The Mutually Mediated Chloride Intracellular Channel Protein 1 (CLIC1) Relationship between Malignant Cells and Tumor Blood Vessel Endothelium Exhibits a Significant Impact on Tumor Angiogenesis, Progression, and Metastasis in Clear Cell Renal Cell Carcinoma (ccRCC)
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Biopsies
2.2. Immunohistochemistry of CLIC1
2.3. Acquisition of Images and Data Interpretation
2.4. Statistical Analysis
2.5. CLIC1 Role and Expression in ccRCC and Bioinformatic Analysis
3. Results
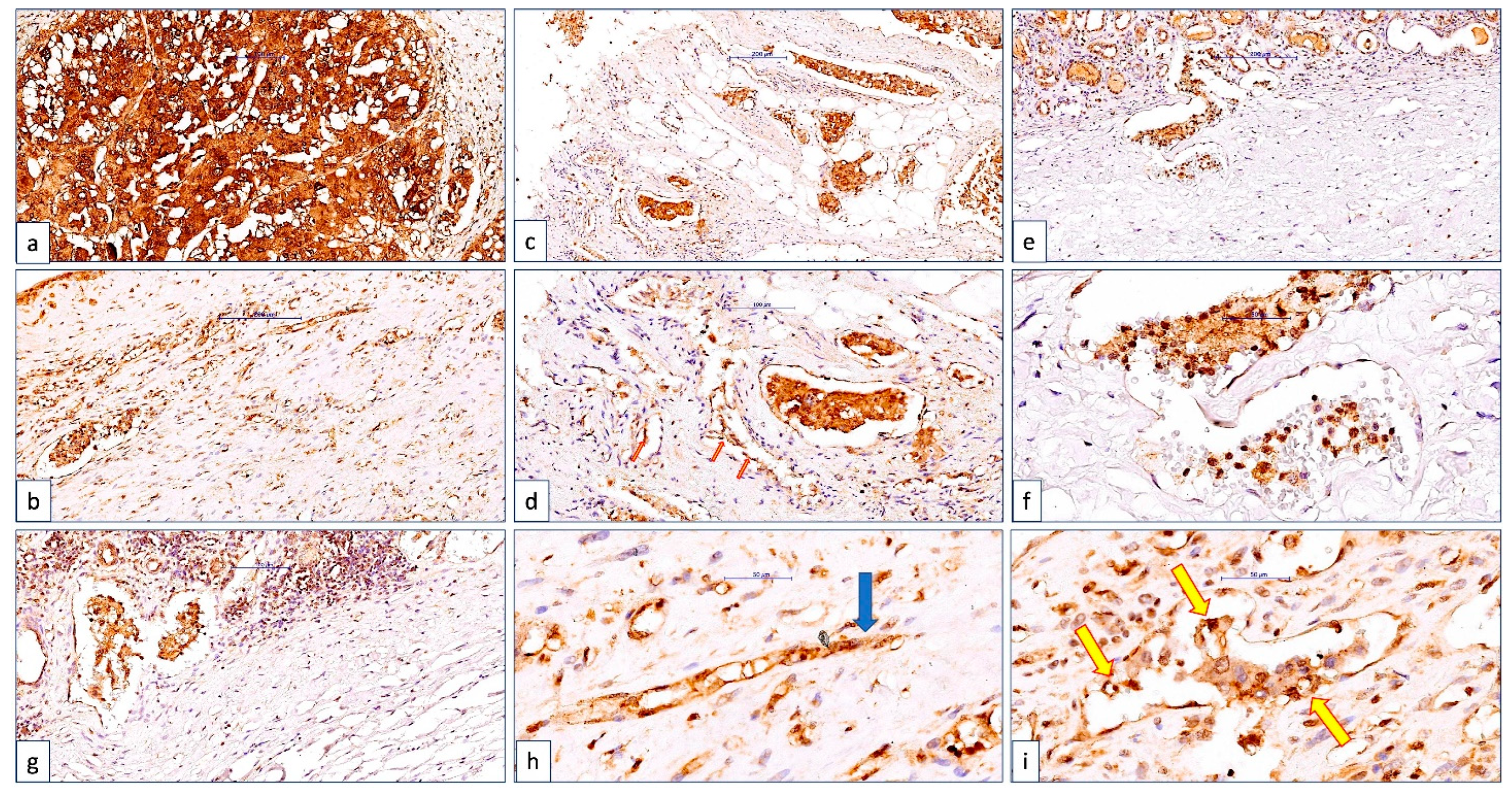
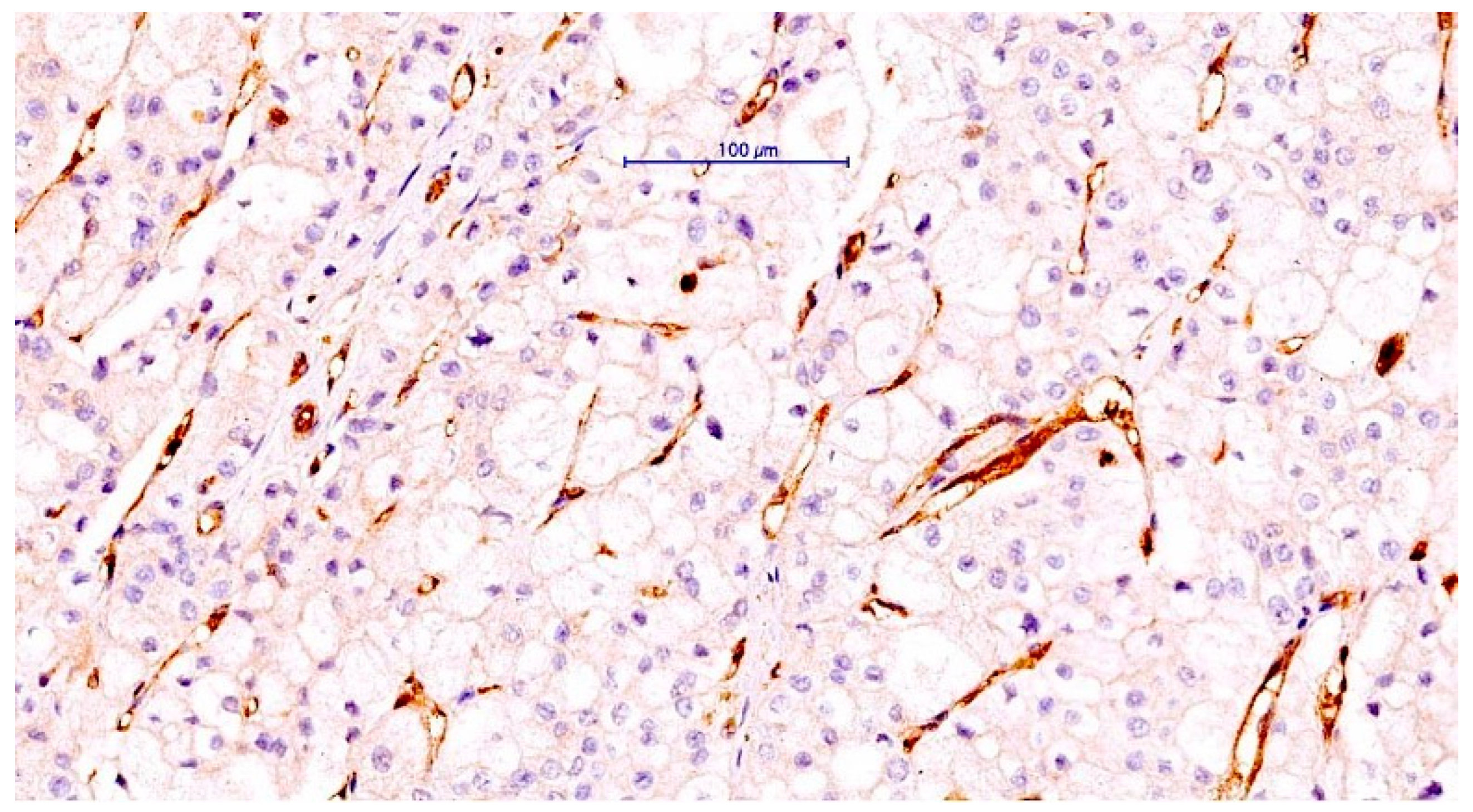
3.1. ccRCC Cases Classification according to CLIC1 Expression in ccRCC Tumor Cells and Tumor Blood Vessels Endothelium
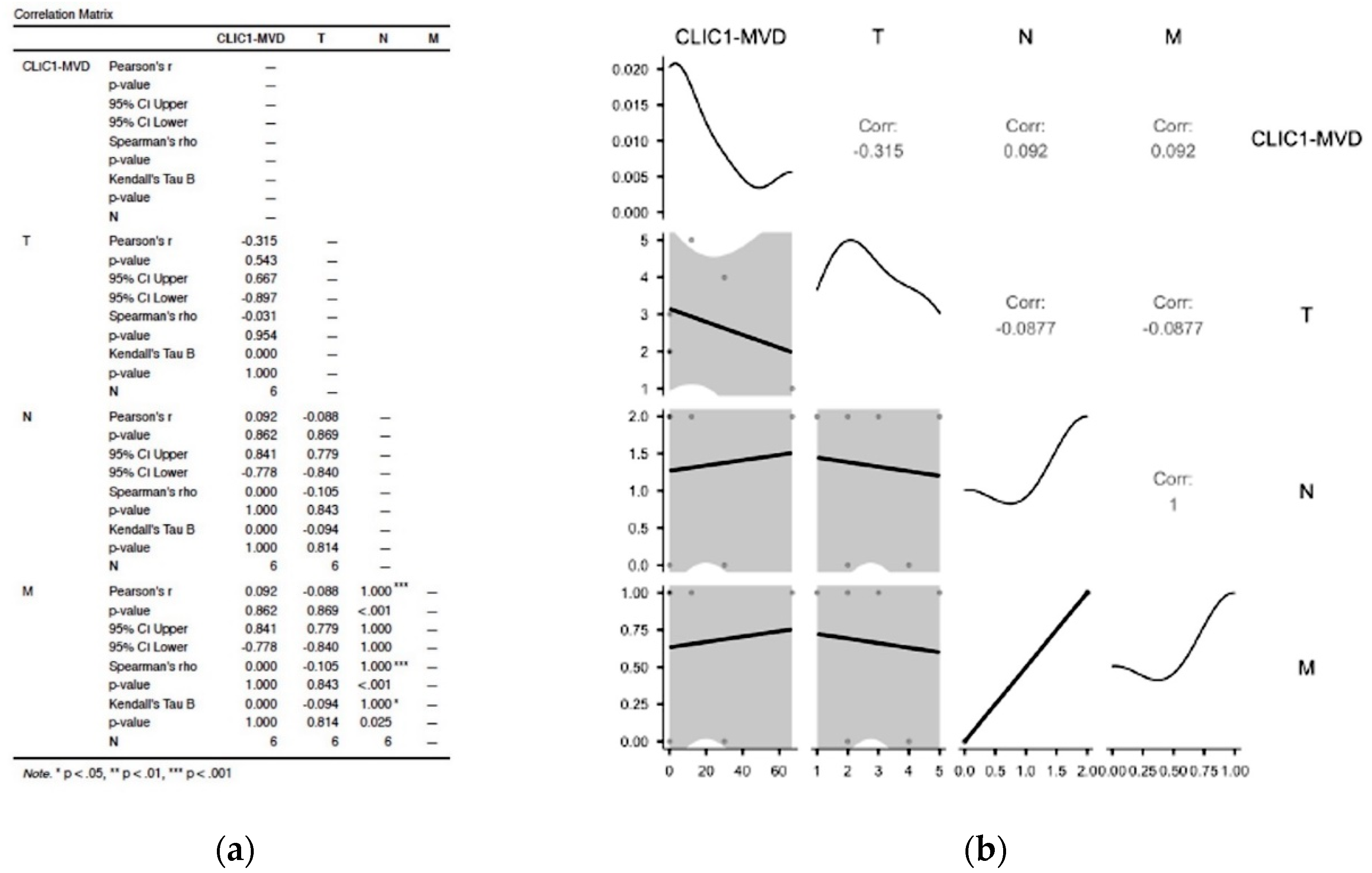
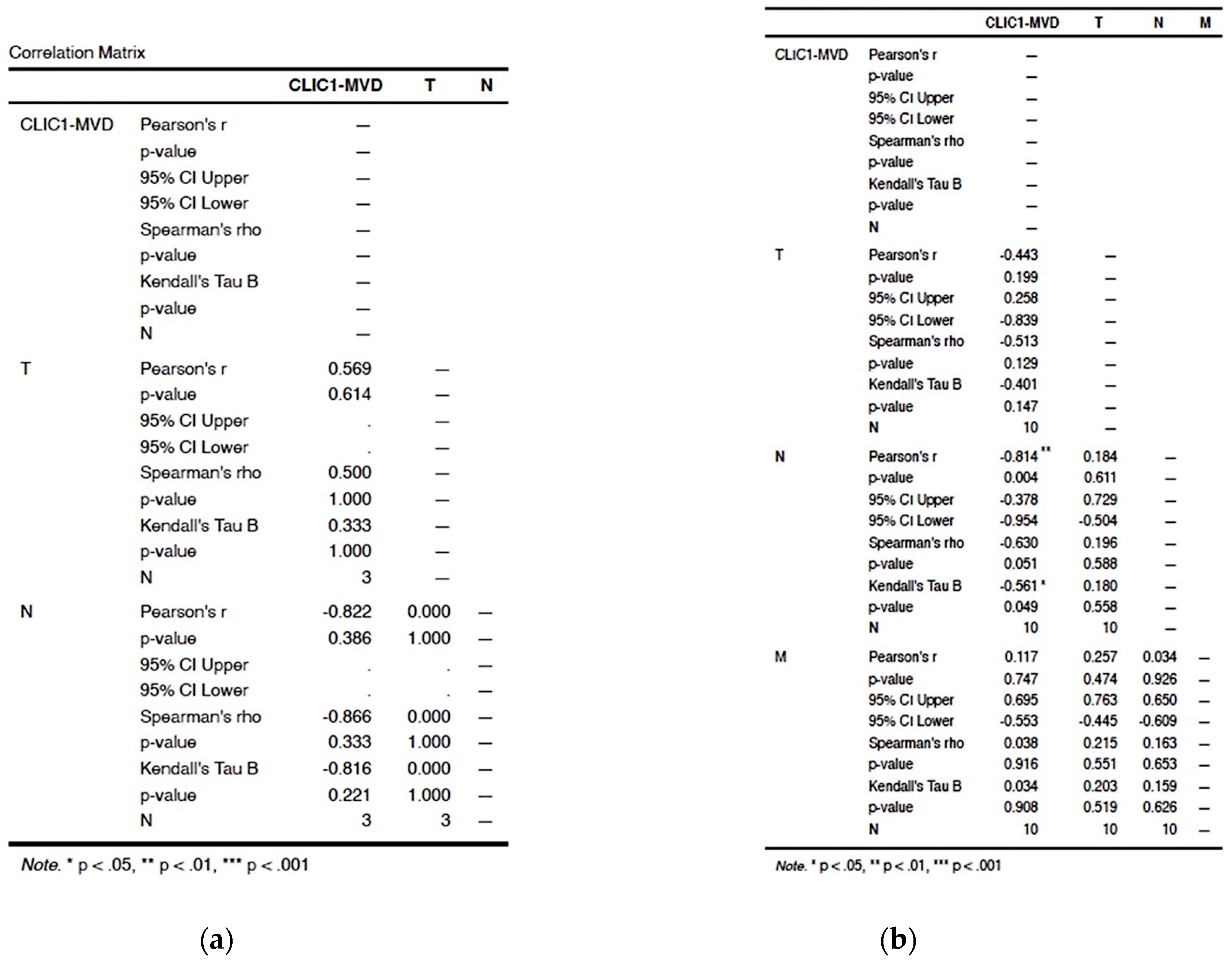
3.2. Statistical Analysis of Correlation between Different Subgroups to the pTNM Staging Parameters
3.2.1. Assessment of Class 0 Cases Did Not Show Any Significant Correlation in between TNM Parameters and CLIC1-MVD
3.2.2. Analysis of Cases Grouped as Class1 Have Shown no Significant Influence on TNM Staging Parameters
3.2.3. Increased CLIC1 Expression in ccRCC Tumor Cells May Influence Nodal Metastasis
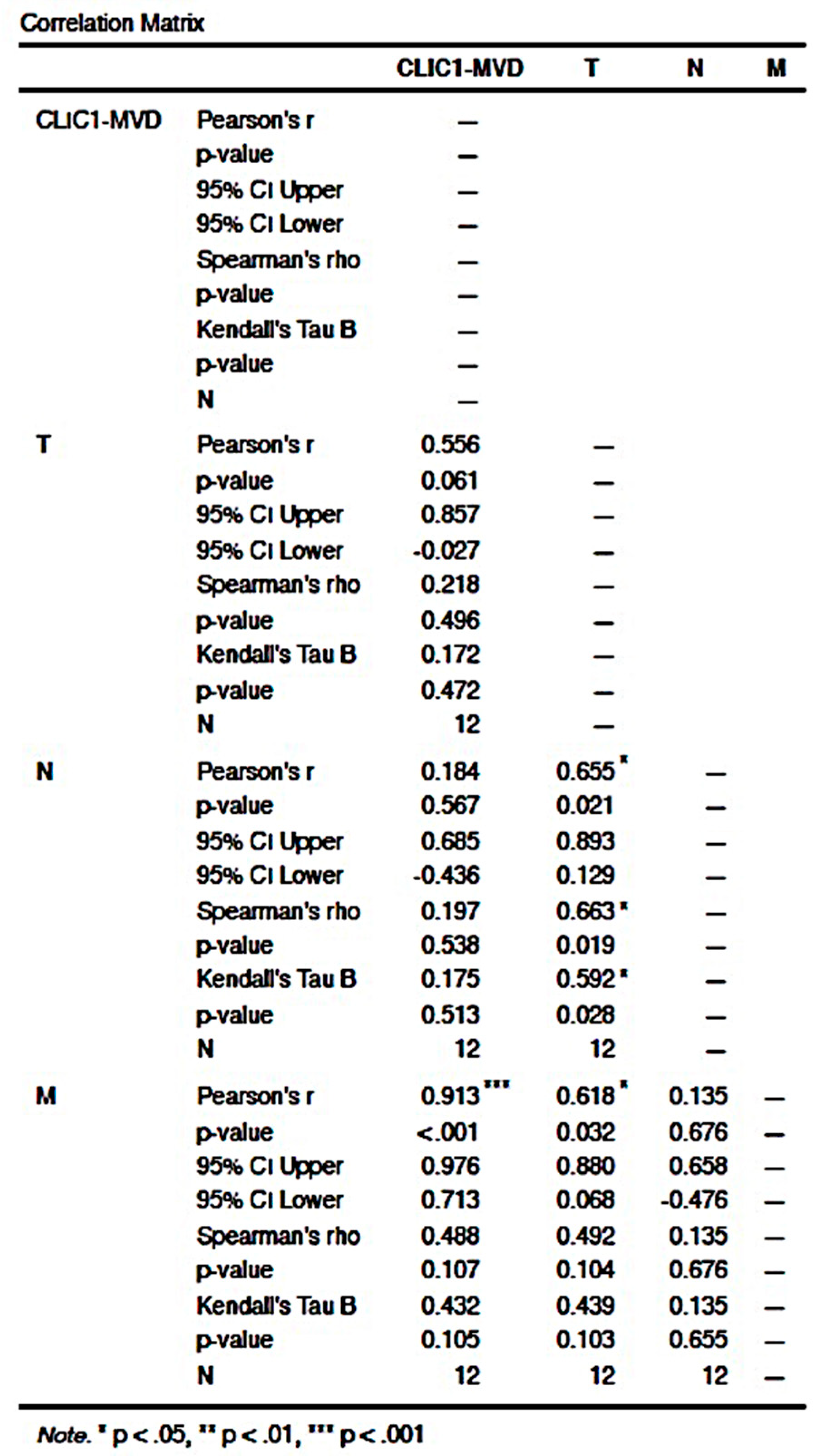
3.2.4. CLIC1 Expression Was Highest for Class 3 Subgroup Tumor Cells and Significantly Influenced CLIC1-MVD, Tumor Stage, Nodal and Distant Metastasis
3.3. The ccRCC Assessment According to CLIC1 Expression Pattern in Tumor Cells, Its Expression in Tumor Blood Vessels, and TNM Staging Parameters
3.4. Analysis of Human Protein Atlas and the Cancer Genome Atlas Data Related to CLIC1 Expression in ccRCC and the Tumor Endothelium
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lenzi, P.; Bocci, G.; Natale, G. John Hunter and the origin of the term “angiogenesis”. Angiogenesis 2016, 19, 255–256. [Google Scholar] [CrossRef]
