Long Intergenic Non-Protein Coding RNA 173 in Human Cancers
Abstract
Simple Summary
Abstract
1. Introduction
2. Aberrant Expression and Tumorigenesis of LINC00173 in Human Cancer
2.1. Oncogenic
2.1.1. Lung Cancer
2.1.2. Hepatocellular Carcinoma
2.1.3. Colorectal Cancer
2.1.4. Esophageal Squamous Cell Carcinoma
2.1.5. Glioma
2.1.6. Breast Cancer
2.1.7. Prostate Cancer
2.1.8. Wilms’ Tumor
2.2. Tumor Suppressor
2.2.1. Non-small Cell Lung Cancer
2.2.2. Pancreatic Cancer
2.2.3. Cervical Cancer
2.2.4. Acute Myeloid Leukemia
3. Biological Roles of LINC00173
3.1. LINC00173 and Chemoresistance
3.2. Regulatory Targets of LINC00173
3.2.1. LINC00173 and Competing Endogenous RNAs (ceRNAs)
3.2.2. Other Targets of LINC00173
4. Conclusions and Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Kopp, F.; Mendell, J.T. Functional Classification and Experimental Dissection of Long Noncoding RNAs. Cell 2018, 172, 393–407. [Google Scholar] [CrossRef] [PubMed]
- Matsui, M.; Corey, D.R. Non-coding RNAs as drug targets. Nat. Rev. Drug Discov. 2017, 16, 167–179. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, G.; Guo, X.; Yao, H.; Wang, G.; Li, C. Non-coding RNA in bladder cancer. Cancer Lett. 2020, 485, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Jathar, S.; Kumar, V.; Srivastava, J.; Tripathi, V. Technological Developments in lncRNA Biology. Adv. Exp. Med. Biol. 2017, 1008, 283–323. [Google Scholar] [CrossRef] [PubMed]
- Bhan, A.; Soleimani, M.; Mandal, S.S. Long Noncoding RNA and Cancer: A New Paradigm. Cancer Res. 2017, 77, 3965–3981. [Google Scholar] [CrossRef]
- Zhang, R.-N.; Wu, D.-M.; Wu, L.-P.; Gao, G.-W. LncRNA LINC00337 sponges mir-1285-3p to promote proliferation and metastasis of lung adenocarcinoma cells by upregulating YTHDF1. Cancer Cell Int. 2021, 21, 550. [Google Scholar] [CrossRef]
- Li, Z.; Li, Y.; Wang, X.; Liang, Y.; Luo, D.; Han, D.; Li, C.; Chen, T.; Zhang, H.; Liu, Y.; et al. LINC01977 Promotes Breast Cancer Progression and Chemoresistance to Doxorubicin by Targeting miR-212-3p/GOLM1 Axis. Front. Oncol. 2021, 11, 657094. [Google Scholar] [CrossRef]
- Qin, Y.-R.; Ma, C.-Q.; Wang, D.-P.; Zhang, Q.-Q.; Liu, M.-R.; Zhao, H.-R.; Jiang, J.-H.; Fang, Q. Bilobalide alleviates neuroinflammation and promotes autophagy in Alzheimer’s disease by upregulating lincRNA-p21. Am. J. Transl. Res. 2021, 13, 2021–2040. [Google Scholar]
- Schwarzer, A.; Emmrich, S.; Schmidt, F.; Beck, D.; Ng, M.; Reimer, C.; Adams, F.F.; Grasedieck, S.; Witte, D.; Käbler, S.; et al. The non-coding RNA landscape of human hematopoiesis and leukemia. Nat. Commun. 2017, 8, 218. [Google Scholar] [CrossRef]
- Volders, P.-J.; Verheggen, K.; Menschaert, G.; Vandepoele, K.; Martens, L.; Vandesompele, J.; Mestdagh, P. An update on LNCipedia: A database for annotated human lncRNA sequences. Nucleic Acids Res. 2015, 43, D174–D180. [Google Scholar] [CrossRef]
