A Patient-Specific Fracture Risk Assessment Tool for Femoral Bone Metastases: Using the Bone Strength (BOS) Score in Clinical Practice
Abstract
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Study Design
2.2. Requesting a BOS Score
2.3. FE Model and BOS Score Calculation
2.4. BOS Score Report
- Did you use the BOS score (yes or no)?
- Are you satisfied with the BOS score, and why?
- Was the BOS score of added value for the treatment decision, and why?
- Was the patient satisfied regarding the use of the BOS score, and why?
- Do you have additional points for improvement or other comments, for example, regarding the application form or the BOS report?
2.5. Analysis
3. Results
3.1. Patients
3.2. Effect on Treatment Decisions
3.3. Experiences of Physicians with the Use of the BOS Score
| Case A: A female patient (age 77 years, salivary duct carcinoma, KPS 60) was referred to the orthopedic surgeon because of a large painful lytic metastasis in her femur (pain score 8 on a scale of 0 to 10). The orthopedic surgeon suggested planning a prophylactic stabilizing surgery. However, the patient hesitated; hence, a BOS score was requested. The BOS score indicated a high fracture risk, which was discussed with the patient by the orthopedic surgeon and the radiation oncologist. It convinced the patient to undergo the prophylactic stabilizing surgery. One day prior to the scheduled surgery, she fractured her femur while stepping out of her bed. The location of the fracture was similar to the weakest location of the bone indicated by the BOS score. The fracture was treated during surgery. Two months later, she passed away. | Case B: A female patient (age 76 years, breast cancer, KPS 80) visited the radiation oncologist because of a painful lytic metastasis in the femoral head (pain score 5 on a scale of 0 to 10). The radiation oncologist estimated the fracture risk as low and requested a BOS score, which also indicated a low fracture risk. The patient was treated with 1 × 8 Gy radiotherapy. After a follow-up of six months, the patient had not developed a fracture and was still alive. | Case C: A female patient (63 years, breast cancer, KPS 80) visited the radiation oncologist because of a painful metastasis in the femoral shaft (pain score 9 on a scale of 0 to 10). The metastases had a mixed appearance but were predominantly lytic. The radiation oncologists initially estimated the fracture risk as low, and the initial plan was to treat the patient with a radiotherapy dose of 1 × 8 Gy. However, the BOS score indicated a moderate risk of fracture. The patient was, therefore, treated with 2 × 8 Gy radiotherapy with the aim to induce remineralization of the bone. After six months, the patient had not developed a fracture and was still alive. |
BOS score of Femur A depicted relative to patients in the BOS database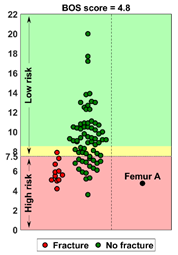 | BOS score of Femur B depicted relative to patients in the BOS database | BOS score of Femur C depicted relative to patients in the BOS database |
| Weakest location of the bone ATTENTION: Fracture localization by experimental fracturing in computer model does not necessarily coincide with metastatic location  | Weakest location of the bone ATTENTION: Fracture localization by experimental fracturing in computer model does not necessarily coincide with metastatic location 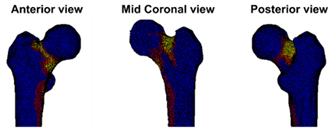 | Weakest location of the bone ATTENTION: Fracture localization by experimental fracturing in computer model does not necessarily coincide with metastatic location 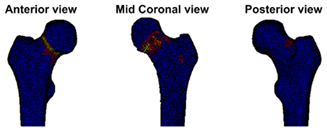 |
