Near-Infrared Photoimmunotherapy for Oropharyngeal Cancer
Abstract
Simple Summary
Abstract
1. Introduction
2. Mechanism of NIR-PIT Cytotoxicity
3. Clinical Studies
3.1. RM-1929-101 Study
3.2. RM-1929-102 Study
4. Treatment Flow of HN-PIT in Clinical Practice
4.1. Day 1: Administration of RM-1929
4.2. Day 2: Laser Illumination
5. Treatment-Specific Adverse Events
6. Treatment Indications
7. Case Presentation
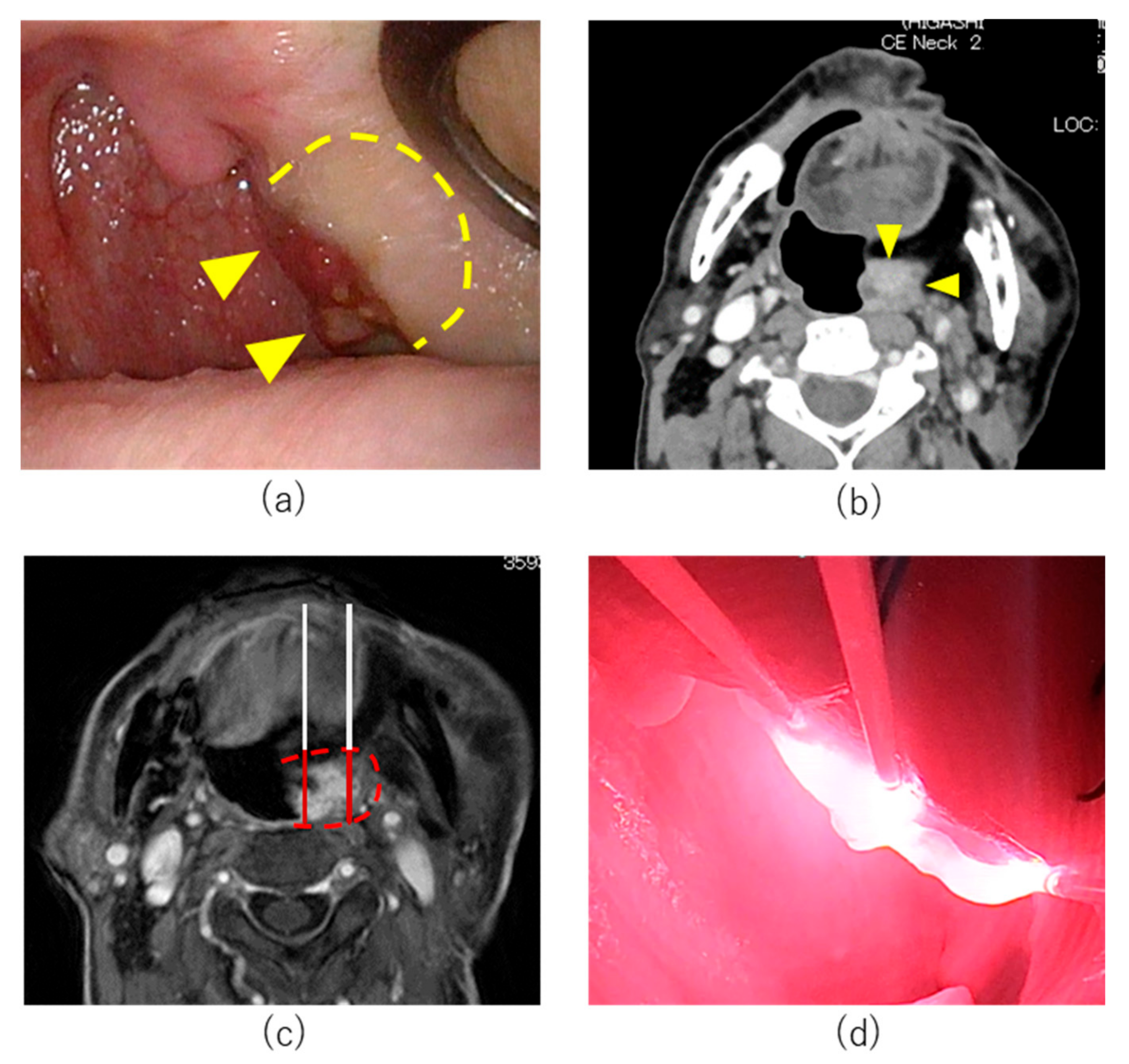
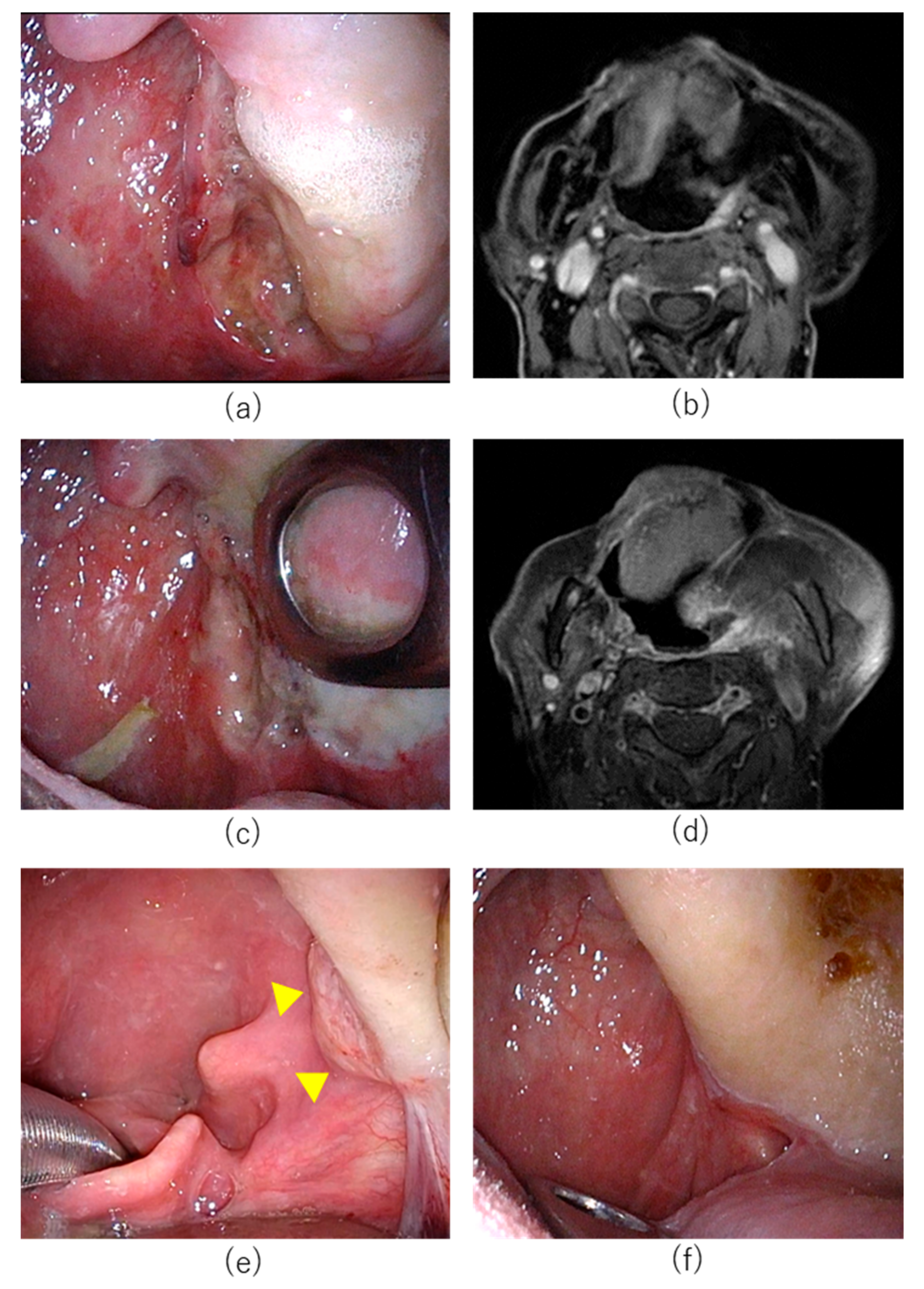
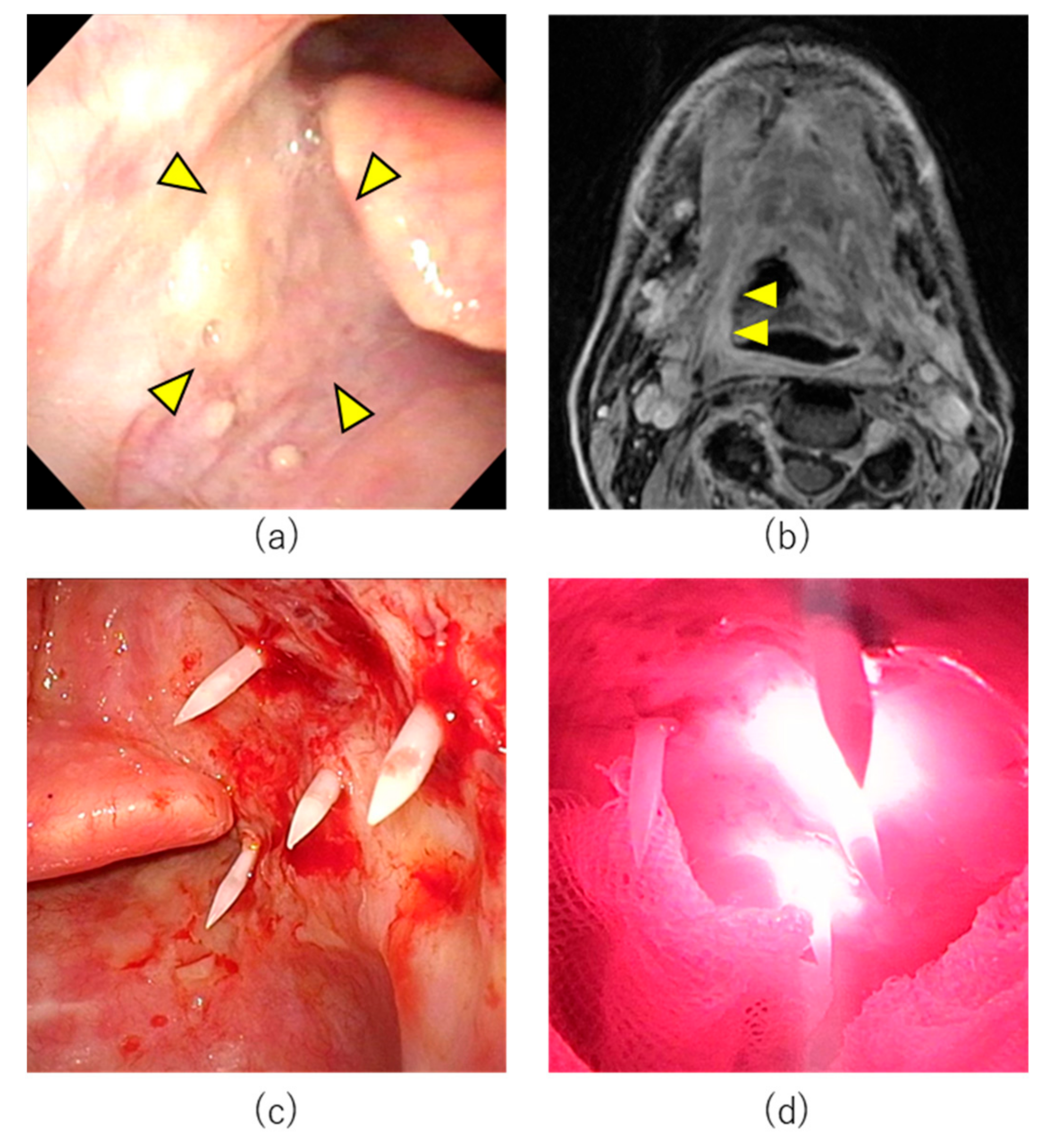
7.1. Case 1
7.2. Case 2
8. Immune Activation
9. Combined Treatment with ICI
10. Laser Illumination Approach
11. Impact on Quality of Life
12. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chow, L.Q.M. Head and Neck Cancer. Reply. N. Engl. J. Med. 2020, 382, e57. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, A.K.; Engels, E.A.; Pfeiffer, R.M.; Hernandez, B.Y.; Xiao, W.; Kim, E.; Jiang, B.; Goodman, M.T.; Sibug-Saber, M.; Cozen, W.; et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J. Clin. Oncol. 2011, 29, 4294–4301. [Google Scholar] [CrossRef] [PubMed]
- Chenevert, J.; Chiosea, S. Incidence of human papillomavirus in oropharyngeal squamous cell carcinomas: Now and 50 years ago. Hum. Pathol. 2012, 43, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Ang, K.K.; Harris, J.; Wheeler, R.; Weber, R.; Rosenthal, D.I.; Nguyen-Tan, P.F.; Westra, W.H.; Chung, C.H.; Jordan, R.C.; Lu, C.; et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N. Engl. J. Med. 2010, 363, 24–35. [Google Scholar] [CrossRef]
- Mellin, H.; Friesland, S.; Lewensohn, R.; Dalianis, T.; Munck-Wikland, E. Human papillomavirus (HPV) DNA in tonsillar cancer: Clinical correlates, risk of relapse, and survival. Int. J. Cancer 2000, 89, 300–304. [Google Scholar] [CrossRef]
- Temam, S.; Pape, E.; Janot, F.; Wibault, P.; Julieron, M.; Lusinchi, A.; Mamelle, G.; Marandas, P.; Luboinski, B.; Bourhis, J. Salvage surgery after failure of very accelerated radiotherapy in advanced head-and-neck squamous cell carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 2005, 62, 1078–1083. [Google Scholar] [CrossRef]