- Li, Q.; Li, X.; Yang, X.; Zhang, B.; Gu, Y.; Gu, G.; Xiong, J.; Li, Y.; Qian, Z. Long Intergenic Nonprotein Coding RNA 173 Inhibits Tumor Growth and Promotes Apoptosis by Repressing Sphingosine Kinase 1 Protein Expression in Pancreatic Cancer. DNA Cell Biol. 2021, 40, 757–775. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, M.; Zhao, X.; Wang, G.; Li, J. Long noncoding RNA LINC00173 is downregulated in cervical cancer and inhibits cell proliferation and invasion by modulating the miR-182-5p/FBXW7 axis. Pathol. Res. Pract. 2020, 216, 152994. [Google Scholar] [CrossRef]
- Alfano, V.; Zeisel, M.B.; Levrero, M.; Guerrieri, F. The lncRNAs in HBV-Related HCCs: Targeting Chromatin Dynamics and Beyond. Cancers 2021, 13, 3115. [Google Scholar] [CrossRef]
- Sun, Q.; Hao, Q.; Prasanth, K.V. Nuclear Long Noncoding RNAs: Key Regulators of Gene Expression. Trends Genet. 2018, 34, 142–157. [Google Scholar] [CrossRef]
- Rashid, F.; Shah, A.; Shan, G. Long Non-coding RNAs in the Cytoplasm. Genomics Proteomics Bioinform. 2016, 14, 73–80. [Google Scholar] [CrossRef]
- Postler, T.S.; Pantry, S.N.; Desrosiers, R.C.; Ghosh, S. Identification and characterization of a long non-coding RNA up-regulated during HIV-1 infection. Virology 2017, 511, 30–39. [Google Scholar] [CrossRef]
- Yang, Q.; Tang, Y.; Tang, C.; Cong, H.; Wang, X.; Shen, X.; Ju, S. Diminished LINC00173 expression induced miR-182-5p accumulation promotes cell proliferation, migration and apoptosis inhibition via AGER/NF-κB pathway in non-small-cell lung cancer. Am. J. Transl. Res. 2019, 11, 4248–4262. [Google Scholar]
- Mao, Y.; Fu, Z.; Zhang, Y.; Dong, L.; Zhang, Y.; Zhang, Q.; Li, X.; Liu, J. A seven-lncRNA signature predicts overall survival in esophageal squamous cell carcinoma. Sci. Rep. 2018, 8, 8823. [Google Scholar] [CrossRef]
- Hu, Z.; Yang, D.; Tang, Y.; Zhang, X.; Wei, Z.; Fu, H.; Xu, J.; Zhu, Z.; Cai, Q. Five-long non-coding RNA risk score system for the effective prediction of gastric cancer patient survival. Oncol. Lett. 2019, 17, 4474–4486. [Google Scholar] [CrossRef]
- Li, Q.; Chen, X.; Chen, L.; Yan, H.; Li, J. LINC00173 promotes the apoptosis of hypertrophic scar fibroblasts through increasing β-catenin expression. Mol. Cell. Biochem. 2021, 476, 1005–1014. [Google Scholar] [CrossRef]
- Chen, L.; Kong, C. LINC00173 regulates polycystic ovarian syndrome progression by promoting apoptosis and repressing proliferation in ovarian granulosa cells via the microRNA-124-3p (miR-124-3p)/jagged canonical Notch ligand 1 (JAG1) pathway. Bioengineered 2022, 13, 10373–10385. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Ren, J.; Zhang, D.; Li, Y.; Huang, X.; Hu, Q.; Wang, H.; Song, Y.; Ni, Y.; Hou, Y. A novel stromal lncRNA signature reprograms fibroblasts to promote the growth of oral squamous cell carcinoma via LncRNA-CAF/interleukin-33. Carcinogenesis 2018, 39, 397–406. [Google Scholar] [CrossRef] [PubMed]
