Systemic Therapy for Patients with HER2-Positive Breast Cancer and Brain Metastases: A Systematic Review and Meta-Analysis
Abstract
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Search Strategy and Selection Criteria
2.2. Data Analysis
3. Results

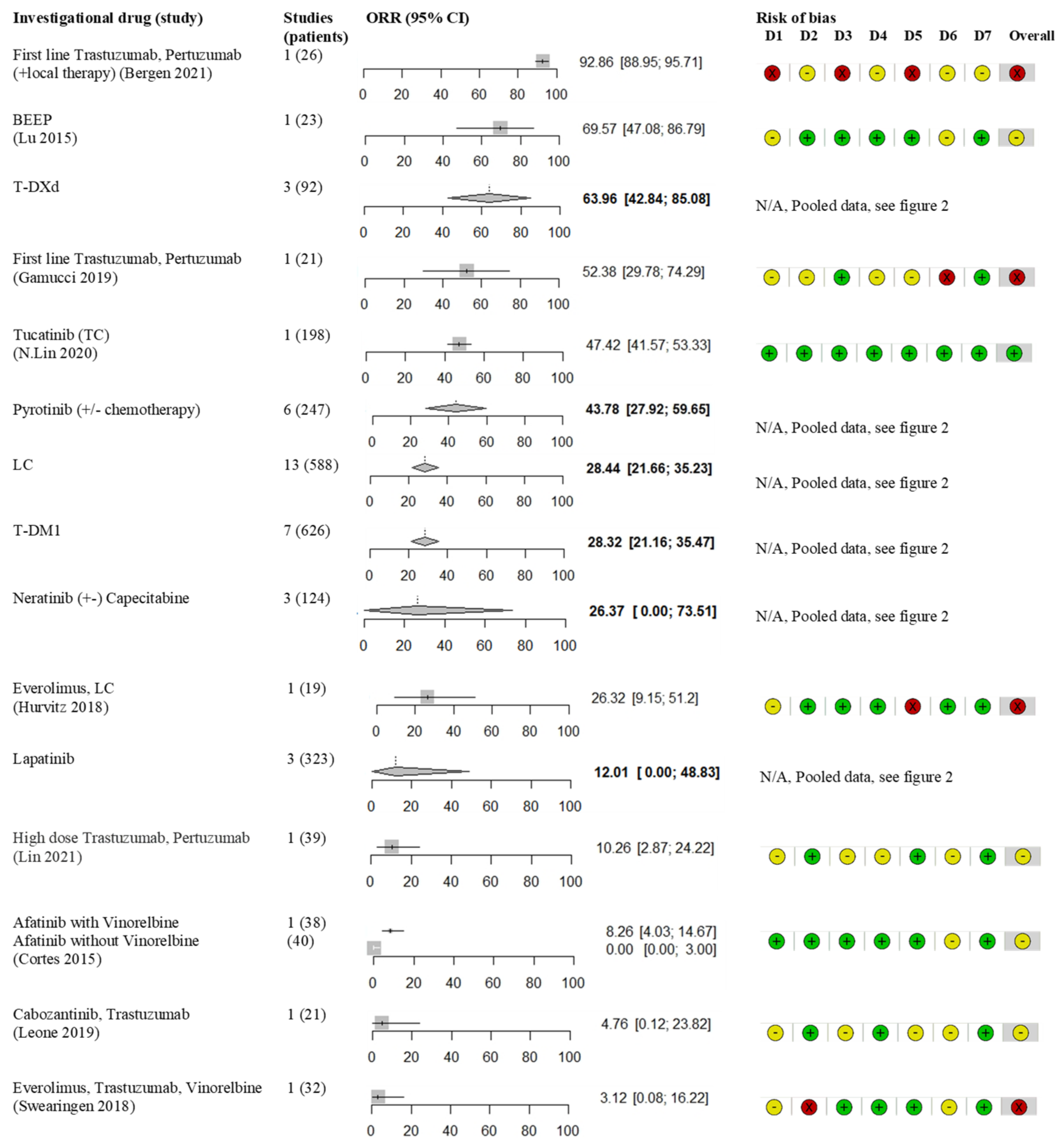
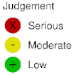
3.1. Monoclonal Antibodies
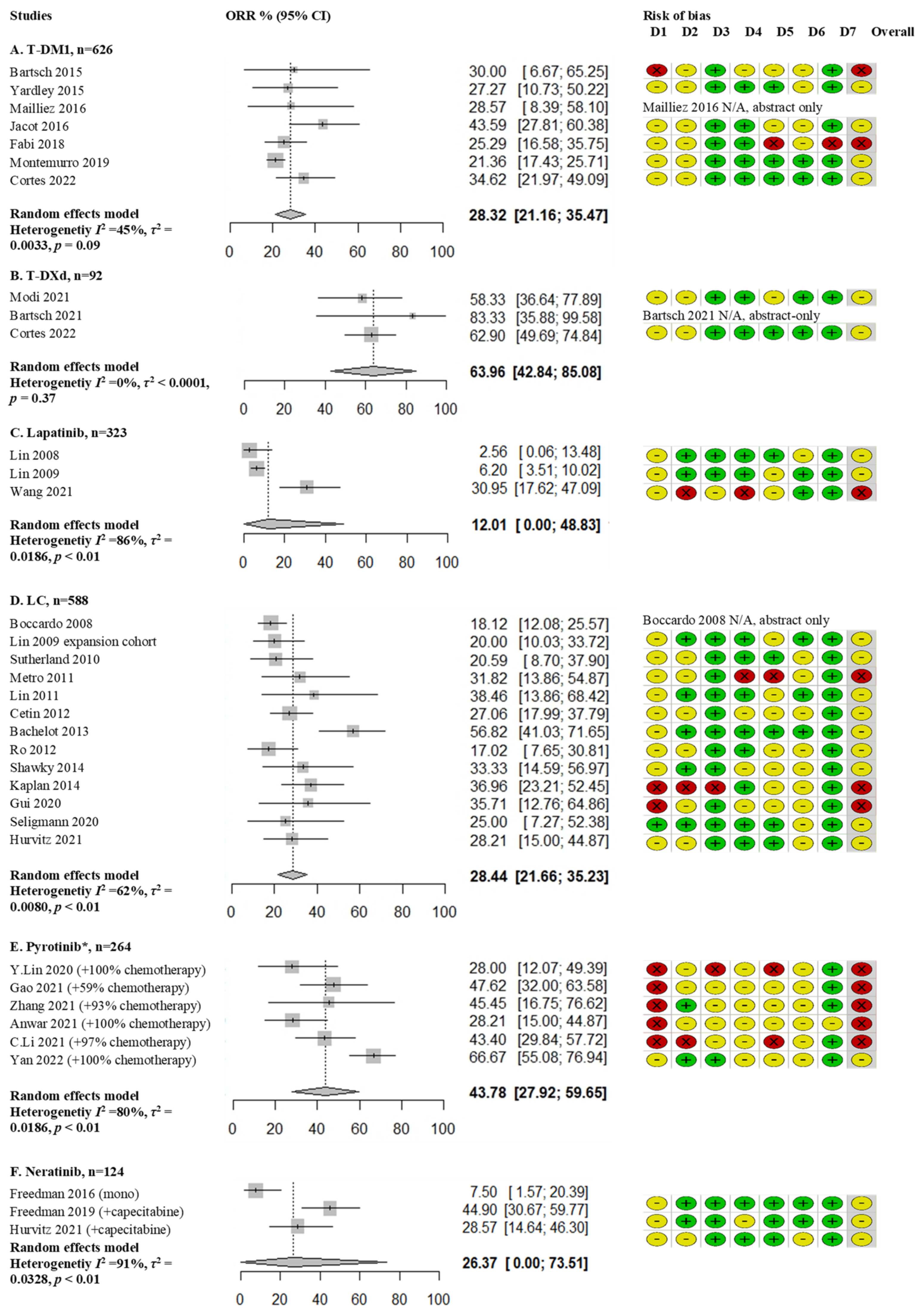
3.2. Antibody Drug Conjugates
3.3. Tyrosine Kinase Inhibitors
3.4. Other Treatments
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Grinda, T.; Antoine, A.; Jacot, W.; Blaye, C.; Cottu, P.-H.; Diéras, V.; Dalenc, F.; Gonçalves, A.; Debled, M.; Patsouris, A.; et al. Evolution of overall survival and receipt of new therapies by subtype among 20 446 metastatic breast cancer patients in the 2008-2017 ESME cohort. ESMO Open 2021, 6, 100114. [Google Scholar] [CrossRef] [PubMed]
- Kuksis, M.; Gao, Y.; Tran, W.; Hoey, C.; Kiss, A.; Komorowski, A.S.; Dhaliwal, A.J.; Sahgal, A.; Das, S.; Chan, K.K.; et al. The incidence of brain metastases among patients with metastatic breast cancer: A systematic review and meta-analysis. Neuro-Oncology 2021, 23, 894–904. [Google Scholar] [CrossRef] [PubMed]
- Sperduto, P.W.; Mesko, S.; Li, J.; Cagney, D.; Aizer, A.; Lin, N.U.; Nesbit, E.; Kruser, T.J.; Chan, J.; Braunstein, S.; et al. Beyond an Updated Graded Prognostic Assessment (Breast GPA): A Prognostic Index and Trends in Treatment and Survival in Breast Cancer Brain Metastases From 1985 to Today. Int. J. Radiat. Oncol. Biol. Phys. 2020, 107, 334–343. [Google Scholar] [CrossRef] [PubMed]
- Distefano, A.; Yap, H.Y.; Hortobagyi, G.N.; Blumenschein, G.R. The natural history of breast cancer patients with brain metastases. Cancer 1979, 44, 1913–1918. [Google Scholar] [CrossRef]
- Brufsky, A.M.; Mayer, M.; Rugo, H.S.; Kaufman, P.A.; Tan-Chiu, E.; Tripathy, D.; Tudor, I.C.; Wang, L.I.; Brammer, M.G.; Shing, M.; et al. Central Nervous System Metastases in Patients with HER2-Positive Metastatic Breast Cancer: Incidence, Treatment, and Survival in Patients from registHER. Clin. Cancer Res. 2011, 17, 4834–4843. [Google Scholar] [CrossRef]
- Sperduto, P.W.; Mesko, S.; Li, J.; Cagney, D.; Aizer, A.; Lin, N.U.; Nesbit, E.; Kruser, T.J.; Chan, J.; Braunstein, S.; et al. Estrogen/progesterone receptor and HER2 discordance between primary tumor and brain metastases in breast cancer and its effect on treatment and survival. Neuro-Oncology 2020, 22, 1359–1367. [Google Scholar] [CrossRef]
