Prognosis of Anaplastic Thyroid Cancer with Distant Metastasis
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Ethics Statement
2.3. Patients
2.4. Treatment
2.5. Outcomes
2.6. Statistics
3. Results
3.1. Demographic Characteristics
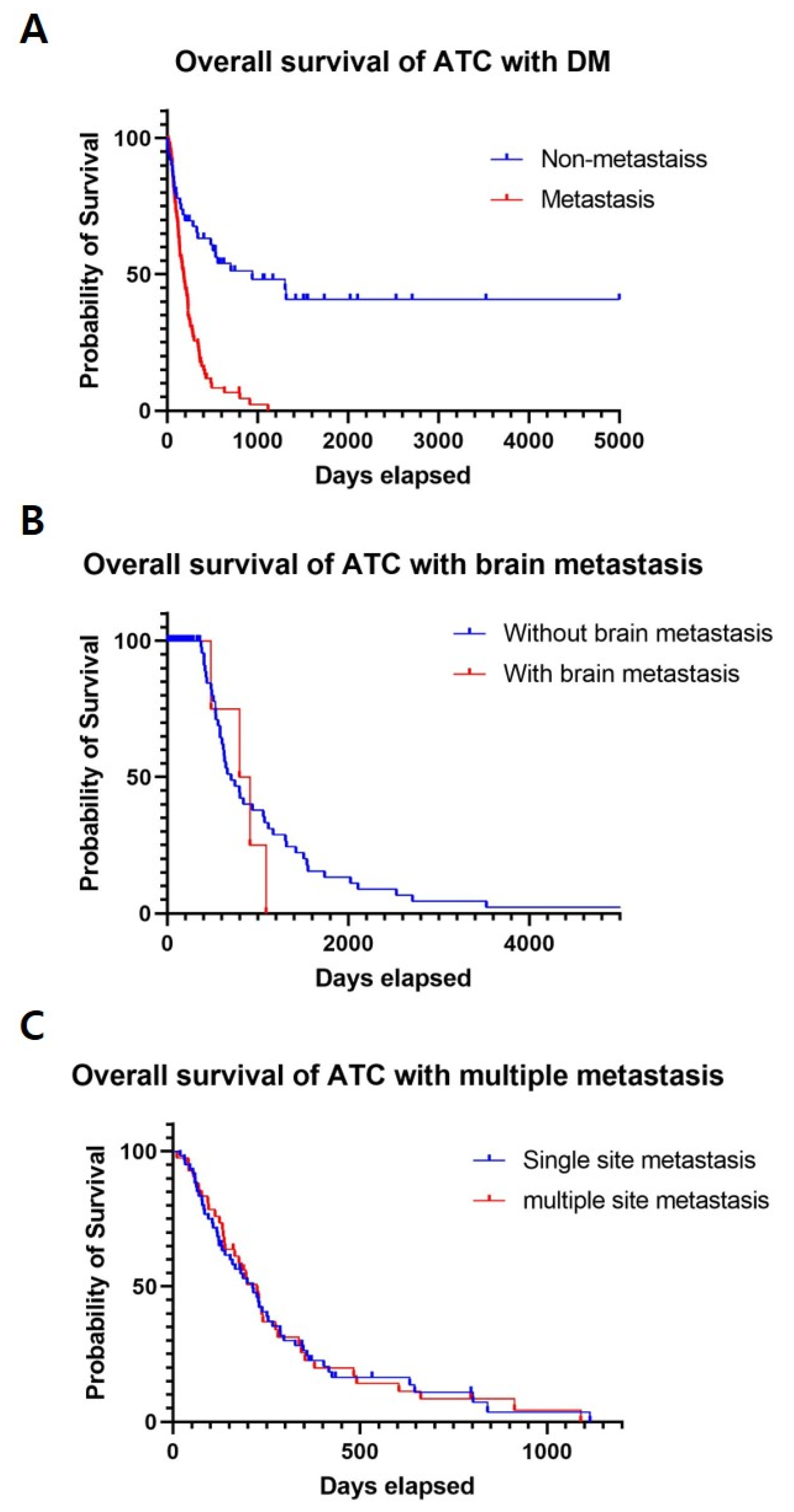
3.2. ATC with Distant Metastasis
3.3. ATC with Brain Metastasis
3.4. ATC with Multiple-Site Distant Metastasis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lim, H.; Devesa, S.S.; Sosa, J.A.; Check, D.; Kitahara, C.M. Trends in thyroid cancer incidence and mortality in the united states, 1974–2013. JAMA 2017, 317, 1338–1348. [Google Scholar] [CrossRef]
- Casella, C.; Fusco, M. Thyroid cancer. Epidemiol. Prev. 2004, 28, 88–91. [Google Scholar] [PubMed]
- Siegel, R.; Ma, J.; Zou, Z.; Jemal, A. Cancer statistics, 2014. CA Cancer J. Clin. 2014, 64, 9–29. [Google Scholar] [CrossRef] [PubMed]
- Londero, S.C.; Krogdahl, A.; Bastholt, L.; Overgaard, J.; Trolle, W.; Pedersen, H.B.; Bentzen, J.; Schytte, S.; Christiansen, P.; Godballe, C. Papillary thyroid microcarcinoma in denmark 1996-2008: A national study of epidemiology and clinical significance. Thyroid 2013, 23, 1159–1164. [Google Scholar] [CrossRef] [PubMed]
- Smallridge, R.C.; Copland, J.A. Anaplastic thyroid carcinoma: Pathogenesis and emerging therapies. Clin. Oncol. 2010, 22, 486–497. [Google Scholar] [CrossRef]
- Neff, R.L.; Farrar, W.B.; Kloos, R.T.; Burman, K.D. Anaplastic thyroid cancer. Endocrinol. Metab. Clin. N. Am. 2008, 37, 525–538. [Google Scholar] [CrossRef]
- Bible, K.C.; Kebebew, E.; Brierley, J.; Brito, J.P.; Cabanillas, M.E.; Clark, T.J., Jr.; Di Cristofano, A.; Foote, R.; Giordano, T.; Kasperbauer, J.; et al. 2021 american thyroid association guidelines for management of patients with anaplastic thyroid cancer. Thyroid 2021, 31, 337–386. [Google Scholar] [CrossRef]
- Ranganath, R.; Shah, M.A.; Shah, A.R. Anaplastic thyroid cancer. Curr. Opin. Endocrinol. Diabetes Obes. 2015, 22, 387–391. [Google Scholar] [CrossRef]
- Kim, S.Y.; Kim, S.-M.; Kim, J.W.; Lee, I.J.; Jeon, T.J.; Chang, H.; Kim, B.-W.; Lee, Y.S.; Chang, H.-S.; Park, C.S. Survival with lenvatinib for the treatment of progressive anaplastic thyroid cancer: A single-center, retrospective analysis. Front. Endocrinol. 2020, 11, 599. [Google Scholar] [CrossRef]
- Maniakas, A.; Dadu, R.; Busaidy, N.L.; Wang, J.R.; Ferrarotto, R.; Lu, C.; Williams, M.D.; Gunn, G.B.; Hofmann, M.-C.; Cote, G.; et al. Evaluation of overall survival in patients with anaplastic thyroid carcinoma, 2000–2019. JAMA Oncol. 2020, 6, 1397–1404. [Google Scholar] [CrossRef]