- Acha-Sagredo, A.; Uko, B.; Pantazi, P.; Bediaga, N.G.; Moschandrea, C.; Rainbow, L.; Marcus, M.W.; Davies, M.P.A.; Field, J.K.; Liloglou, T. Long non-coding RNA dysregulation is a frequent event in non-small cell lung carcinoma pathogenesis. Br. J. Cancer 2020, 122, 1050–1058. [Google Scholar] [CrossRef] [PubMed]
- Yousefi, H.; Maheronnaghsh, M.; Molaei, F.; Mashouri, L.; Reza Aref, A.; Momeny, M.; Alahari, S.K. Long noncoding RNAs and exosomal lncRNAs: Classification, and mechanisms in breast cancer metastasis and drug resistance. Oncogene 2020, 39, 953–974. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Kong, S.; Zheng, M.; Hong, Y.; Sun, J.; Ming, X.; Gu, Y.; Shen, X.; Ju, S. Long intergenic noncoding RNA LINC00173 as a potential serum biomarker for diagnosis of non-small-cell lung cancer. Cancer Biomark. 2020, 29, 441–451. [Google Scholar] [CrossRef]
- Chen, J.; Liu, A.; Wang, Z.; Wang, B.; Chai, X.; Lu, W.; Cao, T.; Li, R.; Wu, M.; Lu, Z.; et al. LINC00173.v1 promotes angiogenesis and progression of lung squamous cell carcinoma by sponging miR-511-5p to regulate VEGFA expression. Mol. Cancer 2020, 19, 98. [Google Scholar] [CrossRef]
- Wang, S.; Zeng, F.; Liang, S.; Wang, Q.; Wen, Y.; Wang, Q.; Zhang, J.; Li, M.; Fang, S.; Wei, T.; et al. lncRNA Linc00173 modulates glucosemetabolism and multidrug chemoresistancein SCLC: Potential molecular panel for targeted therapy. Mol. Ther. 2022, 30, 1787. [Google Scholar] [CrossRef]
- Zeng, F.; Wang, Q.; Wang, S.; Liang, S.; Huang, W.; Guo, Y.; Peng, J.; Li, M.; Zhu, W.; Guo, L. Linc00173 promotes chemoresistance and progression of small cell lung cancer by sponging miR-218 to regulate Etk expression. Oncogene 2020, 39, 293–307. [Google Scholar] [CrossRef]
- Zhao, G.; Zhang, A.; Sun, S.; Ding, Y. Long non-coding RNA LINC00173 enhances cisplatin resistance in hepatocellular carcinoma via the microRNA-641/RAB14 axis. Oncol. Lett. 2021, 21, 371. [Google Scholar] [CrossRef]
- Yu, Y.; Lu, X.; Yang, C.; Yin, F. Long Noncoding RNA LINC00173 Contributes to the Growth, Invasiveness and Chemo-Resistance of Colorectal Cancer Through Regulating miR-765/PLP2 Axis. Cancer Manag. Res. 2020, 12, 3363–3369. [Google Scholar] [CrossRef]
- Du, Q.; Liu, J.; Tian, D.; Zhang, X.; Zhu, J.; Qiu, W.; Wu, J. Long Noncoding RNA LINC00173 Promotes NUTF2 Expression Through Sponging miR-765 and Facilitates Tumorigenesis in Glioma. Cancer Manag. Res. 2020, 12, 7211–7217. [Google Scholar] [CrossRef]