- Khan, N.; Clemens, M.; Liu, J.; Garden, A.S.; Lawyer, A.; Weber, R.; Gunn, G.B.; Morrison, W.H.; Kupferman, M.E. The role of salvage surgery with interstitial brachytherapy for the Management of Regionally Recurrent Head and Neck Cancers. Cancers Head Neck 2019, 4, 4. [Google Scholar] [CrossRef]
- Lee, N.; Chan, K.; Bekelman, J.E.; Zhung, J.; Mechalakos, J.; Narayana, A.; Wolden, S.; Venkatraman, E.S.; Pfister, D.; Kraus, D.; et al. Salvage re-irradiation for recurrent head and neck cancer. Int. J. Radiat. Oncol. Biol. Phys. 2007, 68, 731–740. [Google Scholar] [CrossRef]
- Suzuki, H.; Hanai, N.; Nishikawa, D.; Fukuda, Y.; Hasegawa, Y. Complication and surgical site infection for salvage surgery in head and neck cancer after chemoradiotherapy and bioradiotherapy. Auris Nasus Larynx 2017, 44, 596–601. [Google Scholar] [CrossRef]
- Vermorken, J.B.; Mesia, R.; Rivera, F.; Remenar, E.; Kawecki, A.; Rottey, S.; Erfan, J.; Zabolotnyy, D.; Kienzer, H.R.; Cupissol, D.; et al. Platinum-based chemotherapy plus cetuximab in head and neck cancer. N. Engl. J. Med. 2008, 359, 1116–1127. [Google Scholar] [CrossRef] [PubMed]
- Ferris, R.L.; Blumenschein, G., Jr.; Fayette, J.; Guigay, J.; Colevas, A.D.; Licitra, L.; Harrington, K.; Kasper, S.; Vokes, E.E.; Even, C.; et al. Nivolumab for Recurrent Squamous-Cell Carcinoma of the Head and Neck. N. Engl. J. Med. 2016, 375, 1856–1867. [Google Scholar] [CrossRef] [PubMed]
- Burtness, B.; Harrington, K.J.; Greil, R.; Soulieres, D.; Tahara, M.; de Castro, G., Jr.; Psyrri, A.; Baste, N.; Neupane, P.; Bratland, A.; et al. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): A randomised, open-label, phase 3 study. Lancet 2019, 394, 1915–1928. [Google Scholar] [CrossRef]
