Pelvic Lymphadenectomy May Not Improve Biochemical Recurrence-Free Survival in Patients with Prostate Cancer Treated with Robot-Assisted Radical Prostatectomy in Japan (The MSUG94 Group)
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Population
2.2. Pathological Analysis
2.3. Follow-Up Schedule
2.4. Endpoints and Statistical Analyses
3. Results
3.1. Patient Characteristics
3.2. Surgical and Pathological Outcomes
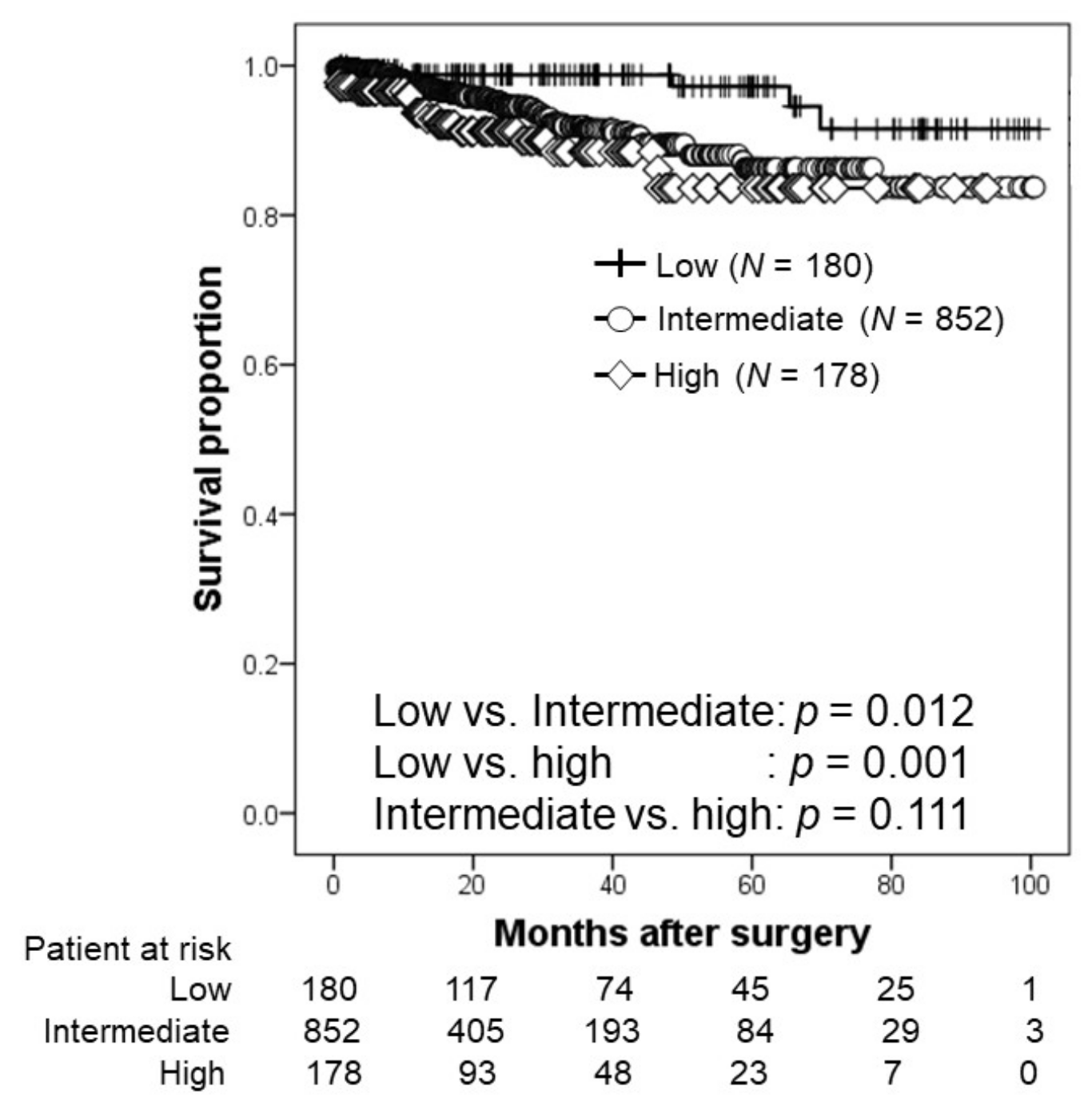
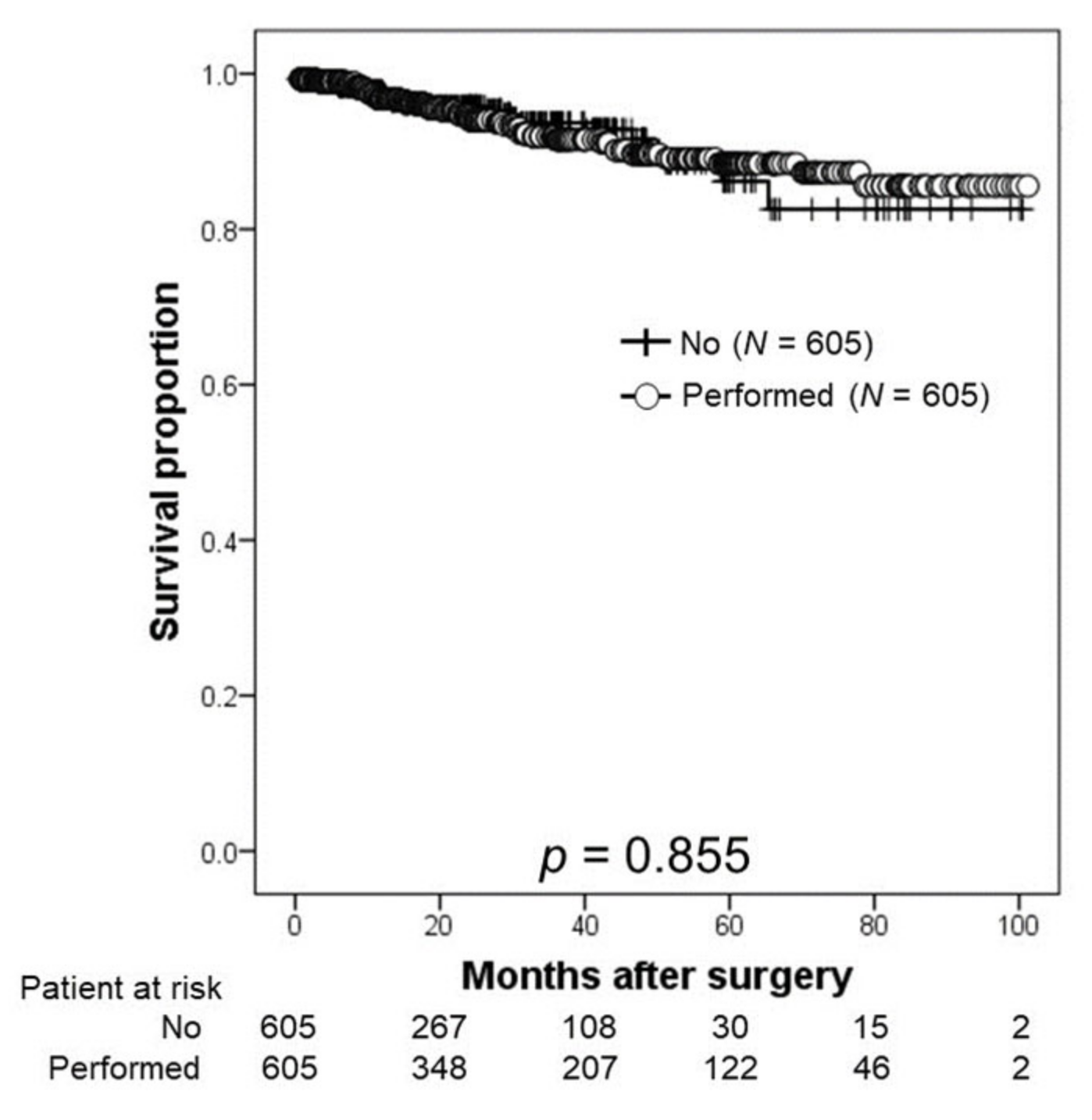
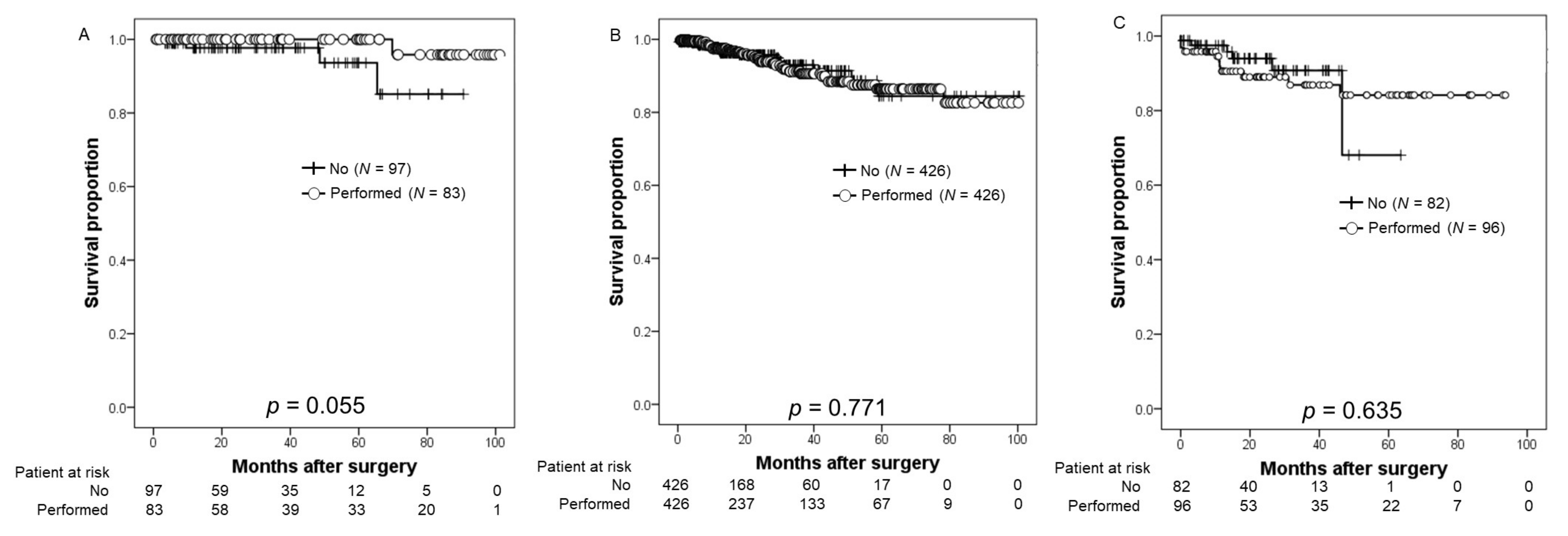
3.3. Oncological Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mottet, N.; van den Bergh, R.C.N.; Briers, E.; Van den Broeck, T.; Cumberbatch, M.G.; Santis, M.D.; Fnati, S.; Fossati, N.; Gandaglia, G.; Gillessen, S.; et al. EAU-EANM-ESTRO-ESUR-SIOG Guidelines on Prostate Cancer-2020 Update. Part 1: Screening, Diagnosis, and Local Treatment with Curative Intent. Eur. Urol. 2021, 79, 243–262. [Google Scholar] [CrossRef] [PubMed]
- Prostate Cancer (2022) NCCN Guidelines®. Available online: https://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf (accessed on 5 October 2022).
