Simple Summary
Metformin exerts anti-cancer effects but its effect on multiple myeloma requires investigation. This study used the nationwide database of Taiwan’s National Health Insurance to examine whether metformin use in patients with type 2 diabetes mellitus would have a reduced risk of multiple myeloma. Intention-to-treat analyses showed that patients who receive metformin treatment within the first 12 months of prescription of antidiabetic drugs have an approximately 30% lower risk than those who do not. In the per-protocol analyses, patients who adhere to metformin treatment will have an even lower risk reduction of approximately 65%. The findings of this study support an anti-cancer effect of metformin on multiple myeloma and provide a good reason for the recommendation of metformin as the first-line antidiabetic drug for patients with type 2 diabetes mellitus. In patients without contraindications, patients should be advised to maintain on metformin use because of its multiple pleiotropic benefits.
Abstract
Background: Whether metformin might reduce the risk of multiple myeloma (MM) has not been extensively researched in humans. Methods: The study subjects were enrolled from the reimbursement database of Taiwan’s National Health Insurance. A total of 739,553 patients who had a new diagnosis of type 2 diabetes mellitus during 1999–2009 were identified. They were categorized as metformin initiators (metformin (+)) and non-metformin initiators (metformin (−)) based on the prescriptions of antidiabetic drugs that included metformin and did not include metformin within the initial 12 months, respectively. MM incidence was calculated after the initial 12 months of treatment group assignment until 31 December 2011. Hazard ratios based on intention-to-treat (ITT) and per-protocol (PP) approaches were estimated by Cox regression weighted by propensity scores. Results: In the ITT analyses, the respective incidence rates for 497,248 metformin (+) and 242,305 metformin (−) were 9.97 and 14.33 per 100,000 person-years. The hazard ratio that compared metformin (+) to metformin (−) in the ITT analysis was 0.710 (95% confidence interval 0.593–0.850). In the PP analysis, the respective incidence rates were 5.14 and 13.98 per 100,000 person-years, and the hazard ratio was 0.355 (95% confidence interval, 0.270–0.466). The lower risk of MM among metformin (+) was supported by subgroup and sensitivity analyses. Conclusions: Type 2 diabetes patients who are initiated with metformin treatment have a significantly lower risk of MM, especially when they adhere to metformin treatment.
1. Introduction
Multiple myeloma (MM) is the second most common hematological malignancy after lymphoma [1]. It accounts for 1% of all cancers and represents approximately 10% of all hematological cancers [2]. MM is characterized by bone marrow plasmacytosis with clinical manifestations of hypercalcemia, renal failure, anemia or lytic bone lesions [2]. Although the etiology remains unknown, it is associated with some gene mutations and linked to diabetes mellitus, metabolic syndrome and obesity [2,3,4,5,6]. Ionizing radiation can also be a risk factor for MM [7]. Most patients develop MM from an asymptomatic premalignant stage called monoclonal gammopathy of undetermined significance (MGUS), which can be present in approximately 5% of the population above the age of 50 [2]. Approximately 1% of the population with MGUS progresses to MM per year [1,2]. Smoldering MM is a more advanced premalignant stage, which progresses to MM at a rate of approximately 10% per year over the first year of diagnosis [1,2]. In the USA, the median age at diagnosis of MM is 69 years, and African Americans have twice the incidence of MM compared to European Americans [1].
The incidence of MM is lower in Asian populations than in westerners [8]. In Taiwan, the average age at the diagnosis of MM is 67.6 years, and the age-adjusted incidence has increased from 1.41 per 100,000 population in 2007 to 1.59 per 100,000 population in 2012 (p = 0.01) [8]. On the other hand, the age-standardized incidence in western countries is approximately 5 per 100,000 population [1].
Diabetes mellitus and MM are closely related [4]. An early meta-analysis that included 10 observational studies suggested a non-significantly higher risk of MM while comparing diabetes patients to non-diabetic people with an estimated odds ratio of 1.22 (95% confidence interval: 0.98–1.53, p = 0.08) [9]. Another recent meta-analysis that included 13 studies estimated an odds ratio of 1.60 (1.13–2.26, p < 0.001) [10]. A recent population-based study published after the latest meta-analysis that used healthcare databases from Ontario, Canada, suggested a significant 15% higher risk of MM in diabetes patients [11]. The estimated incidence was 19.0 per 100,000 non-diabetic people and 25.7 per 100,000 diabetes patients, and the estimated hazard ratio was 1.15 (95% confidence interval: 1.09–1.20, p < 0.0001) [11].
Metformin reduces the risk of several types of cancer [12,13,14,15,16]. In our previous study, we also demonstrated a significantly lower risk of non-Hodgkin lymphoma (another blood cancer that is associated with obesity) among metformin users in patients with type 2 diabetes mellitus [17].
In recent years, although a large number of basic research has suggested a promising effect of metformin on the inhibition of the proliferation of MM cells either via 5’ adenosine monophosphate-activated protein kinase (AMPK)-dependent or AMPK-independent mechanisms [18], only a few studies have investigated such an effect in humans. In a large study that included male US military veterans, metformin use was associated with a reduced risk of progression of MGUS to MM [19]. However, this could not be supported by later nested case-control studies conducted in the UK [20,21]. To our knowledge, there has not been any previous human population-based study that investigated whether metformin could be preventive for the development of MM in patients with type 2 diabetes mellitus. In this study, from the nationwide database of Taiwan’s National Health Insurance (NHI), we enrolled patients with a new diagnosis of type 2 diabetes mellitus to compare the risk of MM between metformin initiators (metformin (+)) and non-metformin initiators (metformin (−)).
