Identification of Altered Primary Immunodeficiency-Associated Genes and Their Implications in Pediatric Cancers
Abstract
Simple Summary
Abstract
1. Introduction
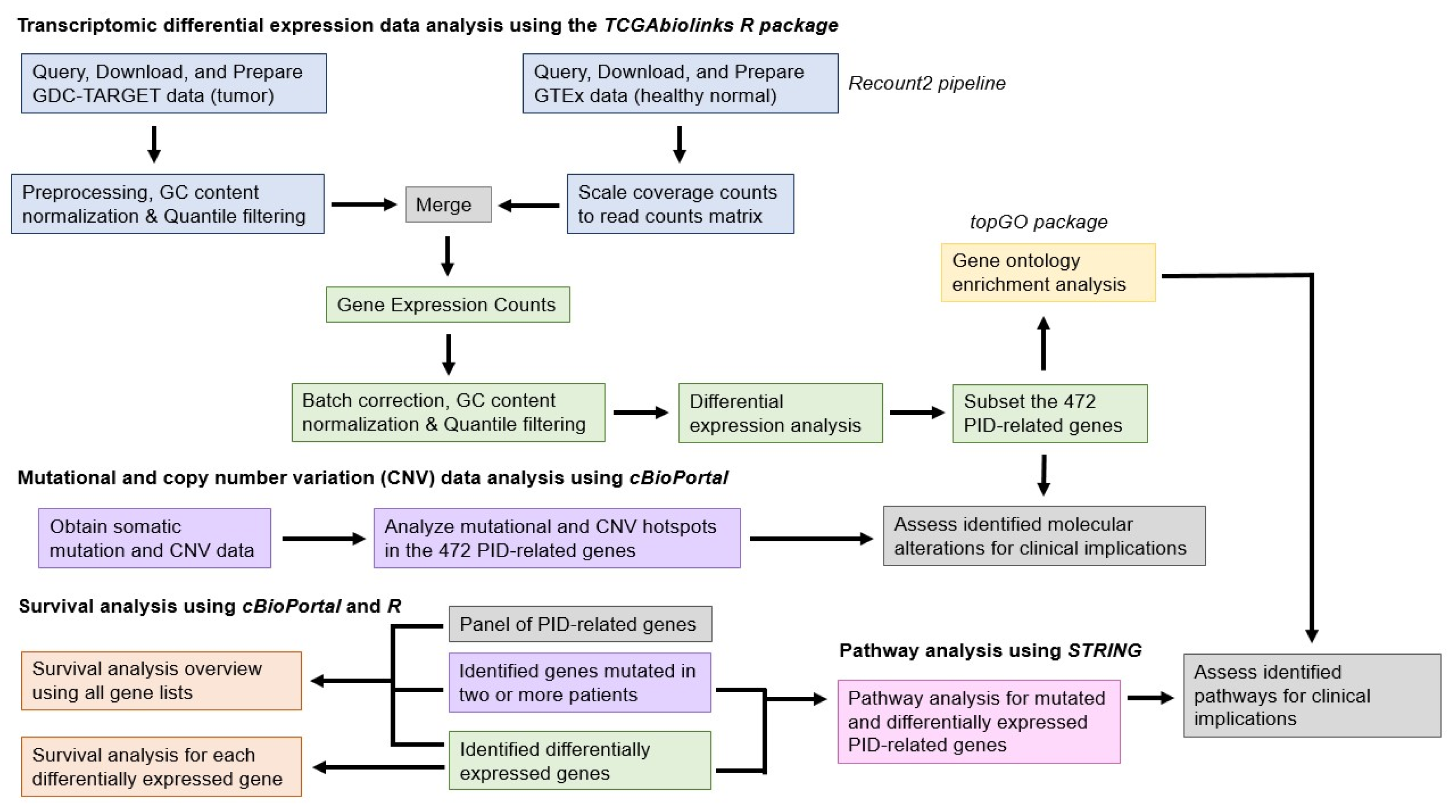
2. Materials and Methods
2.1. Data Collection and Processing
2.2. Differential Gene Expression Analysis of Unified TARGET-GTEx Datasets
2.3. Mutational and Copy Number Variation Analysis
2.4. Functional Enrichment Analysis
2.5. Survival Analysis
3. Results
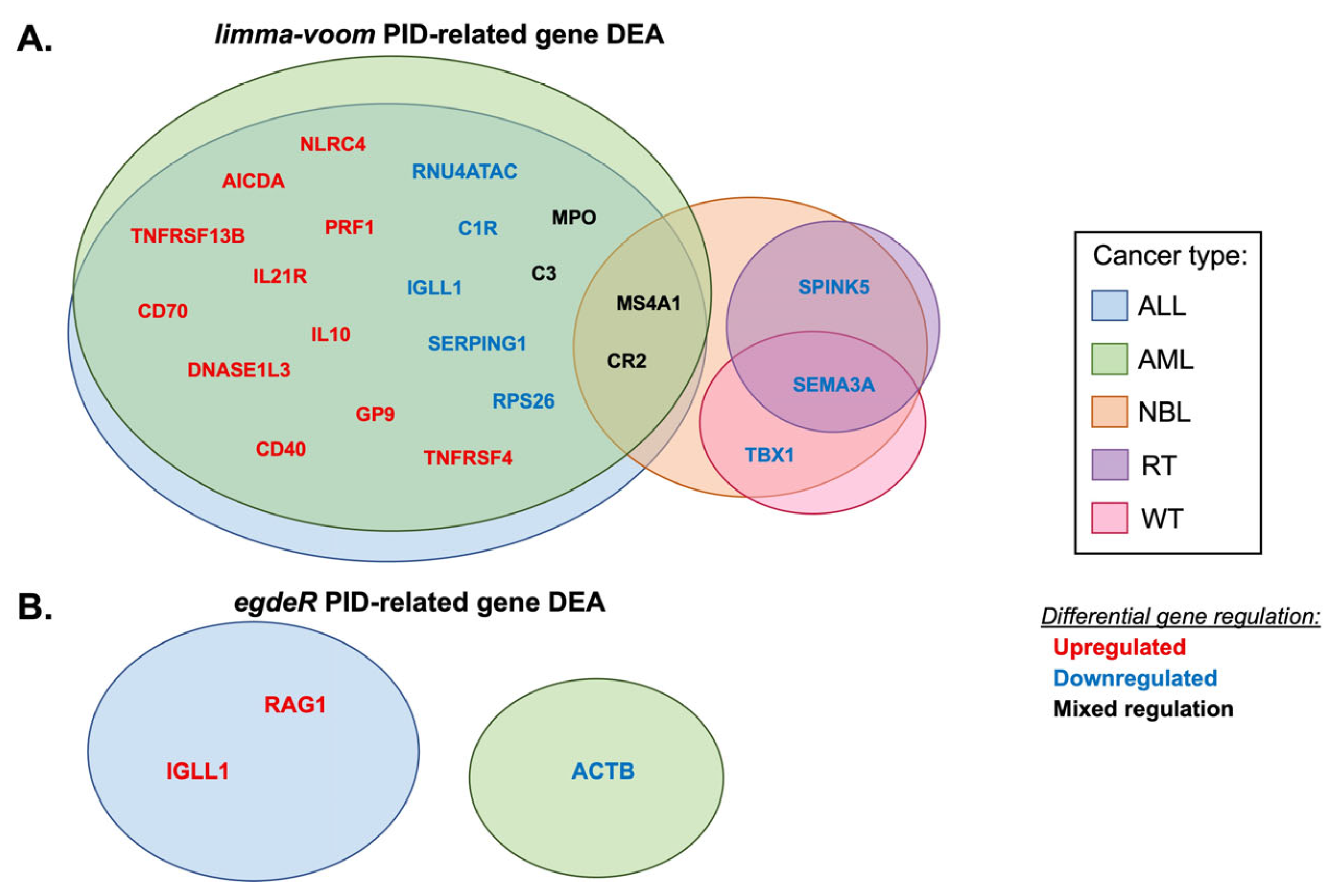
3.1. Differential Expression of PID-Related Genes in Pediatric Cancer Datasets
3.2. PID-Related DEGs Are Associated with Various Immunological and Oncologic Pathways
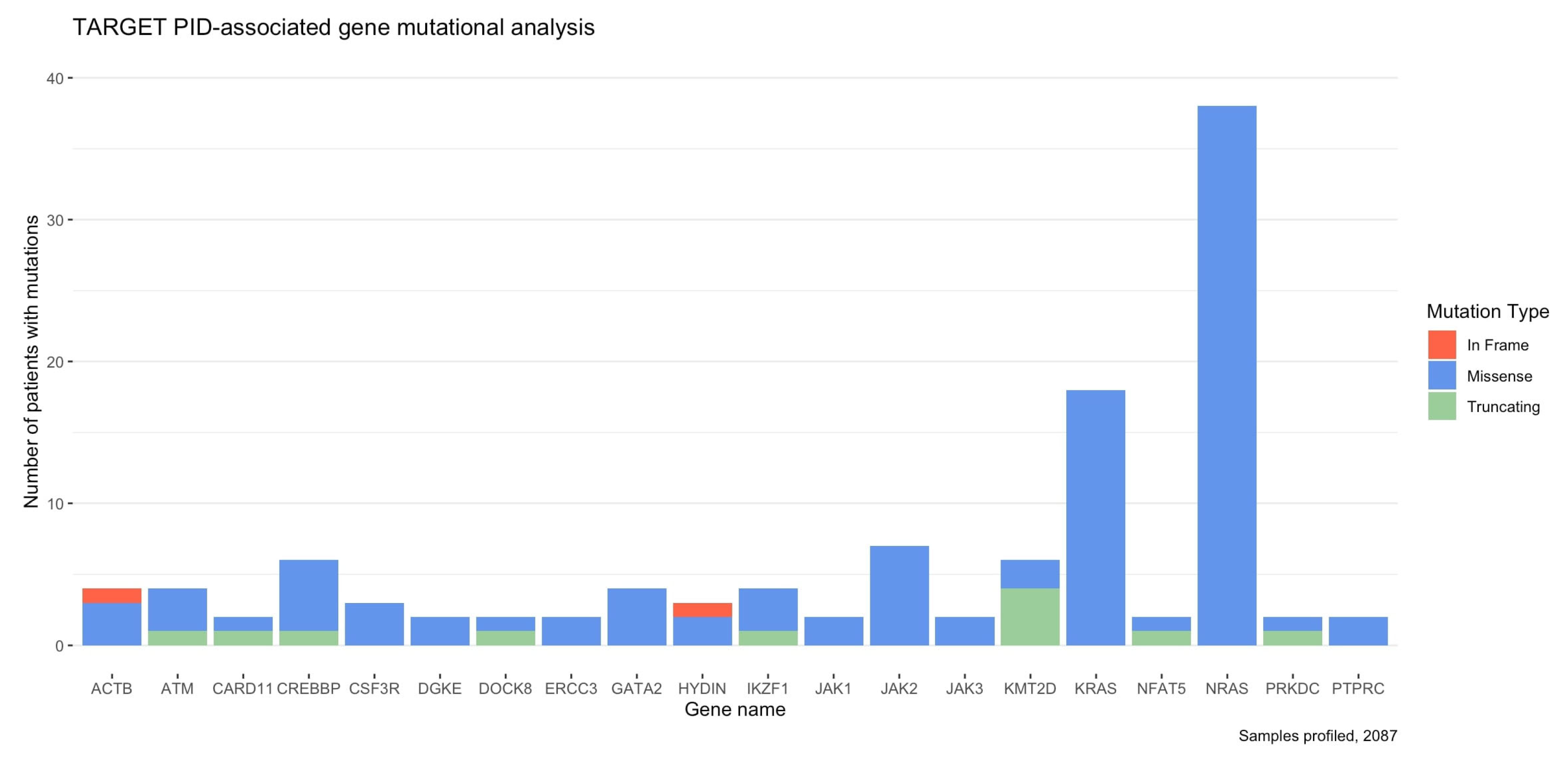
3.3. Pediatric Cancers Harbor Mutations in PID-Related Genes
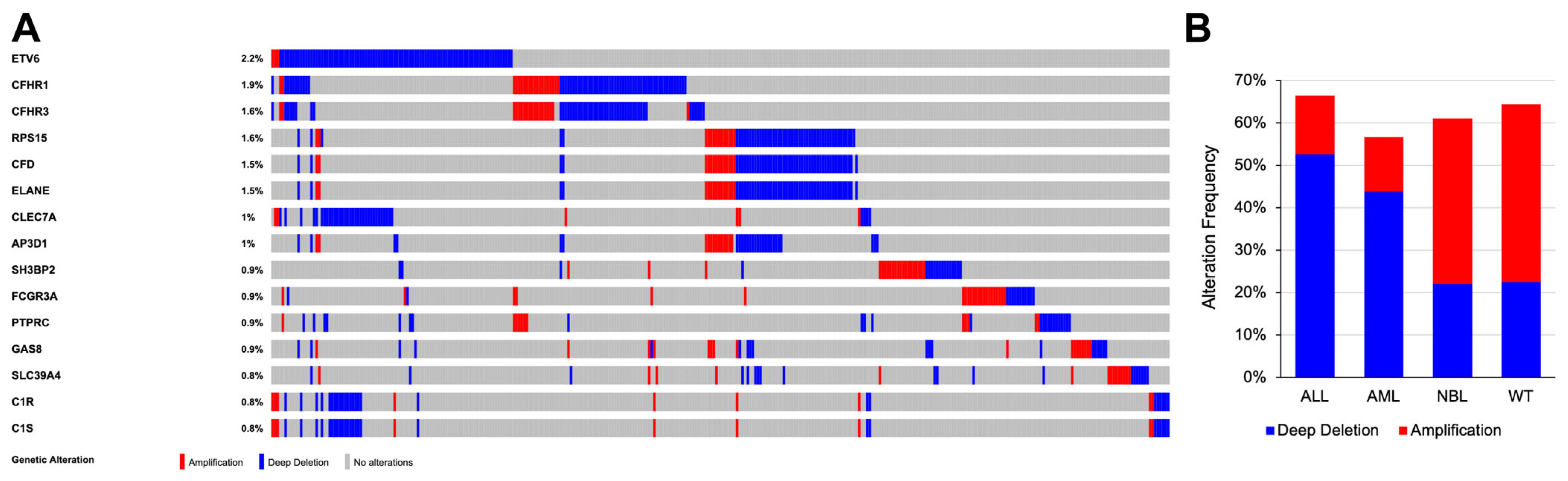
3.4. Copy Number Alterations in PID-Related Genes among Pediatric Cancers
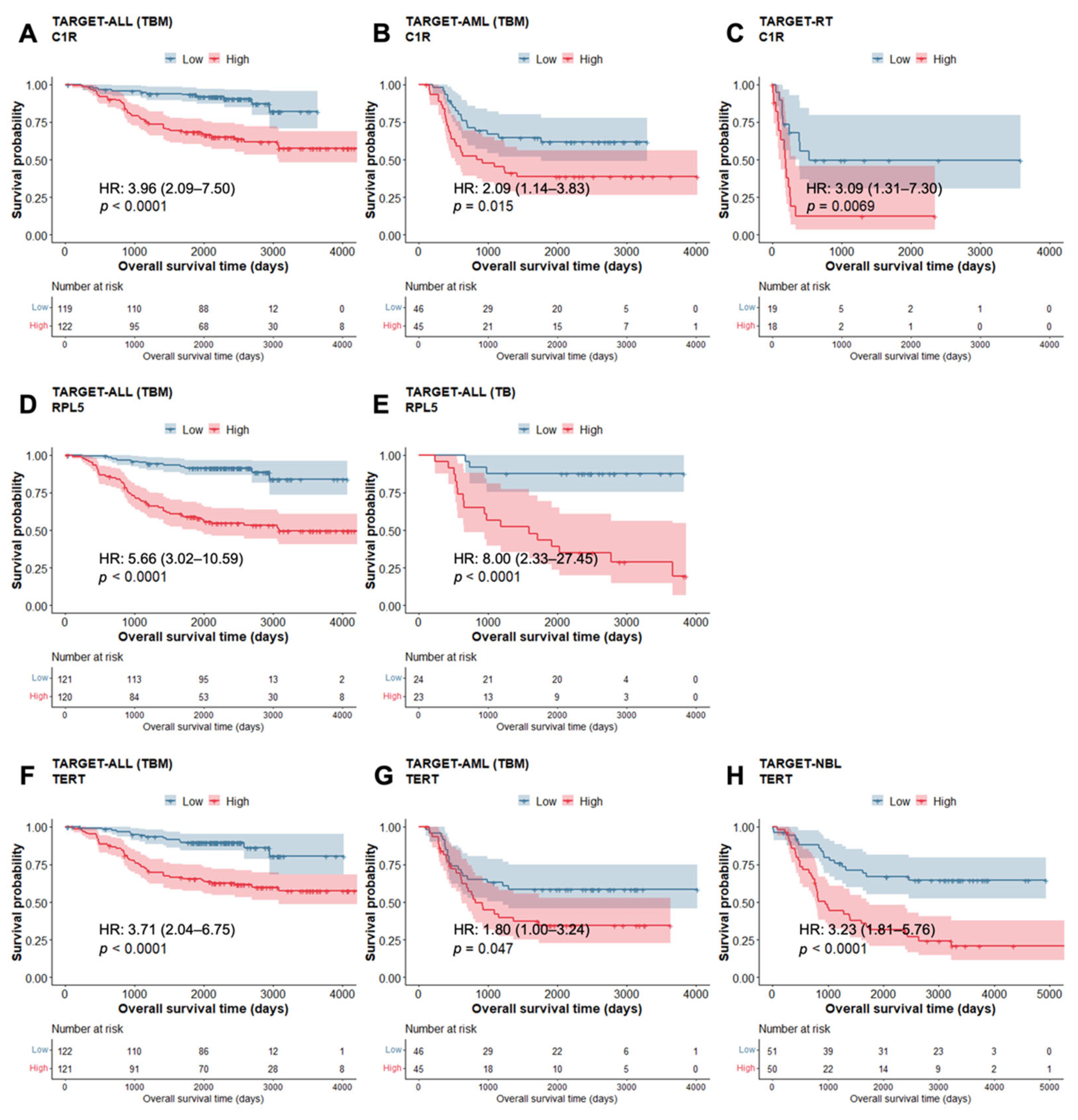
3.5. Altered PID-Related Genes Affect Overall Survival in Pediatric Cancer Cohorts
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Canadian Cancer Society’s Advisory Committee on Cancer Statistics. Canadian Cancer Statistics 2015; Canadian Cancer Society: Calgary, AB, Canada, 2015; pp. 1–151. [Google Scholar]
- Derpoorter, C.; Bordon, V.; Laureys, G.; Haerynck, F.; Lammens, T. Genes at the Crossroad of Primary Immunodeficiencies and Cancer. Front. Immunol. 2018, 9, 2544. [Google Scholar] [CrossRef] [PubMed]
- Hauck, F.; Voss, R.; Urban, C.; Seidel, M.G. Intrinsic and extrinsic causes of malignancies in patients with primary immunodeficiency disorders. J. Allergy Clin. Immunol. 2018, 141, 59–68.e4. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. The Hallmarks of Cancer. Cell 2000, 100, 57–70. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Tangye, S.G.; Al-Herz, W.; Bousfiha, A.; Cunningham-Rundles, C.; Franco, J.L.; Holland, S.M.; Klein, C.; Morio, T.; Oksenhendler, E.; Picard, C.; et al. Human inborn errors of immunity: 2022 update on the classification from the International Union of Immunological Societies Expert Committee. J. Clin. Immunol. 2022, 42, 1473–1507. [Google Scholar] [CrossRef]
- Proceedings of the Canadian Society of Allergy and Clinical Immunology Annual Scientific Meeting 2019. Allergy Asthma Clin Immunol. 2020, 16, 47. [CrossRef]
- Proceedings of the Canadian Society of Allergy and Clinical Immunology Annual Scientific Meeting 2020. Allergy Asthma Clin. Immunol. 2021, 17, 33. [CrossRef]
- Mounir, M.; Lucchetta, M.; Silva, T.C.; Olsen, C.; Bontempi, G.; Chen, X.; Noushmehr, H.; Colaprico, A.; Papaleo, E. New functionalities in the TCGAbiolinks package for the study and integration of cancer data from GDC and GTEx. PLoS Comput. Biol. 2019, 15, e1006701. [Google Scholar] [CrossRef]