- Fan, H.; Yuan, J.; Li, X.; Ma, Y.; Wang, X.; Xu, B.; Li, X. LncRNA LINC00173 enhances triple-negative breast cancer progression by suppressing miR-490-3p expression. Biomed. Pharmacother. 2020, 125, 109987. [Google Scholar] [CrossRef]
- Hu, C.H.; Yang, X.J.; Yu, L.; Wang, L.Y.; Zhao, X.C.; Han, C.H. Long non-coding RNA LINC00173 serves as sponge for miR-338-3p to promote prostate cancer progression via regulating Rab25. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 9290–9302. [Google Scholar] [CrossRef]
- Zhu, Q.; Zhan, D.; Yang, Y.; Chong, Y.; Xue, H.; Zhu, P. LINC00173 Promotes Wilms’ Tumor Progression through MGAT1-mediated MUC3A N-glycosylation. Cell Cycle 2022, 21, 1795–1810. [Google Scholar] [CrossRef]
- Zhang, H.; Pan, Z.; Ling, X.; Tan, Q.; Yuan, Q.; Qin, J.; Zhong, B.; Li, H.; Chen, J.; Zhang, H.; et al. LINC00173 Interacts With DNMT1 to Regulate LINC00173 Expression via Promoter Methylation in Hydroquinone-Induced Malignantly Transformed TK6 Cells and Benzene-Exposed Workers. Toxicol. Sci. 2022, 187, 311–324. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Jandura, A.; Krause, H.M. The New RNA World: Growing Evidence for Long Noncoding RNA Functionality. Trends Genet. 2017, 33, 665–676. [Google Scholar] [CrossRef]
- Hirsch, F.R.; Scagliotti, G.V.; Mulshine, J.L.; Kwon, R.; Curran, W.J.; Wu, Y.-L.; Paz-Ares, L. Lung cancer: Current therapies and new targeted treatments. Lancet 2017, 389, 299–311. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef]
- Wang, J.-F.; Xi, Z.-N.; Su, H.-J.; Bao, Z.; Qiao, Y.-H. SP1-induced overexpression of LINC00520 facilitates non-small cell lung cancer progression through miR-577/CCNE2 pathway and predicts poor prognosis. Hum. Cell 2021, 34, 952–964. [Google Scholar] [CrossRef]
- lncRNA Linc00173 modulates glucose metabolism and multidrug chemoresistance inSCLC:Potentialmolecular panel for targeted therapy. Mol. Ther. 2022, 30, 2868. [CrossRef] [PubMed]
- Torimura, T.; Iwamoto, H. Treatment and the prognosis of hepatocellular carcinoma in Asia. Liver Int. 2022, 42, 2042–2054. [Google Scholar] [CrossRef]
- Jin, X.-H.; Hong, Y.-G.; Li, P.; Hao, L.-Q.; Chen, M. Long noncoding RNA LINC00520 accelerates the progression of colorectal cancer by serving as a competing endogenous RNA of microRNA-577 to increase HSP27 expression. Hum. Cell 2020, 33, 683–694. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Ping, W.; Zhang, R.; Hao, Z.; Zhang, N. DEPDC1B collaborates with GABRD to regulate ESCC progression. Cancer Cell Int. 2022, 22, 214. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Qi, D.; Gan, Q.; Wang, F.; Qin, B.; Li, J.; Wang, H.; Wang, D. Studies on the Regulatory Roles and Related Mechanisms of lncRNAs in the Nervous System. Oxid. Med. Cell. Longev. 2021, 2021, 6657944. [Google Scholar] [CrossRef]