2. Materials and Methods
2.1. The Nationwide Database of NHI
Since 1 March 1995, Taiwan has started to implement a nationwide and compulsory healthcare system, the NHI. The coverage rate is very high and includes over 99% of Taiwan’s population. The Bureau of the NHI signs contracts with all hospitals and >93% of all medical settings across the country to provide medical services to the covered insurants. The NHI database contains all information on disease diagnoses, medication prescriptions and clinical procedures being submitted for reimbursement purposes. The database can be used for academic research if the proposal is reviewed and approved by an Ethics Review Board. The present study was reviewed and approved by the Ethics Review Board of the National Health Research Institutes with an approval number of 99274. The database was described in more detail previously [22].
2.2. Disease Codes
During the study period, the NHI used the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) as the coding system for disease diagnoses. The disease diagnoses and their corresponding ICD-9-CM codes used in the study are shown in Supplementary Table S1. The accuracy of disease diagnoses in the NHI database has been investigated, which showed moderate to substantial agreements between claim data and medical records, with kappa values ranging from 0.55 to 0.86 [23].
Patients were classified as metformin (+) or metformin (−) based on the prescriptions of antidiabetic drugs after diabetes diagnosis during the initial 12 months as described in our previous studies [17,24,25]. Metformin (+) referred to patients whose prescription during the initial 12 months included metformin. Metformin (−) was assigned to patients who had not been prescribed metformin during the initial 12 months.
2.3. Enrollment of Study Subjects
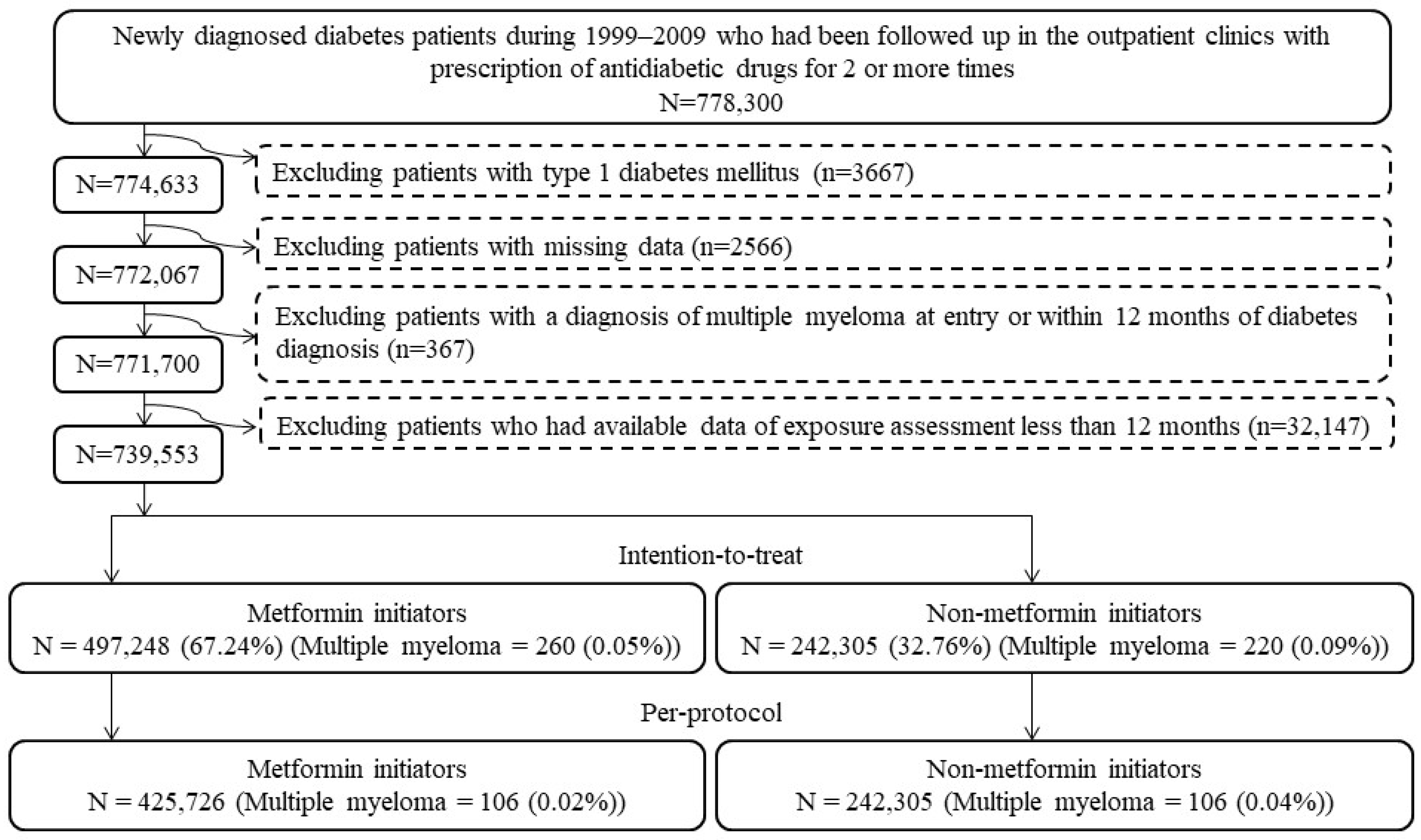
Figure 1 shows the step-by-step procedures followed in enrolling metformin (+) and metformin (−) patients from the database. A total of 778,300 patients were first identified based on the following two criteria: (1) the patients should have had a new diagnosis of diabetes mellitus from 1999 to 2009 (patients with a diagnosis of diabetes mellitus made during the period from 1995 to 1998 were not included); and (2) they should have been treated at the outpatient clinics with at least two incidences of prescriptions of antidiabetic drugs. We then excluded stepwise the following ineligible patients: (1) patients with a diagnosis of type 1 diabetes mellitus (n = 3667); (2) patients having missing data (n = 2566); (3) patients having been diagnosed with MM before follow-up or within 12 months of diabetes diagnosis (n = 367); and (4) patients who had available data of exposure assessment of less than 12 months (n = 32,147). As a result, 497,248 metformin (+) and 242,305 metformin (−) subjects were used for the intention-to-treat (ITT) analyses, and 425,726 metformin (+) and 242,305 metformin (−) subjects who adhered to the initial assignments were used in the per-protocol (PP) analyses.
