Site-Coded Oral Squamous Cell Carcinoma Evaluation by Optical Coherence Tomography (OCT): A Descriptive Pilot Study
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Entry Criteria
- Age ≥ 18 years
- Ability to provide informed consent
- Clinical diagnosis strongly suggestive of OSCC
- No previous history of OSCC or previous anti-cancer therapy for OSCC
2.3. OSCCs Evaluations
3. Results
- -
- six case of OSCC on the dorsal surface of the tongue (code C02.0)
- -
- six cases of OSCC on the border of the tongue (code C02.1)
- -
- five case of OSCC on the ventral surface of the tongue (code C02.2)
- -
- six cases of OSCC on anterior alveolar mucosa (code C03.1)
- -
- seven cases of OSCC on the buccal mucosa (code C06.0)
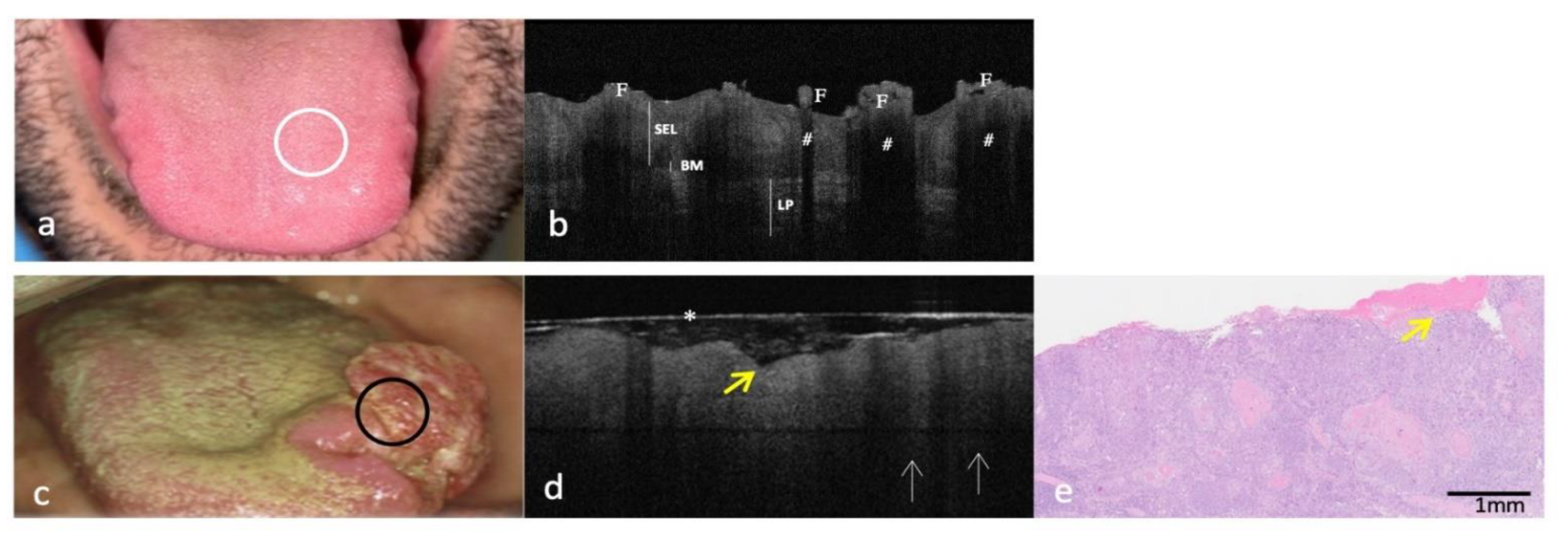
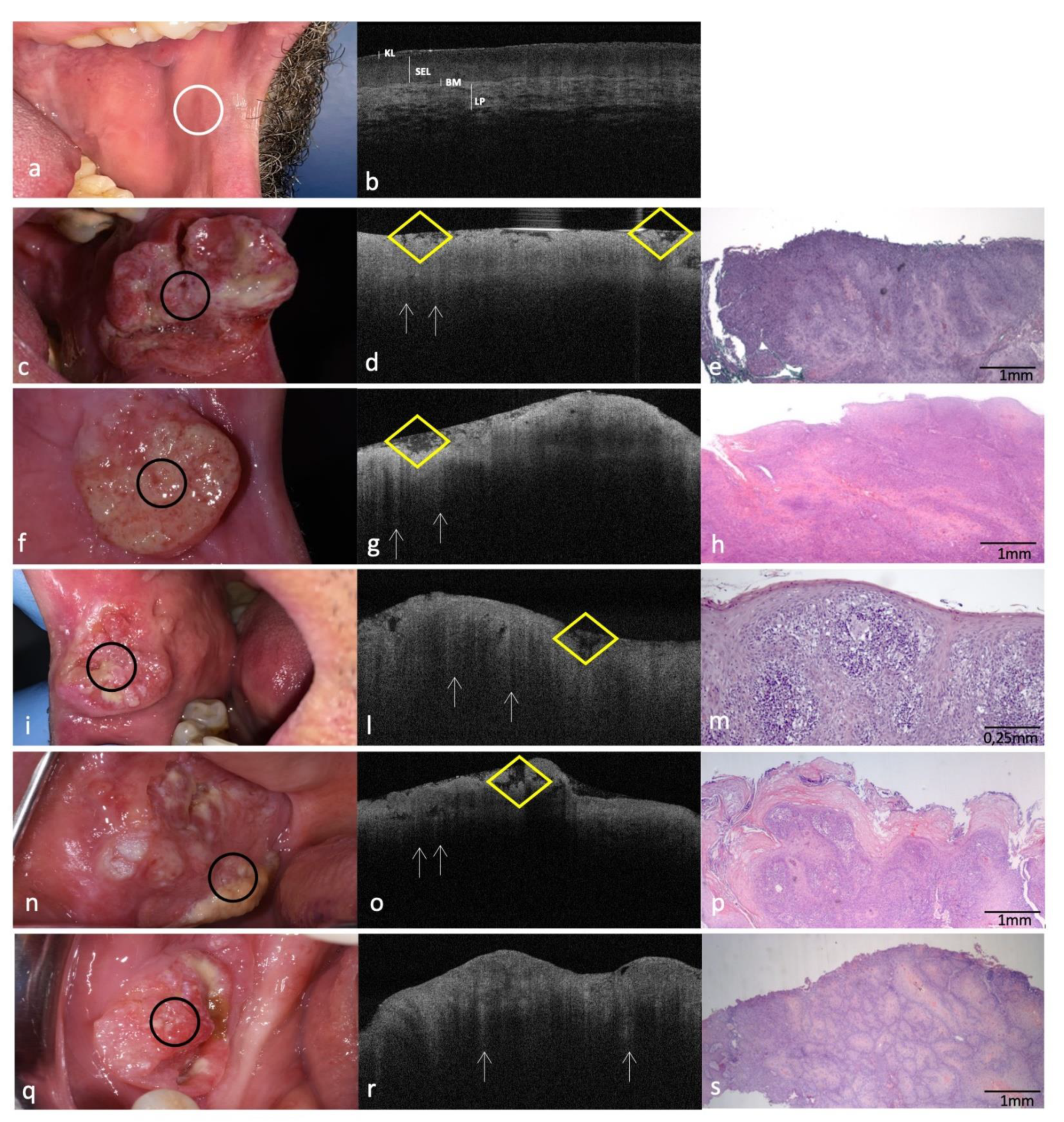
3.1. Clinical, OCT and Histopathology Images of OSCC on the Dorsal Surface of Tongue Compared to the Same-Site Healthy Mucosa (Site Code C02.0)
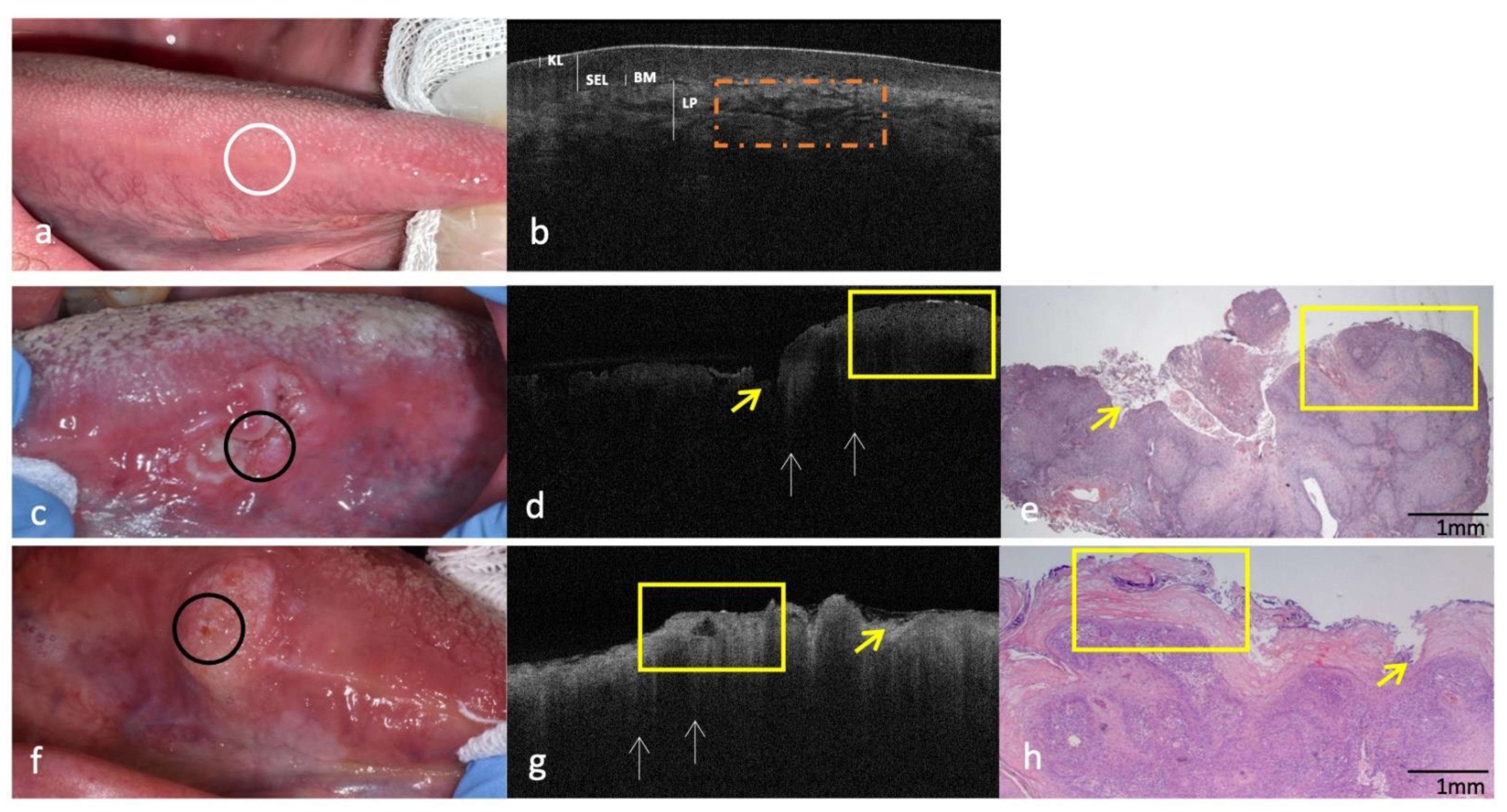
3.2. Clinical, OCT and Histopathology of OSCC on Lateral Borders of Tongue Compared to the Same-Site Healthy Mucosa (Site Code C02.1)
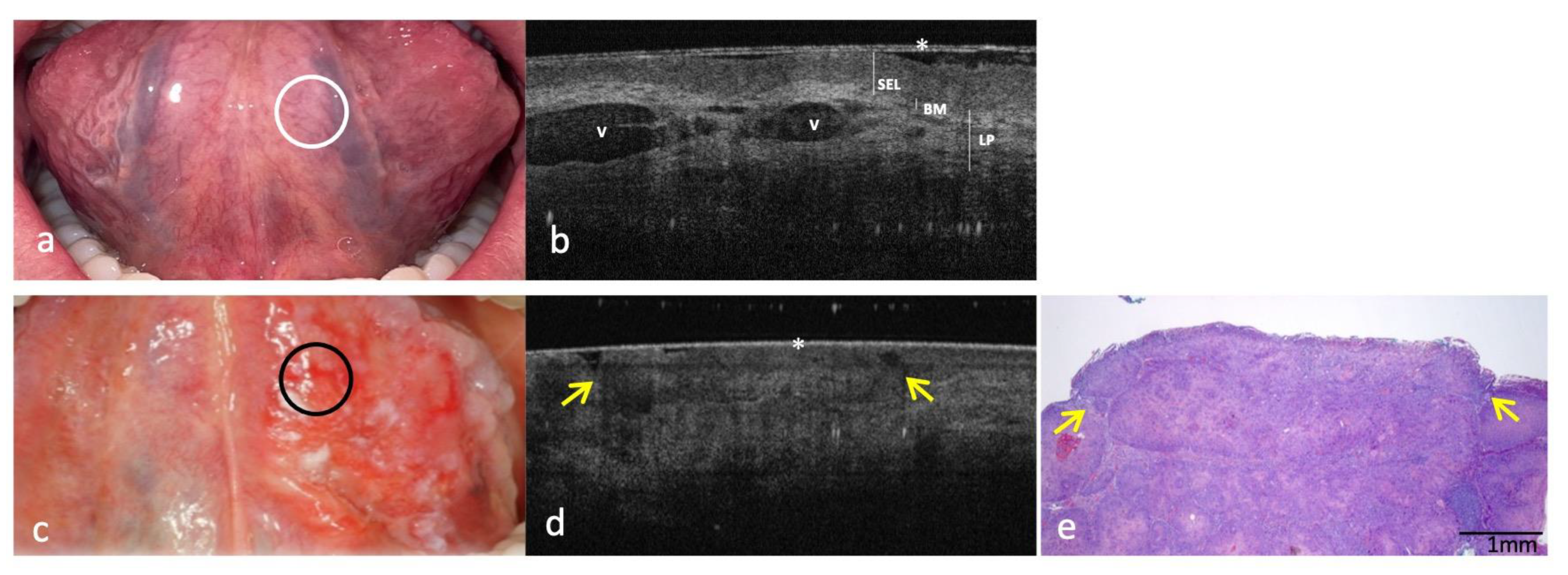
3.3. Clinical, OCT and Histopathology Images of OSCC on Ventral Surface of Tongue Compared to the Same-Site Healthy Mucosa (Site Code C02.2)