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Coleman, R.E.; Croucher, P.I.; Padhani, A.R.; Clezardin, P.; Chow, E.; Fallon, M.; Guise, T.; Colangeli, S.; Capanna, R.; Costa, L. Bone metastases. Nat. Rev. Dis. Prim. 2020, 6, 83. [Google Scholar] [CrossRef] [PubMed]
- Ratasvuori, M.; Wedin, R.; Keller, J.; Nottrott, M.; Zaikova, O.; Bergh, P.; Kalen, A.; Nilsson, J.; Jonsson, H.; Laitinen, M. Insight opinion to surgically treated metastatic bone disease: Scandinavian Sarcoma Group Skeletal Metastasis Registry report of 1195 operated skeletal metastasis. Surg. Oncol. 2013, 22, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Mavrogenis, A.F.; Pala, E.; Romagnoli, C.; Romantini, M.; Calabro, T.; Ruggieri, P. Survival analysis of patients with femoral metastases. J. Surg. Oncol. 2012, 105, 135–141. [Google Scholar] [CrossRef]
- Blank, A.T.; Lerman, D.M.; Patel, N.M.; Rapp, T.B. Is Prophylactic Intervention More Cost-Effective Than the Treatment of Pathologic Fractures in Metastatic Bone Disease? Clin. Orthop. Relat. Res. 2016, 474, 1563–1570. [Google Scholar] [CrossRef]
- Ward, W.G.; Holsenbeck, S.; Dorey, F.J.; Spang, J.; Howe, D. Metastatic disease of the femur: Surgical treatment. Clin. Orthop. Relat. Res. 2003, S230–S244. [Google Scholar] [CrossRef]
- Van der Linden, Y.M.; Kroon, H.M.; Dijkstra, S.P.; Lok, J.J.; Noordijk, E.M.; Leer, J.W.; Marijnen, C.A. Simple radiographic parameter predicts fracturing in metastatic femoral bone lesions: Results from a randomised trial. Radiother. Oncol. 2003, 69, 21–31. [Google Scholar] [CrossRef]
- Van der Linden, Y.M.; Dijkstra, P.D.; Kroon, H.M.; Lok, J.J.; Noordijk, E.M.; Leer, J.W.; Marijnen, C.A. Comparative analysis of risk factors for pathological fracture with femoral metastases. J. Bone Jt. Surg. Br. 2004, 86, 566–573. [Google Scholar] [CrossRef]
- Mirels, H. Metastatic disease in long bones. A proposed scoring system for diagnosing impending pathologic fractures. Clin. Orthop. 1989, 256–264. [Google Scholar] [CrossRef]
- Harrington, K.D. New trends in the management of lower extremity metastases. Clin. Orthop. Relat. Res. 1982, 53–61. [Google Scholar] [CrossRef]
- van der Wal, C.W.P.G.; Eggermont, F.E.; Fiocco, M.; Kroon, H.M.; Ayu, O.; Slot, A.; Snyers, A.; Rozema, T.; Verdonschot, N.J.J.; Dijkstra, P.D.S.; et al. Axial cortical involvement of metastatic lesions to identify impending femoral fractures; a clinical validation study. Radiother. Oncol. 2019, 144, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Tatar, Z.; Soubrier, M.; Dillies, A.F.; Verrelle, P.; Boisgard, S.; Lapeyre, M. Assessment of the risk factors for impending fractures following radiotherapy for long bone metastases using CT scan-based virtual simulation: A retrospective study. Radiat. Oncol. 2014, 9, 227. [Google Scholar] [CrossRef] [PubMed]
- Damron, T.A.; Morgan, H.; Prakash, D.; Grant, W.; Aronowitz, J.; Heiner, J. Critical evaluation of Mirels’ rating system for impending pathologic fractures. Clin. Orthop. Relat. Res. 2003, S201–S207. [Google Scholar] [CrossRef] [PubMed]
- Shimoyama, T.; Katagiri, H.; Harada, H.; Murata, H.; Wasa, J.; Hosaka, S.; Suzuki, T.; Takahashi, M.; Asakura, H.; Nishimura, T.; et al. Fracture after radiation therapy for femoral metastasis: Incidence, timing and clinical features. J. Radiat. Res. 2017, 58, 661–668. [Google Scholar] [CrossRef]
- Damron, T.A.; Mann, K.A. Fracture risk assessment and clinical decision making for patients with metastatic bone disease. J. Orthop. Res. 2020, 38, 1175–1190. [Google Scholar] [CrossRef]
- Tanck, E.; van Aken, J.B.; van der Linden, Y.M.; Schreuder, H.W.; Binkowski, M.; Huizenga, H.; Verdonschot, N. Pathological fracture prediction in patients with metastatic lesions can be improved with quantitative computed tomography based computer models. Bone 2009, 45, 777–783. [Google Scholar] [CrossRef]