- Witzel, I.; Laakmann, E.; Weide, R.; Neunhöffer, T.; Park-Simon, T.-J.; Schmidt, M.; Fasching, P.A.; Hesse, T.; Polasik, A.; Mohrmann, S.; et al. Treatment and outcomes of patients in the Brain Metastases in Breast Cancer Network Registry. Eur. J. Cancer 2018, 102, 1–9. [Google Scholar] [CrossRef]
- Hurvitz, S.A.; O’Shaughnessy, J.; Mason, G.; Yardley, D.A.; Jahanzeb, M.; Brufsky, A.M.; Rugo, H.S.; Swain, S.M.; Kaufman, P.A.; Tripathy, D.; et al. Central Nervous System Metastasis in Patients with HER2-Positive Metastatic Breast Cancer: Patient Characteristics, Treatment, and Survival from SystHERs. Clin. Cancer Res. 2019, 25, 2433–2441. [Google Scholar] [CrossRef]
- Steeg, P.S. The blood–tumour barrier in cancer biology and therapy. Nat. Rev. Clin. Oncol. 2021, 18, 696–714. [Google Scholar] [CrossRef]
- Yu, T.; Cho, B.J.; Choi, E.J.; Park, J.M.; Kim, D.H.; Kim, I.A. Radiosensitizing effect of lapatinib in human epidermal growth factor receptor 2-positive breast cancer cells. Oncotarget 2016, 7, 79089–79100. [Google Scholar] [CrossRef]
- Tian, W.; Hao, S.; Wang, L.; Chen, Y.; Li, Z.; Luo, D. Pyrotinib treatment enhances the radiosensitivity in HER2-positive brain metastatic breast cancer patients. Anti-Cancer Drugs 2022, 33, e622–e627. [Google Scholar] [CrossRef]
- Geraud, A.; Xu, H.P.; Beuzeboc, P.; Kirova, Y.M. Preliminary experience of the concurrent use of radiosurgery and T-DM1 for brain metastases in HER2-positive metastatic breast cancer. J. Neuro-Oncology 2017, 131, 69–72. [Google Scholar] [CrossRef] [PubMed]
- Mills, M.N.; Walker, C.; Thawani, C.; Naz, A.; Figura, N.B.; Yu, M.; Robinson, T.J.; Etame, A.B.; Liu, J.K.; Vogelbaum, M.A.; et al. Clinical Outcomes of Breast Cancer Patients with HER2-positive Brain Metastases Treated with Stereotactic Radiation and Trastuzumab Emtansine. Int. J. Radiat. Oncol. Biol. Phys. 2020, 108, e679–e680. [Google Scholar] [CrossRef]
- Swain, S.M.; Kim, S.B.; Cortés, J.; Ro, J.; Semiglazov, V.; Campone, M.; Ciruelos, E.; Ferrero, J.M.; Schneeweiss, A.; Knott, A.; et al. Per-tuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA study): Overall survival re-sults from a randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol. 2013, 14, 461–471. [Google Scholar] [CrossRef]
- Swain, S.M.; Miles, D.; Kim, S.B.; Im, Y.H.; Im, S.A.; Semiglazov, V.; Ciruelos, E.; Schneeweiss, A.; Loi, S.; Monturus, E.; et al. Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA): End-of-study results from a dou-ble-blind, randomised, placebo-controlled, phase 3 study. Lancet Oncol. 2020, 21, 519–530. [Google Scholar] [CrossRef]