- Elice, F.; Rodeghiero, F. Side effects of anti-angiogenic drugs. Thromb. Res. 2012, 129 (Suppl. S1), S50–S53. [Google Scholar] [CrossRef] [PubMed]
- Pietrzyk, Ł. Biomarkers Discovery for Colorectal Cancer. A Review on Tumor Endothelial Markers as Perspective Candidates. Dis. Markers 2016, 2016, 4912405. [Google Scholar] [CrossRef] [PubMed]
- Høye, A.M.; Tolstrup, S.D.; Horton, E.R.; Nicolau, M.; Frost, H.; Woo, J.H.; Mauldin, J.P.; Frankel, A.E.; Cox, T.R.; Erler, J.T. Tumor endothelial marker 8 promotes cancer progression and metastasis. Oncotarget 2018, 9, 30173–30188. [Google Scholar] [CrossRef] [PubMed]
- Ho, Y.J.; Wang, T.C.; Fan, C.H.; Yeh, C.K. Current progress in antivascular tumor therapy. Drug Discov. Today 2017, 22, 1503–1515. [Google Scholar] [CrossRef] [PubMed]
- Kargahi, N.; Torabinia, N.; Razavi, S.M.; Tahririan, D.; Kamani, H.; Nazari, M. Immunohistochemically Detection of Angiogenesis in Oral Pre-Cancerous Lesions Compared with Oral Invasive Carcinomas. Asian Pac. J. Cancer Prev. 2018, 19, 1805–1808. [Google Scholar] [CrossRef] [PubMed]
- Opławski, M.; Dziobek, K.; Adwent, I.; Dąbruś, D.; Grabarek, B.; Zmarzły, N.; Plewka, A.; Boroń, D. Expression Profile of Endoglin in Different Grades of Endometrial Cancer. Curr. Pharm. Biotechnol. 2018, 19, 990–995. [Google Scholar] [CrossRef]
- Mikkelsen, V.E.; Stensjøen, A.L.; Granli, U.S.; Berntsen, E.M.; Salvesen, Ø.; Solheim, O.; Torp, S.H. Angiogenesis and radiological tumor growth in patients with glioblastoma. BMC Cancer 2018, 18, 862. [Google Scholar] [CrossRef]
- Szafarowski, T.; Sierdzinski, J.; Szczepanski, M.J.; Whiteside, T.L.; Ludwig, N.; Krzeski, A. Microvessel density in head and neck squamous cell carcinoma. Eur. Arch. Otorhinolaryngol. 2018, 275, 1845–1851. [Google Scholar] [CrossRef]