- Grossman, R.L.; Heath, A.P.; Ferretti, V.; Varmus, H.E.; Lowy, D.R.; Kibbe, W.A.; Staudt, L.M. Toward a Shared Vision for Cancer Genomic Data. N. Engl. J. Med. 2016, 375, 1109–1112. [Google Scholar] [CrossRef]
- Collado-Torres, L.; Nellore, A.; Kammers, K.; Ellis, S.E.; Taub, M.A.; Hansen, K.D.; Jaffe, A.E.; Langmead, B.; Leek, J.T. Reproducible RNA-seq analysis using recount2. Nat. Biotechnol. 2017, 35, 319–321. [Google Scholar] [CrossRef]
- Bartha, Á.; Győrffy, B. TNMplot.com: A Web Tool for the Comparison of Gene Expression in Normal, Tumor and Metastatic Tissues. Int. J. Mol. Sci. 2021, 22, 2622. [Google Scholar] [CrossRef]
- Zeng, W.Z.D.; Glicksberg, B.S.; Li, Y.; Chen, B. Selecting precise reference normal tissue samples for cancer research using a deep learning approach. BMC Med. Genom. 2019, 12, 179–189. [Google Scholar] [CrossRef]
- Ritchie, M.E.; Belinda, P.; Wu, D.; Hu, Y.; Law, C.W.; Shi, W.; Smyth, G.K. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015, 43, e47. [Google Scholar] [CrossRef]
- Lucchetta, M.; Da Piedade, I.; Mounir, M.; Vabistsevits, M.; Terkelsen, T.; Papaleo, E. Distinct signatures of lung cancer types: Aberrant mucin O-glycosylation and compromised immune response. BMC Cancer 2019, 19, 824. [Google Scholar] [CrossRef]
- Comprehensive Primary Immunodeficiency NGS Panel. Fulgent Genetics. Available online: https://www.fulgentgenetics.com/Comprehensive-Primary-Immunodeficiency (accessed on 21 July 2022).
- Gao, J.; Aksoy, B.A.; Dogrusoz, U.; Dresdner, G.; Gross, B.E.; Sumer, S.O.; Sun, Y.; Jacobsen, A.; Sinha, R.; Larsson, E.; et al. Integrative Analysis of Complex Cancer Genomics and Clinical Profiles Using the cBioPortal. Sci. Signal. 2013, 6, pl1. [Google Scholar] [CrossRef]
- Alexa, A.; Rahnenführer, J. Gene set enrichment analysis with topGO. Bioconduct. Improv. 2009, 27, 1–26. [Google Scholar]
- Szklarczyk, D.; Gable, A.L.; Lyon, D.; Junge, A.; Wyder, S.; Huerta-Cepas, J.; Simonovic, M.; Doncheva, N.T.; Morris, H.J.; Bork, P.; et al. STRING v11: Protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2019, 47, D607–D613. [Google Scholar] [CrossRef]
- Asokan, R.; Banda, N.K.; Szakonyi, G.; Chen, X.S.; Holers, V.M. Human complement receptor 2 (CR2/CD21) as a receptor for DNA: Implications for its roles in the immune response and the pathogenesis of systemic lupus erythematosus (SLE). Mol. Immunol. 2013, 53, 99–110. [Google Scholar] [CrossRef]
- Pavlasova, G.; Mraz, M. The regulation and function of CD20: An “enigma” of B-cell biology and targeted therapy. Haematologica 2020, 105, 1494–1506. [Google Scholar] [CrossRef]
- Hanchate, N.K.; Giacobini, P.; Lhuillier, P.; Parkash, J.; Espy, C.; Fouveaut, C.; Leroy, C.; Baron, S.; Campagne, C.; Vanacker, C.; et al. SEMA3A, a Gene Involved in Axonal Pathfinding, Is Mutated in Patients with Kallmann Syndrome. PLoS Genet. 2012, 8, e1002896. [Google Scholar] [CrossRef]