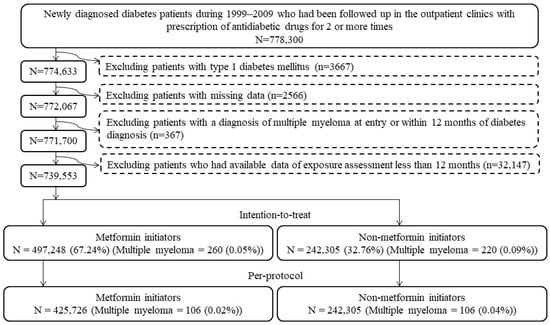
Figure 1.
Flowchart presenting the steps followed to enroll metformin initiators and non-metformin initiators for the intention-to-treat and per-protocol analyses in the study.
2.4. Potential Confounders
Potential confounders are shown in Table 1. The “time without antidiabetic drugs after diabetes diagnosis” was defined as the time when the patients were not treated with any antidiabetic drugs after a diabetes diagnosis. Occupation was classified as class I, II, III and IV [17]. Class I included civil servants, teachers, employees of governmental or private businesses, professionals and technicians. Class II included people without a specific employer, self-employed people and seamen. Class III referred to farmers and fishermen. Class IV included low-income families supported by social welfare and veterans. Use of immunosuppressants was defined as continuous use of ≥90 days of corticosteroids, calcineurin inhibitors and/or inosine-5′-monophosphate dehydrogenase inhibitors.

Table 1.
Characteristics of metformin initiators (metformin (+)) and non-metformin initiators (metformin (−)).
Helicobacter pylori (HP) infection was defined previously by one or two of the following criteria [26]: (1) patients who had received an HP eradication therapy; and (2) patients who had been diagnosed with HP infection.
2.5. Statistical Analyses
We used SAS statistical software version 9.4 (SAS Institute, Cary, NC, USA) for statistical analyses and considered p < 0.05 as statistically significant.
As a test of balance diagnostics, we calculated the standardized difference for each covariate. A value of standardized difference >10% was viewed as an indicator of potential confounding.
In the ITT analyses, we started to follow the patients after the initial 12-month period used for exposure assessment and ended follow-up at a time until 31 December 2011 when any of the following three events occurred, whichever first, with no exclusion according to switching to or adding other antidiabetic drugs thereafter [17]: the last reimbursement record, MM diagnosis or death. The numerator of incidence was the case number of newly diagnosed MM during the follow-up, and the denominator was the person-years of follow-up.
In the PP analyses, we first excluded patients who did not adhere to the assigned treatment within the initial 12-month period of exposure assessment and then followed the rest for the incidence of MM. We started follow-up after the 12-month period as we have previously done in the ITT analyses. Besides the three events (the last reimbursement record, MM diagnosis or death) to end follow-up at a time until 31 December 2011, follow-up also ended when nonadherence to the assigned treatment occurred, which was defined by the time of addition of metformin in the metformin (−) group, and by the time of addition of non-metformin antidiabetic drugs in the metformin (+) group [17].
We used logistic regression to create propensity scores (PS) from independent variables that included all variables listed in Table 1 plus the date of the start of follow-up. The inclusion of the starting date of follow-up was expected to partly account for some unknown risk factors that might have occurred during the long inclusion period, such as changes in treatment guidelines or the introduction of novel therapeutic drugs. We then estimated hazard ratios and their 95% confidence intervals that compared metformin (+) to metformin (−) by Cox regression constructed with the inverse probability of treatment-weighting using PS. This method for the estimation of PS-weighted hazard ratios is recommended by Austin to reduce the potential confounding by indication because of the differences in baseline characteristics [27].
Age was categorized into two subgroups of <60 and ≥60 years, and subgroup analyses were conducted for each subgroup of age and sex.
To examine the consistency of the findings, we conducted four sensitivity analyses: (1) patients receiving two consecutive prescriptions of metformin spanning a period of more than 6 months were excluded; (2) patients having been treated with incretin-based therapies during follow-up were excluded (the NHI did not reimburse incretin-based therapies until after 1 March 2009); (3) patients having been treated with thiazolidinediones were excluded because thiazolidinediones may cause bone loss and fractures [28] leading to a differential detection rate of MM; and (4) patients with a diagnosis of any cancer other than MM during follow-up were excluded.
3. Results
Table 1 shows the baseline characteristics of the study subjects. Metformin (−) and metformin (+) varied in six characteristics with values of standardized difference >10%: time without antidiabetic drugs after diabetes diagnosis, occupation, dyslipidemia, obesity, eye disease and statins.
In the ITT analyses, for metformin (−) and metformin (+) subjects, respectively, the median follow-up time was 6.35 years and 4.81 years. The respective follow-up times in the PP analyses were 2.34 and 4.35 years. The incidence of MM and the hazard ratios comparing metformin (+) to metformin (−) are shown in Table 2. Both the ITT and PP analyses favored a lower risk of MM in the metformin (+) group. The hazard ratio (95% confidence intervals) was 0.710 (0.593–0.850) in the ITT analysis and was 0.355 (0.270–0.466) in the PP analysis.

Table 2.
Incidence of multiple myeloma and hazard ratios comparing metformin initiators (metformin (+)) to non-metformin initiators (metformin (−)) in the intention-to-treat and per-protocol analyses.