- Kumagai, Y.; Tachikawa, T.; Higashi, M.; Sobajima, J.; Takahashi, A.; Amano, K.; Fukuchi, M.; Ishibashi, K.I.; Mochiki, E.; Yakabi, K.; et al. Thymidine phosphorylase and angiogenesis in early stage esophageal squamous cell carcinoma. Esophagus 2018, 15, 19–26. [Google Scholar] [CrossRef]
- Clara, C.A.; Marie, S.K.; de Almeida, J.R.; Wakamatsu, A.; Oba-Shinjo, S.M.; Uno, M.; Neville, M.; Rosemberg, S. Angiogenesis and expression of PDGF-C, VEGF, CD105 and HIF-1α in human glioblastoma. Neuropathology 2014, 34, 343–352. [Google Scholar] [CrossRef] [PubMed]
- Mehta, C.R.; Liu, L.; Theuer, C. An adaptive population enrichment phase III trial of TRC105 and pazopanib versus pazopanib alone in patients with advanced angiosarcoma (TAPPAS trial). Ann. Oncol. 2019, 30, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Nunes, T.; Hamdan, D.; Leboeuf, C.; El Bouchtaoui, M.; Gapihan, G.; Nguyen, T.T.; Meles, S.; Angeli, E.; Ratajczak, P.; Lu, H.; et al. Targeting Cancer Stem Cells to Overcome Chemoresistance. Int. J. Mol. Sci. 2018, 19, 4036. [Google Scholar] [CrossRef] [PubMed]
- Corraliza-Gorjón, I.; Somovilla-Crespo, B.; Santamaria, S.; Garcia-Sanz, J.A.; Kremer, L. New Strategies Using Antibody Combinations to Increase Cancer Treatment Effectiveness. Front. Immunol. 2017, 8, 1804. [Google Scholar] [CrossRef]
- Kasprzak, A.; Adamek, A. Role of Endoglin (CD105) in the Progression of Hepatocellular Carcinoma and Anti-Angiogenic Therapy. Int. J. Mol. Sci. 2018, 19, 3887. [Google Scholar] [CrossRef]
- Dorff, T.B.; Longmate, J.A.; Pal, S.K.; Stadler, W.M.; Fishman, M.N.; Vaishampayan, U.N.; Rao, A.; Pinksi, J.K.; Hu, J.S.; Quinn, D.I.; et al. Bevacizumab alone or in combination with TRC105 for patients with refractory metastatic renal cell cancer. Cancer 2017, 123, 4566–4573. [Google Scholar] [CrossRef]