- Baselga, J.; Cortés, J.; Kim, S.-B.; Im, S.-A.; Hegg, R.; Im, Y.-H.; Roman, L.; Pedrini, J.L.; Pienkowski, T.; Knott, A.; et al. Pertuzumab plus Trastuzumab plus Docetaxel for Metastatic Breast Cancer. N. Engl. J. Med. 2012, 366, 109–119. [Google Scholar] [CrossRef]
- Swain, S.M.; Baselga, J.; Miles, D.; Im, Y.-H.; Quah, C.; Lee, L.F.; Cortes, J. Incidence of central nervous system metastases in patients with HER2-positive metastatic breast cancer treated with pertuzumab, trastuzumab, and docetaxel: Results from the randomized phase III study CLEOPATRA. Ann. Oncol. 2014, 25, 1116–1121. [Google Scholar] [CrossRef]
- Dijkers, E.C.; Oude Munnink, T.H.; Kosterink, J.G.; Brouwers, A.H.; Jager, P.L.; De Jong, J.R.; Van Dongen, G.A.; Schröder, C.P.; Lub-De Hooge, M.N.; De Vries, E.G. Biodistribution of 89 Zr-trastuzumab and PET Imaging of HER2-Positive Lesions in Patients with Metastatic Breast Cancer. Clin. Pharmacol. Ther. 2010, 87, 586–592. [Google Scholar] [CrossRef]
- Saleem, A.; Searle, G.E.; Kenny, L.M.; Huiban, M.; Kozlowski, K.; Waldman, A.D.; Woodley, L.; Palmieri, C.; Lowdell, C.; Kaneko, T.; et al. Lapatinib access into normal brain and brain metastases in patients with Her-2 overexpressing breast cancer. EJNMMI Res. 2015, 5, 30. [Google Scholar] [CrossRef]
- Ulaner, G.A.; Lyashchenko, S.K.; Riedl, C.; Ruan, S.; Zanzonico, P.B.; Lake, D.; Jhaveri, K.; Zeglis, B.; Lewis, J.S.; O’Donoghue, J. First-in-Human Human Epidermal Growth Factor Receptor 2-Targeted Imaging Using 89Zr-Pertuzumab PET/CT: Dosimetry and Clinical Application in Patients with Breast Cancer. J. Nucl. Med. 2018, 59, 900–906. [Google Scholar]
- Galanti, D.; Inno, A.; La Vecchia, M.; Borsellino, N.; Incorvaia, L.; Russo, A.; Gori, S.C. Current treatment options for HER2-positive breast cancer patients with brain metastases. Crit. Rev. Oncol. Hematol. 2021, 161, 103329. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Alvarez, A.; Papakonstantinou, A.; Oliveira, M. Brain Metastases in HER2-Positive Breast Cancer: Current and Novel Treatment Strategies. Cancers 2021, 13, 2927. [Google Scholar] [CrossRef] [PubMed]
- Larsen, P.B.; Kümler, I.; Nielsen, D.L. A systematic review of trastuzumab and lapatinib in the treatment of women with brain metastases from HER2-positive breast cancer. Cancer Treat. Rev. 2013, 39, 720–727. [Google Scholar] [CrossRef] [PubMed]