- Massin, J.P.; Savoie, J.C.; Garnier, H.; Guiraudon, G.; Leger, F.A.; Bacourt, F. Pulmonary metastases in differentiated thyroid carcinoma. Study of 58 cases with implications for the primary tumor treatment. Cancer 1984, 53, 982–992. [Google Scholar] [CrossRef] [PubMed]
- Ruegemer, J.J.; Hay, I.D.; Bergstralh, E.J.; Ryan, J.J.; Offord, K.P.; Gorman, C.A. Distant metastases in differentiated thyroid carcinoma: A multivariate analysis of prognostic variables. J. Clin. Endocrinol. Metab. 1988, 67, 501–508. [Google Scholar] [CrossRef] [PubMed]
- Casara, D.; Rubello, D.; Saladini, G.; Gallo, V.; Masarotto, G.; Busnardo, B. Distant metastases in differentiated thyroid cancer: Long-term results of radioiodine treatment and statistical analysis of prognostic factors in 214 patients. Tumori 1991, 77, 432–436. [Google Scholar] [CrossRef] [PubMed]
- Samaan, N.A.; Schultz, P.N.; Haynie, T.P.; Ordonez, N.G. Pulmonary metastasis of differentiated thyroid carcinoma: Treatment results in 101 patients. J. Clin. Endocrinol. Metab. 1985, 60, 376–380. [Google Scholar] [CrossRef] [PubMed]
- Jonklaas, J.; Sarlis, N.J.; Litofsky, D.; Ain, K.B.; Bigos, S.T.; Brierley, J.D.; Cooper, D.S.; Haugen, B.R.; Ladenson, P.W.; Magner, J.; et al. Outcomes of patients with differentiated thyroid carcinoma following initial therapy. Thyroid 2006, 16, 1229–1242. [Google Scholar] [CrossRef]
- Lang, B.H.; Wong, K.P.; Cheung, C.Y.; Wan, K.Y.; Lo, C.Y. Evaluating the prognostic factors associated with cancer-specific survival of differentiated thyroid carcinoma presenting with distant metastasis. Ann. Surg Oncol. 2013, 20, 1329–1335. [Google Scholar] [CrossRef]
- Haq, M.S.; Harmer, C. Non-surgical management of thyroid cancer. In Practical Management of Thyroid Cancer: A Multidisciplinary Approach; Mazzaferri, E.L., Harmer, C., Mallick, U.K., Kendall-Taylor, P., Eds.; Springer: London, UK, 2006; pp. 171–191. [Google Scholar] [CrossRef]
- Cho, S.W.; Choi, H.S.; Yeom, G.J.; Lim, J.A.; Moon, J.H.; Park, D.J.; Chung, J.K.; Cho, B.Y.; Yi, K.H.; Park, Y.J. Long-term prognosis of differentiated thyroid cancer with lung metastasis in korea and its prognostic factors. Thyroid 2014, 24, 277–286. [Google Scholar] [CrossRef]
- Carcangiu, M.L.; Steeper, T.; Zampi, G.; Rosai, J. Anaplastic thyroid carcinoma. A study of 70 cases. Am. J. Clin. Pathol. 1985, 83, 135–158. [Google Scholar] [CrossRef]