- Derikx, L.C.; van Aken, J.B.; Janssen, D.; Snyers, A.; van der Linden, Y.M.; Verdonschot, N.; Tanck, E. The assessment of the risk of fracture in femora with metastatic lesions: Comparing case-specific finite element analyses with predictions by clinical experts. J. Bone Jt. Surg. Br. 2012, 94, 1135–1142. [Google Scholar] [CrossRef]
- Eggermont, F.; Derikx, L.C.; Verdonschot, N.; Van der Geest, I.C.M.; De Jong, M.A.A.; Snyers, A.; Van der Linden, Y.M.; Tanck, E. Can patient-specific finite element models better predict fractures in metastatic bone disease than experienced clinicians? Towards introducing computational modelling into daily clinical practice. Bone Jt. Res. 2018, 7, 430–439. [Google Scholar] [CrossRef]
- Eggermont, F.; van der Wal, G.; Westhoff, P.; Laar, A.; de Jong, M.; Rozema, T.; Kroon, H.M.; Ayu, O.; Derikx, L.; Dijkstra, S.; et al. Patient-specific finite element computer models improve fracture risk assessments in cancer patients with femoral bone metastases compared to clinical guidelines. Bone 2020, 130, 115101. [Google Scholar] [CrossRef]
- EuroQol, G. EuroQol—A new facility for the measurement of health-related quality of life. Health Policy 1990, 16, 199–208. [Google Scholar] [CrossRef]
- Eggermont, F.; Verdonschot, N.; Van der Linden, Y.; Tanck, E. Calibration with or without phantom for fracture risk prediction in cancer patients with femoral bone metastases using CT-based finite element models. PLoS ONE 2019, 14, e0220564. [Google Scholar] [CrossRef] [PubMed]
- Fang, Q.Q.; Boas, D.A. Tetrahedral Mesh Generation from Volumetric Binary and Gray-Scale Images. In Proceedings of the 2009 IEEE International Symposium on Biomedical Imaging: From Nano to Macro, Boston, MA, USA, 28 June–1 July 2009; Volumes 1–2, pp. 1142–1145. [Google Scholar] [CrossRef]
- Powell, D.; Abel, T. An exact general remeshing scheme applied to physically conservative voxelization. J. Comput. Phys. 2015, 297, 340–356. [Google Scholar] [CrossRef]
- Powell, D.M. r3d: Software for Fast, Robust Geometric Operations in 3D and 2D; Report of Los Alamos National Laboratory, LA-UR-15-26964; Los Alamos National Laboratory: Los Alamos, NM, USA, 2015. Available online: https://github.com/devonmpowell/r3d (accessed on 20 September 2017).
- Keyak, J.H.; Kaneko, T.S.; Tehranzadeh, J.; Skinner, H.B. Predicting proximal femoral strength using structural engineering models. Clin. Orthop. 2005, 437, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Braun, V.; Clarke, V. Using thematic analysis in psychology. Qual. Res. Psychol. 2006, 3, 77–101. [Google Scholar] [CrossRef]
- Keaveny, T.M.; Clarke, B.L.; Cosman, F.; Orwoll, E.S.; Siris, E.S.; Khosla, S.; Bouxsein, M.L. Biomechanical Computed Tomography analysis (BCT) for clinical assessment of osteoporosis. Osteoporos. Int. 2020, 31, 1025–1048. [Google Scholar] [CrossRef] [PubMed]
- Benemerito, I.; Griffiths, W.; Allsopp, J.; Furnass, W.; Bhattacharya, P.; Li, X.; Marzo, A.; Wood, S.; Viceconti, M.; Narracott, A. Delivering computationally-intensive digital patient applications to the clinic: An exemplar solution to predict femoral bone strength from CT data. Comput. Methods Programs Biomed. 2021, 208, 106200. [Google Scholar] [CrossRef]
- Kimura, T. Multidisciplinary Approach for Bone Metastasis: A Review. Cancers 2018, 10, 156. [Google Scholar] [CrossRef]
- Agarwal, M.G.; Nayak, P. Management of skeletal metastases: An orthopaedic surgeon’s guide. Indian J. Orthop. 2015, 49, 83–100. [Google Scholar] [CrossRef]
- Shay, L.A.; Lafata, J.E. Where Is the Evidence? A Systematic Review of Shared Decision Making and Patient Outcomes. Med. Decis. Making 2015, 35, 114–131. [Google Scholar] [CrossRef]
