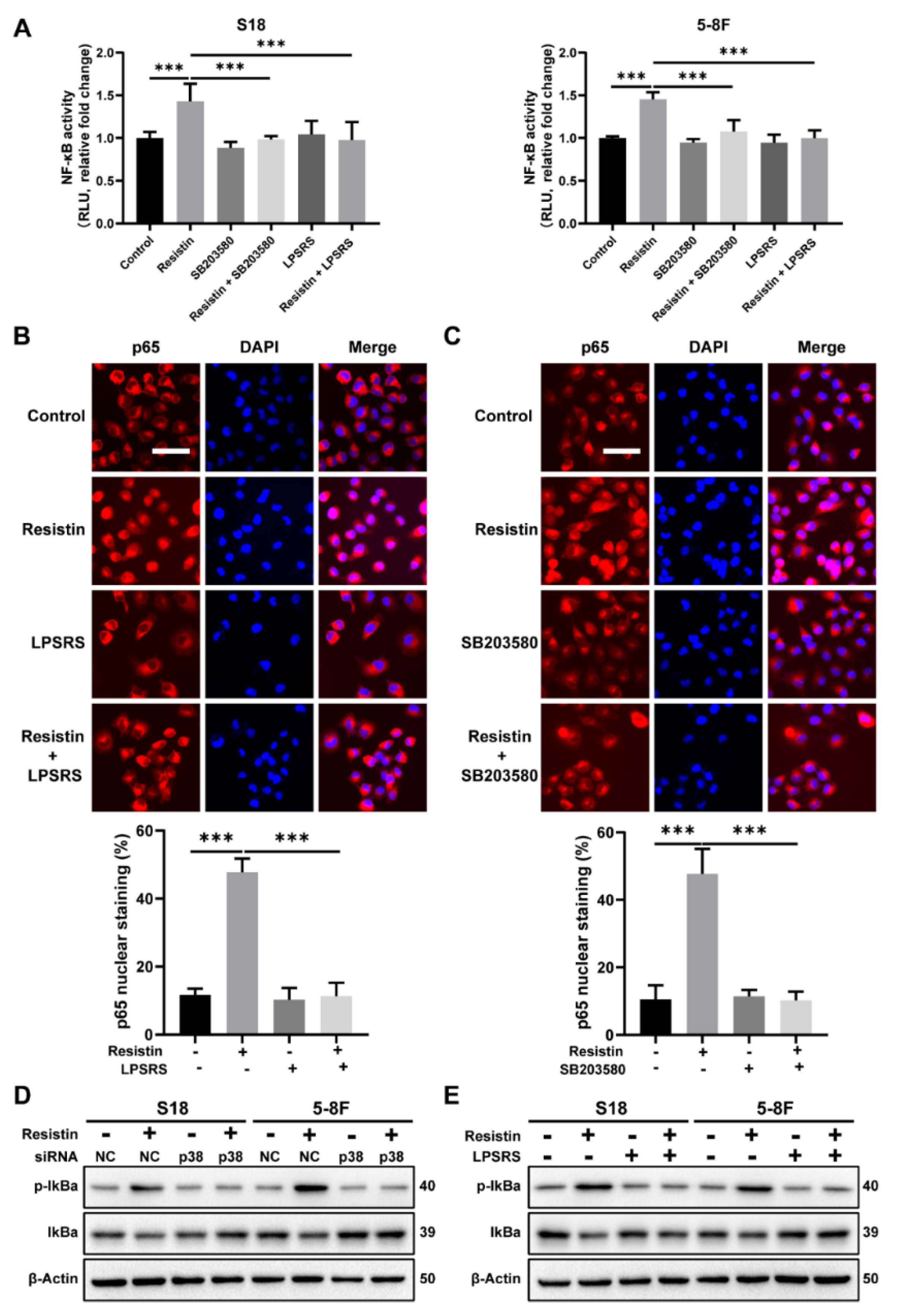
3.4. The p38 MAPK Signaling Pathway Is Involved in Resistin-Induced Migration in NPC Cells
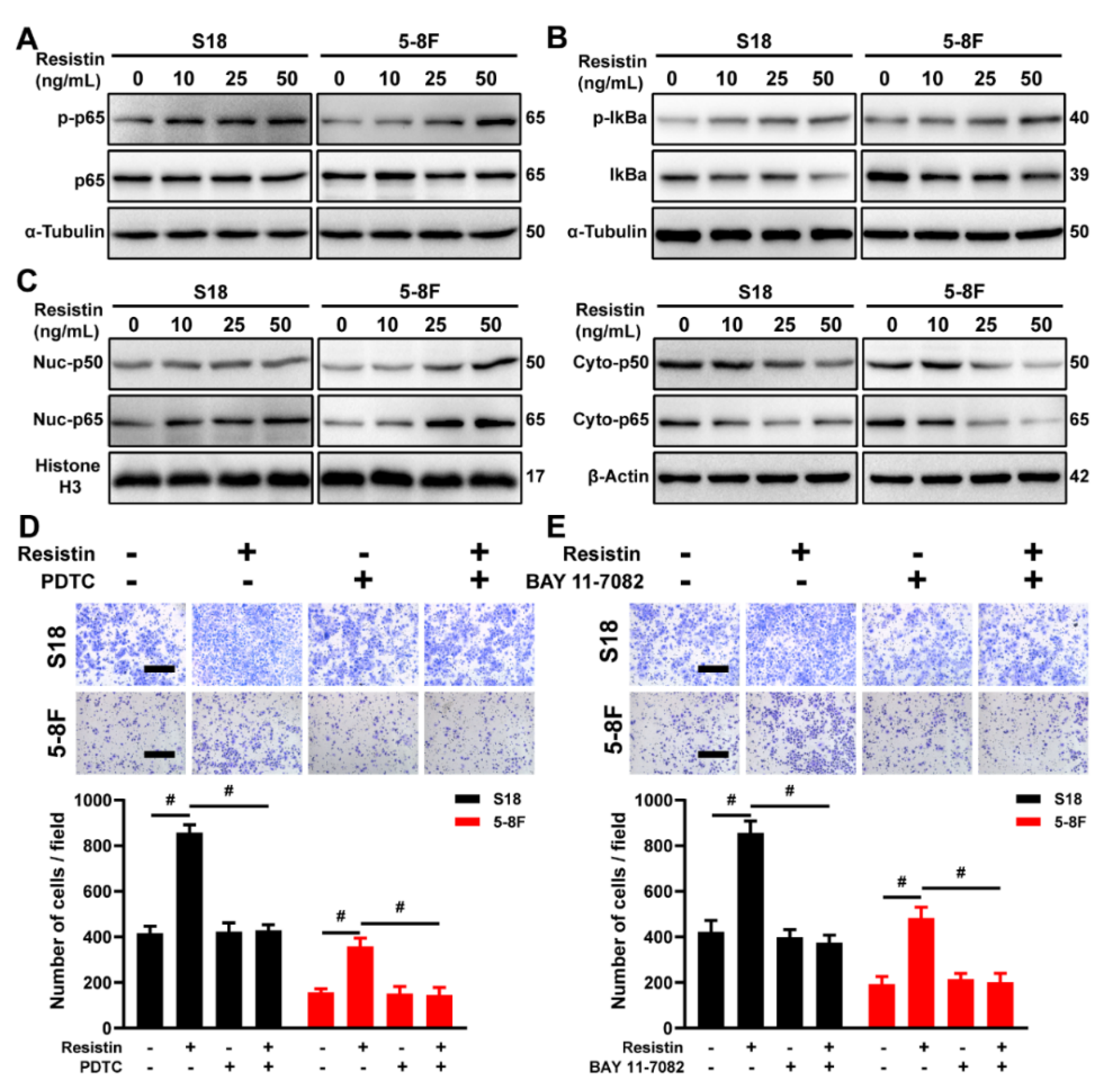
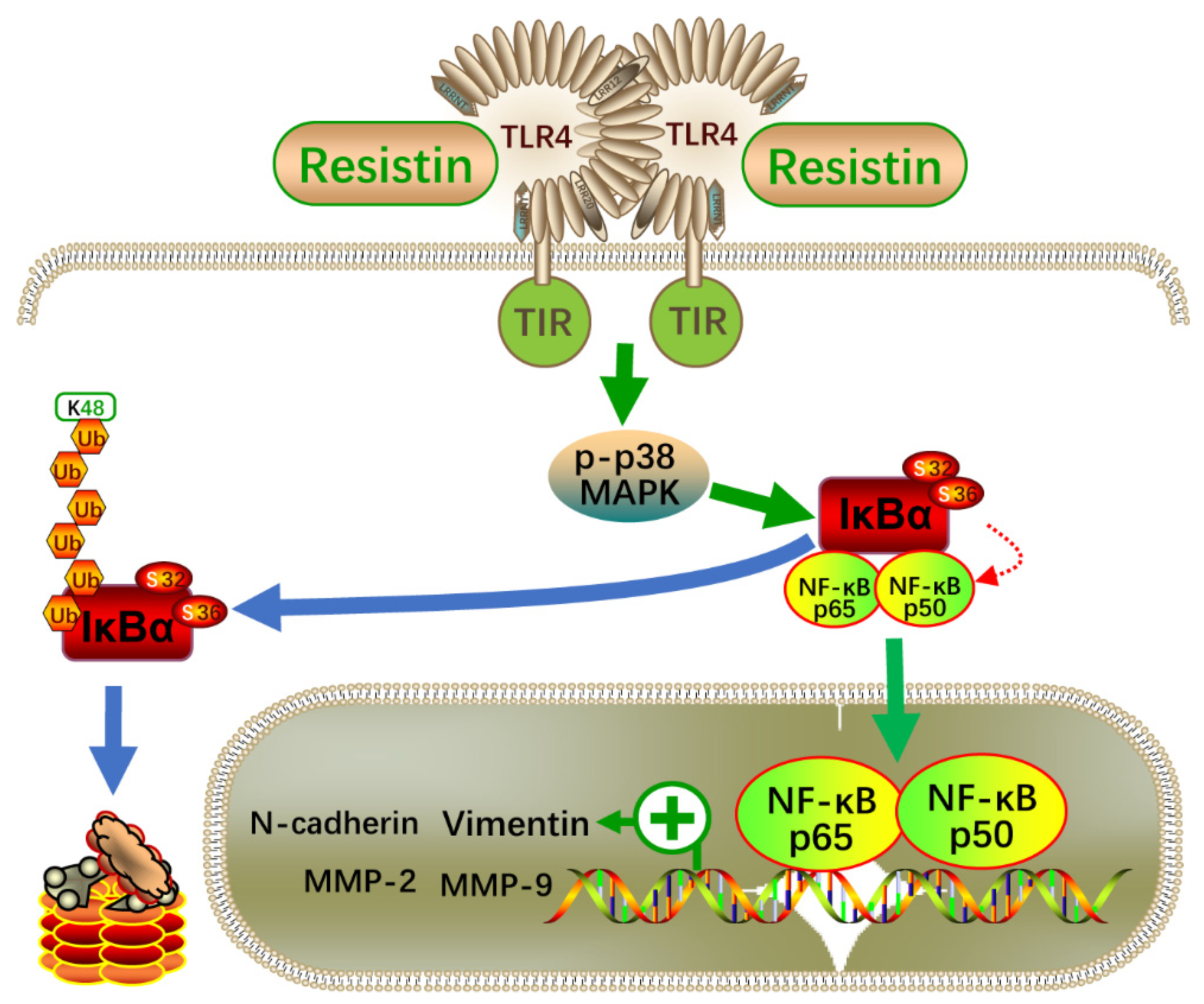
3.5. Resistin Regulates Expression of EMT-Related Protein via NF-κB
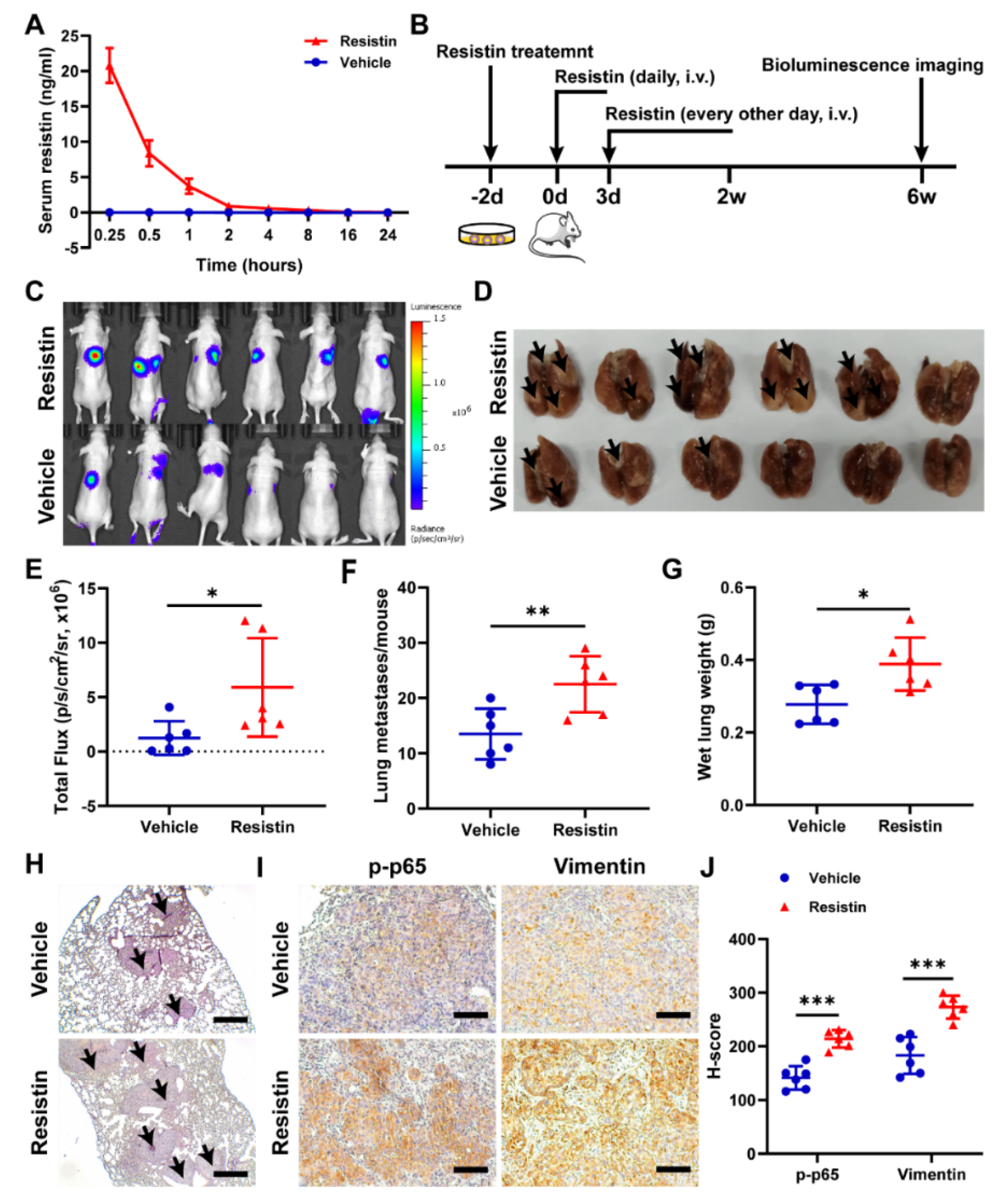
3.6. Resistin Promotes NPC Tumor Metastasis in Animal Models
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, Y.P.; Chan AT, C.; Le, Q.T.; Blanchard, P.; Sun, Y.; Ma, J. Nasopharyngeal carcinoma. Lancet 2019, 394, 64–80. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Sun, K.; Zheng, R.; Zeng, H.; Wang, S.; Chen, R.; Wei, W.; He, J. Cancer incidence and mortality in China, 2015. J. Nat. Cancer Cent. 2021, 1, 2–11. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, Q.; Liu, W.; Liu, Q.; Jia, W.; Chang, E.; Chen, F.; Liu, Z.; Guo, X.; Mo, H.; et al. Establishment of VCA and EBNA1 IgA-based combination by enzyme-linked immunosorbent assay as preferred screening method for nasopharyngeal carcinoma: A two-stage design with a preliminary performance study and a mass screening in southern China. Int. J. Cancer 2012, 131, 406–416. [Google Scholar] [CrossRef]
- Chang, E.T.; Adami, H.O. The enigmatic epidemiology of nasopharyngeal carcinoma. Cancer Epidemiol. Biomarkers Prev. 2006, 15, 1765–1777. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Johnson, R.C.; Deng, H.; Liao, J.; Guan, L.; Nelson, G.W.; Tang, M.; Zheng, Y.; de The, G.; O’Brien, S.J.; et al. Evaluation of nonviral risk factors for nasopharyngeal carcinoma in a high-risk population of Southern China. Int. J. Cancer 2009, 124, 2942–2947. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.P.; Chang, Y.T.; Wu, C.C.; Liu, Y.L.; Chen, M.C.; Tsang, N.M.; Hsu, C.L.; Chang, Y.S.; Yu, J.S. Multiplexed immunobead-based profiling of cytokine markers for detection of nasopharyngeal carcinoma and prognosis of patient survival. Head Neck 2011, 33, 886–897. [Google Scholar] [CrossRef] [PubMed]