- Eastham, J.A.; Auffenberg, G.B.; Barocas, D.A.; Chou, R.; Crispino, T.; Davis, J.W.; Eggener, S.; Horwitz, E.M.; Kane, C.J.; Kirkby, E.; et al. Clinically Localized Prostate Cancer: AUA/ASTRO Guideline, Part II: Principles of Active Surveillance, Principles of Surgery, and Follow-Up. J. Urol. 2022, 208, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Preisser, F.; van den Bergh, R.C.N.; Gandaglia, G.; Ost, P.; Surcel, C.I.; Sooriakumaran, P.; Montorsi, F.; Graefen, M.; van der Poel, H.; de la Taille, A.; et al. Effect of Extended Pelvic Lymph Node Dissection on Oncologic Outcomes in Patients with D’Amico Intermediate and High Risk Prostate Cancer Treated with Radical Prostatectomy: A Multi-Institutional Study. J. Urol. 2020, 203, 338–343. [Google Scholar] [CrossRef] [PubMed]
- Choo, M.S.; Kim, M.; Ku, J.H.; Kwak, C.; Kim, H.H.; Jeong, C.W. Extended versus Standard Pelvic Lymph Node Dissection in Radical Prostatectomy on Oncological and Functional Outcomes: A Systematic Review and Meta-Analysis. Ann. Surg. Oncol. 2017, 24, 2047–2054. [Google Scholar] [CrossRef] [PubMed]
- Withrow, D.R.; DeGroot, J.M.; Siemens, D.R.; Groome, P.A. Therapeutic value of lymph node dissection at radical prostatectomy: A population-based case-cohort study. BJU Int. 2011, 108, 209–216. [Google Scholar] [CrossRef]
- Briganti, A.; Larcher, A.; Abdollah, F.; Capitanio, U.; Gallina, A.; Suardi, N.; Bianchi, M.; Sun, M.; Freschi, M.; Salonia, A.; et al. Updated nomogram predicting lymph node invasion in patients with prostate cancer undergoing extended pelvic lymph node dissection: The essential importance of percentage of positive cores. Eur. Urol. 2012, 61, 480–487. [Google Scholar] [CrossRef]
- Diamand, R.; Oderda, M.; Albisinni, S.; Fourcade, A.; Fournier, G.; Benamran, D.; Iselin, C.; Fiard, G.; Descotes, J.L.; Assenmacher, G.; et al. External validation of the Briganti nomogram predicting lymph node invasion in patients with intermediate and high-risk prostate cancer diagnosed with magnetic resonance imaging-targeted and systematic biopsies: A European multicenter study. Urol. Oncol. 2020, 38, 847.e9–847.e16. [Google Scholar] [CrossRef]
- Touijer, K.A.; Sjoberg, D.D.; Benfante, N.; Laudone, V.P.; Ehdaie, B.; Eastham, J.A.; Scardino, P.T.; Vickers, A. Limited versus Extended Pelvic Lymph Node Dissection for Prostate Cancer: A Randomized Clinical Trial. Eur. Urol. Oncol. 2021, 4, 532–539. [Google Scholar] [CrossRef] [PubMed]
- Lestingi, J.F.P.; Guglielmetti, G.B.; Trinh, Q.D.; Coelho, R.F.; Pontes, J., Jr.; Bastos, D.A.; Cordeiro, M.D.; Sarkis, A.S.; Faraj, S.F.; Mitre, A.I.; et al. Extended Versus Limited Pelvic Lymph Node Dissection During Radical Prostatectomy for Intermediate- and High-risk Prostate Cancer: Early Oncological Outcomes from a Randomized Phase 3 Trial. Eur. Urol. 2021, 79, 595–604. [Google Scholar] [CrossRef]
- Cacciamani, G.E.; Maas, M.; Nassiri, N.; Ortega, D.; Gill, K.; Dell’Oglio, P.; Thalmann, G.N.; Heidenreich, A.; Eastham, J.A.; Evans, C.P.; et al. Impact of Pelvic Lymph Node Dissection and Its Extent on Perioperative Morbidity in Patients Undergoing Radical Prostatectomy for Prostate Cancer: A Comprehensive Systematic Review and Meta-analysis. Eur. Urol. Oncol. 2021, 4, 134–149. [Google Scholar] [CrossRef]