- Corti, C.; Antonarelli, G.; Criscitiello, C.; Lin, N.U.; Carey, L.A.; Cortés, J.; Poortmans, P.; Curigliano, G. Targeting brain metastases in breast cancer. Cancer Treat. Rev. 2022, 103, 102324. [Google Scholar] [CrossRef] [PubMed]
- Petrelli, F.; Ghidini, M.; Lonati, V.; Tomasello, G.; Borgonovo, K.; Ghilardi, M.; Cabiddu, M.; Barni, S. The efficacy of lapatinib and capecitabine in HER-2 positive breast cancer with brain metastases: A systematic review and pooled analysis. Eur. J. Cancer 2017, 84, 141–148. [Google Scholar] [CrossRef]
- Balduzzi, S.; Rücker, G.; Schwarzer, G. How to perform a meta-analysis with R: A practical tutorial. EÉvid. Based Ment. Health 2019, 22, 153–160. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomized studies of interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- McGuinness, L.; Higgins, J. Risk-of-bias VISualization (robvis): An R package and Shiny web app for visualizing risk-of-bias assessments. Res. Syn. Meth. 2020, 12, 55–61. [Google Scholar] [CrossRef]
- Lu, Y.S.; Chen, T.W.; Lin, C.H.; Yeh, D.C.; Tseng, L.M.; Wu, P.F.; Rau, K.M.; Chen, B.B.; Chao, T.C.; Huang, S.M.; et al. Bevacizumab precon-ditioning followed by Etoposide and Cisplatin is highly effective in treating brain metastases of breast cancer progressing from whole-brain radiotherapy. Clin. Cancer Res. 2015, 21, 1851–1858. [Google Scholar] [CrossRef]
- Cortés, J.; Dieras, V.; Ro, J.; Barriere, J.; Bachelot, T.; Hurvitz, S.; Le Rhun, E.; Espié, M.; Kim, S.B.; Schneeweiss, A.; et al. Afatinib alone or afatinib plus vinorelbine versus investigator’s choice of treatment for HER2-positive breast cancer with progressive brain metastases after trastuzumab, lapatinib, or both (LUX-Breast 3): A randomised, open-label, multicentre, phase 2 trial. Lancet Oncol. 2015, 16, 1700–1710. [Google Scholar]
- Freedman, R.A.; Gelman, R.S.; Wefel, J.S.; Melisko, M.E.; Hess, K.R.; Connolly, R.M.; Van Poznak, C.H.; Niravath, P.A.; Puhalla, S.L.; Ibrahim, N.; et al. Translational Breast Cancer Research Consortium (TBCRC) 022: A Phase II Trial of Neratinib for Patients With Human Epidermal Growth Factor Receptor 2–Positive Breast Cancer and Brain Metastases. J. Clin. Oncol. 2016, 34, 945–952. [Google Scholar] [CrossRef] [PubMed]
- Freedman, R.A.; Gelman, R.S.; Anders, C.K.; Melisko, M.E.; Parsons, H.A.; Cropp, A.M.; Silvestri, K.; Cotter, C.M.; Componeschi, K.P.; Marte, J.M.; et al. TBCRC 022: A Phase II Trial of Neratinib and Capecitabine for Patients With Human Epidermal Growth Factor Receptor 2–Positive Breast Cancer and Brain Metastases. J. Clin. Oncol. 2019, 37, 1081–1089. [Google Scholar] [CrossRef] [PubMed]