- Du, Q.; Lin, Y.; Zhang, W.; He, F.; Xu, Y.; Chen, Z. Bioinformatics analysis of LMAN1 expression, clinical characteristics, and its effects on cell proliferation and invasion in glioma. Brain Res. 2022, 1789, 147952. [Google Scholar] [CrossRef]
- Noblejas-López, M.D.M.; Nieto-Jimenez, C.; Burgos, M.; Gómez-Juárez, M.; Montero, J.C.; Esparís-Ogando, A.; Pandiella, A.; Galán-Moya, E.M.; Ocaña, A. Activity of BET-proteolysis targeting chimeric (PROTAC) compounds in triple negative breast cancer. J. Exp. Clin. Cancer Res. 2019, 38, 383. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, J.; Chen, J.; Ye, M.; Jin, X. Functional roles of E3 ubiquitin ligases in prostate cancer. J. Mol. Med. 2022, 100, 1125–1144. [Google Scholar] [CrossRef]
- Oroojalian, F.; Charbgoo, F.; Hashemi, M.; Amani, A.; Yazdian-Robati, R.; Mokhtarzadeh, A.; Ramezani, M.; Hamblin, M.R. Recent advances in nanotechnology-based drug delivery systems for the kidney. J. Control. Release 2020, 321, 442–462. [Google Scholar] [CrossRef]
- Mahamdallie, S.; Yost, S.; Poyastro-Pearson, E.; Holt, E.; Zachariou, A.; Seal, S.; Elliott, A.; Clarke, M.; Warren-Perry, M.; Hanks, S.; et al. Identification of new Wilms tumour predisposition genes: An exome sequencing study. Lancet Child Adolesc. Health 2019, 3, 322–331. [Google Scholar] [CrossRef]
- Lee, S.H.; Hwang, H.K.; Lee, W.J.; Kang, C.M. MCT4 as a potential therapeutic target to augment gemcitabine chemosensitivity in resected pancreatic cancer. Cell. Oncol. 2021, 44, 1363–1371. [Google Scholar] [CrossRef]
- Paskeh, M.D.A.; Mirzaei, S.; Gholami, M.H.; Zarrabi, A.; Zabolian, A.; Hashemi, M.; Hushmandi, K.; Ashrafizadeh, M.; Aref, A.R.; Samarghandian, S. Cervical cancer progression is regulated by SOX transcription factors: Revealing signaling networks and therapeutic strategies. Biomed. Pharmacother. 2021, 144, 112335. [Google Scholar] [CrossRef]
- Carter, B.Z.; Mak, P.Y.; Tao, W.; Warmoes, M.; Lorenzi, P.L.; Mak, D.; Ruvolo, V.; Tan, L.; Cidado, J.; Drew, L.; et al. Targeting MCL-1 dysregulates cell metabolism and leukemia-stroma interactions and resensitizes acute myeloid leukemia to BCL-2 inhibition. Haematologica 2022, 107, 58–76. [Google Scholar] [CrossRef]
- Mirzaei, S.; Gholami, M.H.; Hashemi, F.; Zabolian, A.; Hushmandi, K.; Rahmanian, V.; Entezari, M.; Girish, Y.R.; Sharath Kumar, K.S.; Aref, A.R.; et al. Employing siRNA tool and its delivery platforms in suppressing cisplatin resistance: Approaching to a new era of cancer chemotherapy. Life Sci. 2021, 277, 119430. [Google Scholar] [CrossRef]
- To, K.K.W.; Cho, W.C.S. Flavonoids Overcome Drug Resistance to Cancer Chemotherapy by Epigenetically Modulating Multiple Mechanisms. Curr. Cancer Drug Targets 2021, 21, 289–305. [Google Scholar] [CrossRef]