- Gong, D.; Li, Z.; Ding, R.; Cheng, M.; Huang, H.; Liu, A.; Kang, M.; He, H.; Xu, Y.; Shao, J.; et al. Extensive serum biomarker analysis in patients with nasopharyngeal carcinoma. Cytokine 2019, 118, 107–114. [Google Scholar] [CrossRef]
- Jin, Y.B.; Zhang, G.Y.; Lin, K.R.; Chen, X.P.; Cui, J.H.; Wang, Y.J.; Luo, W. Changes of plasma cytokines and chemokines expression level in nasopharyngeal carcinoma patients after treatment with definitive intensity-modulated radiotherapy (IMRT). PLoS ONE 2017, 12, e0172264. [Google Scholar] [CrossRef]
- Lu, K.; Feng, X.; Deng, Q.; Sheng, L.; Liu, P.; Xu, S.; Su, D. Prognostic role of serum cytokines in patients with nasopharyngeal carcinoma. Onkologie 2012, 35, 494–498. [Google Scholar] [CrossRef]
- Yang, M.J.; Guo, J.; Ye, Y.F.; Chen, S.H.; Peng, L.X.; Lin, C.Y.; Hu, T.; Xie, S.H.; Xie, C.B.; Huang, Q.H.; et al. Decreased macrophage inflammatory protein (MIP)-1alpha and MIP-1beta increase the risk of developing nasopharyngeal carcinoma. Cancer Commun. 2018, 38, 7. [Google Scholar] [CrossRef] [PubMed]
- Tilg, H.; Moschen, A.R. Adipocytokines: Mediators linking adipose tissue, inflammation and immunity. Nat. Rev. Immunol. 2006, 6, 772–783. [Google Scholar] [CrossRef] [PubMed]
- Deng, T.; Lyon, C.J.; Bergin, S.; Caligiuri, M.A.; Hsueh, W.A. Obesity, Inflammation, and Cancer. Annu. Rev. Pathol. 2016, 11, 421–449. [Google Scholar] [CrossRef]
- Ouchi, N.; Parker, J.L.; Lugus, J.J.; Walsh, K. Adipokines in inflammation and metabolic disease. Nat. Rev. Immunol. 2011, 11, 85–97. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Shi, H.; Zhao, Z.; Wang, S.; Zhou, S.; Mu, Y.; Ding, N.; Lai, Y.; Zhao, A.Z.; Cheng, L.; et al. Adiponectin deficiency promotes endometrial carcinoma pathogenesis and development via activation of mitogen-activated protein kinase. J. Pathol. 2022, 257, 146–157. [Google Scholar] [CrossRef]
- Curat, C.A.; Wegner, V.; Sengenes, C.; Miranville, A.; Tonus, C.; Busse, R.; Bouloumie, A. Macrophages in human visceral adipose tissue: Increased accumulation in obesity and a source of resistin and visfatin. Diabetologia 2006, 49, 744–747. [Google Scholar] [CrossRef]