Table 3 shows the results of the subgroup analyses. It was noted that the lower risk associated with metformin use could be observed in both sexes in both the ITT and the PP analyses. In the analyses with regards to age subgroups, the significantly lower risk associated with metformin use could be seen in the PP analyses with either younger age (<60 years) or older age (≥60 years). However, the lower risk associated with metformin was borderline significant in the ITT analyses in both age subgroups.

Table 3.
Subgroup analyses by age and sex.
As shown in Table 4, all sensitivity analyses supported a lower risk of MM among metformin (+) in either the ITT analyses or the PP analyses.

Table 4.
Sensitivity analyses.
4. Discussion
4.1. Main Findings
This population-based observational study first investigated the risk of MM with regard to metformin exposure in an Asian population with type 2 diabetes mellitus. A significant risk reduction of 30% in metformin (+) subjects in the ITT analysis and a risk reduction of 65% in the PP analysis (Table 2) were noted. The risk reduction among metformin (+) was supported by subgroup analyses (Table 3) and sensitivity analyses (Table 4). The risk reduction in metformin (+) was more remarkable in the PP analyses than in the ITT analyses in all analyses (Table 2, Table 3 and Table 4).
4.2. Findings in Earlier Studies
The findings of the present study supported a preventive role of metformin in the development of MM, as observed in a previous study conducted on male US military veterans that showed a reduced risk of progression of premyeloma stage to MM [19]. However, the generalizability of the USA study was limited because it was not a population-based study, involved mainly male patients with diabetes (98%), and metformin use was defined as a use of 4 years or longer [19]. The investigators estimated an adjusted hazard ratio of 0.47 (95% confidence interval: 0.25–0.87) [19], which was close to the PS-weighted hazard ratio of 0.425 (95% confidence interval: 0.294–0.616) in the PP analysis for the subgroup of males (Table 3) in our study.
There are two nested case-control studies that were conducted in the UK [20,21]. One showed a null association between metformin use and the incidence of MGUS [20]. In this study, the investigators used a nested case-control study design and selected 4 controls matched on age, sex, practice site and duration of follow-up for each case of incident MGUS [20]. They estimated odds ratios rather than hazard ratios, and the duration of exposure to metformin was not mentioned. Therefore, whether the time of exposure was sufficient for an effect to occur was not known. Though not significant, a 23% lower risk of MGUS (adjusted odds ratio: 0.77, 95% confidence interval: 0.56–1.05) was associated with metformin use.
The second study conducted by the same UK group looked at the progression of MGUS to MM by using a matched case-control study nested within a population-based database of The Health Improvement Network [21]. Among the diabetes patients, there were 11 cases and 127 controls, and the adjusted odds ratio was 1.01 (0.18–5.65) for metformin exposure <24 months and 0.40 (0.08–2.04) for those with metformin exposure >24 months [21]. Though not significant, probably because of the small numbers of cases and controls, an approximately 60% lower risk of progression was observed among patients who had been exposed to metformin for >24 months.
4.3. Mechanisms
Although the mechanisms of this clinical benefit of metformin remain to be explored, findings from basic research provide reasonable explanations for the mode of action either through an AMPK-dependent or an AMPK-independent pathway [18]. These may include: (1) the induction of cell cycle arrest and autophagy in MM cells [29]; (2) the inhibition of the HIF-1 pathway of MM leading to growth arrest without inducing apoptosis [30]; (3) the inhibition of MM serum-induced endothelial cell thrombosis by downregulating miR-532 [31]; (4) the inhibition of IL-6 signaling by decreasing IL-6R expression on MM cells [32]; (5) acting as an oxidative phosphorylation inhibitor [33]; (6) the induction of necrosis and apoptosis in MM cells [34]; (7) suppressing glucose-regulated protein 78, an endoplasmic reticulum chaperone with anti-apoptotic properties [35]; and (8) lowering intracellular pH and enhanced cytotoxicity [36].
Additionally, metformin may target obesity (a major risk factor for MM [6]) and the metabolic pathways of MM cells [37,38]. Research has also suggested that metformin may act synergistically with other chemotherapeutic agents to inhibit the growth of MM [38,39,40,41,42].
However, an in vitro and in vivo study showed that metformin might exert an indirect pro-tumorigenic effect on MM by increasing OPN expression in preosteoblasts and thus increasing myeloma cell adherence [43]. Metformin treatment may also induce resistance to the proteasome inhibitor bortezomib in cancer cells [44]. Therefore, the beneficial effect of metformin on MM requires more extensive research.
4.4. Implications
This study has some clinical implications. First, the protective effect of metformin against MM, as shown in the present study, together with the known extra bonuses beyond its glucose-lowering effect, such as its anti-cancer, anti-inflammatory, anti-microbial and anti-aging effects [45,46,47,48,49,50,51,52,53,54,55], provide a good reason to recommend metformin as the first-line drug to be used to treat patients with type 2 diabetes mellitus.
Second, the finding of a more remarkable risk reduction in the PP analyses than in the ITT analyses (Table 2, Table 3 and Table 4) implied that adherence to metformin treatment may provide more remarkable protection against MM.
Third, metformin is an inexpensive drug, safe and without the risk of hypoglycemia when used as a monotherapy. Therefore, repurposing metformin as a preventive agent or an adjuvant therapeutic agent for MM is worthy of more in-depth investigation.
Fourth, two-thirds of patients with MM may have cardiac events [56], and metformin may exert a prophylactic effect on cardiotoxicity induced by carfilzomib [57] and may have a positive impact on the life expectancy of patients with MM and heart failure [58]. In our previous studies, we also demonstrated a reduced risk of hypertension [59], atrial fibrillation [60] and heart failure [61] among metformin users. Therefore, metformin may exert a protective effect on cardiovascular diseases in the absence or presence of MM.