- Choueiri, T.K.; Michaelson, M.D.; Posadas, E.M.; Sonpavde, G.P.; McDermott, D.F.; Nixon, A.B.; Liu, Y.; Yuan, Z.; Seon, B.K.; Walsh, M.; et al. An Open Label Phase Ib Dose Escalation Study of TRC105 (Anti-Endoglin Antibody) with Axitinib in Patients with Metastatic Renal Cell Carcinoma. Oncologist 2019, 24, 202–210. [Google Scholar] [CrossRef]
- Ferician, O.; Cimpean, A.M.; Avram, S.; Raica, M. Endostatin Effects on Tumor Cells and Vascular Network of Human Renal Cell Carcinoma Implanted on Chick Embryo Chorioallantoic Membrane. Anticancer Res. 2015, 35, 6521–6528. [Google Scholar]
- Saroufim, A.; Messai, Y.; Hasmim, M.; Rioux, N.; Iacovelli, R.; Verhoest, G.; Bensalah, K.; Patard, J.J.; Albiges, L.; Azzarone, B.; et al. Tumoral CD105 is a novel independent prognostic marker for prognosis in clear-cell renal cell carcinoma. Br. J. Cancer 2014, 110, 1778–1784. [Google Scholar] [CrossRef]
- Raica, M.; Cimpean, A.M.; Anghel, A. Immunohistochemical expression of vascular endothelial growth factor (VEGF) does not correlate with microvessel density in renal cell carcinoma. Neoplasma 2007, 54, 278–284. [Google Scholar]
- Zhang, X.; Ji, J.; Zhang, G.; Fang, C.; Jiang, F.; Ma, S.; Hou, J. Expression and significance of B7-H3 and Tie-2 in the tumor vasculature of clear cell renal carcinoma. Onco Targets Ther. 2017, 10, 5417–5424. [Google Scholar] [CrossRef] [PubMed]
- Sarrazin, S.; Maurage, C.A.; Delmas, D.; Lassalle, P.; Delehedde, M. Endocan as a Biomarker of Endothelial Dysfunction in Cancer. J. Cancer Sci. Ther. 2010, 2, 047–052. [Google Scholar]
- Nesiu, A.; Cimpean, A.M.; Ceausu, R.A.; Adile, A.; Ioiart, I.; Porta, C.; Mazzanti, M.; Camerota, T.C.; Raica, M. Intracellular Chloride Ion Channel Protein-1 Expression in Clear Cell Renal Cell Carcinoma. Cancer Genom. Proteom. 2019, 16, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Tung, J.J.; Kitajewski, J. Chloride intracellular channel 1 functions in endothelial cell growth and migration. J. Angiogenesis Res. 2010, 2, 23. [Google Scholar] [CrossRef]