- Hanai, N.; Shimizu, Y.; Kariya, S.; Yasumatsu, R.; Yokota, T.; Fujii, T.; Tsukahara, K.; Yoshida, M.; Hanyu, K.; Ueda, T.; et al. Effectiveness and safety of nivolumab in patients with head and neck cancer in Japanese real-world clinical practice: A multicenter retrospective clinical study. Int. J. Clin. Oncol. 2021, 26, 494–506. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, D.; Suzuki, H.; Beppu, S.; Terada, H.; Sawabe, M.; Kadowaki, S.; Sone, M.; Hanai, N. Eosinophil prognostic scores for patients with head and neck squamous cell carcinoma treated with nivolumab. Cancer Sci. 2021, 112, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Mitsunaga, M.; Ogawa, M.; Kosaka, N.; Rosenblum, L.T.; Choyke, P.L.; Kobayashi, H. Cancer cell-selective in vivo near infrared photoimmunotherapy targeting specific membrane molecules. Nat. Med. 2011, 17, 1685–1691. [Google Scholar] [CrossRef]
- Kishimoto, S.; Bernardo, M.; Saito, K.; Koyasu, S.; Mitchell, J.B.; Choyke, P.L.; Krishna, M.C. Evaluation of oxygen dependence on in vitro and in vivo cytotoxicity of photoimmunotherapy using IR-700-antibody conjugates. Free Radic. Biol. Med. 2015, 85, 24–32. [Google Scholar] [CrossRef]
- Sato, K.; Ando, K.; Okuyama, S.; Moriguchi, S.; Ogura, T.; Totoki, S.; Hanaoka, H.; Nagaya, T.; Kokawa, R.; Takakura, H.; et al. Photoinduced Ligand Release from a Silicon Phthalocyanine Dye Conjugated with Monoclonal Antibodies: A Mechanism of Cancer Cell Cytotoxicity after Near-Infrared Photoimmunotherapy. ACS Cent. Sci. 2018, 4, 1559–1569. [Google Scholar] [CrossRef]
- Cognetti, D.M.; Johnson, J.M.; Curry, J.M.; Kochuparambil, S.T.; McDonald, D.; Mott, F.; Fidler, M.J.; Stenson, K.; Vasan, N.R.; Razaq, M.A.; et al. Phase 1/2a, open-label, multicenter study of RM-1929 photoimmunotherapy in patients with locoregional, recurrent head and neck squamous cell carcinoma. Head Neck 2021, 43, 3875–3887. [Google Scholar] [CrossRef]
- Mitsunaga, M.; Nakajima, T.; Sano, K.; Kramer-Marek, G.; Choyke, P.L.; Kobayashi, H. Immediate in vivo target-specific cancer cell death after near infrared photoimmunotherapy. BMC Cancer 2012, 12, 345. [Google Scholar] [CrossRef]