- Steppan, C.M.; Bailey, S.T.; Bhat, S.; Brown, E.J.; Banerjee, R.R.; Wright, C.M.; Patel, H.R.; Ahima, R.S.; Lazar, M.A. The hormone resistin links obesity to diabetes. Nature 2001, 409, 307–312. [Google Scholar] [CrossRef]
- Tripathi, D.; Kant, S.; Pandey, S.; Ehtesham, N.Z. Resistin in metabolism, inflammation, and disease. FEBS J. 2020, 287, 3141–3149. [Google Scholar] [CrossRef]
- Sudan, S.K.; Deshmukh, S.K.; Poosarla, T.; Holliday, N.P.; Dyess, D.L.; Singh, A.P.; Singh, S. Resistin: An inflammatory cytokine with multi-faceted roles in cancer. Biochim. Biophys. Acta Rev. Cancer 2020, 1874, 188419. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Hung, A.C.; Lo, S.; Yuan, S.F. Adipocytokines visfatin and resistin in breast cancer: Clinical relevance, biological mechanisms, and therapeutic potential. Cancer Lett. 2021, 498, 229–239. [Google Scholar] [CrossRef]
- Georgiou, G.P.; Provatopoulou, X.; Kalogera, E.; Siasos, G.; Menenakos, E.; Zografos, G.C.; Gounaris, A. Serum resistin is inversely related to breast cancer risk in premenopausal women. Breast 2016, 29, 163–169. [Google Scholar] [CrossRef]
- Nakajima, T.E.; Yamada, Y.; Hamano, T.; Furuta, K.; Gotoda, T.; Katai, H.; Kato, K.; Hamaguchi, T.; Shimada, Y. Adipocytokine levels in gastric cancer patients: Resistin and visfatin as biomarkers of gastric cancer. J. Gastroenterol. 2009, 44, 685–690. [Google Scholar] [CrossRef]
- Ilhan, T.T.; Kebapcilar, A.; Yilmaz, S.A.; Ilhan, T.; Kerimoglu, O.S.; Pekin, A.T.; Akyurek, F.; Unlu, A.; Celik, C. Relations of Serum Visfatin and Resistin Levels with Endometrial Cancer and Factors Associated with its Prognosis. Asian Pac. J. Cancer Prev. 2015, 16, 4503–4508. [Google Scholar] [CrossRef]
- Nakajima, T.E.; Yamada, Y.; Hamano, T.; Furuta, K.; Matsuda, T.; Fujita, S.; Kato, K.; Hamaguchi, T.; Shimada, Y. Adipocytokines as new promising markers of colorectal tumors: Adiponectin for colorectal adenoma, and resistin and visfatin for colorectal cancer. Cancer Sci. 2010, 101, 1286–1291. [Google Scholar] [CrossRef]