- Thuringer, D.; Chanteloup, G.; Winckler, P.; Garrido, C. The vesicular transfer of CLIC1 from glioblastoma to microvascular endothelial cells requires TRPM7. Oncotarget 2018, 9, 33302–33311. [Google Scholar] [CrossRef]
- Peng, J.M.; Lin, S.H.; Yu, M.C.; Hsieh, S.Y. CLIC1 recruits PIP5K1A/C to induce cell-matrix adhesions for tumor metastasis. J. Clin. Investig. 2021, 131, e133525. [Google Scholar] [CrossRef]
- Li, B.; Chen, Y.; Liang, L.; Wang, Y.; Huang, W.; Zhao, K.; Liu, S.; Deng, G.; Chen, J. Tumor-derived extracellular vesicles shuttle c-Myc to promote gastric cancer growth and metastasis via the KCNQ1OT1/miR-556-3p/CLIC1 axis. Cell Death Dis. 2022, 13, 217. [Google Scholar] [CrossRef]
- Wei, X.; Pan, B.; Yang, M.; Shu, W.; Khan, A.R.; Su, R.; Lin, H.; Xu, X. CLIC1 Drives Angiogenesis in Hepatocellular Carcinoma by Modulating VEGFA. Technol. Cancer Res. Treat. 2022, 21, 15330338221106820. [Google Scholar] [CrossRef]
- Gurski, L.A.; Knowles, L.M.; Basse, P.H.; Maranchie, J.K.; Watkins, S.C.; Pilch, J. Relocation of CLIC1 promotes tumor cell invasion and colonization of fibrin. Mol. Cancer Res. 2015, 13, 273–280. [Google Scholar] [CrossRef]
- Wei, X.; Li, J.; Xie, H.; Wang, H.; Wang, J.; Zhang, X.; Zhuang, R.; Lu, D.; Ling, Q.; Zhou, L.; et al. Chloride intracellular channel 1 participates in migration and invasion of hepatocellular carcinoma by targeting maspin. J. Gastroenterol. Hepatol. 2015, 30, 208–216. [Google Scholar] [CrossRef]
- Raica, M.; Ceausu, A.R.; Cimpean, A.M.; ComŞa, Ş.; Sarb, S. Chloride Intracellular Channel Protein 1 (CLIC1), E-cadherin and P-cadherin Define Distinct Subclasses of HER2, Luminal B and Triple-negative Breast Cancer. Anticancer Res. 2021, 41, 795–802. [Google Scholar] [CrossRef] [PubMed]