- Ogawa, M.; Tomita, Y.; Nakamura, Y.; Lee, M.J.; Lee, S.; Tomita, S.; Nagaya, T.; Sato, K.; Yamauchi, T.; Iwai, H.; et al. Immunogenic cancer cell death selectively induced by near infrared photoimmunotherapy initiates host tumor immunity. Oncotarget 2017, 8, 10425–10436. [Google Scholar] [CrossRef] [PubMed]
- Green, D.R.; Ferguson, T.; Zitvogel, L.; Kroemer, G. Immunogenic and tolerogenic cell death. Nat. Rev. Immunol. 2009, 9, 353–363. [Google Scholar] [CrossRef] [PubMed]
- Obeid, M.; Panaretakis, T.; Tesniere, A.; Joza, N.; Tufi, R.; Apetoh, L.; Ghiringhelli, F.; Zitvogel, L.; Kroemer, G. Leveraging the immune system during chemotherapy: Moving calreticulin to the cell surface converts apoptotic death from “silent” to immunogenic. Cancer Res. 2007, 67, 7941–7944. [Google Scholar] [CrossRef] [PubMed]
- Henderson, T.A.; Morries, L.D. Near-infrared photonic energy penetration: Can infrared phototherapy effectively reach the human brain? Neuropsychiatr. Dis. Treat. 2015, 11, 2191–2208. [Google Scholar] [CrossRef] [PubMed]
- Wakiyama, H.; Kato, T.; Furusawa, A.; Choyke, P.L.; Kobayashi, H. Near infrared photoimmunotherapy of cancer; possible clinical applications. Nanophotonics 2021, 10, 3135–3151. [Google Scholar] [CrossRef]
- Railkar, R.; Krane, L.S.; Li, Q.Q.; Sanford, T.; Siddiqui, M.R.; Haines, D.; Vourganti, S.; Brancato, S.J.; Choyke, P.L.; Kobayashi, H.; et al. Epidermal Growth Factor Receptor (EGFR)-targeted Photoimmunotherapy (PIT) for the Treatment of EGFR-expressing Bladder Cancer. Mol. Cancer Ther. 2017, 16, 2201–2214. [Google Scholar] [CrossRef]
- Maczynska, J.; Da Pieve, C.; Burley, T.A.; Raes, F.; Shah, A.; Saczko, J.; Harrington, K.J.; Kramer-Marek, G. Immunomodulatory activity of IR700-labelled affibody targeting HER2. Cell Death Dis. 2020, 11, 886. [Google Scholar] [CrossRef]
- Kobayashi, H.; Choyke, P.L. Near-Infrared Photoimmunotherapy of Cancer. Acc. Chem. Res. 2019, 52, 2332–2339. [Google Scholar] [CrossRef]