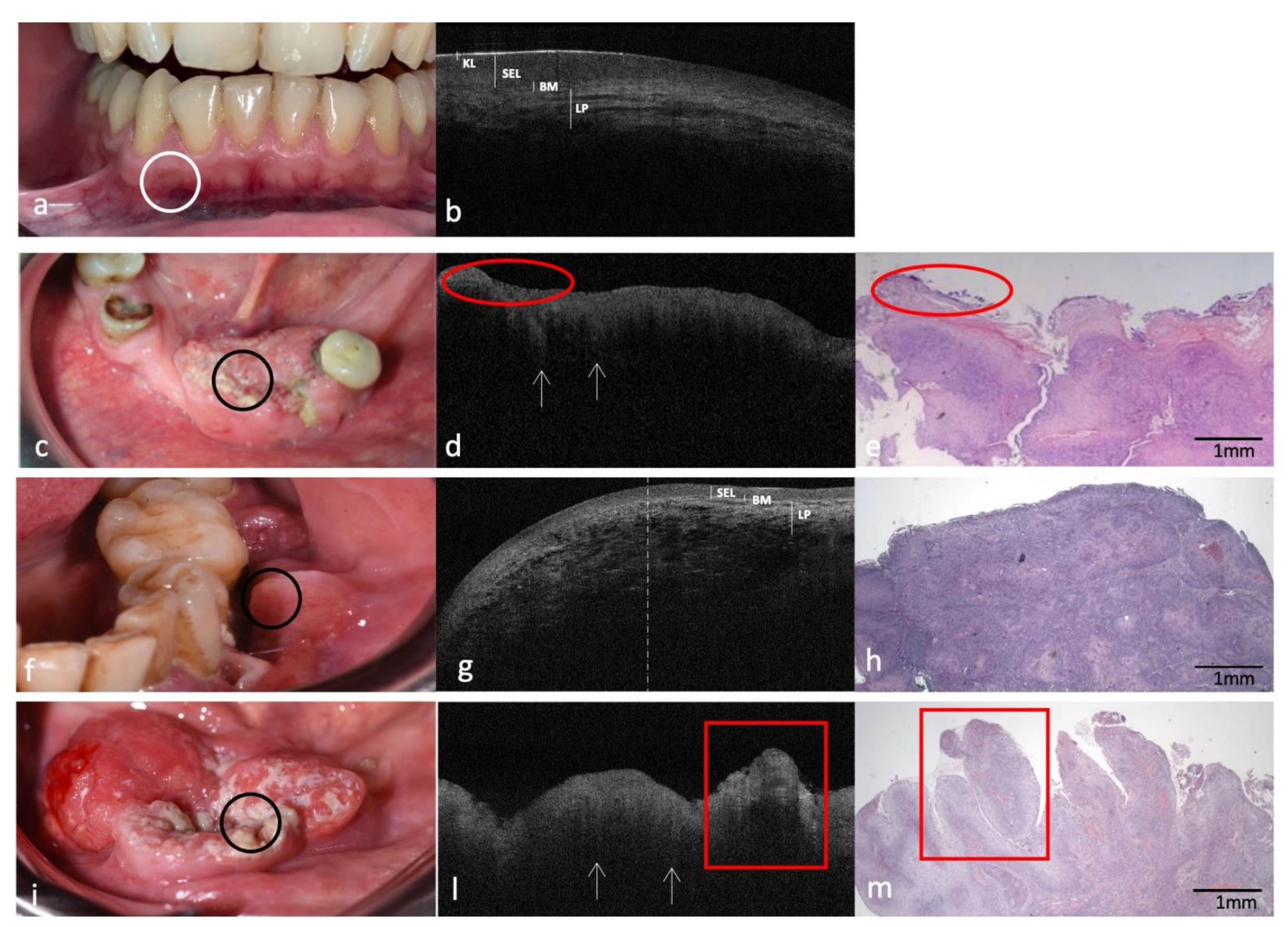
3.4. Clinical, OCT and Histopathology of OSCC on Low Anterior Alveolar Mucosa and Mandibular Gingiva Compared to the Same-Site Healthy Mucosa (Site Code C03.1)
3.5. Clinical, OCT and Histopathology Images of OSCC on Buccal Mucosa Compared to the Same-Site Healthy Mucosa (Site Code C06.0) Aspect
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Warnakulasuriya, S.; Kujan, O.; Aguirre-Urizar, J.M.; Bagan, J.V.; González-Moles, M.Á.; Kerr, A.R.; Lodi, G.; Mello, F.W.; Monteiro, L.; Ogden, G.R.; et al. Oral Potentially Malignant Disorders: A Consensus Report from an International Seminar on Nomenclature and Classification, Convened by the WHO Collaborating Centre for Oral Cancer. Oral. Dis. 2021, 27, 1862–1880. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, M.; Schnabel, C.; Mueller, J.; Golde, J.; Koch, E.; Walther, J. In Vivo Endoscopic Optical Coherence Tomography of the Healthy Human Oral Mucosa: Qualitative and Quantitative Image Analysis. Diagnostics 2020, 10, 827. [Google Scholar] [CrossRef] [PubMed]
- Panzarella, V.; Pizzo, G.; Calvino, F.; Compilato, D.; Colella, G.; Campisi, G. Diagnostic Delay in Oral Squamous Cell Carcinoma: The Role of Cognitive and Psychological Variables. Int. J. Oral Sci. 2013, 6, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Green, B.; Cobb, A.R.M.; Brennan, P.A.; Hopper, C. Optical Diagnostic Techniques for Use in Lesions of the Head and Neck: Review of the Latest Developments. Br. J. Oral Maxillofac. Surg. 2014, 52, 675–680. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Swanson, E.A.; Lin, C.P.; Schuman, J.S.; Stinson, W.G.; Chang, W.; Hee, M.R.; Flotte, T.; Gregory, K.; Puliafito, C.A.; et al. Optical Coherence Tomography. Science 1991, 254, 1178–1181. [Google Scholar] [CrossRef]
- Capocasale, G.; Panzarella, V.; Rodolico, V.; di Fede, O.; Campisi, G. In Vivo Optical Coherence Tomography Imaging in a Case of Mucous Membrane Pemphigoid and a Negative Nikolsky’s Sign. J. Derm. 2018, 45, 603–605. [Google Scholar] [CrossRef]
- Reddy, R.; Sai Praveen, K. Optical Coherence Tomography in Oral Cancer: A Transpiring Domain. J. Cancer Res. 2016, 13, 883–888. [Google Scholar] [CrossRef]
- Romano, A.; di Stasio, D.; Petruzzi, M.; Fiori, F.; Lajolo, C.; Santarelli, A.; Lucchese, A.; Serpico, R.; Contaldo, M. Noninvasive Imaging Methods to Improve the Diagnosis of Oral Carcinoma and Its Precursors: State of the Art and Proposal of a Three-Step Diagnostic Process. Cancers 2021, 13, 2864. [Google Scholar] [CrossRef]