- Zheng, R.; Jia, J.; Guan, L.; Yuan, H.; Liu, K.; Liu, C.; Ye, W.; Liao, Y.; Lin, S.; Huang, O. Long noncoding RNA lnc-LOC645166 promotes adriamycin resistance via NF-κB/GATA3 axis in breast cancer. Aging 2020, 12, 8893–8912. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Dingemans, A.-M.C.; Bootsma, G.; van Baardwijk, A.; Reymen, B.; Wanders, R.; Brans, B.; Das, M.; Hochstenbag, M.; van Belle, A.; Houben, R.; et al. A phase I study of concurrent individualized, isotoxic accelerated radiotherapy and cisplatin-vinorelbine-cetuximab in patients with stage III non-small-cell lung cancer. J. Thorac. Oncol. 2014, 9, 710–716. [Google Scholar] [CrossRef]
- Zhang, Y.-W.; Zheng, Y.; Wang, J.-Z.; Lu, X.-X.; Wang, Z.; Chen, L.-B.; Guan, X.-X.; Tong, J.-D. Integrated analysis of DNA methylation and mRNA expression profiling reveals candidate genes associated with cisplatin resistance in non-small cell lung cancer. Epigenetics 2014, 9, 896–909. [Google Scholar] [CrossRef]
- Ciaffaglione, V.; Modica, M.N.; Pittalà, V.; Romeo, G.; Salerno, L.; Intagliata, S. Mutual Prodrugs of 5-Fluorouracil: From a Classic Chemotherapeutic Agent to Novel Potential Anticancer Drugs. ChemMedChem 2021, 16, 3496–3512. [Google Scholar] [CrossRef]
- Fan, Y.; Zhou, Y.; Li, X.; Lou, M.; Gao, Z.; Tong, J.; Yuan, K. Long Non-Coding RNA AL513318.2 as ceRNA Binding to hsa-miR-26a-5p Upregulates Expression and Predicts Poor Prognosis in Non-Small Lung Cancer. Front. Oncol. 2022, 12, 781903. [Google Scholar] [CrossRef] [PubMed]
- He, B.; Zhao, Z.; Cai, Q.; Zhang, Y.; Zhang, P.; Shi, S.; Xie, H.; Peng, X.; Yin, W.; Tao, Y.; et al. miRNA-based biomarkers, therapies, and resistance in Cancer. Int. J. Biol. Sci. 2020, 16, 2628–2647. [Google Scholar] [CrossRef] [PubMed]
- Su, K.; Wang, N.; Shao, Q.; Liu, H.; Zhao, B.; Ma, S. The role of a ceRNA regulatory network based on lncRNA MALAT1 site in cancer progression. Biomed. Pharmacother. 2021, 137, 111389. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Jiang, X.; Duan, L.; Xiong, Q.; Yuan, Y.; Liu, P.; Jiang, L.; Shen, Q.; Zhao, S.; Yang, C.; et al. LncRNA PKMYT1AR promotes cancer stem cell maintenance in non-small cell lung cancer via activating Wnt signaling pathway. Mol. Cancer 2021, 20, 156. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Shen, L.; Mao, L.; Wang, B.; Li, Y.; Yu, H. miR-92a is upregulated in cervical cancer and promotes cell proliferation and invasion by targeting FBXW7. Biochem. Biophys. Res. Commun. 2015, 458, 63–69. [Google Scholar] [CrossRef]
- Ahmad, S.; Khan, H.; Siddiqui, Z.; Khan, M.Y.; Rehman, S.; Shahab, U.; Godovikova, T.; Silnikov, V.; Moinuddin. AGEs, RAGEs and s-RAGE; friend or foe for cancer. Semin. Cancer Biol. 2018, 49, 44–55. [Google Scholar] [CrossRef]