- Radu-Cosnita, A.D.; Nesiu, A.; Berzava, P.L.; Cerbu, S.; Cosma, A.; Comsa, S.; Sarb, S.; Ferician, A.M.; Ferician, O.C.; Cimpean, A.M. Anti-chloride Intracellular Channel Protein 1 (CLIC1) Antibodies Induce Tumour Necrosis and Angiogenesis Inhibition on In Vivo Experimental Models of Human Renal Cancer. Anticancer Res. 2022, 42, 1313–1325. [Google Scholar] [CrossRef] [PubMed]
- PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/?term=CLIC1+endothelial+cells (accessed on 20 November 2022).
- Knowles, L.M.; Malik, G.; Hood, B.L.; Conrads, T.P.; Pilch, J. CLT1 targets angiogenic endothelium through CLIC1 and fibronectin. Angiogenesis 2012, 15, 115–129. [Google Scholar] [CrossRef]
- Chalothorn, D.; Zhang, H.; Smith, J.E.; Edwards, J.C.; Faber, J.E. Chloride intracellular channel-4 is a determinant of native collateral formation in skeletal muscle and brain. Circ. Res. 2009, 105, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.; Kleinjan, M.L.; Jilishitz, I.; Swaminathan, B.; Obinata, H.; Komarova, Y.A.; Bayless, K.J.; Hla, T.; Kitajewski, J.K. CLIC1 and CLIC4 mediate endothelial S1P receptor signaling to facilitate Rac1 and RhoA activity and function. Sci. Signal. 2021, 14, eabc0425. [Google Scholar] [CrossRef]
- PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/?term=CLIC1+cancer (accessed on 20 November 2022).
- Bordean, L.; Chis, M.; Raica, M.; Cotoi, O.S.; Ceausu, A.R.; Avram, C.; Cimpean, A.M. CLIC1 Expression in Skin Biopsies from Patients with Rheumatoid and Psoriatic Arthritis as a Potential Tool to Predict Therapy Response. In Vivo 2021, 35, 2559–2567. [Google Scholar] [CrossRef] [PubMed]


| CLIC1 | % POSITIVE | % NEGATIVE |
|---|---|---|
| ccRCC TC | 87.5 | 12.5 |
| ccRCC TBvsE | 65.11 | 34.88 |
| ccRCC TC/ccRCCTBvsE | 59 | 41 |
| ccRCC Classes | Cases % | CLIC1-ccRCC TC-/ccRCC TBvsE- | CLIC1-ccRCC TC-/ccRCC TBvsE+ | CLIC1-ccRCC TC+/ccRCC TBvsE- | CLIC1-ccRCC TC+/ccRCC TBvsE+ |
|---|---|---|---|---|---|