- Wang, C.H.; Wang, P.J.; Hsieh, Y.C.; Lo, S.; Lee, Y.C.; Chen, Y.C.; Tsai, C.H.; Chiu, W.C.; Chu-Sung Hu, S.; Lu, C.W.; et al. Resistin facilitates breast cancer progression via TLR4-mediated induction of mesenchymal phenotypes and stemness properties. Oncogene 2018, 37, 589–600. [Google Scholar] [CrossRef]
- Tsai, C.H.; Tsai, H.C.; Huang, H.N.; Hung, C.H.; Hsu, C.J.; Fong, Y.C.; Hsu, H.C.; Huang, Y.L.; Tang, C.H. Resistin promotes tumor metastasis by down-regulation of miR-519d through the AMPK/p38 signaling pathway in human chondrosarcoma cells. Oncotarget 2015, 6, 258–270. [Google Scholar] [CrossRef] [PubMed]
- Su, C.M.; Tang, C.H.; Chi, M.J.; Lin, C.Y.; Fong, Y.C.; Liu, Y.C.; Chen, W.C.; Wang, S.W. Resistin facilitates VEGF-C-associated lymphangiogenesis by inhibiting miR-186 in human chondrosarcoma cells. Biochem. Pharmacol. 2018, 154, 234–242. [Google Scholar] [CrossRef]
- Hsieh, Y.Y.; Shen, C.H.; Huang, W.S.; Chin, C.C.; Kuo, Y.H.; Hsieh, M.C.; Yu, H.R.; Chang, T.S.; Lin, T.H.; Chiu, Y.W.; et al. Resistin-induced stromal cell-derived factor-1 expression through Toll-like receptor 4 and activation of p38 MAPK/ NFkappaB signaling pathway in gastric cancer cells. J. Biomed. Sci. 2014, 21, 59. [Google Scholar] [CrossRef]
- Gong, W.J.; Liu, J.Y.; Yin, J.Y.; Cui, J.J.; Xiao, D.; Zhuo, W.; Luo, C.; Liu, R.J.; Li, X.; Zhang, W.; et al. Resistin facilitates metastasis of lung adenocarcinoma through the TLR4/Src/EGFR/PI3K/NF-kappaB pathway. Cancer Sci. 2018, 109, 2391–2400. [Google Scholar] [CrossRef]
- Chen, S.S.; Tang, C.H.; Chie, M.J.; Tsai, C.H.; Fong, Y.C.; Lu, Y.C.; Chen, W.C.; Lai, C.T.; Wei, C.Y.; Tai, H.C.; et al. Resistin facilitates VEGF-A-dependent angiogenesis by inhibiting miR-16-5p in human chondrosarcoma cells. Cell Death Dis. 2019, 10, 31. [Google Scholar] [CrossRef]
- Zhang, Z.M.; Du, J.N.; Xu, Q.H.; Xing, C.F.; Li, Y.Y.; Zhou, S.J.; Zhao, Z.G.; Mu, Y.P.; Zhao, Z.J.; Cao, S.M.; et al. Adiponectin Suppresses Metastasis of Nasopharyngeal Carcinoma through Blocking the Activation of NF-kappa B and STAT3 Signaling. Int. J. Mol. Sci. 2022, 23, 12729. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Du, J.; Shi, H.; Wang, S.; Yan, Y.; Xu, Q.; Zhou, S.; Zhao, Z.; Mu, Y.; Qian, C.; et al. Adiponectin suppresses tumor growth of nasopharyngeal carcinoma through activating AMPK signaling pathway. J. Transl. Med. 2022, 20, 89. [Google Scholar] [CrossRef]
