Chewing Behavior Attenuates Lung-Metastasis-Promoting Effects of Chronic Stress in Breast-Cancer Lung-Metastasis Model Mice
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Animal Models and Tail Vein Metastasis Assays
2.3. Serum Corticosterone Assay
2.4. Histopathological Image Analysis
2.5. Immunohistochemical Staining
2.6. Western Blot Analyses
2.7. Statistical Analysis
3. Results
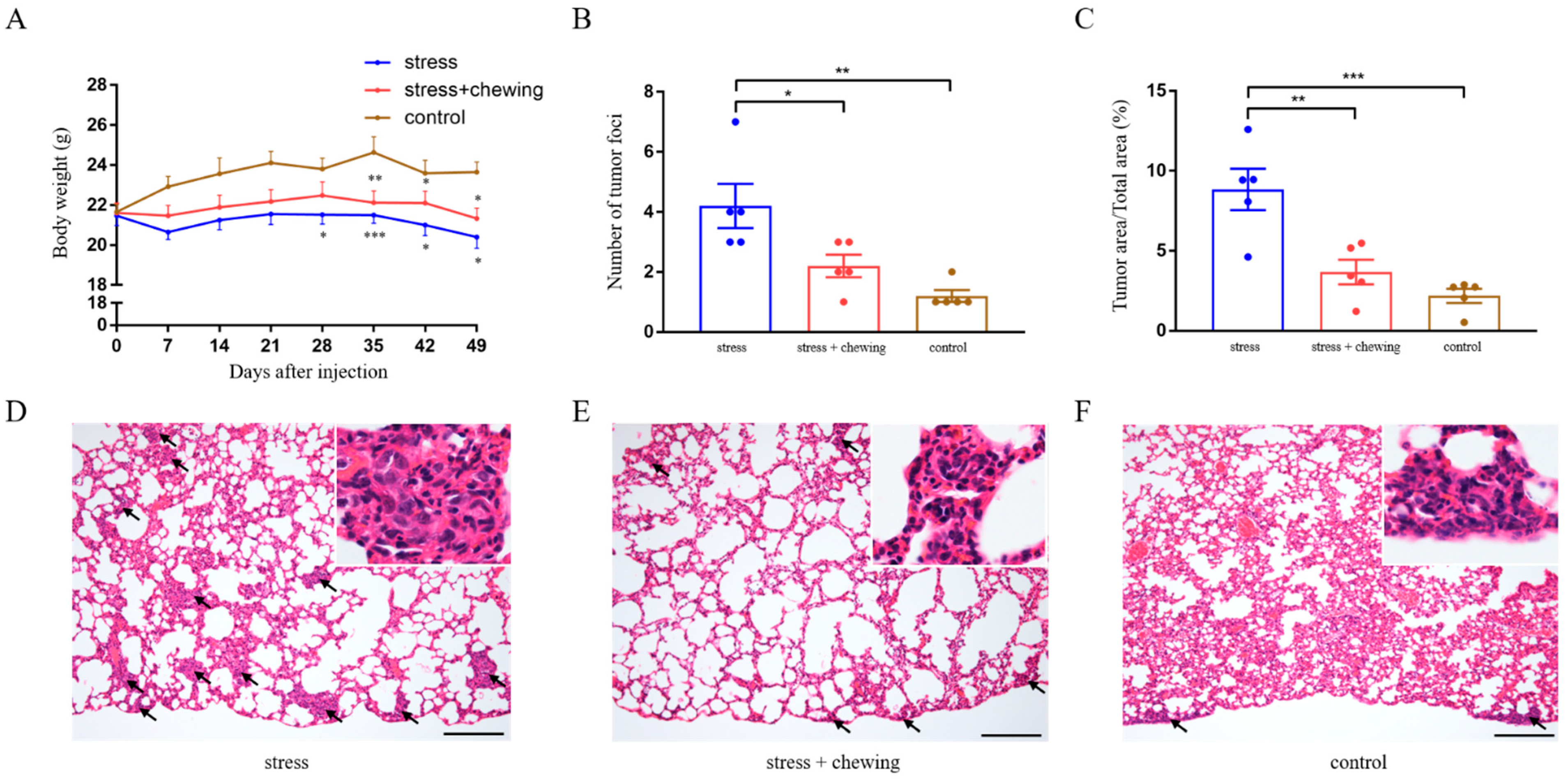
3.1. Chewing Behavior Attenuated the Promoting Effects of Restraint Stress on Lung Metastasis
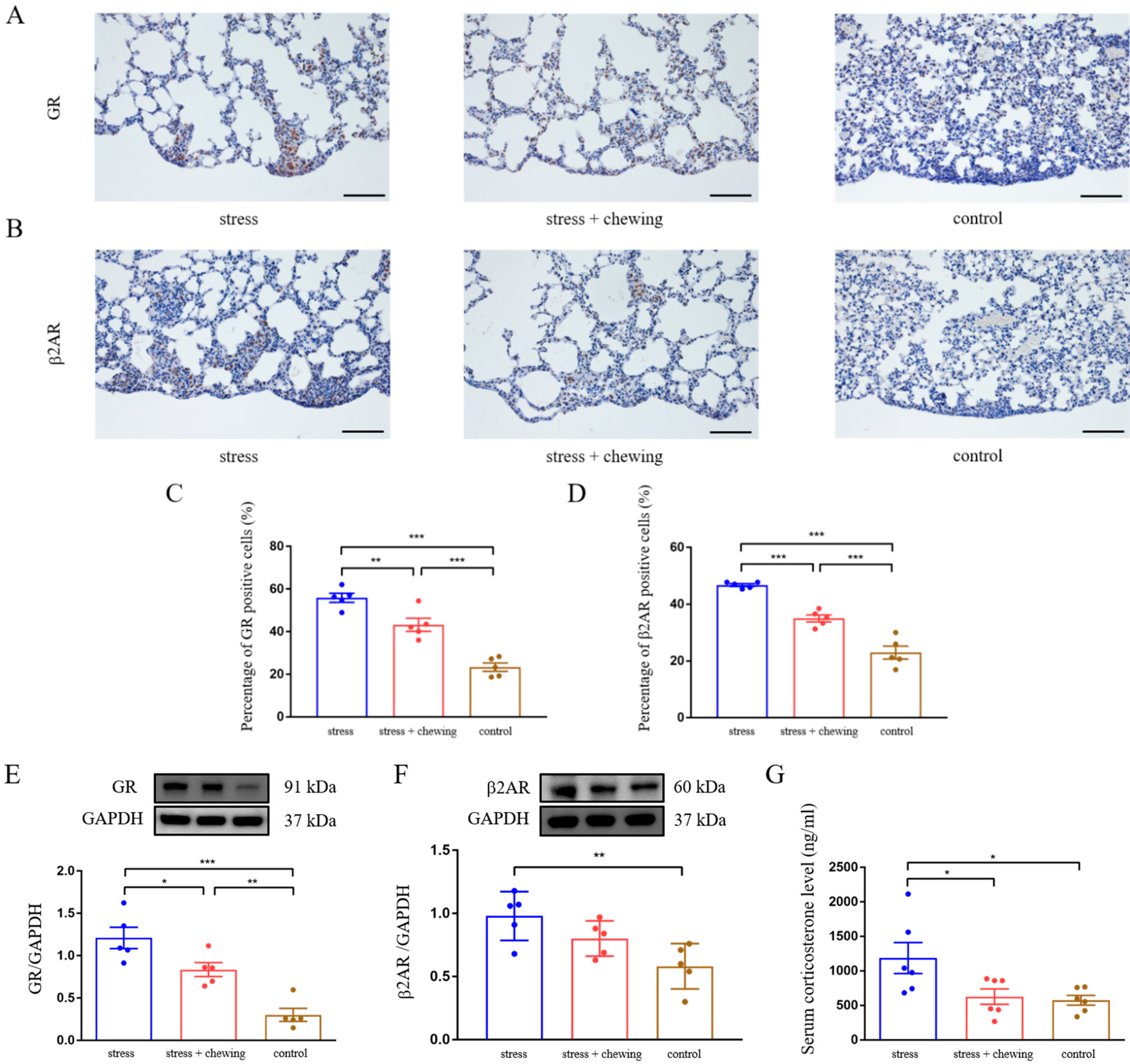
3.2. Chewing Behavior Normalized Restraint Stress-Induced Elevation of GR and β2AR Expression, and Corticosterone Level
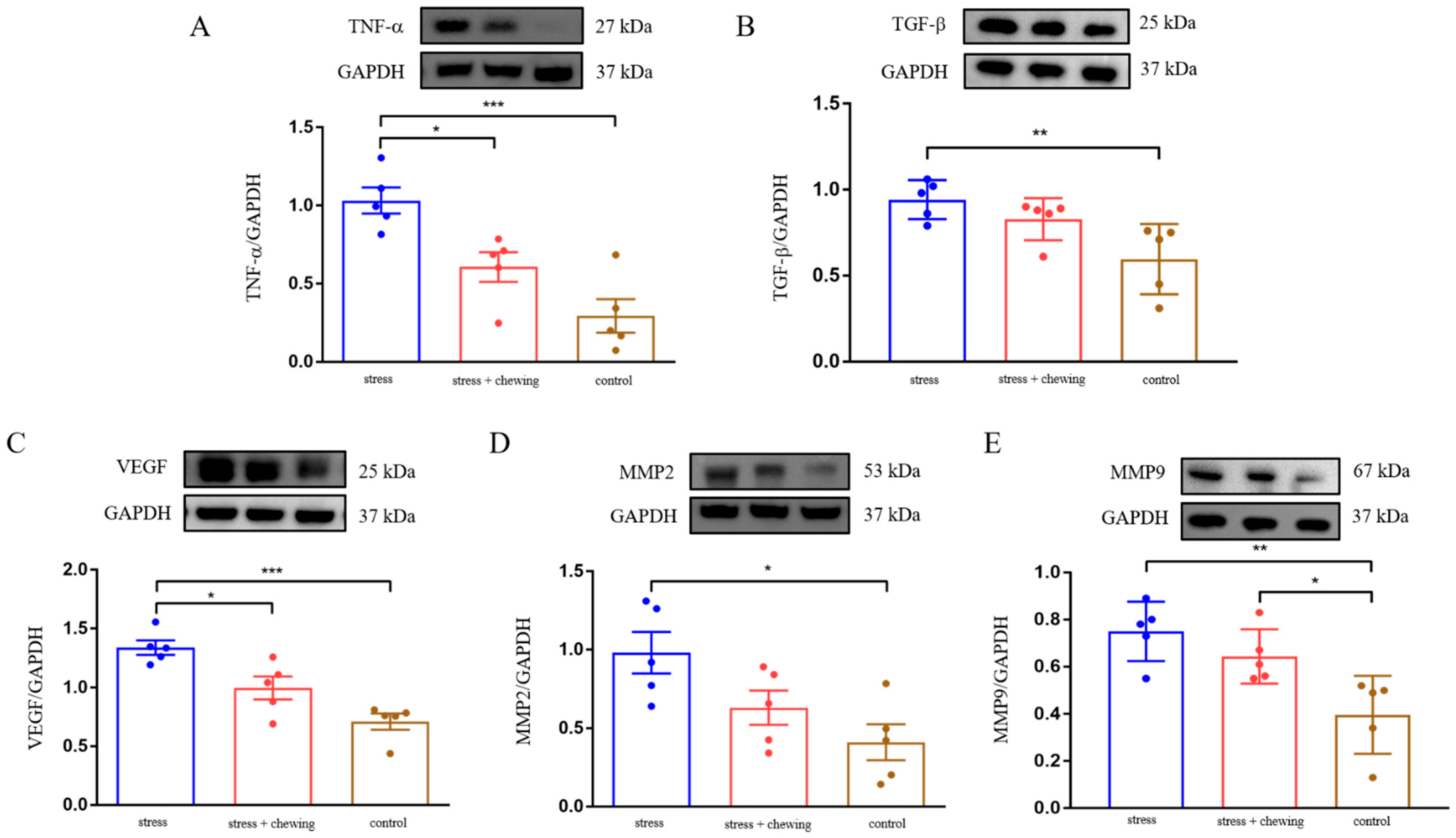
3.3. Chewing Behavior Normalized Restraint Stress-Induced Elevation of TNF-α, TGF-β, VEGF and MMP Expression
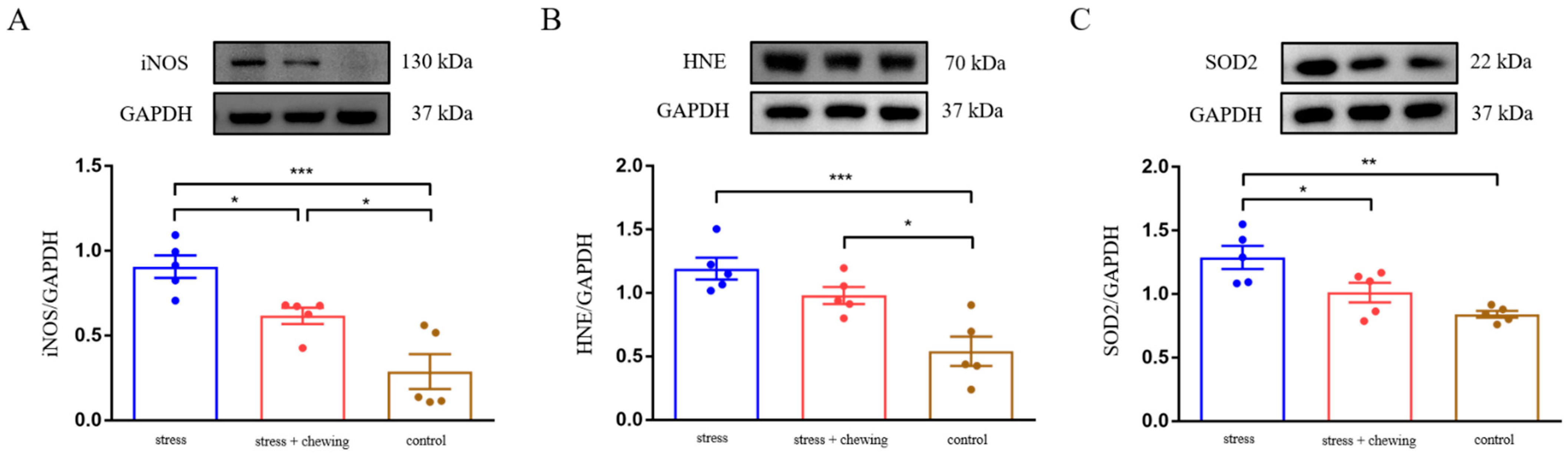
3.4. Chewing Behavior Ameliorated Oxidative Stress Induced by Restraint Stress
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: Globocan Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Pan, J.; Chen, W.; Jiang, J.; Huang, J. Chronic stress-induced immune dysregulation in cancer: Implications for initiation, progression, metastasis, and treatment. Am. J. Cancer Res. 2020, 10, 1294–1307. [Google Scholar] [PubMed]
- Dai, S.; Mo, Y.; Wang, Y.; Xiang, B.; Liao, Q.; Zhou, M.; Li, X.; Li, Y.; Xiong, W.; Li, G.; et al. Chronic Stress Promotes Cancer Development. Front. Oncol. 2020, 10, 1492. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Y.; He, Z.; Yin, K.; Li, B.; Zhang, L.; Xu, Z. Chronic stress promotes gastric cancer progression and metastasis: An essential role for ADRB2. Cell Death Dis. 2019, 10, 788. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Liu, D.; Guo, L.; Cheng, X.; Guo, N.; Shi, M. Chronic psychological stress promotes lung metastatic colonization of circulating breast cancer cells by decorating a pre-metastatic niche through activating beta-adrenergic signaling. J. Pathol. 2018, 244, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Cui, B.; Peng, F.; Lu, J.; He, B.; Su, Q.; Luo, H.; Deng, Z.; Jiang, T.; Su, K.; Huang, Y.; et al. Cancer and stress: NextGen strategies. Brain Behav. Immun. 2021, 93, 368–383. [Google Scholar] [CrossRef]
- Du, P.; Zeng, H.; Xiao, Y.; Zhao, Y.; Zheng, B.; Deng, Y.; Liu, J.; Huang, B.; Zhang, X.; Yang, K.; et al. Chronic stress promotes EMT-mediated metastasis through activation of STAT3 signaling pathway by miR-337-3p in breast cancer. Cell Death Dis. 2020, 11, 761. [Google Scholar] [CrossRef]
- Obradovic, M.M.S.; Hamelin, B.; Manevski, N.; Couto, J.P.; Sethi, A.; Coissieux, M.M.; Munst, S.; Okamoto, R.; Kohler, H.; Schmidt, A.; et al. Glucocorticoids promote breast cancer metastasis. Nature 2019, 567, 540–544. [Google Scholar] [CrossRef]
- Sloan, E.K.; Priceman, S.J.; Cox, B.F.; Yu, S.; Pimentel, M.A.; Tangkanangnukul, V.; Arevalo, J.M.; Morizono, K.; Karanikolas, B.D.; Wu, L.; et al. The sympathetic nervous system induces a metastatic switch in primary breast cancer. Cancer Res. 2010, 70, 7042–7052. [Google Scholar] [CrossRef]
- Liu, Z.W.; Zhang, Y.M.; Zhang, L.Y.; Zhou, T.; Li, Y.Y.; Zhou, G.C.; Miao, Z.M.; Shang, M.; He, J.P.; Ding, N.; et al. Duality of Interactions between T.TGF-beta and TNF-alpha during Tumor Formation. Front. Immunol. 2021, 12, 810286. [Google Scholar] [CrossRef]