- Di Stasio, D.; Lauritano, D.; Romano, A.; Salerno, C.; Minervini, G.; Minervini, G.; Gentile, E.; Serpico, R.; Lucchese, A. In Vivo Characterization of Oral Pemphigus Vulgaris by Optical Coherence Tomography. J. Biol. Regul. Homeost. Agents 2015, 29, 39–41. [Google Scholar]
- Di Stasio, D.; Lauritano, D.; Loffredo, F.; Gentile, E.; della Vella, F.; Petruzzi, M.; Lucchese, A. Optical Coherence Tomography Imaging of Oral Mucosa Bullous Diseases: A Preliminary Study. Dentomaxillofac. Radiol. 2020, 49, 20190071. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-K.; Tsai, M.-T.; Lee, H.-C.; Chen, H.-M.; Chiang, C.-P.; Wang, Y.-M.; Yang, C.C. Diagnosis of Oral Submucous Fibrosis with Optical Coherence Tomography. J. Biomed. Opt. 2009, 14, 054008. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-K.; Chi, T.-T.; Wu, C.-T.; Tsai, M.-T.; Chiang, C.-P.; Yang, C.-C. Diagnosis of Oral Precancer with Optical Coherence Tomography. Biomed. Opt. Express 2012, 3, 1632. [Google Scholar] [CrossRef] [PubMed]
- Hamdoon, Z.; Jerjes, W.; Al-Delayme, R.; McKenzie, G.; Jay, A.; Hopper, C. Structural Validation of Oral Mucosal Tissue Using Optical Coherence Tomography. Head Neck Oncol. 2012, 4, 29. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hamdoon, Z.; Jerjes, W.; Upile, T.; McKenzie, G.; Jay, A.; Hopper, C. Optical Coherence Tomography in the Assessment of Suspicious Oral Lesions: An Immediate Ex Vivo Study. Photodiagn. Photodyn. 2013, 10, 17–27. [Google Scholar] [CrossRef]