- Venkatesh, Y.S.; Ordonez, N.G.; Schultz, P.N.; Hickey, R.C.; Goepfert, H.; Samaan, N.A. Anaplastic carcinoma of the thyroid. A clinicopathologic study of 121 cases. Cancer 1990, 66, 321–330. [Google Scholar] [CrossRef]
- Tan, R.K.; Finley, R.K., III; Driscoll, D.; Bakamjian, V.; Hicks, W.L., Jr.; Shedd, D.P. Anaplastic carcinoma of the thyroid: A 24-year experience. Head Neck 1995, 17, 41–47. [Google Scholar] [CrossRef]
- Salvati, M.; Frati, A.; Rocchi, G.; Masciangelo, R.; Antonaci, A.; Gagliardi, F.M.; Delfini, R. Single brain metastasis from thyroid cancer: Report of twelve cases and review of the literature. J. Neurooncol. 2001, 51, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Chiu, A.C.; Delpassand, E.S.; Sherman, S.I. Prognosis and treatment of brain metastases in thyroid carcinoma. J. Clin. Endocrinol. Metab. 1997, 82, 3637–3642. [Google Scholar] [CrossRef] [PubMed]
- Gomes-Lima, C.J.; Wu, D.; Rao, S.N.; Punukollu, S.; Hritani, R.; Zeymo, A.; Deeb, H.; Mete, M.; Aulisi, E.F.; Van Nostrand, D.; et al. Brain metastases from differentiated thyroid carcinoma: Prevalence, current therapies, and outcomes. J. Endocr. Soc. 2019, 3, 359–371. [Google Scholar] [CrossRef] [PubMed]
- Henriques de Figueiredo, B.; Godbert, Y.; Soubeyran, I.; Carrat, X.; Lagarde, P.; Cazeau, A.L.; Italiano, A.; Sargos, P.; Kantor, G.; Loiseau, H.; et al. Brain metastases from thyroid carcinoma: A retrospective study of 21 patients. Thyroid 2014, 24, 270–276. [Google Scholar] [CrossRef]
- Choi, J.; Kim, J.W.; Keum, Y.S.; Lee, I.J. The largest known survival analysis of patients with brain metastasis from thyroid cancer based on prognostic groups. PLoS ONE 2016, 11, e0154739. [Google Scholar]
- Samuel, A.M.; Shah, D.H. Brain metastases in well-differentiated carcinoma of the thyroid. Tumori 1997, 83, 608–610. [Google Scholar] [CrossRef]
- Are, C.; Shaha, A.R. Anaplastic thyroid carcinoma: Biology, pathogenesis, prognostic factors, and treatment approaches. Ann. Surg. Oncol. 2006, 13, 453–464. [Google Scholar] [CrossRef]
- Keutgen, X.M.; Sadowski, S.M.; Kebebew, E. Management of anaplastic thyroid cancer. Gland Surg. 2015, 4, 44–51. [Google Scholar]
- Wang, J.R.; Montierth, M.; Xu, L.; Goswami, M.; Zhao, X.; Cote, G.; Wang, W.; Iyer, P.; Dadu, R.; Busaidy, N.L.; et al. Impact of somatic mutations on survival outcomes in patients with anaplastic thyroid carcinoma. JCO Precis. Oncol. 2022, 6, e2100504. [Google Scholar] [CrossRef]
| Characteristics | Values (%) |
|---|---|
| Sex (n = 152) | |
| Male | 66 (43%) |
| Female | 86 (57%) |
| Age, mean ± SD, years | 64 ± 11.44 |