- Chen, J.; Wang, Z.; Zhao, J.; Zhu, S.; Sun, G.; Liu, J.; Zhang, H.; Zhang, X.; Shen, P.; Shi, M.; et al. Pelvic lymph node dissection and its extent on survival benefit in prostate cancer patients with a risk of lymph node invasion >5%: A propensity score matching analysis from SEER database. Sci. Rep. 2019, 9, 17985. [Google Scholar] [CrossRef]
- Fujimoto, N.; Shiota, M.; Tomisaki, I.; Minato, A.; Yahara, K. Reconsideration on Clinical Benefit of Pelvic Lymph Node Dissection during Radical Prostatectomy for Clinically Localized Prostate Cancer. Urol. Int. 2019, 103, 125–136. [Google Scholar] [CrossRef]
- Colicchia, M.; Sharma, V.; Abdollah, F.; Briganti, A.; Jeffrey Karnes, R. Therapeutic Value of Standard Versus Extended Pelvic Lymph Node Dissection During Radical Prostatectomy for High-Risk Prostate Cancer. Curr. Urol. Rep. 2017, 18, 51. [Google Scholar] [CrossRef] [PubMed]
- Briganti, A.; Blute, M.L.; Eastham, J.H.; Graefen, M.; Heidenreich, A.; Karnes, J.R.; Montorsi, F.; Studer, U.E. Pelvic lymph node dissection in prostate cancer. Eur. Urol. 2009, 55, 1251–1265. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Xie, J.; Guo, Y.; Yuan, J.; Wang, R.; Guo, C.; Peng, B.; Yao, X.; Yang, B. Identifying the Candidates Who Will Benefit From Extended Pelvic Lymph Node Dissection at Radical Prostatectomy Among Patients With Prostate Cancer. Front. Oncol. 2022, 11, 790183. [Google Scholar] [CrossRef] [PubMed]
- Buyyounouski, M.K.; Choyke, P.L.; McKenney, J.K.; Sartor, O.; Sandler, H.M.; Amin, M.B.; Kattan, M.W.; Lin, D.W. Prostate cancer—Major changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer. J. Clin. 2017, 67, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Epstein, J.I.; Egevad, L.; Amin, M.B.; Delahunt, B.; Srigley, J.; Humphrey, P.A.; Committee, G. The 2014 International Society of Urological Pathology (ISUP) Consensus Conference on Gleason Grading of Prostatic Carcinoma: Definition of Grading Patterns and Proposal for a New Grading System. Am. J. Surg. Pathol. 2016, 40, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Clark, T.; Parekh, D.J.; Cookson, M.S.; Chang, S.S.; Smith, E.R., Jr.; Wells, N.; Smith, J.A., Jr. Randomized prospective evaluation of extended versus limited lymph node dissection in patients with clinically localized prostate cancer. J. Urol. 2003, 169, 145–148. [Google Scholar] [CrossRef] [PubMed]
- Furubayashi, N.; Negishi, T.; Iwai, H.; Nagase, K.; Taguchi, K.; Shimokawa, M.; Nakamura, M. Determination of adequate pelvic lymph node dissection range for Japanese males undergoing radical prostatectomy. Mol. Clin. Oncol. 2017, 6, 775–781. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Epstein, J.I.; Allsbrook, W.C.; Amin, M.B.; Egevad, L.L.; ISUP Grading Committee. The 2005 International Society of Urological Pathology (ISUP) Consensus Conference on Gleason Grading of Prostatic Carcinoma. Am. J. Surg. Pathol. 2005, 29, 1228–1242. [Google Scholar] [CrossRef] [PubMed]
- Clavien, P.A.; Barkun, J.; de Oliveira, M.L.; Vauthey, J.N.; Dindo, D.; Schulick, R.D.; de Santibañes, E.; Pekolj, J.; Slankamenac, K.; Bassi, C.; et al. The Clavien-Dindo classification of surgical complications: Five-year experience. Ann. Surg. 2009, 250, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Prostate Cancer Nomograms: Dynamic Prostate Cancer Nomogram: Coefficients (2017) Memorial Sloan Kettering Cancer Center. Available online: https://www.mskcc.org/nomograms/prostate/pre_op/coefficients (accessed on 5 October 2022).