- Jerjes, W.; Hamdoon, Z.; Yousif, A.A.; Al-Rawi, N.H.; Hopper, C. Epithelial Tissue Thickness Improves Optical Coherence Tomography’s Ability in Detecting Oral Cancer. Photodiagn. Photodyn. 2019, 28, 69–74. [Google Scholar] [CrossRef]
- Obade, A.Y.; Pandarathodiyil, A.K.; Oo, A.L.; Warnakulasuriya, S.; Ramanathan, A. Application of Optical Coherence Tomography to Study the Structural Features of Oral Mucosa in Biopsy Tissues of Oral Dysplasia and Carcinomas. Clin. Oral Investig. 2021, 25, 5411–5419. [Google Scholar] [CrossRef]
- O’Leary, S.; Fotouhi, A.; Turk, D.; Sriranga, P.; Rajabi-Estarabadi, A.; Nouri, K.; Daveluy, S.; Mehregan, D.; Nasiriavanaki, M. OCT Image Atlas of Healthy Skin on Sun-Exposed Areas. Ski. Res. Technol. 2018, 24, 570–586. [Google Scholar] [CrossRef]
- Ulrich, M.; Themstrup, L.; de Carvalho, N.; Manfredi, M.; Grana, C.; Ciardo, S.; Kästle, R.; Holmes, J.; Whitehead, R.; Jemec, G.B.E.; et al. Dynamic Optical Coherence Tomography in Dermatology. Dermatology 2016, 232, 298–311. [Google Scholar] [CrossRef]
- James, B.; Sunny, S.; Heidari, A.; Ramanjinappa, R.; Lam, T.; Tran, A.; Kankanala, S.; Sil, S.; Tiwari, V.; Patrick, S.; et al. Validation of a Point-of-Care Optical Coherence Tomography with Machine Learning Algorithm for Detection of Oral Malignant and Malignant Lesions. Cancers 2021, 13, 3583. [Google Scholar] [CrossRef]