4.5. Strengths
There are some merits to this study. First, we can be more confident in generalizing the findings because of the use of a nationwide database that covers >99% of the population.
Second, the risk of self-reporting bias could be avoided because of the use of existing medical records.
Third, different socioeconomic statuses may lead to a serious problem of detection bias in other countries. However, this would not be the case in our healthcare system. Cancer is considered a catastrophic illness, and most medical copayments can be waived for patients with a certified diagnosis of cancer. Additionally, many medical expenses can be waived for veterans and patients with low incomes or receiving drug refills for chronic disease.
4.6. Limitations
There are some limitations. First, we did not have information on radiation exposure for adjustment. We tried to balance radiation by using ocular pterygium as a surrogate diagnosis for exposure to UV sunlight (Table 1). Because the standardized difference of ocular pterygium was <10%, potential confounding from radiation might be minimal.
Second, obesity is a well-recognized risk factor for MM [6], but we did not have anthropometric data of body height and body weight in the database for analyses. Although we used a diagnosis of obesity rather than an actual measurement of body height and body weight in the analyses, the prevalence rates of obesity in metformin (−) and metformin (+) subjects were 1.88% and 4.38%, respectively (Table 1). In our earlier epidemiologic survey, the prevalence rates of obesity in diabetes patients defined by a body mass index of ≥25 and ≥30 kg/m2 were 33.5% and 7.1%, respectively [62]. Therefore, a diagnosis of obesity might only have been labelled in patients with severe obesity, and the use of an ICD-9-CM diagnosis of obesity might have underestimated the true prevalence rates of obesity. It is worth pointing out that metformin is always recommended for obese patients, and this was truly reflected by the higher prevalence of such a diagnosis among metformin initiators (Table 1). The higher prevalence of obesity among metformin (+) subjects would only have underestimated the true beneficial effect of metformin on MM.
Third, statins [63,64] and aspirin [65,66] exhibit anti-cancer activity in MM cells. Although the distribution of aspirin between metformin (+) and metformin (−) was balanced, more patients were using statins in the metformin (+) group (33.11% versus 25.92%, Table 1). This imbalance in the use of statins might have exerted a residual confounding even though we had weighted the hazard ratios by PS. To further confirm that the lower risk of MM among the metformin (+) group would not be impacted by the use of statins, we additionally conducted a sensitivity analysis after excluding those who had used any statin. The respective hazard ratios comparing the metformin (+) to the metformin (−) groups were 0.653 (95% confidence interval: 0.532–0.800) in the ITT analysis and 0.350 (95% confidence interval: 0.257–0.478) in the PP analysis. The consistency of the results supported the robustness of the findings.
Fourth, MM is an insidious disease with asymptomatic premalignant stages [1,2]. If the delayed diagnosis of MM differed significantly between the metformin (+) and metformin (−) groups, this might have caused a biased estimate. Therefore, more future studies are required to investigate the possible roles of other confounders.
Fifth, because MM related to genetic mutations are not studied, their potential confounding could not be excluded. However, if the MM-related genetic mutations did not distribute differentially between the metformin (−) and the metformin (+) groups, it was expected that the estimated hazard ratios would only bias toward the null.
Sixth, we did not have the pathology of bone marrow biopsies and/or aspiration for confirmation of MM diagnosis and for additional analyses.
4.7. Conclusions
Patients with type 2 diabetes mellitus who have been initiated with metformin therapy have a significantly lower risk of MM, especially when they adhere to the treatment.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cancers14225637/s1, Table S1: The disease diagnoses and their corresponding codes of the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) used in the study.
Funding
The author has received financial support from the Ministry of Science and Technology (MOST 107-2221-E-002-129-MY3) of Taiwan for the implementation of the study and conduction of the analyses. The funder did not have a role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board of the National Health Research Institutes (approval number of 99274).
Informed Consent Statement
Patient consent was waived because personal information has been de-identified in the released database and there was no way to contact the patient.
Data Availability Statement
Public availability of the dataset is restricted by local regulations to protect privacy.
Acknowledgments
The author wishes to thank Ting-Ting Chan for her exceptional help in conducting all the statistical analyses.
Conflicts of Interest
The author declares no conflict of interest.