- Yang, Y.; Cao, Y. The impact of VEGF on cancer metastasis and systemic disease. Semin. Cancer Biol. 2022, 86, 251–261. [Google Scholar] [CrossRef] [PubMed]
- Kumagai, M.; Guo, X.; Wang, K.Y.; Izumi, H.; Tsukamoto, M.; Nakashima, T.; Tasaki, T.; Kurose, N.; Uramoto, H.; Sasaguri, Y.; et al. Depletion of WNT10A Prevents Tumor Growth by Suppressing Microvessels and Collagen Expression. Int. J. Med. Sci. 2019, 16, 416–423. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Hamid, N.M.; Abass, S.A. Matrix metalloproteinase contribution in management of cancer proliferation, metastasis and drug targeting. Mol. Biol. Rep. 2021, 48, 6525–6538. [Google Scholar] [CrossRef] [PubMed]
- Flaherty, R.L.; Intabli, H.; Falcinelli, M.; Bucca, G.; Hesketh, A.; Patel, B.A.; Allen, M.C.; Smith, C.P.; Flint, M.S. Stress hormone-mediated acceleration of breast cancer metastasis is halted by inhibition of nitric oxide synthase. Cancer Lett. 2019, 459, 59–71. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.F.; Diers, A.R.; Hogg, N. Cancer cell metabolism and the modulating effects of nitric oxide. Free Radic. Biol. Med. 2015, 79, 324–336. [Google Scholar] [CrossRef]
- Cipak Gasparovic, A.; Milkovic, L.; Dandachi, N.; Stanzer, S.; Pezdirc, I.; Vrancic, J.; Sitic, S.; Suppan, C.; Balic, M. Chronic Oxidative Stress Promotes Molecular Changes Associated with Epithelial Mesenchymal Transition, NRF2, and Breast Cancer Stem Cell Phenotype. Antioxidants 2019, 8, 633. [Google Scholar] [CrossRef]
- Alateyah, N.; Gupta, I.; Rusyniak, R.S.; Ouhtit, A. SOD2, a Potential Transcriptional Target Underpinning CD44-Promoted Breast Cancer Progression. Molecules 2022, 27, 811. [Google Scholar] [CrossRef]