- O’Brien, B.C.; Harris, I.B.; Beckman, T.J.; Reed, D.A.; Cook, D.A. Standards for Reporting Qualitative Research: A Synthesis of Recommendations. Acad. Med. 2014, 89, 1245–1251. [Google Scholar] [CrossRef] [PubMed]
- Panzarella, V.; Bartolone, A.; Coniglio, R.; Rodolico, V.; Maniscalco, L.; Capocasale, G.; Iurato Carbone, M.; Campisi, G. Diagnostic Concordance between Optical Coherence Tomography and Histological Investigations for Immune-Mediated Desquamative Gingivitis: Observational Study. Int. J. Environ. Res. Public Health 2021, 18, 9095. [Google Scholar] [CrossRef] [PubMed]
- Fritz, A.; Percy, C.; Jack, A.; Shanmugaratnam, K.; Sobin, L.; Parkin, M.; Whelan, S. ICD-O International Classification of Diseases for Oncology First Revision; World Health Organization: Geneva, Switzerland, 2013.
- National Cancer Institute. Surveillance, Epidemiology, and E.R.P.-(NIH/SEER). Head and Neck Equivalent Terms and Defini-tions C000-C148, C300-C339, C410, C411, C442, C479 Excludes Lymphoma and Leukemia M9590–M9992 and Kaposi sarcoma M9140. 2021; pp. 1–50. Available online: https://seer.cancer.gov/tools/solidtumor/Head_Neck_STM.pdf (accessed on 27 November 2022).