- Rezaeepoor, M.; Ganjalikhani-Hakemi, M.; Shapoori, S.; Eskandari, N.; Sharifi, M.; Etemadifar, M.; Marjan, M. Semaphorin-3A as an immune modulator is suppressed by microRNA-145-5p. Cell J. 2018, 20, 113–119. [Google Scholar] [PubMed]
- Notarangelo, L.D.; Kim, M.-S.; Walter, J.E.; Lee, Y.N. Human RAG mutations: Biochemistry and clinical implications. Nat. Rev. Immunol. 2016, 16, 234–246. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.; Ma, J.; Gu, Y.; Song, H.; Kapadia, M.; Kawasawa, Y.I.; Dovat, S.; Song, C.; Ge, Z. RAG1 high expression associated with IKZF1 dysfunction in adult B-cell acute lymphoblastic leukemia. J. Cancer 2019, 10, 3842–3850. [Google Scholar] [CrossRef] [PubMed]
- Rommel, P.C.; Oliveira, T.Y.; Nussenzweig, M.C.; Robbiani, D.F. RAG1/2 induces genomic insertions by mobilizing DNA into RAG1/2-independent breaks. J. Exp. Med. 2017, 214, 815–831. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Huang, S.; Luo, S.; Liao, H.; Wang, Y.; Deng, X.; Ma, F.; Ma, C.W.; Zhou, L. Identification of genes underlying the enhancement of immunity by a formula of lentinan, pachymaran and tremelia polysaccharides in immunosuppressive mice. Sci. Rep. 2018, 8, 10082. [Google Scholar] [CrossRef]
- Whichard, Z.L.; Sarkar, C.A.; Kimmel, M.; Corey, S.J. Hematopoiesis and its disorders: A systems biology approach. Blood 2010, 115, 2339–2347. [Google Scholar] [CrossRef]
- Wang, S.; Wu, Z.; Li, T.; Li, Y.; Wang, W.; Hao, Q.; Xie, X.; Wan, D.; Jiang, Z.; Wang, C.; et al. Mutational spectrum and prognosis in NRAS-mutated acute myeloid leukemia. Sci. Rep. 2020, 10, 12152. [Google Scholar] [CrossRef]
- Prior, I.A.; Lewis, P.D.; Mattos, C. A Comprehensive Survey of Ras Mutations in Cancer. Cancer Res 2012, 72, 2457–2467. [Google Scholar] [CrossRef]
- Freitas, R.M.D.; Santos, M.D.O.; Maranduba, C.M.D.C. The JAK2 gene as a protagonist in chronic myeloproliferative neoplasms. Rev. Bras Hematol. Hemoter. 2013, 35, 278–279. [Google Scholar] [CrossRef][Green Version]
- Farrar, W.L.; Brini, A.T.; Harel-Bellan, A.; Korner, M.; Ferris, D.K. Hematopoietic growth-factor signal transduction and regulation of gene expression. Immunol. Ser. 1990, 49, 379–410. [Google Scholar]
- Mullighan, C.G.; Zhang, J.; Kasper, L.H.; Lerach, S.; Payne-Turner, D.; Phillips, L.A.; Heatley, S.L.; Holmfeldt, L.; Collins-Underwood, J.R.; Ma, J.; et al. CREBBP mutations in relapsed acute lymphoblastic leukaemia. Nature 2011, 471, 235–239. [Google Scholar] [CrossRef]
- Chang, Y.; Woessner, D.W.; Lin, W.; Chen, T.; Xu, B.; Fan, Y.; Haiyan, T.; Junmin, P.; Lawryn, K.; Churchman, M.L.; et al. CREBBP histone acetyltransferase domain mutations result in dexamethasone resistance in B-progenitor acute lymphoblastic leukemia. Blood 2017, 130, 560. [Google Scholar]