- Hempel, N.; Carrico, P.M.; Melendez, J.A. Manganese superoxide dismutase (Sod2) and redox-control of signaling events that drive metastasis. Anticancer. Agents Med. Chem. 2011, 11, 191–201. [Google Scholar] [CrossRef]
- Zhao, J.; Ma, Y.; Tanimoto, T.; Ozaki, A.; Chen, W.L.; Wang, J.Y.; Zhang, Y.X.; Chen, L.L.; Wang, J.W.; Yu, J.M. Effects of physical activity and stress on the relationship between social capital and quality of life among breast cancer survivors. Sci. Rep. 2020, 10, 17746. [Google Scholar] [CrossRef]
- Kenne Sarenmalm, E.; Martensson, L.B.; Andersson, B.A.; Karlsson, P.; Bergh, I. Mindfulness and its efficacy for psychological and biological responses in women with breast cancer. Cancer Med. 2017, 6, 1108–1122. [Google Scholar] [CrossRef]
- Kajimoto, K.; Hisada, C.; Ochi, S.; Yoshikawa, E.; Suzuki, A.; Tsugane, H.; Zhang, J.; Iinuma, M.; Kubo, K.Y.; Azuma, K. Maternal chewing improves prenatal stress-induced cognitive deficit and anxiety-like behavior associated with alterations of the apoptotic response and serotonin pathway in mouse offspring. Arch. Oral. Biol. 2021, 130, 105245. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Suzuki, A.; Iinuma, M.; Wang, K.Y.; Kubo, K.Y.; Azuma, K. Effects of Maternal Chewing on Prenatal Stress-Induced Cognitive Impairments in the Offspring via Multiple Molecular Pathways. Int. J. Mol. Sci. 2020, 21, 5627. [Google Scholar] [CrossRef]
- Kubo, K.Y.; Kotachi, M.; Suzuki, A.; Iinuma, M.; Azuma, K. Chewing during prenatal stress prevents prenatal stress-induced suppression of neurogenesis, anxiety-like behavior and learning deficits in mouse offspring. Int. J. Med. Sci. 2018, 15, 849–858. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Katano, M.; Zhang, J.H.; Liu, X.; Wang, K.Y.; Iinuma, M.; Kubo, K.Y.; Azuma, K. Chewing Behavior Attenuates the Tumor Progression-Enhancing Effects of Psychological Stress in a Breast Cancer Model Mouse. Brain Sci. 2021, 11, 479. [Google Scholar] [CrossRef] [PubMed]