References
- Kazandjian, D. Multiple myeloma epidemiology and survival: A unique malignancy. Semin. Oncol. 2016, 43, 676–681. [Google Scholar] [CrossRef]
- Rajkumar, S.V. Multiple myeloma: 2022 update on diagnosis, risk stratification, and management. Am. J. Hematol. 2022, 97, 1086–1107. [Google Scholar] [CrossRef] [PubMed]
- Cowan, A.J.; Green, D.J.; Kwok, M.; Lee, S.; Coffey, D.G.; Holmberg, L.A.; Tuazon, S.; Gopal, A.K.; Libby, E.N. Diagnosis and management of multiple myeloma: A review. JAMA 2022, 327, 464–477. [Google Scholar] [CrossRef] [PubMed]
- Tentolouris, A.; Ntanasis-Stathopoulos, I.; Eleftheriadou, I.; Malandrakis, P.; Tzeravini, E.; Gavriatopoulou, M. Diabetes mellitus and multiple myeloma; common features of two distinct entities. Diabetes Metab. Res. Rev. 2022, 38, e3535. [Google Scholar] [CrossRef] [PubMed]
- Ragbourne, S.C.; Maghsoodi, N.; Streetly, M.; Crook, M.A. The association between metabolic syndrome and multiple myeloma. Acta Haematol. 2021, 144, 24–33. [Google Scholar] [CrossRef] [PubMed]
- da Cunha Júnior, A.D.; Zanette, D.L.; Pericole, F.V.; Olalla Saad, S.T.; Barreto Campello Carvalheira, J. Obesity as a possible risk factor for progression from monoclonal gammopathy of undetermined significance progression into multiple myeloma: Could myeloma be prevented with metformin treatment? Adv. Hematol. 2021, 2021, 6615684. [Google Scholar] [CrossRef] [PubMed]
- Harbron, R.W.; Pasqual, E. Ionising radiation as a risk factor for lymphoma: A review. J. Radiol. Prot. 2020, 40, R151–R185. [Google Scholar] [CrossRef]
- Tang, C.H.; Liu, H.Y.; Hou, H.A.; Qiu, H.; Huang, K.C.; Siggins, S.; Rothwell, L.A.; Liu, Y. Epidemiology of multiple myeloma in Taiwan, a population based study. Cancer Epidemiol. 2018, 55, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Castillo, J.J.; Mull, N.; Reagan, J.L.; Nemr, S.; Mitri, J. Increased incidence of non-Hodgkin lymphoma, leukemia, and myeloma in patients with diabetes mellitus type 2: A meta-analysis of observational studies. Blood 2012, 119, 4845–4850. [Google Scholar] [CrossRef]
- Zhang, C.; Sha, Y.; Liu, H.; Guo, D.; Jiang, Y.; Hong, L.; Shi, L.; Huang, H. Type 2 diabetes mellitus does not increase the risk of multiple myeloma: A systematic review and meta-analysis. Transl. Cancer Res. 2020, 9, 2884–2894. [Google Scholar] [CrossRef]
- Gong, I.Y.; Cheung, M.C.; Read, S.; Na, Y.; Lega, I.C.; Lipscombe, L.L. Association between diabetes and haematological malignancies: A population-based study. Diabetologia 2021, 64, 540–551. [Google Scholar] [CrossRef]
- Tseng, C.H. Metformin significantly reduces incident prostate cancer risk in Taiwanese men with type 2 diabetes mellitus. Eur. J. Cancer 2014, 50, 2831–2837. [Google Scholar] [CrossRef] [PubMed]
- Tseng, C.H. Metformin and risk of hepatocellular carcinoma in patients with type 2 diabetes. Liver Int. 2018, 38, 2018–2027. [Google Scholar] [CrossRef] [PubMed]
- Tseng, C.H. Metformin and endometrial cancer risk in Chinese women with type 2 diabetes mellitus in Taiwan. Gynecol. Oncol. 2015, 138, 147–153. [Google Scholar] [CrossRef]
- Tseng, C.H. Metformin reduces thyroid cancer risk in Taiwanese patients with type 2 diabetes. PLoS ONE 2014, 9, e109852. [Google Scholar] [CrossRef] [PubMed]
- Tseng, C.H. Metformin and biliary tract cancer in patients with type 2 diabetes. Front. Oncol. 2020, 10, 587666. [Google Scholar] [CrossRef] [PubMed]
- Tseng, C.H. Metformin is associated with a lower risk of non-Hodgkin lymphoma in patients with type 2 diabetes. Diabetes Metab. 2019, 45, 458–464. [Google Scholar] [CrossRef]
- Podhorecka, M. Metformin—Its anti-cancer effects in hematologic malignancies. Oncol. Rev. 2021, 15, 514. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.H.; Luo, S.; O’Brian, K.K.; Thomas, T.S.; Colditz, G.A.; Carlsson, N.P.; Carson, K.R. Association between metformin use and progression of monoclonal gammopathy of undetermined significance to multiple myeloma in US veterans with diabetes mellitus: A population-based retrospective cohort study. Lancet Haematol. 2015, 2, e30–e36. [Google Scholar] [CrossRef]
- Boursi, B.; Weiss, B.M.; Haynes, K.; Mamtani, R.; Yang, Y.X. Reappraisal of risk factors for monoclonal gammopathy of undetermined significance. Am. J. Hematol. 2016, 91, 581–584. [Google Scholar] [CrossRef] [PubMed]
- Boursi, B.; Mamtani, R.; Yang, Y.X.; Weiss, B.M. Impact of metformin on the progression of MGUS to multiple myeloma. Leuk. Lymphoma 2017, 58, 1265–1267. [Google Scholar] [CrossRef] [PubMed]
- Tseng, C.H. Metformin is associated with a lower risk of colorectal cancer in Taiwanese patients with type 2 diabetes: A retrospective cohort analysis. Diabetes Metab. 2017, 43, 438–445. [Google Scholar] [CrossRef] [PubMed]
- Chang, L. A Study of Validation on Comorbidity Derived from Claims Data. Master’s Thesis, National Yang-Ming University, Taipei, Taiwan, 2004. Available online: http://etd.lib.nctu.edu.tw/cgi-bin/gs32/ymgsweb.cgi/ccd=ji3XTg/search#result (accessed on 22 February 2020).