- Maxson, J.E.; Gotlib, J.; Pollyea, D.A.; Fleischman, A.G.; Agarwal, A.; Eide, C.A.; Bottomly, D.; Wilmot, B.; McWeeney, S.K.; Tognon, C.E.; et al. Oncogenic CSF3R mutations in chronic neutrophilic leukemia and atypical CML. N. Engl. J. Med. 2013, 368, 1781–1790. [Google Scholar] [CrossRef]
- Steeghs, E.M.; Jerchel, I.S.; de Goffau-Nobel, W.; Hoogkamer, A.Q.; Boer, J.M.; Boeree, A.; van de Ven, C.; Koudijs, M.J.; Besselink, N.J.; de Groot-Kruseman, H.A.; et al. JAK2 aberrations in childhood B-cell precursor acute lymphoblastic leukemia. Oncotarget 2017, 8, 89923–89938. [Google Scholar] [CrossRef]
- Shan, Y.; Gnanasambandan, K.; Ungureanu, D.; Kim, E.T.; Hammarén, H.; Yamashita, K.; Silvennoinen, O.; Shaw, D.E.; Hubbard, S.R. Molecular basis for pseudokinase-dependent autoinhibition of JAK2 tyrosine kinase. Nat. Struct. Mol. Biol. 2014, 21, 579–584. [Google Scholar] [CrossRef]
- Greaves, S.A.; Peterson, J.N.; Torres, R.M.; Pelanda, R. Corrigendum: Activation of the MEK-ERK Pathway Is Necessary but Not Sufficient for Breaking Central B Cell Tolerance. Front. Immunol. 2018, 9, 707. [Google Scholar] [CrossRef]
- Alabdulaali, M.K. The role of JAK2 abnormalities in hematologic neoplasms. Hematol. Rep. 2009, 1, e10. [Google Scholar] [CrossRef]
- Waters, A.M.; Der, C.J. KRAS: The critical driver and therapeutic target for pancreatic cancer. Cold Spring Harb. Perspect. Med. 2018, 8, a031435. [Google Scholar] [CrossRef]
- Rabara, D.; Tran, T.H.; Dharmaiah, S.; Stephens, R.M.; McCormick, F.; Simanshu, D.K.; Holderfield, M. KRAS G13D sensitivity to neurofibromin-mediated GTP hydrolysis. Proc. Natl. Acad. Sci. USA 2019, 116, 22122–22131. [Google Scholar] [CrossRef]
- Smith, M.J.; Neel, B.G.; Ikura, M. NMR-based functional profiling of RASopathies and oncogenic RAS mutations. Proc. Natl. Acad. Sci. USA 2013, 110, 4574–4579. [Google Scholar] [CrossRef]
- Burd, C.E.; Liu, W.; Huynh, M.V.; Waqas, M.A.; Gillahan, J.E.; Clark, K.S.; Fu, K.; Martin, B.L.; Jeck, W.R.; Souroullas, G.P.; et al. Mutation-Specific RAS Oncogenicity Explains NRAS Codon 61 Selection in Melanoma. Cancer Discov. 2014, 4, 1418–1429. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.; Szankasi, P.; Sederberg, M.; Schumacher, J.; Frizzell, K.A.; Gee, E.P.; Patel, J.L.; South, S.T.; Xu, X.; Kelley, T.W. Concurrent detection of targeted copy number variants and mutations using a myeloid malignancy next generation sequencing panel allows comprehensive genetic analysis using a single testing strategy. Br. J. Haematol. 2016, 173, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Forero-Castro, M.; Robledo, C.; Benito, R.; Abáigar, M.; Martín, A.; Arefi, M.; Fuster, J.L.; Heras, N.D.L.; Rodríguez, J.N.; Quintero, J.; et al. Genome-Wide DNA Copy Number Analysis of Acute Lymphoblastic Leukemia Identifies New Genetic Markers Associated with Clinical Outcome. PLoS ONE 2016, 11, e0148972. [Google Scholar] [CrossRef] [PubMed]