- Mittal, V. Epithelial Mesenchymal Transition in Tumor Metastasis. Annu. Rev. Pathol. 2018, 13, 395–412. [Google Scholar] [CrossRef]
- Loh, C.Y.; Chai, J.Y.; Tang, T.F.; Wong, W.F.; Sethi, G.; Shanmugam, M.K.; Chong, P.P.; Looi, C.Y. The E-Cadherin and N-Cadherin Switch in Epithelial-to-Mesenchymal Transition: Signaling, Therapeutic Implications, and Challenges. Cells 2019, 8, 1118. [Google Scholar] [CrossRef] [PubMed]
- Madonna, G.; Ullman, C.D.; Gentilcore, G.; Palmieri, G.; Ascierto, P.A. NF-kappaB as potential target in the treatment of melanoma. J. Transl. Med. 2012, 10, 53. [Google Scholar] [CrossRef]
- Huber, M.A.; Azoitei, N.; Baumann, B.; Grunert, S.; Sommer, A.; Pehamberger, H.; Kraut, N.; Beug, H.; Wirth, T. NF-kappaB is essential for epithelial-mesenchymal transition and metastasis in a model of breast cancer progression. J. Clin. Investig. 2004, 114, 569–581. [Google Scholar] [CrossRef]
- Patel, L.; Buckels, A.C.; Kinghorn, I.J.; Murdock, P.R.; Holbrook, J.D.; Plumpton, C.; Macphee, C.H.; Smith, S.A. Resistin is expressed in human macrophages and directly regulated by PPAR gamma activators. Biochem. Biophys. Res. Commun. 2003, 300, 472–476. [Google Scholar] [CrossRef]
- Song, C.; Chen, L.Z.; Zhang, R.H.; Yu, X.J.; Zeng, Y.X. Functional variant in the 3’-untranslated region of Toll-like receptor 4 is associated with nasopharyngeal carcinoma risk. Cancer Biol. Ther. 2006, 5, 1285–1291. [Google Scholar] [CrossRef][Green Version]
- Yang, Z.H.; Dai, Q.; Gu, Y.J.; Guo, Q.X.; Gong, L. Cytokine and chemokine modification by Toll-like receptor polymorphisms is associated with nasopharyngeal carcinoma. Cancer Sci. 2012, 103, 653–658. [Google Scholar] [CrossRef]
- Ruuskanen, M.; Leivo, I.; Minn, H.; Vahlberg, T.; Haglund, C.; Hagstrom, J.; Irjala, H. Expression of toll-like receptors in non-endemic nasopharyngeal carcinoma. BMC Cancer 2019, 19, 624. [Google Scholar] [CrossRef]