- Furuzawa, M.; Chen, H.; Fujiwara, S.; Yamada, K.; Kubo, K.Y. Chewing ameliorates chronic mild stress-induced bone loss in senescence-accelerated mouse (SAMP8), a murine model of senile osteoporosis. Exp. Gerontol. 2014, 55, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Mahauad-Fernandez, W.D.; Naushad, W.; Panzner, T.D.; Bashir, A.; Lal, G.; Okeoma, C.M. BST-2 promotes survival in circulation and pulmonary metastatic seeding of breast cancer cells. Sci. Rep. 2018, 8, 17608. [Google Scholar] [CrossRef]
- Azuma, K.; Zhou, Q.; Niwa, M.; Kubo, K.Y. Association between Mastication, the Hippocampus, and the HPA Axis: A Comprehensive Review. Int. J. Mol. Sci. 2017, 18, 1687. [Google Scholar] [CrossRef]
- He, L.; Yuan, L.; Sun, Y.; Wang, P.; Zhang, H.; Feng, X.; Wang, Z.; Zhang, W.; Yang, C.; Zeng, Y.A.; et al. Glucocorticoid Receptor Signaling Activates TEAD4 to Promote Breast Cancer Progression. Cancer Res. 2019, 79, 4399–4411. [Google Scholar] [CrossRef]
- Puhr, M.; Hoefer, J.; Eigentler, A.; Ploner, C.; Handle, F.; Schaefer, G.; Kroon, J.; Leo, A.; Heidegger, I.; Eder, I.; et al. The Glucocorticoid Receptor Is a Key Player for Prostate Cancer Cell Survival and a Target for Improved Antiandrogen Therapy. Clin. Cancer Res. 2018, 24, 927–938. [Google Scholar] [CrossRef]
- Kubo, K.Y.; Iinuma, M.; Chen, H. Mastication as a Stress-Coping Behavior. Biomed. Res. Int. 2015, 2015, 876409. [Google Scholar] [CrossRef]