- Hurvitz, S.A.; Saura, C.; Oliveira, M.; Trudeau, M.E.; Moy, B.; Delaloge, S.; Gradishar, W.; Kim, S.-B.; Haley, B.; Ryvo, L.; et al. Efficacy of Neratinib Plus Capecitabine in the Subgroup of Patients with Central Nervous System Involvement from the NALA Trial. Oncology 2021, 26, e1327–e1338. [Google Scholar] [CrossRef] [PubMed]
- Van Swearingen, A.E.D.; Siegel, M.B.; Deal, A.M.; Sambade, M.J.; Hoyle, A.; Hayes, D.N.; Jo, H.; Little, P.; Dees, E.C.; Muss, H.; et al. LCCC 1025: A phase II study of everolimus, trastuzumab, and vinorelbine to treat progressive HER2-positive breast cancer brain metastases. Breast Cancer Res. Treat. 2018, 171, 637–648. [Google Scholar] [CrossRef]
- Hurvitz, S.; Singh, R.; Adams, B.; Taguchi, J.A.; Chan, D.; Dichmann, R.A.; Castrellon, A.; Hu, E.; Berkowitz, J.; Mani, A.; et al. Phase Ib/II single-arm trial evaluating the combination of everolimus, lapatinib and capecitabine for the treatment of HER2-positive breast cancer with brain metastases (TRIO-US B-09). Ther. Adv. Med Oncol. 2018, 10. [Google Scholar] [CrossRef] [PubMed]
- Leone, J.P.; Duda, D.G.; Hu, J.; Barry, W.T.; Trippa, L.; Gerstner, E.R.; Jain, R.K.; Tan, S.; Lawler, E.; Winer, E.P.; et al. A phase II study of cabozantinib alone or in combination with trastuzumab in breast cancer patients with brain metastases. Breast Cancer Res. Treat. 2020, 179, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Lin, N.U.; Borges, V.; Anders, C.; Murthy, R.K.; Paplomata, E.; Hamilton, E.; Hurvitz, S.; Loi, S.; Okines, A.; Abramson, V.; et al. Intra-cranial Efficacy and Survival With Tucatinib Plus Trastuzumab and Capecitabine for Previously Treated HER2-Positive Breast Cancer With Brain Metastases in the HER2CLIMB Trial. J. Clin. Oncol. 2020, 38, 2610–2619. [Google Scholar] [CrossRef]
- Jerusalem, G.H.M.; Park, Y.H.; Yamashita, T.; Hurvitz, S.A.; Modi, S.; Andre, F.; Krop, I.E.; Gonzalez, X.; Hall, P.S.; You, B.; et al. Trastuzumab deruxtecan (T-DXd) in patients with HER2+ metastatic breast cancer with brain metastases: A subgroup analysis of the DESTINY-Breast01 trial. J. Clin. Oncol. 2021, 39, 526. [Google Scholar] [CrossRef]
- Bartsch, R.; Berghoff, A.; Furtner, J.; Bergen, E.; Roider-Schur, S.; Marhold, M.; Starzer, A.; Forstner, H.; Rottenmanner, B.; Dieckmann, K.; et al. 280P Intracranial activity of trastuzumab-deruxtecan (T-DXd) in HER2-positive breast cancer patients with active brain metastases: Results from the first stage of the phase II TUXEDO-1 trial. Ann. Oncol. 2021, 32, S486. [Google Scholar] [CrossRef]