- Gambichler, T.; Regeniter, P.; Bechara, F.G.; Orlikov, A.; Vasa, R.; Moussa, G.; Stücker, M.; Altmeyer, P.; Hoffmann, K. Characterization of Benign and Malignant Melanocytic Skin Lesions Using Optical Coherence Tomography In Vivo. J. Am. Acad. Derm. 2007, 57, 629–637. [Google Scholar] [CrossRef]
- Garbarino, F.; Migliorati, S.; Farnetani, F.; de Pace, B.; Ciardo, S.; Manfredini, M.; Reggiani Bonetti, L.; Kaleci, S.; Chester, J.; Pellacani, G. Nodular Skin Lesions: Correlation of Reflectance Confocal Microscopy and Optical Coherence Tomography Features. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 101–111. [Google Scholar] [CrossRef]
- Wilder-Smith, P.; Lee, K.; Guo, S.; Zhang, J.; Osann, K.; Chen, Z.; Messadi, D. In Vivo Diagnosis of Oral Dysplasia and Malignancy Using Optical Coherence Tomography: Preliminary Studies in 50 Patients. Lasers Surg. Med. 2009, 41, 353–357. [Google Scholar] [CrossRef] [PubMed]
- Gambino, A.; Cafaro, A.; Broccoletti, R.; Turotti, L.; Karimi, D.; El Haddad, G.; Hopper, C.; Porter, S.R.; Chiusa, L.; Arduino, P.G. In Vivo Evaluation of Traumatic and Malignant Oral Ulcers with Optical Coherence Tomography: A Comparison between Histopathological and Ultrastructural Findings. Photodiagn. Photodyn. 2022, 39, 103019. [Google Scholar] [CrossRef]
- Gentile, E.; Maio, C.; Romano, A.; Laino, L.; Lucchese, A. The Potential Role of in Vivo Optical Coherence Tomography for Evaluating Oral Soft Tissue: A Systematic Review. J. Oral Pathol. Med. 2017, 46, 864–876. [Google Scholar] [CrossRef] [PubMed]
- Schuh, S.; Kaestle, R.; Sattler, E.; Welzel, J. Comparison of Different Optical Coherence Tomography Devices for Diagnosis of Non-Melanoma Skin Cancer. Ski. Res. Technol. 2016, 22, 395–405. [Google Scholar] [CrossRef]
- Prestin, S.; Rothschild, S.I.; Betz, C.S.; Kraft, M. Measurement of Epithelial Thickness within the Oral Cavity Using Optical Coherence Tomography. Head Neck 2012, 34, 1777–1781. [Google Scholar] [CrossRef]
- Walther, J. In Vivo Imaging in the Oral Cavity by Endoscopic Optical Coherence Tomography. J. Biomed. Opt. 2018, 23, 071207. [Google Scholar] [CrossRef]
- Walther, J.; Golde, J.; Albrecht, M.; Quirk, B.C.; Scolaro, L.; Kirk, R.W.; Gruda, Y.; Schnabel, C.; Tetschke, F.; Joehrens, K.; et al. A Handheld Fiber-Optic Probe to Enable Optical Coherence Tomography of Oral Soft Tissue. IEEE Trans. Biomed. Eng. 2022, 69, 2276–2282. [Google Scholar] [CrossRef] [PubMed]
- Tsai, M.-T.; Chen, Y.; Lee, C.-Y.; Huang, B.-H.; Trung, N.H.; Lee, Y.-J.; Wang, Y.-L. Noninvasive Structural and Microvascular Anatomy of Oral Mucosae Using Handheld Optical Coherence Tomography. Biomed. Opt. Express 2017, 8, 5001. [Google Scholar] [CrossRef] [PubMed]
- Ridgway, J.M.; Armstrong, W.B.; Guo, S.; Mahmood, U.; Su, J.; Jackson, R.P.; Shibuya, T.; Crumley, R.L.; Gu, M.; Chen, Z.; et al. In Vivo Optical Coherence Tomography of the Human Oral Cavity and Oropharynx. Arch. Otolaryngol. Head Neck Surg. 2006, 132, 1074–1081. [Google Scholar] [CrossRef] [PubMed]
- Heidari, A.E.; Suresh, A.; Kuriakose, M.A.; Chen, Z.; Wilder-Smith, P.; Sunny, S.P.; James, B.L.; Lam, T.M.; Tran, A.V.; Yu, J.; et al. Optical Coherence Tomography as an Oral Cancer Screening Adjunct in a Low Resource Settings. IEEE J. Sel. Top. Quantum Electron. 2019, 25, 1–8. [Google Scholar] [CrossRef]
- Ramezani, K.; Tofangchiha, M. Oral Cancer Screening by Artificial Intelligence-Oriented Interpretation of Optical Coherence Tomography Images. Radiol. Res. Pract. 2022, 2022, 1614838. [Google Scholar] [CrossRef]
| Parameters of the OCT Image Analysis | |
|---|---|
| Parameter | Evaluation |
| Keratin layer (KL) | Assessable/Hyper-reflective Not assessable |
| Squamous epithelial layer (SEL) | Assessable/Hypo-reflective Not assessable |
| Basement membrane (BM) | Continuously assessable Discontinuously assessable Not assessable |