- Bokemeyer, A.; Eckert, C.; Meyr, F.; Koerner, G.; von Stackelberg, A.; Ullmann, R.; Türkmen, S.; Henze, G.; Seeger, K. Copy number genome alterations are associated with treatment response and outcome in relapsed childhood ETV6/RUNX1-positive acute lymphoblastic leukemia. Haematologica 2014, 99, 706–714. [Google Scholar] [CrossRef] [PubMed]
- Järviaho, T.; Bang, B.; Zachariadis, V.; Taylan, F.; Moilanen, J.; Möttönen, M.; Smith, C.I.E.; Harila-Saari, A.; Niinimäki, R.; Nordgren, A. Predisposition to childhood acute lymphoblastic leukemia caused by a constitutional translocation disrupting ETV6. Blood Adv. 2019, 3, 2722–2731. [Google Scholar] [CrossRef]
- Panda, A.; Yadav, A.; Yeerna, H.; Singh, A.; Biehl, M.; Lux, M.; Schulz, A.; Klecha, T.; Doniach, S.; Khiabanian, H.; et al. Tissue- and development-stage-specific mRNA and heterogeneous CNV signatures of human ribosomal proteins in normal and cancer samples. Nucleic Acids Res. 2020, 48, 7079–7098. [Google Scholar] [CrossRef]
- Jodele, S.; Licht, C.; Goebel, J.; Dixon, B.P.; Zhang, K.; Sivakumaran, T.A.; Davies, S.M.; Pluthero, F.G.; Lu, L.; Laskin, B.L. Abnormalities in the alternative pathway of complement in children with hematopoietic stem cell transplant-associated thrombotic microangiopathy. Blood 2013, 122, 2003–2007. [Google Scholar] [CrossRef]
- Cheng, G.; Ozgonenel, B.; Bhambhani, K.; Kapur, G.; Smith, R.; Savaşan, S. Recurrent Atypical Hemolytic Uremic Syndrome in Children With Acute Lymphoblastic Leukemia Undergoing Maintenance Chemotherapy. J. Pediatr. Hematol. 2018, 40, 560–562. [Google Scholar] [CrossRef]
- Nozawa, A.; Ozeki, M.; Hori, T.; Kawamoto, N.; Hirayama, M.; Azuma, E.; Fukao, T. A Heterozygous CFHR3-CFHR1 Gene Deletion in a Pediatric Patient With Transplant-associated Thrombotic Microangiopathy Who was Treated with Eculizumab. J. Pediatr. Hematol. 2018, 40, e544–e546. [Google Scholar] [CrossRef]
- Witjes, L.; Van Troys, M.; Verhasselt, B.; Ampe, C. Prevalence of Cytoplasmic Actin Mutations in Diffuse Large B-Cell Lymphoma and Multiple Myeloma: A Functional Assessment Based on Actin Three-Dimensional Structures. Int. J. Mol. Sci. 2020, 21, 3093. [Google Scholar] [CrossRef]
- Castro, E.; Cortes-Santiago, N.; Ferguson, L.M.S.; Rao, P.H.; Venkatramani, R.; López-Terrada, D. Translocation t(7;12) as the sole chromosomal abnormality resulting in ACTB-GLI1 fusion in pediatric gastric pericytoma. Hum. Pathol. 2016, 53, 137–141. [Google Scholar] [CrossRef]
- Kerr, D.A.; Pinto, A.; Subhawong, T.K.; Wilky, B.A.; Schlumbrecht, M.P.; Antonescu, C.R.; Nielsen, G.P.; Rosenberg, A.E. Pericytoma with t(7;12) and ACTB-GLI1 fusion: Reevaluation of an unusual entity and its relationship to the spectrum of GLI1 fusion-related neoplasms. Am. J. Surg. Pathol. 2019, 43, 1682–1692. [Google Scholar] [CrossRef]