- Cortés, J.; Kim, S.-B.; Chung, W.-P.; Im, S.-A.; Park, Y.H.; Hegg, R.; Kim, M.H.; Tseng, L.-M.; Petry, V.; Chung, C.-F.; et al. Trastuzumab Deruxtecan versus Trastuzumab Emtansine for Breast Cancer. N. Engl. J. Med. 2022, 386, 1143–1154. [Google Scholar] [CrossRef] [PubMed]
- Lin, N.U.; Pegram, M.; Sahebjam, S.; Ibrahim, N.; Fung, A.; Cheng, A.; Nicholas, A.; Kirschbrown, W.; Kumthekar, P. Pertuzumab Plus High-Dose Trastuzumab in Patients With Progressive Brain Metastases and HER2-Positive Metastatic Breast Cancer: Primary Analysis of a Phase II Study. J. Clin. Oncol. 2021, 39, 2667–2675. [Google Scholar] [CrossRef] [PubMed]
- Bergen, E.S.; Binter, A.; Starzer, A.M.; Heller, G.; Kiesel, B.; Tendl-Schulz, K.; Bago-Horvath, Z.; Furtner, J.; Leitner, J.; Exner, R.; et al. Favourable outcome of patients with breast cancer brain metastases treated with dual HER2 blockade of trastuzumab and pertuzumab. Ther. Adv. Med Oncol. 2021, 13. [Google Scholar] [CrossRef] [PubMed]
- Gamucci, T.; Pizzuti, L.; Natoli, C.; Mentuccia, L.; Sperduti, I.; Barba, M.; Sergi, D.; Iezzi, L.; Maugeri-Saccà, M.; Vaccaro, A.; et al. A multicenter REtrospective observational study of first-line treatment with PERtuzumab, trastuzumab and taxanes for advanced HER2 positive breast cancer patients. RePer Study. Cancer Biol. Ther. 2019, 20, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Krop, I.E.; Lin, N.U.; Blackwell, K.; Guardino, E.; Huober, J.; Lu, M.; Miles, D.; Samant, M.; Welslau, M.; Diéras, V. Trastuzumab emtansine (T-DM1) versus lapatinib plus capecitabine in patients with HER2-positive metastatic breast cancer and central nervous system metastases: A retrospective, exploratory analysis in EMILIA. Ann. Oncol. 2015, 26, 113–119. [Google Scholar] [CrossRef]
- Bartsch, R.; Berghoff, A.S.; Vogl, U.; Rudas, M.; Bergen, E.; Dubsky, P.; Dieckmann, K.; Pinker-Domenig, K.; Bago-Horvath, Z.; Galid, A.; et al. Activity of T-DM1 in Her2-positive breast cancer brain metastases. Clin. Exp. Metastasis 2015, 32, 729–737. [Google Scholar] [CrossRef]
- Yardley, D.A.; Krop, I.E.; LoRusso, P.M.; Mayer, M.; Barnett, B.; Yoo, B.; Perez, E.A. Trastuzumab emtansine (T-DM1) in patients With HER2-positive metastatic breast cancer previously treated with chemotherapy and 2 or more HER2-targeted agents: Results from the T-PAS expanded access study. Cancer J. 2015, 21, 357–364. [Google Scholar] [CrossRef]