- Tseng, C.H. Metformin use and leukemia risk in patients with type 2 diabetes mellitus. Front. Endocrinol. (Lausanne) 2020, 11, 541090. [Google Scholar] [CrossRef] [PubMed]
- Tseng, C.H. Metformin and primary bone cancer risk in Taiwanese patients with type 2 diabetes mellitus. Bone 2021, 151, 116037. [Google Scholar] [CrossRef]
- Tseng, C.H. Diabetes, insulin use and Helicobacter pylori eradication: A retrospective cohort study. BMC Gastroenterol. 2012, 12, 46. [Google Scholar] [CrossRef] [PubMed]
- Austin, P.C. The performance of different propensity score methods for estimating marginal hazard ratios. Stat. Med. 2013, 32, 2837–2849. [Google Scholar] [CrossRef]
- Nien, F.J.; Tseng, C.H. A review on the clinical safety of thiazolidinediones. Formos. J. Endocrinol. Metab. 2014, 5 (Suppl. 1), 2–14. [Google Scholar]
- Wang, Y.; Xu, W.; Yan, Z.; Zhao, W.; Mi, J.; Li, J.; Yan, H. Metformin induces autophagy and G0/G1 phase cell cycle arrest in myeloma by targeting the AMPK/mTORC1 and mTORC2 pathways. J. Exp. Clin. Cancer Res. 2018, 37, 63. [Google Scholar] [CrossRef] [PubMed]
- Kocemba-Pilarczyk, K.A.; Trojan, S.; Ostrowska, B.; Lasota, M.; Dudzik, P.; Kusior, D.; Kot, M. Influence of metformin on HIF-1 pathway in multiple myeloma. Pharm. Rep. 2020, 72, 1407–1417. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Li, L.; Hu, J.; Li, G.; Zhang, Y.; Dai, X.; De, Z.; Xu, F. Metformin inhibits multiple myeloma serum-induced endothelial cell thrombosis by down-regulating miR-532. Ann. Vasc. Surg. 2022, 85, 347–357.e2. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.K.; Dingli, D. Metformin inhibits IL-6 signaling by decreasing IL-6R expression on multiple myeloma cells. Leukemia 2019, 33, 2695–2709. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Zhou, T.; Doh, H.M.; Trinh, K.R.; Catapang, A.; Lee, J.T.; Braas, D.; Bayley, N.A.; Yamada, R.E.; Vasuthasawat, A.; et al. An HK2 antisense oligonucleotide induces synthetic lethality in HK1-HK2+ multiple myeloma. Cancer Res. 2019, 79, 2748–2760. [Google Scholar] [CrossRef]
- Wu, Z.; Wu, L.; Zou, L.; Wang, M.; Liu, X. Metformin induces myeloma cells necrosis and apoptosis and it is considered for therapeutic use. J. Chemother. 2022, 15, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Abdel Malek, M.A.; Jagannathan, S.; Malek, E.; Sayed, D.M.; Elgammal, S.A.; Abd El-Azeem, H.G.; Thabet, N.M.; Driscoll, J.J. Molecular chaperone GRP78 enhances aggresome delivery to autophagosomes to promote drug resistance in multiple myeloma. Oncotarget 2015, 6, 3098–3110. [Google Scholar] [CrossRef] [PubMed]
- Hanson, D.J.; Nakamura, S.; Amachi, R.; Hiasa, M.; Oda, A.; Tsuji, D.; Itoh, K.; Harada, T.; Horikawa, K.; Teramachi, J.; et al. Effective impairment of myeloma cells and their progenitors by blockade of monocarboxylate transportation. Oncotarget 2015, 6, 33568–33586. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Chen, S.; Xiang, W.; Xiao, M.; Xiao, H. The mechanism of treatment of multiple myeloma with metformin by way of metabolism. Arch. Med. Sci. 2020, 17, 1056–1063. [Google Scholar] [CrossRef] [PubMed]
- Dalva-Aydemir, S.; Bajpai, R.; Martinez, M.; Adekola, K.U.; Kandela, I.; Wei, C.; Singhal, S.; Koblinski, J.E.; Raje, N.S.; Rosen, S.T.; et al. Targeting the metabolic plasticity of multiple myeloma with FDA-approved ritonavir and metformin. Clin. Cancer Res. 2015, 21, 1161–1171. [Google Scholar] [CrossRef]
- Nathwani, N.; Palmer, J.; Synold, T.W.; Salehian, B.; Rosenzweig, M.; Sanchez, J.F.; Hammond, S.N.; Adekola, K.; Tomarchio, V.; Chowdhury, A.; et al. Toxicities associated with metformin/ritonavir combination treatment in relapsed/refractory multiple myeloma. Clin. Lymphoma Myeloma Leuk. 2020, 20, e667–e672. [Google Scholar] [CrossRef] [PubMed]
- Zi, F.M.; He, J.S.; Li, Y.; Wu, C.; Yang, L.; Yang, Y.; Wang, L.J.; He, D.H.; Zhao, Y.; Wu, W.J.; et al. Metformin displays anti-myeloma activity and synergistic effect with dexamethasone in in vitro and in vivo xenograft models. Cancer Lett. 2015, 356, 443–453. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhao, Y.; Zhang, E.; Yan, H.; Lv, N.; Cai, Z. The synergistic effect of PFK15 with metformin exerts anti-myeloma activity via PFKFB3. Biochem. Biophys. Res. Commun. 2019, 515, 332–338. [Google Scholar] [CrossRef] [PubMed]
- Mishra, R.K.; Wei, C.; Hresko, R.C.; Bajpai, R.; Heitmeier, M.; Matulis, S.M.; Nooka, A.K.; Rosen, S.T.; Hruz, P.W.; Schiltz, G.E.; et al. In silico modeling-based identification of glucose transporter 4 (GLUT4)-selective inhibitors for cancer therapy. J. Biol. Chem. 2015, 290, 14441–14453. [Google Scholar] [CrossRef] [PubMed]
- Gámez, B.; Morris, E.V.; Olechnowicz, S.W.Z.; Webb, S.; Edwards, J.R.; Sowman, A.; Turner, C.J.; Edwards, C.M. The antidiabetic drug metformin acts on the bone microenvironment to promote myeloma cell adhesion to preosteoblasts and increase myeloma tumour burden in vivo. Transl. Oncol. 2022, 15, 101301. [Google Scholar] [CrossRef] [PubMed]