| Lamina propria (LP) | Well demarcated/Distinguishable from SEL Not demarcated/Indistinguishable from SEL |
| OSCC Cases by Code | Female (n) | Male (n) | Total (n) | Mean Age (years) |
|---|---|---|---|---|
| C02.0 (tongue dorsum) | 3 | 4 | 6 | 75.3 |
| C02.1 (tongue lateral border) | 4 | 3 | 6 | 65.3 |
| C02.2 (tongue ventral surface) | 3 | 2 | 5 | 67.4 |
| C03.1 (anterior inferior alveolar mucosa) | 2 | 4 | 6 | 74.8 |
| C06.0 (buccal mucosa) | 3 | 4 | 7 | 65.6 |
| Total | 15 | 15 | 30 | 69.6 |
| Parameter | Healthy Tissue OCT | OSCC Lesion OCT |
|---|---|---|
| Dorsal surface of tongue (site code C02.0) | ||
| KL | Physiological not assessable | Not assessable |
| SEL | Assessable and physiologically irregular Papillae visualized as hyper-reflective oval round reliefs | Not assessable and pathologically homogeneous to light reflection Papillae are completely indistinguishable |
| BM | Continuously assessable | Not assessable |
| LP | Well demarcated/Distinguishable from SEL | Not demarcated/Indistinguishable from SEL Presence of “icicle-like” structures |
| Lateral border of tongue (site code C02.1) | ||
| KL | Assessable | Not assessable |
| SEL | Not assessable | |
| Assessable/Hypo-reflective | ||
| BM | Continuously assessable | Not assessable |
| LP | Well demarcated/Distinguishable from SEL Streaking pattern | Not demarcated/Indistinguishable from SEL Presence of “icicle-like” structures |
| Ventral surface of tongue (site code C02.2) | ||
| KL | Physiologically not present | Not present |
| SEL | Assessable/Hypo-reflective | Not assessable |
| BM | Continuously assessable | Not assessable |
| LP | Well demarcated/Distinguishable from SEL Hyper-reflective and lax reticular structure surrounding blood vessels | Not demarcated/Indistinguishable from SEL |
| Inferior alveolar mucosa and gingiva (site code C03.1) | ||
| KL | Assessable and continuously hyper-reflective | Not assessable |
| SEL | Assessable/Hypo-reflective | Not assessable |
| BM | Continuously assessable | Not assessable |
| LP | Well demarcated with linear-mottled pattern | Not demarcated/Indistinguishable from SEL Presence of “icicle-like” structures |
| Buccal mucosa (site code C06.0) | ||
| KL | Physiologically less assessable | Not assessable |
| SEL | Clear and continuous hypo-reflective homogeneous band-shaped area | Not assessable |
| BM | Continuously assessable | Not assessable |
| LP | Well demarcated/Distinguishable from SEL Mottled hyper-reflective network | Not demarcated/Indistinguishable from SEL Presence of “icicle-like” structures |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panzarella, V.; Buttacavoli, F.; Gambino, A.; Capocasale, G.; Di Fede, O.; Mauceri, R.; Rodolico, V.; Campisi, G. Site-Coded Oral Squamous Cell Carcinoma Evaluation by Optical Coherence Tomography (OCT): A Descriptive Pilot Study. Cancers 2022, 14, 5916. https://doi.org/10.3390/cancers14235916
Panzarella V, Buttacavoli F, Gambino A, Capocasale G, Di Fede O, Mauceri R, Rodolico V, Campisi G. Site-Coded Oral Squamous Cell Carcinoma Evaluation by Optical Coherence Tomography (OCT): A Descriptive Pilot Study. Cancers. 2022; 14(23):5916. https://doi.org/10.3390/cancers14235916
Chicago/Turabian StylePanzarella, Vera, Fortunato Buttacavoli, Alessio Gambino, Giorgia Capocasale, Olga Di Fede, Rodolfo Mauceri, Vito Rodolico, and Giuseppina Campisi. 2022. "Site-Coded Oral Squamous Cell Carcinoma Evaluation by Optical Coherence Tomography (OCT): A Descriptive Pilot Study" Cancers 14, no. 23: 5916. https://doi.org/10.3390/cancers14235916
APA StylePanzarella, V., Buttacavoli, F., Gambino, A., Capocasale, G., Di Fede, O., Mauceri, R., Rodolico, V., & Campisi, G. (2022). Site-Coded Oral Squamous Cell Carcinoma Evaluation by Optical Coherence Tomography (OCT): A Descriptive Pilot Study. Cancers, 14(23), 5916. https://doi.org/10.3390/cancers14235916