| Tumor size, mean ± SD, centimeters | 4.9 ± 2.23 |
| T4, n | 119 (74%) |
| N1, n | 128 (84%) |
| M1, n | 88 (58%) |
| Operation (n = 104) | |
| Excisional biopsy (R2) | 16 (15%) |
| Debulking (R1) | 39 (38%) |
| Complete resection (R0) | 49 (47%) |
| Chemotherapy (n = 124) | |
| Paclitaxel | 105 (85%) |
| Others | 19 (15%) |
| Target therapy (n = 74) | |
| Lenvatinib | 60 (80%) |
| Sorafenib | 14 (20%) |
| Radiation therapy, n | 124 (82%) |
| Death, n (%) | 116 (76%) |
| Median survival, median (min–max), days | 228.5 (2–5074) |
| Variable | ATC (n = 152) | ATC with Distant Metastasis (n = 88) | p-Value |
|---|---|---|---|
| Male | 66 (43%) | 37 (42%) | |
| Age, mean ± SD, years | 64 ± 11.44 | 67 ± 9.38 | 0.06 |
| Tumor size, mean ± SD, centimeters | 4.9 ± 2.23 | 5.2 ± 2.35 | 0.34 |
| T4 | 119 (74%) | 43 (83%) | 0.63 |
| N1 | 128 (84%) | 76 (86%) | 0.65 |
| R0 | 49 (47%) | 20 (40%) | 0.68 |
| R1 or R2 | 55 (53%) | 30 (60%) | 0.68 |
| Chemotherapy | 124 (82%) | 81 (92%) | 0.043 * |
| Targeted therapy | 74 (49%) | 59 (67%) | 0.009 * |
| Radiation therapy | 124 (82%) | 80 (91%) | 0.078 |
| Death | 116 (76%) | 79 (90%) | 0.01 * |
| Survival > 1 year | 49 (32%) | 13 (15%) | 0.003 * |
| Median survival, days (range) | 228.5 (2–5074) | 171 (10–1115) | 0.01 * |
| Variable | ATC with Brain Metastasis (n = 17) | ATC without Brain Metastasis (n = 135) | p-Value |
|---|---|---|---|
| Male | 9 (53%) | 57 (42%) | |
| Age, mean ± SD, years | 63 ± 6.68 | 64 ± 11.91 | 0.64 |
| Tumor size, mean ± SD, centimeters | 4.3 ± 1.77 | 5.0 ± 2.28 | 0.16 |
| T4 | 13 (77%) | 106 (79%) | 0.76 |
| N1 | 16 (94%) | 112 (83%) | 0.24 |
| R0 | 5 (38%) | 44 (51%) | 0.61 |
| R1 or R2 | 8 (62%) | 43 (49%) | 0.61 |
| Chemotherapy | 13 (76%) | 111 (82%) | 0.81 |
| Targeted therapy | 14 (82%) | 60 (44%) | 0.007 * |
| Radiation therapy | 14 (82%) | 110 (81%) | 0.93 |
| Death | 15 (88%) | 101 (75%) | 0.22 |
| Survival > 1 year | 4 (24%) | 45 (33%) | 0.42 |
| Median survival, days (range) | 227 (10–1090) | 228 (2–5074) | 0.89 |
| Case ID | Sex | Age | T | N | R0/R1 or R2 | Time to Brain Metastasis, Days | CTx/TKI | Brain RTx Dose, cGy | Surgical Removal of Brain Tumor | Death | Survival, Days |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 67 | 2 | 1 | R0 | 1 059 | –/– | – | – | Death | 1090 |
| 2 | F | 59 | 2 | 1 | R0 | – | –/– | – | – | Death | 10 |
| 3 | F | 77 | 4 | 1 | R1 or R2 | 135 | –/– | – | – | Death | 198 |
| 4 | F | 70 | 4 | 1 | – | – | Paclitaxel /Nexavar | – | – | Death | 195 |
| 5 | M | 58 | 3b | 1 | R0 | – | –/Nexavar | 3500 | + | Death | 913 |