- Schlesser, C.; Meul, T.; Stathopoulos, G.; Meiners, S. Metformin induces resistance of cancer cells to the proteasome inhibitor bortezomib. Biomolecules 2022, 12, 756. [Google Scholar] [CrossRef] [PubMed]
- Maniar, K.; Moideen, A.; Mittal, A.; Patil, A.; Chakrabarti, A.; Banerjee, D. A story of metformin-butyrate synergism to control various pathological conditions as a consequence of gut microbiome modification: Genesis of a wonder drug? Pharmacol. Res. 2017, 117, 103–128. [Google Scholar] [CrossRef]
- Tseng, C.H. The relationship between diabetes mellitus and gastric cancer and the potential benefits of metformin: An extensive review of the literature. Biomolecules 2021, 11, 1022. [Google Scholar] [CrossRef]
- Tseng, C.H. Metformin and risk of malignant brain tumors in patients with type 2 diabetes mellitus. Biomolecules 2021, 11, 1226. [Google Scholar] [CrossRef]
- Tseng, C.H. Metformin and risk of gingival/periodontal diseases in diabetes patients: A retrospective cohort study. Front. Endocrinol. 2022, 13, 1036885. [Google Scholar] [CrossRef]
- Tseng, C.H. Metformin use is associated with a lower risk of inflammatory bowel disease in patients with type 2 diabetes mellitus. J. Crohns Colitis 2021, 15, 64–73. [Google Scholar] [CrossRef]
- Tseng, C.H. Metformin use is associated with a reduced risk of acute appendicitis in Taiwanese patients with type 2 diabetes mellitus. Sci. Rep. 2021, 11, 12400. [Google Scholar] [CrossRef]
- Tseng, C.H. Metformin reduces the risk of diverticula of intestine in Taiwanese patients with type 2 diabetes mellitus. Front. Pharmacol. 2021, 12, 739141. [Google Scholar] [CrossRef]
- Tseng, C.H. Metformin decreases risk of tuberculosis infection in type 2 diabetes patients. J. Clin. Med. 2018, 7, 264. [Google Scholar] [CrossRef] [PubMed]
- Tseng, C.H. Metformin and Helicobacter pylori infection in patients with type 2 diabetes. Diabetes Care 2018, 41, e42–e43. [Google Scholar] [CrossRef] [PubMed]
- Tseng, C.H. The effect of metformin on male reproductive function and prostate: An updated review. World J. Mens Health 2022, 40, 11–29. [Google Scholar] [CrossRef]
- Tseng, C.H. Metformin’s effects on varicocele, erectile dysfunction, infertility and prostate-related diseases: A retrospective cohort study. Front. Pharmacol. 2022, 13, 799290. [Google Scholar] [CrossRef] [PubMed]
- Kistler, K.D.; Rajangam, K.; Faich, G.; Lanes, S. Cardiac event rates in patients with newly diagnosed and relapsed multiple myeloma in US clinical practice. Blood 2012, 120, 2916. [Google Scholar] [CrossRef]
- Efentakis, P.; Psarakou, G.; Varela, A.; Papanagnou, E.D.; Chatzistefanou, M.; Nikolaou, P.E.; Davos, C.H.; Gavriatopoulou, M.; Trougakos, I.P.; Dimopoulos, M.A.; et al. Elucidating carfilzomib’s induced cardiotoxicity in an in vivo model of aging: Prophylactic potential of metformin. Int. J. Mol. Sci. 2021, 22, 10956. [Google Scholar] [CrossRef] [PubMed]
- Proskuriakova, E.; Jada, K.; Kakieu Djossi, S.; Khedr, A.; Neupane, B.; Mostafa, J.A. Mechanisms and potential treatment options of heart failure in patients with multiple myeloma. Cureus 2021, 13, e15943. [Google Scholar] [CrossRef] [PubMed]
- Tseng, C.H. Metformin and risk of hypertension in Taiwanese patients with type 2 diabetes mellitus. J. Am. Heart Assoc. 2018, 7, e008860. [Google Scholar] [CrossRef]
- Tseng, C.H. Metformin use is associated with a lower incidence of hospitalization for atrial fibrillation in patients with type 2 diabetes mellitus. Front. Med. 2021, 7, 592901. [Google Scholar] [CrossRef]
- Tseng, C.H. Metformin use is associated with a lower risk of hospitalization for heart failure in patients with type 2 diabetes mellitus: A retrospective cohort analysis. J. Am. Heart Assoc. 2019, 8, e011640. [Google Scholar] [CrossRef]
- Tseng, C.H. Body mass index and blood pressure in adult type 2 diabetic patients in Taiwan. Circ. J. 2007, 71, 1749–1754. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Liu, B. Statin use and the risk of multiple myeloma: A PRISMA-compliant meta-analysis. Ann. Hematol. 2020, 99, 1805–1812. [Google Scholar] [CrossRef] [PubMed]
- Grabarek, B.O.; Boroń, D.; Morawiec, E.; Michalski, P.; Palazzo-Michalska, V.; Pach, Ł.; Dziuk, B.; Świder, M.; Zmarzły, N. Crosstalk between statins and cancer prevention and therapy: An update. Pharmaceuticals 2021, 14, 1220. [Google Scholar] [CrossRef] [PubMed]
- Marinac, C.R.; Lee, D.H.; Colditz, G.A.; Rebbeck, T.R.; Rosner, B.; Bustoros, M.; Ghobrial, I.M.; Birmann, B.M. Regular aspirin use and mortality in patients with multiple myeloma. Cancer Epidemiol. Biomark. Prev. 2022, 31, 479–485. [Google Scholar] [CrossRef]
- Liu, H.; Xiong, C.; Liu, J.; Sun, T.; Ren, Z.; Li, Y.; Geng, J.; Li, X. Aspirin exerts anti-tumor effect through inhibiting Blimp1 and activating ATF4/CHOP pathway in multiple myeloma. Biomed. Pharmacother. 2020, 125, 110005. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).