- García-Perdomo, H.A.; Correa-Ochoa, J.J.; Contreras-García, R.; Daneshmand, S. Effectiveness of extended pelvic lymphadenectomy in the survival of prostate cancer: A systematic review and meta-analysis. Cent. Eur. J. Urol. 2018, 71, 262–269. [Google Scholar]
- Gandaglia, G.; De Lorenzis, E.; Novara, G.; Fossati, N.; De Groote, R.; Dovey, Z.; Suardi, N.; Montorsi, F.; Briganti, A.; Rocco, B.; et al. Robot-assisted Radical Prostatectomy and Extended Pelvic Lymph Node Dissection in Patients with Locally-advanced Prostate Cancer. Eur. Urol. 2017, 71, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Abdollah, F.; Gandaglia, G.; Suardi, N.; Capitanio, U.; Salonia, A.; Nini, A.; Moschini, M.; Sun, M.; Karakiewicz, P.I.; Shariat, S.F. More extensive pelvic lymph node dissection improves survival in patients with node-positive prostate cancer. Eur. Urol. 2015, 67, 212–219. [Google Scholar]
- Bivalacqua, T.J.; Pierorazio, P.M.; Gorin, M.A.; Allaf, M.E.; Carter, H.B.; Walsh, P.C. Anatomic extent of pelvic lymph node dissection: Impact on long-term cancer-specific outcomes in men with positive lymph nodes at time of radical prostatectomy. Urology 2013, 82, 653–658. [Google Scholar] [CrossRef] [PubMed]
- Miki, J.; Yanagisawa, T.; Tsuzuki, S.; Mori, K.; Urabe, F.; Kayano, S.; Yorozu, T.; Sato, S.; Kimura, T.; Takahashi, H.; et al. Anatomical localization and clinical impact of sentinel lymph nodes based on patterns of pelvic lymphatic drainage in clinically localized prostate cancer. Prostate 2018, 78, 419–425. [Google Scholar] [CrossRef]
- Fossati, N.; Willemse, P.M.; Van den Broeck, T.; van den Bergh, R.C.N.; Yuan, C.Y.; Briers, E.; Bellmunt, J.; Bolla, M.; Cornford, P.; De Santis, M.; et al. The Benefits and Harms of Different Extents of Lymph Node Dissection During Radical Prostatectomy for Prostate Cancer: A Systematic Review. Eur. Urol. 2017, 72, 84–109. [Google Scholar] [CrossRef] [PubMed]
- Harbin, A.C.; Eun, D.D. The role of extended pelvic lymphadenectomy with radical prostatectomy for high-risk prostate cancer. Urol. Oncol. 2015, 33, 208–216. [Google Scholar] [CrossRef]
| Covariates | Non-PLND Group | PLND Group | p |
|---|---|---|---|
| Number | 605 | 605 | |
| Age (median, year, IQR) | 69 (64–73) | 68 (65–72) | 0.967 |
| Body mass index (median, kg/m2, IQR) | 23.4 (21.7–25.2) | 23.5 (21.7–25.6) | 0.200 |
| Initial PSA (median, ng/mL, IQR) | 7.002 (5.280–10.220) | 7.230 (5.300–10.344) | 0.454 |
| Biopsy Grade group (number, %) | 0.541 | ||
| 1 | 170 (28.1) | 168 (27.8) | |
| 2 | 272 (45.0) | 265 (43.7) | |
| 3 | 115 (19.0) | 121 (20.0) | |
| 4 | 40 (6.6) | 39 (6.4) | |
| 5 | 8 (1.3) | 13 (2.1) | |
| Clinical T stage (number, %) | 0.751 | ||
| T1 | 132 (21.8) | 135 (22.3) | |
| T2 | 463 (76.5) | 453 (74.7) | |
| T3 | 10 (1.7) | 18 (3.0) | |
| NCCN risk classification (number, %) | 0.139 | ||