| Class 0 (0–9%) | 12% | 6% | 6% | 0% | 0% |
| Class 1 (10–29%) | 6% | 0% | 0% | 0% | 6% |
| Class 2 (30–59%) | 20% | 0% | 6% | 0% | 14% |
| Class 3 (60–100%) | 62% | 0% | 0% | 23.00% | 39.00% |
| TOTAL | 100% | 6% | 12% | 23.00% | 59.00% |
| Classes | pTNM | CLIC1 TC/BvsE Expression Patterns | ||||
|---|---|---|---|---|---|---|
| CLIC1-ccRCC TC-/ccRCC TBvsE- | CLIC1-ccRCC TC-/ccRCC TBvsE+ | CLIC1-ccRCC TC+/ccRCC TBvsE- | CLIC1-ccRCC TC+/ccRCC TBvsE+ | |||
| CLASS 0 12% | pT | 1a | 0 | 1 | 0 | 0 |
| 1b | 1 | 0 | 0 | 0 | ||
| 2a | 2 | 0 | 0 | 0 | ||
| 2b | 0 | 1 | 0 | 0 | ||
| 3a | 0 | 1 | 0 | 0 | ||
| 3b | 0 | 0 | 0 | 0 | ||
| 4 | 0 | 0 | 0 | 0 | ||
| N | X | 3 | 2 | 0 | 0 | |
| 0 | 0 | 1 | 0 | 0 | ||
| 1 | 0 | 0 | 0 | 0 | ||
| M | X | 3 | 2 | 0 | 0 | |
| 0 | 0 | 1 | 0 | 0 | ||
| 1 | 0 | 0 | 0 | 0 | ||
| CLASS 1 6% | pT | 1a | 0 | 0 | 0 | 0 |
| 1b | 0 | 0 | 0 | 1 | ||
| 2a | 0 | 0 | 0 | 1 | ||
| 2b | 0 | 0 | 0 | 0 | ||
| 3a | 0 | 0 | 0 | 0 | ||
| 3b | 0 | 0 | 0 | 0 | ||
| 4 | 0 | 0 | 0 | 1 | ||
| N | X | 0 | 0 | 0 | 1 | |
| 0 | 0 | 0 | 0 | 2 | ||
| 1 | 0 | 0 | 0 | 0 | ||
| M | X | 0 | 0 | 0 | 3 | |
| 0 | 0 | 0 | 0 | 0 | ||
| 1 | 0 | 0 | 0 | 0 | ||
| CLASS 2 20% | pT | 1a | 0 | 0 | 0 | 1 |
| 1b | 0 | 0 | 1 | 4 | ||
| 2a | 0 | 0 | 1 | 1 | ||
| 2b | 0 | 0 | 0 | 1 | ||
| 3a | 0 | 0 | 1 | 0 | ||
| 3b | 0 | 0 | 0 | 0 | ||
| 4 | 0 | 0 | 0 | 0 | ||
| N | X | 0 | 0 | 3 | 5 | |
| 0 | 0 | 0 | 0 | 1 | ||
| 1 | 0 | 0 | 0 | 1 | ||
| M | X | 0 | 0 | 2 | 6 | |
| 0 | 0 | 0 | 1 | 1 | ||
| 1 | 0 | 0 | 0 | 0 | ||
| CLASS 3 62% | pT | 1a | 0 | 0 | 2 | 2 |
| 1b | 0 | 0 | 5 | 6 | ||
| 2a | 0 | 0 | 2 | 1 | ||
| 2b | 0 | 0 | 0 | 3 | ||
| 3a | 0 | 0 | 0 | 5 | ||
| 3b | 0 | 0 | 0 | 0 | ||
| 4 | 0 | 0 | 0 | 2 | ||
| N | X | 0 | 0 | 9 | 13 | |
| 0 | 0 | 0 | 2 | 5 | ||
| 1 | 0 | 0 | 0 | 1 | ||
| M | X | 0 | 0 | 9 | 16 | |
| 0 | 0 | 0 | 2 | 0 | ||