- Bruzzese, A.; Leardini, D.; Masetti, R.; Strocchio, L.; Girardi, K.; Algeri, M.; Del Baldo, G.; Locatelli, F.; Mastronuzzi, A. GATA2 Related Conditions and Predisposition to Pediatric Myelodysplastic Syndromes. Cancers 2020, 12, 2962. [Google Scholar] [CrossRef]
- Riihilä, P.; Viiklepp, K.; Nissinen, L.; Farshchian, M.; Kallajoki, M.; Kivisaari, A.; Meri, S.; Peltonen, J.; Kähäri, V.; Peltonen, S. Tumour-cell-derived complement components C1r and C1s promote growth of cutaneous squamous cell carcinoma. Br. J. Dermatol. 2019, 182, 658–670. [Google Scholar] [CrossRef]
- Fancello, L.; Kampen, K.R.; Hofman, I.J.; Verbeeck, J.; De Keersmaecker, K. The ribosomal protein gene RPL5 is a haploinsufficient tumor suppressor in multiple cancer types. Oncotarget 2017, 8, 14462–14478. [Google Scholar] [CrossRef]
- Dolezal, J.M.; Dash, A.P.; Prochownik, E.V. Diagnostic and prognostic implications of ribosomal protein transcript expression patterns in human cancers. BMC Cancer 2018, 18, 275. [Google Scholar] [CrossRef]
- Gay-Bellile, M.; Véronèse, L.; Combes, P.; Eymard-Pierre, E.; Kwiatkowski, F.; Dauplat, M.-M.; Cayre, A.; Privat, M.; Abrial, C.; Bignon, Y.-J.; et al. TERT promoter status and gene copy number gains: Effect on TERT expression and association with prognosis in breast cancer. Oncotarget 2017, 8, 77540–77551. [Google Scholar] [CrossRef]
- Zhang, X.; Klamer, B.; Li, J.; Fernandez, S.; Li, L. A pan-cancer study of class-3 semaphorins as therapeutic targets in cancer. BMC Med. Genom. 2020, 13, 45. [Google Scholar] [CrossRef]
| Gene Mutated | Cancer Type | No. of Patients | Mutation Type | Protein Change | Avg. Allele Freq. | OncoKB Status |
|---|---|---|---|---|---|---|
| ACTB | ALL | 1 | MISSENSE | E205K | 0.36 | |
| WT | 1 | MISSENSE | R147L | 0.35 | ||
| WT | 1 | IF DEL | I282del | 0.34 | ||
| WT | 1 | MISSENSE | G146V | 0.55 | ||
| ADAM17 | ALL | 1 | MISSENSE | C506F | 0.38 | |
| ATM | ALL | 1 | MISSENSE | Y1248H | 0.48 | |
| NBL | 1 | NONSENSE | Q3038* | 0.49 | LO | |
| NBL | 1 | MISSENSE | A911S | 0.35 | ||
| C5 | WT | 1 | MISSENSE | V1108I | 0.28 | |
| CARD11 | NBL | 1 | MISSENSE | Y963C | 0.25 | |
| NBL | 1 | SPLICE | X1007_splice | 0.40 | ||
| CARD9 | AML | 1 | MISSENSE | L54M | 0.42 | |
| CREBBP | ALL | 3 | MISSENSE | R1446C | 0.65 | LO |
| ALL | 1 | MISSENSE | R1446H | 0.11 | LO | |
| ALL | 1 | NONSENSE | R1360* | 0.40 | LO | |
| ALL | 1 | MISSENSE | P1494A | 0.34 | ||
| CSF3R | ALL | 1 | MISSENSE | T618I | 0.55 | O |
| AML | 2 | MISSENSE | T618I | 0.29 | O | |
| CXCR4 | AML | 1 | NONSENSE | R334* | 0.30 | LO |
| DGKE | ALL | 1 | MISSENSE | M123T | 0.41 | |
| WT | 1 | MISSENSE | E460G | 0.11 | ||
| DNAAF2 | AML | 1 | MISSENSE | V141I | 0.67 | |