- Mailliez, A.; Girard, E.; Boulanger, T.; Giraud, C.; Bonneterre, J.; Le Rhun, E. Response to ado-trastuzumab emtansine according to RANO criteria in central nervous system metastases of HER2 positive breast cancers. J. Clin. Oncol. 2016, 34, 605. [Google Scholar] [CrossRef]
- Jacot, W.; Pons, E.; Frenel, J.-S.; Guiu, S.; Levy, C.; Heudel, P.E.; Bachelot, T.; D’Hondt, V.; Darlix, A.; Firmin, N.; et al. Efficacy and safety of trastuzumab emtansine (T-DM1) in patients with HER2-positive breast cancer with brain metastases. Breast Cancer Res. Treat. 2016, 157, 307–318. [Google Scholar] [CrossRef]
- Okines, A.; Irfan, T.; Khabra, K.; Smith, I.; O’Brien, M.; Parton, M.; Noble, J.; Stanway, S.; Somaiah, N.; Ring, A.; et al. Development and responses of brain metastases during treatment with trastuzumab emtansine (T-DM1) for HER2 positive advanced breast cancer: A single institution experience. Breast J. 2018, 24, 253–259. [Google Scholar] [CrossRef]
- Fabi, A.; Alesini, D.; Valle, E.; Moscetti, L.; Caputo, R.; Caruso, M.; Carbognin, L.; Ciccarese, M.; La Verde, N.; Arpino, G.; et al. T-DM1 and brain metastases: Clinical outcome in HER2-positive metastatic breast cancer. Breast 2018, 41, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Montemurro, F.; Ellis, P.; Anton, A.; Wuerstlein, R.; Delaloge, S.; Bonneterre, J.; Quenel-Tueux, N.; Linn, S.C.; Irahara, N.; Donica, M.; et al. Safety of trastuzumab emtansine (T-DM1) in patients with HER2-positive advanced breast cancer: Primary results from the KAMILLA study cohort 1. Eur. J. Cancer 2019, 109, 92–102. [Google Scholar] [CrossRef] [PubMed]
- Bahçeci, A.; Paydaş, S.; Ak, N.; Ferhatoğlu, F.; Saip, P.M.; Seydaoğlu, G.; Bilici, M.; Şimşek, M.; Tekin, S.B.; Çalikuşu, Z.; et al. Efficacy and Safety of Trastuzumab Emtansine in Her2 Positive Metastatic Breast Cancer: Real-World Experience. Cancer Investig. 2021, 39, 473–481. [Google Scholar] [CrossRef] [PubMed]
- Lin, N.U.; Carey, L.A.; Liu, M.C.; Younger, J.; Come, S.E.; Ewend, M.; Harris, G.J.; Bullitt, E.; Van den Abbeele, A.D.; Henson, J.W.; et al. Phase II Trial of Lapatinib for Brain Metastases in Patients With Human Epidermal Growth Factor Receptor 2–Positive Breast Cancer. J. Clin. Oncol. 2008, 26, 1993–1999. [Google Scholar] [CrossRef]
- Lin, N.U.; Diéras, V.; Paul, D.; Lossignol, D.; Christodoulou, C.; Stemmler, H.-J.; Roché, H.; Liu, M.C.; Greil, R.; Ciruelos, E.; et al. Multicenter Phase II Study of Lapatinib in Patients with Brain Metastases from HER2-Positive Breast Cancer. Clin. Cancer Res. 2009, 15, 1452–1459. [Google Scholar] [CrossRef]