| Low | 97 (16.0) | 84 (13.9) | |
| Intermediate | 426 (70.4) | 426 (70.2) | |
| High | 82 (13.6) | 96 (15.9) | |
| ECOG-PS (number, %) | 0.244 | ||
| 0 | 591 (97.7) | 586 (96.7) | |
| 1 | 14 (2.3) | 19 (3.1) | |
| 2 | 0 | 1 (0.2) | |
| Follow-up period (median, months, IQR) | 17.2 (8.9–32.6) | 24.7 (11.4–53.6) | <0.001 |
| Covariates | Non-PLND Group | PLND Group | p |
|---|---|---|---|
| Number | 605 | 605 | |
| Console time (median, minutes, IQR) | 153 (116–213) | 157 (120–201) | 0.218 |
| Estimates blood loss (median, mL, IQR) | 50 (10–200) | 50 (10–168) | 0.517 |
| Pathological Grade group (number, %) | 0.756 | ||
| 1 | 55 (9.1) | 62 (10.2) | |
| 2 | 309 (51.0) | 306 (50.5) | |
| 3 | 167 (27.6) | 167 (27.6) | |
| 4 | 50 (8.3) | 43 (7.1) | |
| 5 | 24 (4.0) | 28 (4.6) | |
| Pathological T stage (number, %) | 0.763 | ||
| T2 | 133 (22.0) | 125 (20.7) | |
| T3 | 472 (78.0) | 481 (79.3) | |
| LNI (number, %) | Not applicable | 6 (1.0) | |
| Lymph node count (number, median, IQR) | Not applicable | 5 (3–9) | |
| Positive surgical margin (number, %) | 125 (20.7) | 122 (19.8) | 0.831 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Namiki, S.; Kawase, M.; Ebara, S.; Tatenuma, T.; Sasaki, T.; Ikehata, Y.; Nakayama, A.; Toide, M.; Yoneda, T.; Sakaguchi, K.; et al. Pelvic Lymphadenectomy May Not Improve Biochemical Recurrence-Free Survival in Patients with Prostate Cancer Treated with Robot-Assisted Radical Prostatectomy in Japan (The MSUG94 Group). Cancers 2022, 14, 5803. https://doi.org/10.3390/cancers14235803
Namiki S, Kawase M, Ebara S, Tatenuma T, Sasaki T, Ikehata Y, Nakayama A, Toide M, Yoneda T, Sakaguchi K, et al. Pelvic Lymphadenectomy May Not Improve Biochemical Recurrence-Free Survival in Patients with Prostate Cancer Treated with Robot-Assisted Radical Prostatectomy in Japan (The MSUG94 Group). Cancers. 2022; 14(23):5803. https://doi.org/10.3390/cancers14235803
Chicago/Turabian StyleNamiki, Sanae, Makoto Kawase, Shin Ebara, Tomoyuki Tatenuma, Takeshi Sasaki, Yoshinori Ikehata, Akinori Nakayama, Masahiro Toide, Tatsuaki Yoneda, Kazushige Sakaguchi, and et al. 2022. "Pelvic Lymphadenectomy May Not Improve Biochemical Recurrence-Free Survival in Patients with Prostate Cancer Treated with Robot-Assisted Radical Prostatectomy in Japan (The MSUG94 Group)" Cancers 14, no. 23: 5803. https://doi.org/10.3390/cancers14235803
APA StyleNamiki, S., Kawase, M., Ebara, S., Tatenuma, T., Sasaki, T., Ikehata, Y., Nakayama, A., Toide, M., Yoneda, T., Sakaguchi, K., Teishima, J., Makiyama, K., Inoue, T., Kitamura, H., Saito, K., Koga, F., Urakami, S., & Koie, T. (2022). Pelvic Lymphadenectomy May Not Improve Biochemical Recurrence-Free Survival in Patients with Prostate Cancer Treated with Robot-Assisted Radical Prostatectomy in Japan (The MSUG94 Group). Cancers, 14(23), 5803. https://doi.org/10.3390/cancers14235803