| DNAAF3 | WT | 1 | MISSENSE | E292G | 0.50 | |
| DNAH1 | ALL | 1 | MISSENSE | S3418P | 0.69 | |
| DNAH11 | ALL | 1 | MISSENSE | S3079L | 0.17 | |
| DNAH5 | WT | 1 | MISSENSE | T179A | 0.09 | |
| DOCK8 | NBL | 1 | MISSENSE | P711T | 0.29 | |
| NBL | 1 | SPLICE | X179_splice | 0.65 | ||
| ERCC3 | ALL | 1 | MISSENSE | G130E | 0.55 | |
| AML | 1 | MISSENSE | I220S | 0.46 | ||
| ETV6 | AML | 1 | MISSENSE | F102L | 0.20 | |
| AML | 1 | MISSENSE | R103G | 0.20 | ||
| F7 | WT | 1 | MISSENSE | Y104H | 0.16 | |
| FANCA | ALL | 1 | MISSENSE | V6D | 0.59 | |
| FANCE | AML | 1 | MISSENSE | S52G | 0.39 | |
| FANCM | NBL | 1 | MISSENSE | E1744K | 0.33 | |
| FAT4 | WT | 1 | MISSENSE | K4272N | 0.32 | |
| GAS8 | AML | 1 | MISSENSE | R369H | 0.26 | |
| GATA1 | AML | 1 | MISSENSE | P73L | 0.37 | |
| GATA2 | AML | 1 | MISSENSE | L321P | 0.16 | |
| AML | 1 | MISSENSE | N317I | 0.28 | ||
| AML | 1 | MISSENSE | A318V | 0.35 | ||
| AML | 1 | MISSENSE | A318T | 0.21 | LN | |
| AML | 1 | MISSENSE | R396Q | 0.45 | ||
| HYDIN | WT | 1 | MISSENSE | T2521P | 0.36 | |
| WT | 1 | MISSENSE | K2518R | 0.08 | ||
| WT | 1 | IF DEL | T2521_R2525del | 0.26 | ||
| IKBKB | ALL | 1 | MISSENSE | N109S | 0.21 | |
| IKZF1 | AML | 1 | MISSENSE | R502W | 0.42 | |
| AML | 1 | MISSENSE | C175Y | 0.52 | ||
| AML | 1 | MISSENSE | N159S | 0.33 | ||
| AML | 1 | NONSENSE | K380* | 0.42 | ||
| IL7R | ALL | 1 | MISSENSE | S185C | 0.31 | O |
| JAK1 | ALL | 1 | MISSENSE | V658F | 0.26 | O |
| ALL | 1 | MISSENSE | Q572L | 0.16 | ||
| JAK2 | ALL | 1 | MISSENSE | R683G | 0.33 | O |
| ALL | 3 | MISSENSE | R683S | 0.20 | O | |
| ALL | 2 | MISSENSE | D873N | 0.30 | ||
| AML | 1 | MISSENSE | V617F | 0.17 | O | |
| AML | 1 | MISSENSE | M535I | 0.20 | LO | |
| JAK3 | AML | 1 | MISSENSE | M511I | 0.14 | O |
| AML | 1 | MISSENSE | L857P | 0.18 | LO | |
| KDM6A | AML | 1 | MISSENSE | R1111P | 0.28 | |
| KMT2D | ALL | 1 | MISSENSE | R5432W | 0.20 | LO |
| ALL | 1 | FS INS | K287Ffs*2 | N/A | LO | |
| ALL | 1 | FS DEL | Q170Afs*49 | 0.31 | LO | |
| WT | 1 | MISSENSE | I1336V | 0.48 | ||
| AML | 1 | NONSENSE | Q2416* | 0.44 | LO | |
| AML | 1 | NONSENSE | R4960* | 0.21 | LO | |
| AML | 1 | MISSENSE | G5410E | 0.47 | ||
| KRAS | ALL | 2 | MISSENSE | G13D | 0.36 | |
| ALL | 1 | MISSENSE | G12S | 0.12 | ||
| ALL | 4 | MISSENSE | G12D | 0.33 | O | |
| ALL | 1 | MISSENSE | G12V | 0.41 | O | |
| WT | 1 | MISSENSE | G12D | 0.47 | O | |
| AML | 1 | MISSENSE | Q61H | 0.46 | O | |
| AML | 2 | MISSENSE | G13D | 0.26 | O | |
| AML | 2 | MISSENSE | G12V | 0.38 | O | |
| AML | 3 | MISSENSE | G12D | 0.49 | O | |
| AML | 1 | MISSENSE | K117N | 0.26 | O | |
| MLPH | ALL | 1 | MISSENSE | P429L | 0.28 | |
| MSN | WT | 1 | MISSENSE | E41G | 0.14 | |
| NBN | RT | 1 | MISENSE | Q291H | N/A | |
| NFAT5 | WT | 1 | NONSENSE | Q36* | 0.44 | |
| NBL | 1 | MISSENSE | L390M | 0.31 | ||