- Kroon, L.L.; van Roij, J.; Korfage, I.J.; Reyners, A.K.L.; Van den Beuken-van Everdingen, M.H.J.; den Boer, M.O.; Creemers, G.J.; de Graeff, A.; Hendiks, M.P.; Hunting, J.C.B.; et al. Perceptions of involvement in advance care planning and emotional functioning in patients with advanced cancer. J. Cancer Surviv. 2021, 15, 380–385. [Google Scholar] [CrossRef]
- Kehl, K.L.; Landrum, M.B.; Arora, N.K.; Ganz, P.A.; van Ryn, M.; Mack, J.W.; Keating, N.L. Association of Actual and Preferred Decision Roles with Patient-Reported Quality of Care Shared Decision Making in Cancer Care. JAMA Oncol. 2015, 1, 50–58. [Google Scholar] [CrossRef]
- Kashaf, M.S.; McGill, E. Does Shared Decision Making in Cancer Treatment Improve Quality of Life? A Systematic Literature Review. Med. Decis. Making 2015, 35, 1037–1048. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.J.; Roldan, C.S.; Nichipor, A.N.; Balboni, T.A.; Krishnan, M.S.; Revette, A.C.; Hertan, L.M.; Chen, A.B. Patient-Provider Communication, Decision-Making, and Psychosocial Burdens in Palliative Radiotherapy: A Qualitative Study on Patients’ Perspectives. J. Pain Symptom Manag. 2021, 62, 512–522. [Google Scholar] [CrossRef]
- Hipp, J.A.; Rosenberg, A.E.; Hayes, W.C. Mechanical properties of trabecular bone within and adjacent to osseous metastases. J. Bone Miner. Res. 1992, 7, 1165–1171. [Google Scholar] [CrossRef] [PubMed]
- Ataei, A.; Eikhout, J.; van Leeuwen, R.G.H.; Tanck, E.; Eggermont, F. The effect of variations in CT scan protocol on femoral finite element failure load assessment using phantomless calibration. PLoS ONE 2022, 17, e0265524. [Google Scholar] [CrossRef]
- Eggermont, F.; Derikx, L.C.; Free, J.; Van Leeuwen, R.; Van der Linden, Y.M.; Verdonschot, N.; Tanck, E. Effect of Different CT Scanners and Settings on Femoral Failure Loads Calculated by Finite Element Models. J. Orthop. Res. 2018, 36, 2288–2295. [Google Scholar] [CrossRef] [PubMed]
- Giambini, H.; Dragomir-Daescu, D.; Nassr, A.; Yaszemski, M.J.; Zhao, C. Quantitative Computed Tomography Protocols Affect Material Mapping and Quantitative Computed Tomography-Based Finite-Element Analysis Predicted Stiffness. J. Biomech. Eng. 2016, 138, 091003. [Google Scholar] [CrossRef]
- Carpenter, R.D.; Saeed, I.; Bonaretti, S.; Schreck, C.; Keyak, J.H.; Streeper, T.; Harris, T.B.; Lang, T.F. Inter-scanner differences in in vivo QCT measurements of the density and strength of the proximal femur remain after correction with anthropomorphic standardization phantoms. Med. Eng. Phys. 2014, 36, 1225–1232. [Google Scholar] [CrossRef]
- Dragomir-Daescu, D.; Salas, C.; Uthamaraj, S.; Rossman, T. Quantitative computed tomography-based finite element analysis predictions of femoral strength and stiffness depend on computed tomography settings. J. Biomech. 2015, 48, 153–161. [Google Scholar] [CrossRef]
| Age in Years, Mean (Range) | 65.3 (41–89) | |||
|---|---|---|---|---|
| Sex, n (%) | ||||
| M | 18 (46%) | |||
| F | 21 (54%) | |||
| Pain score, mean (range) on a scale of 0–10 (0 = no pain to 10 = worst pain imaginable) | 5.4 (0–9) | |||
| Karnofsky Performance Score (KPS), mean (range) on a scale of 0–100 (0 = dead to 100 = Normal; no complaints, no evidence of disease) | 76.3 (60–100) | |||
| Primary tumor, n (%) | ||||
| Breast | 10 (26%) | |||
| Lung | 9 (23%) | |||
| Prostate | 7 (17%) | |||
| Kidney | 2 (3%) | |||
| Colorectal | 1 (2%) | |||
| Multiple Myeloma | 0 (0%) | |||
| Melanoma | 3 (8%) | |||
| Other | 7 (18%) | |||
| Type of bone metastases, n (%) | ||||
| Lytic | 31 (79%) | |||
| Mixed | 8 (21%) | |||
| Weight in kg, mean (range) | 79 (45–136) | |||