- Chang, A.; Le, C.P.; Walker, A.K.; Creed, S.J.; Pon, C.K.; Albold, S.; Carroll, D.; Halls, M.L.; Lane, J.R.; Riedel, B.; et al. beta2-Adrenoceptors on tumor cells play a critical role in stress-enhanced metastasis in a mouse model of breast cancer. Brain Behav. Immun. 2016, 57, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Zhao, H.; Liu, Y.; Zuo, Y.; Xu, Q.; Liu, L.; Li, X.; Zhu, H.; Zhang, Y.; Zhang, S.; et al. Chronic Stress Activates PlexinA1/VEGFR2-JAK2-STAT3 in Vascular Endothelial Cells to Promote Angiogenesis. Front. Oncol. 2021, 11, 709057. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Lu, X.; Shi, P.; Yang, G.; Zhou, Z.; Li, W.; Mao, X.; Jiang, D.; Chen, C. TNF-alpha increases breast cancer stem-like cells through up-regulating TAZ expression via the non-canonical NF-kappaB pathway. Sci. Rep. 2020, 10, 1804. [Google Scholar] [CrossRef]
- Padua, D.; Zhang, X.H.; Wang, Q.; Nadal, C.; Gerald, W.L.; Gomis, R.R.; Massague, J. TGFbeta primes breast tumors for lung metastasis seeding through angiopoietin-like 4. Cell 2008, 133, 66–77. [Google Scholar] [CrossRef]
- Niland, S.; Riscanevo, A.X.; Eble, J.A. Matrix Metalloproteinases Shape the Tumor Microenvironment in Cancer Progression. Int. J. Mol. Sci. 2021, 23, 146. [Google Scholar] [CrossRef]
- Li, H.; Qiu, Z.; Li, F.; Wang, C. The relationship between MMP-2 and MMP-9 expression levels with breast cancer incidence and prognosis. Oncol. Lett. 2017, 14, 5865–5870. [Google Scholar] [CrossRef]
- Yousef, E.M.; Tahir, M.R.; St-Pierre, Y.; Gaboury, L.A. MMP-9 expression varies according to molecular subtypes of breast cancer. BMC Cancer 2014, 14, 609. [Google Scholar] [CrossRef] [PubMed]
- Zhong, H.; Yin, H. Role of lipid peroxidation derived 4-hydroxynonenal (4-HNE) in cancer: Focusing on mitochondria. Redox Biol. 2015, 4, 193–199. [Google Scholar] [CrossRef] [PubMed]