- Pathania, R.; Ramachandran, S.; Elangovan, S.; Padia, R.; Yang, P.; Cinghu, S.; Veeranan-Karmegam, R.; Arjunan, P.; Gnana-Prakasam, J.P.; Sadanand, F.; et al. DNMT1 is essential for mammary and cancer stem cell maintenance and tumorigenesis. Nat. Commun. 2015, 6, 6910. [Google Scholar] [CrossRef]
- Qin, W.; Leonhardt, H.; Spada, F. Usp7 and Uhrf1 control ubiquitination and stability of the maintenance DNA methyltransferase Dnmt1. J. Cell. Biochem. 2011, 112, 439–444. [Google Scholar] [CrossRef]
- Yin, Q.; Zheng, M.; Luo, Q.; Jiang, D.; Zhang, H.; Chen, C. YB-1 as an Oncoprotein: Functions, Regulation, Post-Translational Modifications, and Targeted Therapy. Cells 2022, 11, 1217. [Google Scholar] [CrossRef]
- Song, L.; Xiong, H.; Li, J.; Liao, W.; Wang, L.; Wu, J.; Li, M. Sphingosine kinase-1 enhances resistance to apoptosis through activation of PI3K/Akt/NF-κB pathway in human non-small cell lung cancer. Clin. Cancer Res. 2011, 17, 1839–1849. [Google Scholar] [CrossRef]
- Russell, B.; Johnston, J.J.; Biesecker, L.G.; Kramer, N.; Pickart, A.; Rhead, W.; Tan, W.-H.; Brownstein, C.A.; Kate Clarkson, L.; Dobson, A.; et al. Clinical management of patients with ASXL1 mutations and Bohring-Opitz syndrome, emphasizing the need for Wilms tumor surveillance. Am. J. Med. Genet. Part A 2015, 167, 2122–2131. [Google Scholar] [CrossRef]
- Yang, S.A.-O.; Wang, J.; Ng, R.T. Inferring RNA sequence preferences for poorly studied RNA-binding proteins based on co-evolution. BMC Bioinform. 2018, 19, 96. [Google Scholar] [CrossRef]
- Xu, S.; Wang, T.; Lu, X.; Zhang, H.; Liu, L.; Kong, X.; Li, S.; Wang, X.; Gao, H.; Wang, J.; et al. Identification of LINC00173 in Myasthenia Gravis by Integration Analysis of Aberrantly Methylated- Differentially Expressed Genes and ceRNA Networks. Front. Genet. 2021, 12, 726751. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, X.; Zhu, H.; Liu, Y.; Cao, J.; Li, D.; Ding, B.; Yan, W.; Jin, H.; Wang, S. Identification of Potential Prognostic Long Non-Coding RNA Biomarkers for Predicting Recurrence in Patients with Cervical Cancer. Cancer Manag. Res. 2020, 12, 719–730. [Google Scholar] [CrossRef]
- Wang, M.; Liu, W.; Liu, W.; Wang, C. Diagnostic and prognostic significance of long noncoding RNA LINC00173 in patients with melanoma. Rev. Assoc. Med. Bras. 2022, 68, 170–175. [Google Scholar] [CrossRef]
- Wang, L.; Fan, H.; Zou, Y.; Yuan, Q.; Hu, X.; Chen, X.; Zhu, C.; Zhang, X.; Cui, H. Aberrant Expression of Long Non-coding RNAs in Exosomes in Follicle Fluid from PCOS Patients. Front. Genet. 2020, 11, 608178. [Google Scholar] [CrossRef]
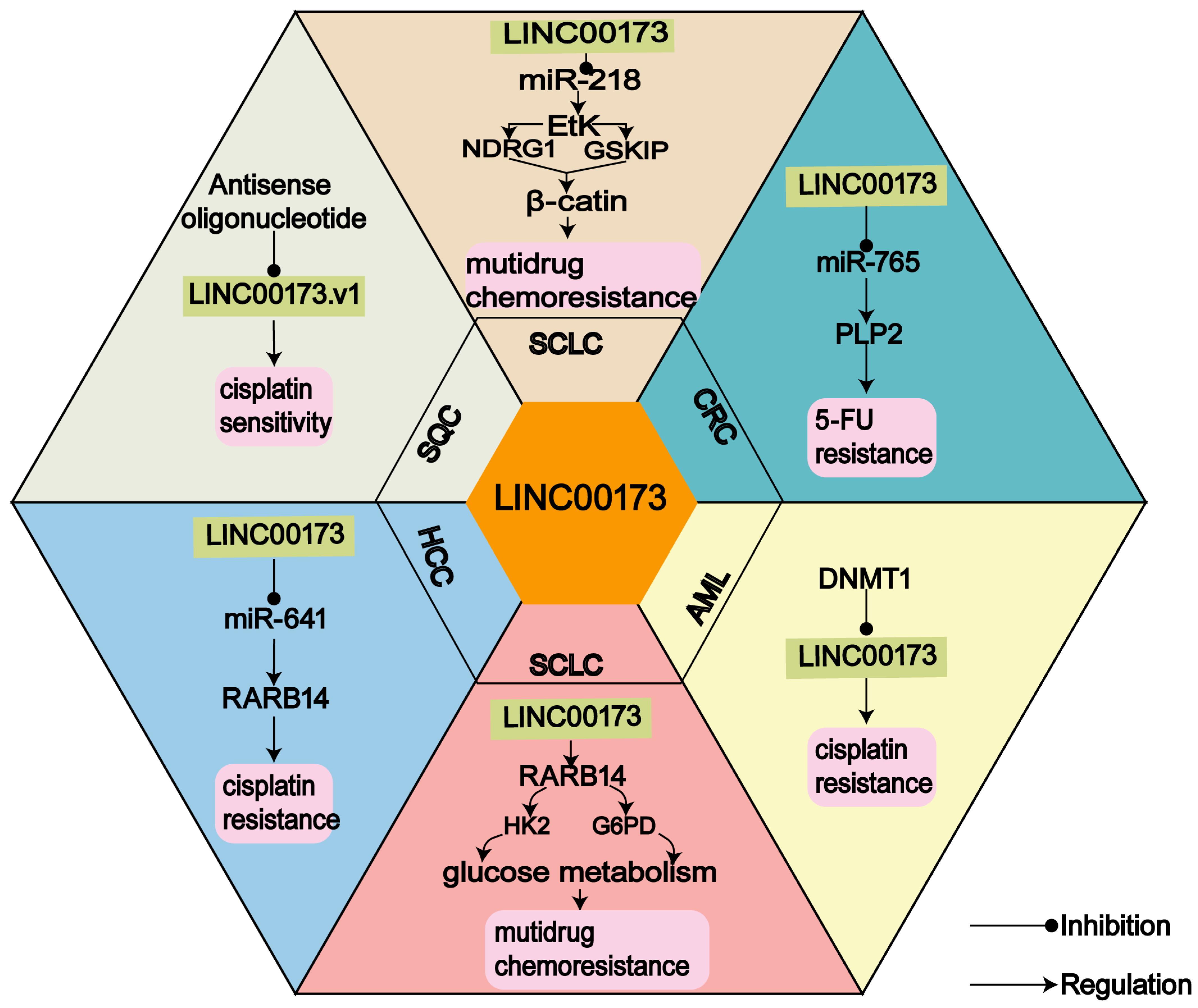
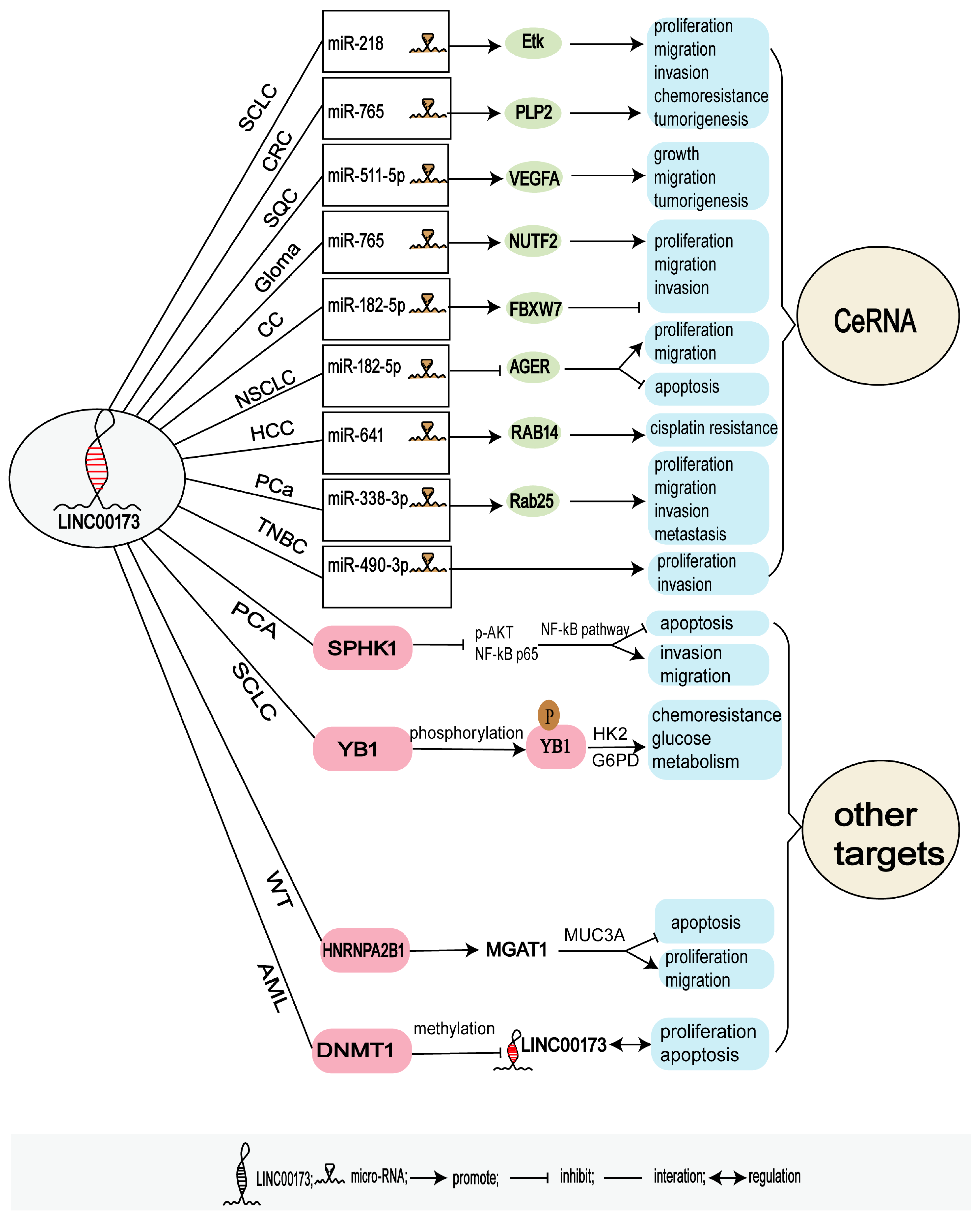