| Length in cm, mean (range) | 171 (150–196) | |||
| Patient reported quality of life | ||||
| EQ-5D-3L *, n (%) | Level 1 No problems | Level 2 Some problems | Level 3 Severe problems | |
| Mobility | 4 (10%) | 33 (85%) | 2 (5%) | |
| Selfcare | 24 (61%) | 12 (31%) | 3 (8%) | |
| Usual activities | 12 (31%) | 19 (49%) | 8 (20%) | |
| Pain and Discomfort | 1 (3%) | 27 (69%) | 11 (28%) | |
| Anxiety and Depression | 14 (36%) | 23 (59%) | 2 (5%) | |
| EQ visual analogue scale (VAS) on patient’s self-rated health: mean (range) on a scale of 0–100 (0 = worst health imaginable, 100 = best health imaginable) | 60.8 (10–90) | |||
| Risk Estimation Physician | Risk BOS Score | N | Fractures | No Treatment | Radiotherapy | Elective Surgery | |
|---|---|---|---|---|---|---|---|
| Single Dose | Multiple Fractions $ | ||||||
| low | low | 6 | 1 | 4 | 1 | ||
| low | moderate | 7 | 1 | 6 | |||
| low | high | 15 | 1 | 3 | 11 * | 1 | |
| high | low | 7 | 2 | 5 | |||
| high | moderate | 2 | 1 | 1 | |||
| high | high | 5 | 2 | 5 ^ | |||
| Legend: | Fracture risk estimation of physician and BOS score are the same | Treatment probably adapted because of BOS score | Treatment may have been adapted because of BOS score # | ||||
| Theme | Narrative Answers | Number of Times Mentioned on the Separate Questionnaires * (n = 42) |
|---|---|---|
| Clarity of BOS score | The BOS score is a clear result | 16 |
| The BOS score gives insight into fracture risk | 11 | |
| If the BOS score indicates moderate risk, this is difficult to interpret | 3 | |
| The BOS score is difficult to interpret if the weakest location of the bone is not clinically expected or is untreatable | 2 | |
| Effect on decision of treatment | The BOS score caused a change in treatment plan | 17 |
| The BOS score resulted in a well-substantiated treatment decision | 10 | |
| Due to the BOS score, multidisciplinary consultation with orthopedic surgeon was performed | 8 | |
| Reassurance | The BOS score can give an extra confirmation of clinical estimation | 16 |
| The BOS score can be reassuring and result in a feeling of security for the patient | 8 | |
| The BOS score can provide confirmation to the patient | 6 | |
| Shared decision making | The BOS score helped to start a conversation between patient and physician | 10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eggermont, F.; van der Linden, Y.; Verdonschot, N.; Dierselhuis, E.; Ligthert, S.; Bitter, T.; Westhoff, P.; Tanck, E. A Patient-Specific Fracture Risk Assessment Tool for Femoral Bone Metastases: Using the Bone Strength (BOS) Score in Clinical Practice. Cancers 2022, 14, 5904. https://doi.org/10.3390/cancers14235904
Eggermont F, van der Linden Y, Verdonschot N, Dierselhuis E, Ligthert S, Bitter T, Westhoff P, Tanck E. A Patient-Specific Fracture Risk Assessment Tool for Femoral Bone Metastases: Using the Bone Strength (BOS) Score in Clinical Practice. Cancers. 2022; 14(23):5904. https://doi.org/10.3390/cancers14235904
Chicago/Turabian StyleEggermont, Florieke, Yvette van der Linden, Nico Verdonschot, Edwin Dierselhuis, Steven Ligthert, Thom Bitter, Paulien Westhoff, and Esther Tanck. 2022. "A Patient-Specific Fracture Risk Assessment Tool for Femoral Bone Metastases: Using the Bone Strength (BOS) Score in Clinical Practice" Cancers 14, no. 23: 5904. https://doi.org/10.3390/cancers14235904
APA StyleEggermont, F., van der Linden, Y., Verdonschot, N., Dierselhuis, E., Ligthert, S., Bitter, T., Westhoff, P., & Tanck, E. (2022). A Patient-Specific Fracture Risk Assessment Tool for Femoral Bone Metastases: Using the Bone Strength (BOS) Score in Clinical Practice. Cancers, 14(23), 5904. https://doi.org/10.3390/cancers14235904