- Kawai, T.; Akira, S. Signaling to NF-kappaB by Toll-like receptors. Trends Mol. Med. 2007, 13, 460–469. [Google Scholar] [CrossRef]
- Kawasaki, T.; Kawai, T. Toll-like receptor signaling pathways. Front. Immunol. 2014, 5, 461. [Google Scholar] [CrossRef] [PubMed]
- Chung, G.T.; Lou, W.P.; Chow, C.; To, K.F.; Choy, K.W.; Leung, A.W.; Tong, C.Y.; Yuen, J.W.; Ko, C.W.; Yip, T.T.; et al. Constitutive activation of distinct NF-kappaB signals in EBV-associated nasopharyngeal carcinoma. J. Pathol. 2013, 231, 311–332. [Google Scholar] [CrossRef]
- Sengupta, S.; den Boon, J.A.; Chen, I.H.; Newton, M.A.; Dahl, D.B.; Chen, M.; Cheng, Y.J.; Westra, W.H.; Chen, C.J.; Hildesheim, A.; et al. Genome-wide expression profiling reveals EBV-associated inhibition of MHC class I expression in nasopharyngeal carcinoma. Cancer Res. 2006, 66, 7999–8006. [Google Scholar] [CrossRef]
- Bao, Y.N.; Cao, X.; Luo, D.H.; Sun, R.; Peng, L.X.; Wang, L.; Yan, Y.P.; Zheng, L.S.; Xie, P.; Cao, Y.; et al. Urokinase-type plasminogen activator receptor signaling is critical in nasopharyngeal carcinoma cell growth and metastasis. Cell Cycle 2014, 13, 1958–1969. [Google Scholar] [CrossRef]
- Bo, H.; Gong, Z.; Zhang, W.; Li, X.; Zeng, Y.; Liao, Q.; Chen, P.; Shi, L.; Lian, Y.; Jing, Y.; et al. Upregulated long non-coding RNA AFAP1-AS1 expression is associated with progression and poor prognosis of nasopharyngeal carcinoma. Oncotarget 2015, 6, 20404–20418. [Google Scholar] [CrossRef]
- Fan, C.; Wang, J.; Tang, Y.; Zhang, S.; Xiong, F.; Guo, C.; Zhou, Y.; Li, Z.; Li, X.; Li, Y.; et al. Upregulation of long non-coding RNA LOC284454 may serve as a new serum diagnostic biomarker for head and neck cancers. BMC Cancer 2020, 20, 917. [Google Scholar] [CrossRef]
- Bose, S.; Yap, L.F.; Fung, M.; Starzcynski, J.; Saleh, A.; Morgan, S.; Dawson, C.; Chukwuma, M.B.; Maina, E.; Buettner, M.; et al. The ATM tumour suppressor gene is down-regulated in EBV-associated nasopharyngeal carcinoma. J. Pathol. 2009, 217, 345–352. [Google Scholar] [CrossRef]