| Cancer | Dysregulated | Tumorigenesis | Subcellular Localization | Key Factors | Function | Pmid |
|---|---|---|---|---|---|---|
| NSCLC | Downregulated | Tumor suppressor | / | miR-182-5p, AGER, NF-kB | Promote proliferation, migration, inhibit apoptosis | 31396332 |
| Upregulated | / | / | / | Potential diagnosis biomarker | 32623390 | |
| SQC | Upregulated | Oncogenic | Cytoplasm | miR-511-5p, ΔNp63 | Promote angiogenesis and tumorigenesis | 32473645 |
| SCLC | Upregulated | Oncogenic | Nucleus and Cytoplasm | YB1, HK2, G6PD, PPP | Promote chemoresistance and regulate glucose metabolism | 34763086 |
| Upregulated | Oncogenic | Nucleus and Cytoplasm | miR-218, Etk, β-catenin GSKIP, NDRG1 | Promote proliferation, migration, invasion, chemoresistance, and tumorigenesis | 31477834 | |
| HCC | Upregulated | Oncogenic | / | miR-641, RAB14 | Enhance CDDP resistance | 33777195 |
| CRC | Upregulated | Oncogenic | Cytoplasm | miR-765, PLP2 | Promote growth, migration, invasiveness, and chemoresistance | 32494200 |
| ESCC | Upregulated | Oncogenic | / | / | Promote proliferation and cell cycle | 29891973 |
| Glioma | Upregulated | Oncogenic | / | miR-765, NUTF2 | Promote proliferation, migration, and invasion | 34603387 |
| TNBC | Upregulated | Oncogenic | / | miR-490-3p | Promote proliferation and invasion | 33141309 |
| PCa | Upregulated | Oncogenic | Cytoplasm | miR-338-3p, Rab25 | Promote proliferation, migration, invasion, and metastasis | 33015770 |
| WT | Upregulated | Oncogenic | / | MGAT1, MUC3A, HNRNPA2B1 | Promote invasion and migration, inhibit apoptosis | 35491865 |
| PCA | Upregulated | Tumor suppressor | / | SPHK1, AKT, NF-kB | Suppress proliferation, invasion, and tumor growth, promote apoptosis | 33978457 |
| CC | Downregulated | Tumor suppressor | Nucleus and Cytoplasm | miR-182-5p, FBXW7 | Repress proliferation, invasiveness, and migration | 34407056 |
| AML | Downregulated | Tumor suppressor | / | DNMT1 | Enhance proliferation and tumor growth, inhibit apoptosis and chemoresistance to the CDDP | 35135009 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mao, W.; Liao, Y.; Tang, L. Long Intergenic Non-Protein Coding RNA 173 in Human Cancers. Cancers 2022, 14, 5923. https://doi.org/10.3390/cancers14235923
Mao W, Liao Y, Tang L. Long Intergenic Non-Protein Coding RNA 173 in Human Cancers. Cancers. 2022; 14(23):5923. https://doi.org/10.3390/cancers14235923
Chicago/Turabian StyleMao, Wei, Yi Liao, and Liling Tang. 2022. "Long Intergenic Non-Protein Coding RNA 173 in Human Cancers" Cancers 14, no. 23: 5923. https://doi.org/10.3390/cancers14235923
APA StyleMao, W., Liao, Y., & Tang, L. (2022). Long Intergenic Non-Protein Coding RNA 173 in Human Cancers. Cancers, 14(23), 5923. https://doi.org/10.3390/cancers14235923