- Sato, K.; Hanaoka, H.; Watanabe, R.; Nakajima, T.; Choyke, P.L.; Kobayashi, H. Near infrared photoimmunotherapy in the treatment of disseminated peritoneal ovarian cancer. Mol. Cancer Ther. 2015, 14, 141–150. [Google Scholar] [CrossRef]
- Sato, K.; Choyke, P.L.; Kobayashi, H. Photoimmunotherapy of gastric cancer peritoneal carcinomatosis in a mouse model. PLoS ONE 2014, 9, e113276. [Google Scholar] [CrossRef]
- Shirasu, N.; Yamada, H.; Shibaguchi, H.; Kuroki, M.; Kuroki, M. Potent and specific antitumor effect of CEA-targeted photoimmunotherapy. Int. J. Cancer 2014, 135, 2697–2710. [Google Scholar] [CrossRef] [PubMed]
- Tahara, M.; Okano, S.; Enokida, T.; Ueda, Y.; Fujisawa, T.; Shinozaki, T.; Tomioka, T.; Okano, W.; Biel, M.A.; Ishida, K.; et al. A phase I, single-center, open-label study of RM-1929 photoimmunotherapy in Japanese patients with recurrent head and neck squamous cell carcinoma. Int. J. Clin. Oncol. 2021, 26, 1812–1821. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, M.; Jewell, S.D. Evaluation of TGF-alpha and EGFR expression in oral leukoplakia and oral submucous fibrosis by quantitative immunohistochemistry. Oncology 2001, 61, 284–292. [Google Scholar] [CrossRef] [PubMed]
- Mukaida, H.; Toi, M.; Hirai, T.; Yamashita, Y.; Toge, T. Clinical significance of the expression of epidermal growth factor and its receptor in esophageal cancer. Cancer 1991, 68, 142–148. [Google Scholar] [CrossRef]
- Jones, N.R.; Rossi, M.L.; Gregoriou, M.; Hughes, J.T. Epidermal growth factor receptor expression in 72 meningiomas. Cancer 1990, 66, 152–155. [Google Scholar] [CrossRef]
- Mascia, F.; Mariani, V.; Girolomoni, G.; Pastore, S. Blockade of the EGF receptor induces a deranged chemokine expression in keratinocytes leading to enhanced skin inflammation. Am. J. Pathol. 2003, 163, 303–312. [Google Scholar] [CrossRef]
- Zumsteg, Z.S.; Luu, M.; Yoshida, E.J.; Kim, S.; Tighiouart, M.; David, J.M.; Shiao, S.L.; Mita, A.C.; Scher, K.S.; Sherman, E.J.; et al. Combined High-Intensity Local Treatment and Systemic Therapy in Metastatic Head and Neck Squamous Cell Carcinoma: An Analysis of the National Cancer Data Base. Cancer 2017, 123, 4583–4593. [Google Scholar] [CrossRef]