- Tang, X.R.; Li, Y.Q.; Liang, S.B.; Jiang, W.; Liu, F.; Ge, W.X.; Tang, L.L.; Mao, Y.P.; He, Q.M.; Yang, X.J.; et al. Development and validation of a gene expression-based signature to predict distant metastasis in locoregionally advanced nasopharyngeal carcinoma: A retrospective, multicentre, cohort study. Lancet Oncol. 2018, 19, 382–393. [Google Scholar] [CrossRef]
- Lei, Y.; Li, Y.Q.; Jiang, W.; Hong, X.H.; Ge, W.X.; Zhang, Y.; Hu, W.H.; Wang, Y.Q.; Liang, Y.L.; Li, J.Y.; et al. A Gene-Expression Predictor for Efficacy of Induction Chemotherapy in Locoregionally Advanced Nasopharyngeal Carcinoma. J. Natl. Cancer Inst. 2021, 113, 471–480. [Google Scholar] [CrossRef]
- Zhang, L.; MacIsaac, K.D.; Zhou, T.; Huang, P.Y.; Xin, C.; Dobson, J.R.; Yu, K.; Chiang, D.Y.; Fan, Y.; Pelletier, M.; et al. Genomic Analysis of Nasopharyngeal Carcinoma Reveals TME-Based Subtypes. Mol. Cancer Res. 2017, 15, 1722–1732. [Google Scholar] [CrossRef] [PubMed]


| Characteristic | Case | Control | Z/χ2 | pa |
|---|---|---|---|---|
| Total | 100 | 100 | ||
| Gender | ||||
| Male | 87 | 84 | 0.363 | 0.547 |
| Female | 13 | 16 | ||
| Age (M, IQR) | 44.5 (38.0–52.0) | 45.0 (38.0–54.0) | −0.444 | 0.657 |
| Age group | ||||
| ≤40 | 34 | 34 | 0.000 | 1.000 |
| >40 | 66 | 66 | ||
| VCA-IgA | ||||
| Negative | 23 | 46 | 11.705 | 0.001 |
| Positive | 77 | 54 | ||
| EBNA1 | ||||
| Negative | 32 | 78 | 42.747 | <0.001 |
| Positive | 68 | 22 | ||
| Resistin b (M, IQR) | ||||
| Total | 4.12 (3.67–4.50) | 3.59 (3.11–3.98) | −6.998 | <0.001 |
| Female | 4.38 (3.65–4.89) | 3.46 (3.67–4.00) | −2.954 | 0.003 |
| Male | 4.18 (3.67–4.48) | 3.58 (3.67–3.98) | −6.347 | <0.001 |
| Variable | b | SE | Wald | p | OR | OR 95%CI |
|---|---|---|---|---|---|---|
| Resistin | 2.605 | 0.435 | 35.871 | <0.001 | 13.531 | 5.769–31.735 |
| EBNA1 | 1.950 | 0.393 | 24.582 | <0.001 | 7.031 | 3.252–15.199 |
| VCA-IgA | 0.982 | 0.401 | 6.013 | 0.014 | 2.670 | 1.218–5.854 |
| Constant | −11.578 | 1.839 | 39.630 | <0.001 | - | - |
| Characteristic | n | Resistin a (M, IQR) | Z | pb |
|---|---|---|---|---|
| LN metastasis | ||||