| 1 | 0 | 0 | 0 | 2 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferician, A.M.; Ferician, O.C.; Nesiu, A.; Cosma, A.A.; Caplar, B.D.; Melnic, E.; Cimpean, A.M. The Mutually Mediated Chloride Intracellular Channel Protein 1 (CLIC1) Relationship between Malignant Cells and Tumor Blood Vessel Endothelium Exhibits a Significant Impact on Tumor Angiogenesis, Progression, and Metastasis in Clear Cell Renal Cell Carcinoma (ccRCC). Cancers 2022, 14, 5981. https://doi.org/10.3390/cancers14235981
Ferician AM, Ferician OC, Nesiu A, Cosma AA, Caplar BD, Melnic E, Cimpean AM. The Mutually Mediated Chloride Intracellular Channel Protein 1 (CLIC1) Relationship between Malignant Cells and Tumor Blood Vessel Endothelium Exhibits a Significant Impact on Tumor Angiogenesis, Progression, and Metastasis in Clear Cell Renal Cell Carcinoma (ccRCC). Cancers. 2022; 14(23):5981. https://doi.org/10.3390/cancers14235981
Chicago/Turabian StyleFerician, Adela Maria, Ovidiu Catalin Ferician, Alexandru Nesiu, Andrei Alexandru Cosma, Borislav Dusan Caplar, Eugen Melnic, and Anca Maria Cimpean. 2022. "The Mutually Mediated Chloride Intracellular Channel Protein 1 (CLIC1) Relationship between Malignant Cells and Tumor Blood Vessel Endothelium Exhibits a Significant Impact on Tumor Angiogenesis, Progression, and Metastasis in Clear Cell Renal Cell Carcinoma (ccRCC)" Cancers 14, no. 23: 5981. https://doi.org/10.3390/cancers14235981
APA StyleFerician, A. M., Ferician, O. C., Nesiu, A., Cosma, A. A., Caplar, B. D., Melnic, E., & Cimpean, A. M. (2022). The Mutually Mediated Chloride Intracellular Channel Protein 1 (CLIC1) Relationship between Malignant Cells and Tumor Blood Vessel Endothelium Exhibits a Significant Impact on Tumor Angiogenesis, Progression, and Metastasis in Clear Cell Renal Cell Carcinoma (ccRCC). Cancers, 14(23), 5981. https://doi.org/10.3390/cancers14235981