- Kato, T.; Okada, R.; Goto, Y.; Furusawa, A.; Inagaki, F.; Wakiyama, H.; Furumoto, H.; Daar, D.; Turkbey, B.; Choyke, P.L.; et al. Electron Donors Rather Than Reactive Oxygen Species Needed for Therapeutic Photochemical Reaction of Near-Infrared Photoimmunotherapy. ACS Pharmacol. Transl. Sci. 2021, 4, 1689–1701. [Google Scholar] [CrossRef]
- Nagaya, T.; Friedman, J.; Maruoka, Y.; Ogata, F.; Okuyama, S.; Clavijo, P.E.; Choyke, P.L.; Allen, C.; Kobayashi, H. Host Immunity Following Near-Infrared Photoimmunotherapy Is Enhanced with PD-1 Checkpoint Blockade to Eradicate Established Antigenic Tumors. Cancer Immunol. Res. 2019, 7, 401–413. [Google Scholar] [CrossRef]
- Haanen, J.; Ernstoff, M.S.; Wang, Y.; Menzies, A.M.; Puzanov, I.; Grivas, P.; Larkin, J.; Peters, S.; Thompson, J.A.; Obeid, M. Autoimmune diseases and immune-checkpoint inhibitors for cancer therapy: Review of the literature and personalized risk-based prevention strategy. Ann. Oncol. 2020, 31, 724–744. [Google Scholar] [CrossRef]
- Barnett, J.D.; Jin, J.; Penet, M.F.; Kobayashi, H.; Bhujwalla, Z.M. Phototheranostics of Splenic Myeloid-Derived Suppressor Cells and Its Impact on Spleen Metabolism in Tumor-Bearing Mice. Cancers 2022, 14, 3578. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Sato, N.; Xu, B.; Nakamura, Y.; Nagaya, T.; Choyke, P.L.; Hasegawa, Y.; Kobayashi, H. Spatially selective depletion of tumor-associated regulatory T cells with near-infrared photoimmunotherapy. Sci. Transl. Med. 2016, 8, 352ra110. [Google Scholar] [CrossRef] [PubMed]
- Omura, G.; Honma, Y.; Matsumoto, Y.; Shinozaki, T.; Itoyama, M.; Eguchi, K.; Sakai, T.; Yokoyama, K.; Watanabe, T.; Ohara, A.; et al. Transnasal photoimmunotherapy with cetuximab sarotalocan sodium: Outcomes on the local recurrence of nasopharyngeal squamous cell carcinoma. Auris Nasus Larynx 2022. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, I.; Okada, T.; Tokashiki, K.; Tsukahara, K. A Case Treated With Photoimmunotherapy Under a Navigation System for Recurrent Lesions of the Lateral Pterygoid Muscle. In Vivo 2022, 36, 1035–1040. [Google Scholar] [CrossRef]