| No | 68 | 4.06 (3.62–4.41) | −2.949 | 0.003 |
| Yes | 32 | 4.35 (4.04–4.59) | ||
| Recurrence | ||||
| No | 94 | 4.20 (3.66–4.48) | −0.501 | 0.616 |
| Yes | 6 | 4.24 (3.53–4.98) | ||
| Stage c | ||||
| Early (Ⅰ/Ⅱ) | 36 | 4.12 (3.54–4.53) | −1.009 | 0.313 |
| Advance (Ⅲ/Ⅳ) | 64 | 4.24 (3.73–4.49) | ||
| Gender | ||||
| Male | 87 | 4.18 (3.67–4.48) | −1.164 | 0.245 |
| Female | 13 | 4.38 (3.65–4.89) | ||
| Age group | 100 | |||
| ≤40 | 34 | 4.29 (3.74–4.49) | −0.786 | 0.432 |
| >40 | 66 | 4.16 (3.64–4.50) |
| Variable | b | SE | Wald | p | OR | OR 95%CI |
|---|---|---|---|---|---|---|
| Resistin | 1.697 | 0.560 | 9.176 | 0.002 | 5.460 | 1.821–16.374 |
| EBNA1 | −0.006 | 0.517 | 0.000 | 0.990 | 0.994 | 0.361–2.736 |
| VCA-IgA | 0.876 | 0.637 | 1.889 | 0.169 | 2.401 | 0.689–8.374 |
| Constant | −8.534 | 2.593 | 10.829 | 0.001 | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Du, J.; Xu, Q.; Li, Y.; Zhou, S.; Zhao, Z.; Mu, Y.; Zhao, A.Z.; Cao, S.-M.; Li, F. Resistin Promotes Nasopharyngeal Carcinoma Metastasis through TLR4-Mediated Activation of p38 MAPK/NF-κB Signaling Pathway. Cancers 2022, 14, 6003. https://doi.org/10.3390/cancers14236003
Zhang Z, Du J, Xu Q, Li Y, Zhou S, Zhao Z, Mu Y, Zhao AZ, Cao S-M, Li F. Resistin Promotes Nasopharyngeal Carcinoma Metastasis through TLR4-Mediated Activation of p38 MAPK/NF-κB Signaling Pathway. Cancers. 2022; 14(23):6003. https://doi.org/10.3390/cancers14236003
Chicago/Turabian StyleZhang, Zongmeng, Jinlin Du, Qihua Xu, Yuyu Li, Sujin Zhou, Zhenggang Zhao, Yunping Mu, Allan Z. Zhao, Su-Mei Cao, and Fanghong Li. 2022. "Resistin Promotes Nasopharyngeal Carcinoma Metastasis through TLR4-Mediated Activation of p38 MAPK/NF-κB Signaling Pathway" Cancers 14, no. 23: 6003. https://doi.org/10.3390/cancers14236003
APA StyleZhang, Z., Du, J., Xu, Q., Li, Y., Zhou, S., Zhao, Z., Mu, Y., Zhao, A. Z., Cao, S.-M., & Li, F. (2022). Resistin Promotes Nasopharyngeal Carcinoma Metastasis through TLR4-Mediated Activation of p38 MAPK/NF-κB Signaling Pathway. Cancers, 14(23), 6003. https://doi.org/10.3390/cancers14236003