| 6 | F | 67 | 4 | 1 | – | 167 | Palitaxel /Lenvima | – | – | Death | 230 |
| 7 | M | 61 | 4 | 1 | R1 or R2 | 459 | Palitaxel /Lenvima | – | – | Death | 483 |
| 8 | M | 55 | 4 | 1 | R1 or R2 | 167 | Palitaxel /Lenvima | – | – | Death | 353 |
| 9 | F | 63 | 4 | 1 | – | 315 | Palitaxel /Lenvima | 3000 | – | Death | 337 |
| 10 | M | 70 | 4 | 1 | – | – | Palitaxel /Lenvima | 3000 | – | Death | 112 |
| 11 | M | 59 | 4 | 1 | R1 or R2 | 170 | Palitaxel /Lenvima | 4000 | + | Alive | 796 |
| 12 | M | 69 | 4 | 1 | R1 or R2 | – | Palitaxel /Lenvima | – | – | Death | 225 |
| 13 | M | 62 | 4 | 1 | R1 or R2 | 85 | Palitaxel /Lenvima | – | – | Death | 139 |
| 14 | M | 59 | 3b | 0 | R0 | – | Cisplatin /Lenvima | – | – | Death | 41 |
| 15 | F | 67 | 4 | 1 | R1 or R2 | 228 | Palitaxel /Lenvima | 3500 | – | Death | 274 |
| 16 | F | 56 | 4 | 1 | R0 | 215 | Palitaxel /Lenvima | – | – | Death | 229 |
| 17 | M | 51 | 4 | 1 | R1 or R2 | 95 | Palitaxel /Lenvima | – | – | Alive | 161 |
| Variable | ATC with Single Site Metastasis (n = 60) | ATC with Multiple-Site Metastasis (n = 42) | p-Value |
|---|---|---|---|
| Male | 23 (38%) | 21 (50%) | |
| Age, mean ± SD, years | 67 ± 9.73 | 64 ± 9.25 | 0.18 |
| Tumor size, mean ± SD, centimeters | 5.56 ± 2.52 | 4.6 ± 1.80 | 0.02 * |
| T4 | 49 (82%) | 35 (83%) | 0.31 |
| N1 | 53 (88%) | 36 (86%) | 0.70 |
| Death | 53 (88%) | 37 (88%) | 0.97 |
| Survival > 1 year | 12 (20%) | 8 (19%) | 0.91 |
| Median survival, days (min–max) | 192.5 (19–1115) | 192 (10–1090) | 0.95 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.-S.; Lee, J.S.; Yun, H.J.; Chang, H.; Kim, S.M.; Lee, Y.S.; Chang, H.-S.; Park, C.S. Prognosis of Anaplastic Thyroid Cancer with Distant Metastasis. Cancers 2022, 14, 5784. https://doi.org/10.3390/cancers14235784
Lee J-S, Lee JS, Yun HJ, Chang H, Kim SM, Lee YS, Chang H-S, Park CS. Prognosis of Anaplastic Thyroid Cancer with Distant Metastasis. Cancers. 2022; 14(23):5784. https://doi.org/10.3390/cancers14235784
Chicago/Turabian StyleLee, Jin-Seok, Jun Sung Lee, Hyeok Jun Yun, Hojin Chang, Seok Mo Kim, Yong Sang Lee, Hang-Seok Chang, and Cheong Soo Park. 2022. "Prognosis of Anaplastic Thyroid Cancer with Distant Metastasis" Cancers 14, no. 23: 5784. https://doi.org/10.3390/cancers14235784
APA StyleLee, J.-S., Lee, J. S., Yun, H. J., Chang, H., Kim, S. M., Lee, Y. S., Chang, H.-S., & Park, C. S. (2022). Prognosis of Anaplastic Thyroid Cancer with Distant Metastasis. Cancers, 14(23), 5784. https://doi.org/10.3390/cancers14235784