- Koyama, S.; Ehara, H.; Donishi, R.; Morisaki, T.; Ogura, T.; Taira, K.; Fukuhara, T.; Fujiwara, K. Photoimmunotherapy with surgical navigation and computed tomography guidance for recurrent maxillary sinus carcinoma. Auris Nasus Larynx 2022. [Google Scholar] [CrossRef]
- Kraaijenga, S.A.; Oskam, I.M.; van der Molen, L.; Hamming-Vrieze, O.; Hilgers, F.J.; van den Brekel, M.W. Evaluation of long term (10-years+) dysphagia and trismus in patients treated with concurrent chemo-radiotherapy for advanced head and neck cancer. Oral Oncol. 2015, 51, 787–794. [Google Scholar] [CrossRef]
- Ojo, B.; Genden, E.M.; Teng, M.S.; Milbury, K.; Misiukiewicz, K.J.; Badr, H. A systematic review of head and neck cancer quality of life assessment instruments. Oral Oncol. 2012, 48, 923–937. [Google Scholar] [CrossRef]
- Liao, L.J.; Hsu, W.L.; Lo, W.C.; Cheng, P.W.; Shueng, P.W.; Hsieh, C.H. Health-related quality of life and utility in head and neck cancer survivors. BMC Cancer 2019, 19, 425. [Google Scholar] [CrossRef]
- Metcalfe, C.W.; Lowe, D.; Rogers, S.N. What patients consider important: Temporal variations by early and late stage oral, oropharyngeal and laryngeal subsites. J. Craniomaxillofac. Surg. 2014, 42, 641–647. [Google Scholar] [CrossRef]
- Okamoto, I.; Okada, T.; Tokashiki, K.; Tsukahara, K. Quality-of-Life Evaluation of Patients with Unresectable Locally Advanced or Locally Recurrent Head and Neck Carcinoma Treated with Head and Neck Photoimmunotherapy. Cancers 2022, 14, 4413. [Google Scholar] [CrossRef]
- Hammerlid, E.; Adnan, A.; Silander, E. Population-based reference values for the European Organization for Research and Treatment of Cancer Head and Neck module. Head Neck 2017, 39, 2036–2047. [Google Scholar] [CrossRef] [PubMed]