| NFKB1 | WT | 1 | MISSENSE | D544G | 0.09 | |
| NLRC4 | ALL | 1 | MISSENSE | D863G | 0.45 | |
| NLRP12 | WT | 1 | MISSENSE | R138W | 0.56 | |
| NLRP3 | NBL | 1 | MISSENSE | R141I | 0.36 | |
| NRAS | ALL | 6 | MISSENSE | G12D | O | |
| ALL | 1 | MISSENSE | Q61K | 0.41 | O | |
| ALL | 1 | MISSENSE | Q61P | 0.28 | LO | |
| AML | 1 | MISSENSE | G12S | 0.15 | O | |
| AML | 5 | MISSENSE | G13D | 0.42 | O | |
| AML | 1 | MISSENSE | G13V | 0.44 | O | |
| AML | 1 | MISSENSE | G13R | 0.34 | O | |
| AML | 3 | MISSENSE | Q61R | 0.37 | O | |
| AML | 9 | MISSENSE | Q61K | 0.33 | O | |
| AML | 2 | MISSENSE | G12D | 0.22 | O | |
| PCCA | ALL | 1 | MISSENSE | D458H | 0.42 | |
| POLE | WT | 1 | MISSENSE | R924C | 0.30 | |
| PRKDC | WT | 1 | MISSENSE | R2914H | 0.54 | |
| NBL | 1 | SPLICE | X259_splice | 0.61 | ||
| PTPRC | NBL | 1 | MISSENSE | P290S | 0.39 | |
| NBL | 1 | MISSENSE | N176S | 0.38 | ||
| RAC2 | AML | 1 | MISSENSE | G12R | 0.21 | |
| RAD50 | WT | 1 | MISSENSE | T1054A | 0.92 | |
| RNASEH2B | WT | 1 | MISSENSE | E168G | 0.28 | |
| RNF168 | ALL | 1 | MISSENSE | N498D | 0.33 | |
| RNF31 | WT | 1 | MISSENSE | L302F | 0.49 | |
| RORC | NBL | 1 | MISSENSE | L267M | 0.33 | |
| SLC39A4 | AML | 1 | MISSENSE | L561M | 0.51 | |
| SPINK5 | ALL | 1 | MISSENSE | R790Q | 0.47 | |
| STIM1 | ALL | 1 | MISSENSE | C49W | 0.28 | |
| STK4 | ALL | 1 | NONSENSE | W99* | 0.36 | |
| TCF3 | ALL | 1 | FS DEL | G470Afs*16 | 0.43 | LO |
| ALL | 1 | MISSENSE | H460Y | 0.46 | ||
| TERT | WT | 1 | MISSENSE | Q829R | 0.10 | |
| TTC37 | WT | 1 | MISSENSE | R1296S | 0.58 | |
| TYK2 | AML | 1 | MISSENSE | V678L | 0.24 | |
| ZAP70 | ALL | 1 | MISSENSE | R360C | 0.55 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Standing, S.; Tran, S.; Murguia-Favela, L.; Kovalchuk, O.; Bose, P.; Narendran, A. Identification of Altered Primary Immunodeficiency-Associated Genes and Their Implications in Pediatric Cancers. Cancers 2022, 14, 5942. https://doi.org/10.3390/cancers14235942
Standing S, Tran S, Murguia-Favela L, Kovalchuk O, Bose P, Narendran A. Identification of Altered Primary Immunodeficiency-Associated Genes and Their Implications in Pediatric Cancers. Cancers. 2022; 14(23):5942. https://doi.org/10.3390/cancers14235942
Chicago/Turabian StyleStanding, Shaelene, Son Tran, Luis Murguia-Favela, Olga Kovalchuk, Pinaki Bose, and Aru Narendran. 2022. "Identification of Altered Primary Immunodeficiency-Associated Genes and Their Implications in Pediatric Cancers" Cancers 14, no. 23: 5942. https://doi.org/10.3390/cancers14235942
APA StyleStanding, S., Tran, S., Murguia-Favela, L., Kovalchuk, O., Bose, P., & Narendran, A. (2022). Identification of Altered Primary Immunodeficiency-Associated Genes and Their Implications in Pediatric Cancers. Cancers, 14(23), 5942. https://doi.org/10.3390/cancers14235942