| Antibody | CAT# | Source | MW (kDa) | Dilution | Application |
|---|---|---|---|---|---|
| GR | #12041 | CST | 91 | 1:1000/1:400 | WB/IHC |
| Β2AR | Ab135641 | Abcam | 60 | 1:100/1:50 | WB/IHC |
| TNF-α | #L1120 | SCBT | 27 | 1:500 | WB |
| TGF-β | #3711 | CST | 25 | 1:1000 | WB |
| VEGF | GTX74091 | GeneTex | 25 | 1:1000 | WB |
| MMP2 | 10373-2-AP | Proteintech | 53 | 1:500 | WB |
| MMP9 | 10375-2-AP | Proteintech | 67 | 1:500 | WB |
| iNOS | #2982 | CST | 130 | 1:1000 | WB |
| HNE | MHN-100P | JaICA | 70 | 16 μg/mL | WB |
| SOD2 | #13141 | CST | 22 | 1:1000 | WB |
| GAPDH | #2118 | CST | 37 | 1:1000 | WB |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.-H.; Wang, K.-Y.; Kubo, K.-Y.; Azuma, K. Chewing Behavior Attenuates Lung-Metastasis-Promoting Effects of Chronic Stress in Breast-Cancer Lung-Metastasis Model Mice. Cancers 2022, 14, 5950. https://doi.org/10.3390/cancers14235950
Zhang J-H, Wang K-Y, Kubo K-Y, Azuma K. Chewing Behavior Attenuates Lung-Metastasis-Promoting Effects of Chronic Stress in Breast-Cancer Lung-Metastasis Model Mice. Cancers. 2022; 14(23):5950. https://doi.org/10.3390/cancers14235950
Chicago/Turabian StyleZhang, Jia-He, Ke-Yong Wang, Kin-Ya Kubo, and Kagaku Azuma. 2022. "Chewing Behavior Attenuates Lung-Metastasis-Promoting Effects of Chronic Stress in Breast-Cancer Lung-Metastasis Model Mice" Cancers 14, no. 23: 5950. https://doi.org/10.3390/cancers14235950
APA StyleZhang, J.-H., Wang, K.-Y., Kubo, K.-Y., & Azuma, K. (2022). Chewing Behavior Attenuates Lung-Metastasis-Promoting Effects of Chronic Stress in Breast-Cancer Lung-Metastasis Model Mice. Cancers, 14(23), 5950. https://doi.org/10.3390/cancers14235950