| Case | Gender | Age | ECOG PS | Histology | Primary site | Location of Target Lesion | Diffuser | Cycle | Complication | BOR |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 84 | 1 | SCC | Floor of mouth | Cervical skin | Cylindrical, frontal | 3 | Pain G2 Bleeding G2 Edema G1 | PR |
| 2 | M | 84 | 1 | SCC | Upper gingiva | Oropharynx | Cylindrical | 2 | Pain G1 | PR |
| 3 | M | 54 | 0 | SCC | Upper gingiva | Subcutaneous tissue of face | Cylindrical | 2 | Pain G2 Edema G1 Fistula G1 | CR |
| 4 | M | 77 | 0 | SCC | Oropharynx | Oropharynx | Cylindrical | 1 | Pain G2 Edema G1 | PR |
| 5 | M | 68 | 0 | SCC | Larynx | Glottis | Cylindrical, frontal | 3 | Edema G1 | PR |
| 6 | M | 79 | 1 | SCC | Oropharynx | Cervical skin | Cylindrical, frontal | 2 | Pain G2 | PR |
| 7 | M | 42 | 0 | SCC | Buccal mucosa | Tongue | Cylindrical | 1 | Pain G2 Edema G2 | CR |
| 8 | M | 88 | 1 | SCC | Lower gingiva | Lower gingiva | Cylindrical, frontal | 1 | Edema G4 | CR |
| 9 | F | 74 | 1 | SCC | Maxilla | Nasal cavity | Cylindrical | 3 | Pain G1 | PR |
| 10 | M | 80 | 1 | SCC | Oral cavity | Subcutaneous tissue of face | Cylindrical | 1 | Fistula G2 | PR |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nishikawa, D.; Suzuki, H.; Beppu, S.; Terada, H.; Sawabe, M.; Hanai, N. Near-Infrared Photoimmunotherapy for Oropharyngeal Cancer. Cancers 2022, 14, 5662. https://doi.org/10.3390/cancers14225662
Nishikawa D, Suzuki H, Beppu S, Terada H, Sawabe M, Hanai N. Near-Infrared Photoimmunotherapy for Oropharyngeal Cancer. Cancers. 2022; 14(22):5662. https://doi.org/10.3390/cancers14225662
Chicago/Turabian StyleNishikawa, Daisuke, Hidenori Suzuki, Shintaro Beppu, Hoshino Terada, Michi Sawabe, and Nobuhiro Hanai. 2022. "Near-Infrared Photoimmunotherapy for Oropharyngeal Cancer" Cancers 14, no. 22: 5662. https://doi.org/10.3390/cancers14225662
APA StyleNishikawa, D., Suzuki, H., Beppu, S., Terada, H., Sawabe, M., & Hanai, N. (2022). Near-Infrared Photoimmunotherapy for Oropharyngeal Cancer. Cancers, 14(22), 5662. https://doi.org/10.3390/cancers14225662