- Wang, X.; Wang, L.; Yu, Q.; Liu, Z.; Li, C.; Wang, F.; Yu, Z. The Effectiveness of Lapatinib in HER2-Positive Metastatic Breast Cancer Patients Pretreated With Multiline Anti-HER2 Treatment: A Retrospective Study in China. Technol. Cancer Res. Treat. 2021, 20. [Google Scholar] [CrossRef]
- Gavilá, J.; De La Haba, J.; Bermejo, B.; Rodríguez-Lescure, A.A.; Ciruelos, E.; Brunet, J.; Muñoz-Couselo, E.; Santisteban, M.; Rodríguez Sánchez, C.A. A retrospective, multicenter study of the efficacy of lapatinib plus trastuzumab in HER2-positive metastatic breast cancer patients previously treated with trastuzumab, lapatinib, or both: The Trastyvere study. Clin. Transl. Oncol. 2020, 22, 420–428. [Google Scholar] [CrossRef]
- Boccardo, F.M.; Kaufman, B.; Baselga, J.; Dieras, V.; Link, J.; Casey, M.A.; Fittipaldo, A.; Oliva, C.; Zembryki, D.; Rubin, S.D. Evaluation of lapatinib (Lap) plus capecitabine (Cap) in patients with brain metastases (BM) from HER2+ breast cancer (BC) enrolled in the Lapatinib Expanded Access Program (LEAP) and French Authorisation Temporaire d’Utilisation (ATU). J. Clin. Oncol. 2008, 26, 1094. [Google Scholar] [CrossRef]
- Sutherland, S.; Ashley, S.; Miles, D.; Chan, S.; Wardley, A.; Davidson, N.; Bhatti, R.; Shehata, M.; Nouras, H.; Camburn, T.; et al. Treatment of HER2-positive metastatic breast cancer with lapatinib and capecitabine in the lapatinib expanded access pro-gramme, including efficacy in brain metastases—The UK experience. Br. J. Cancer 2010, 102, 995–1002. [Google Scholar] [CrossRef]
- Metro, G.; Foglietta, J.; Russillo, M.; Stocchi, L.; Vidiri, A.; Giannarelli, D.; Crinò, L.; Papaldo, P.; Mottolese, M.; Cognetti, F.; et al. Clinical outcome of patients with brain metastases from HER2-positive breast cancer treated with lapatinib and capecitabine. Ann. Oncol. 2011, 22, 625–630. [Google Scholar] [CrossRef]
- Lin, N.U.; Eierman, W.; Greil, R.; Campone, M.; Kaufman, B.; Steplewski, K.; Lane, S.R.; Zembryki, D.; Rubin, S.D.; Winer, E.P. Randomized phase II study of lapatinib plus capecitabine or lapatinib plus topotecan for patients with HER2-positive breast cancer brain metastases. J. Neuro-Oncology 2011, 105, 613–620. [Google Scholar] [CrossRef] [PubMed]
- Cetin, B.; Benekli, M.; Oksuzoglu, B.; Koral, L.; Ulas, A.; Dane, F.; Turker, I.; Kaplan, M.A.; Koca, D.; Boruban, C.; et al. Lapatinib plus Capecitabine for Brain Metastases in Patients with Human Epidermal Growth Factor Receptor 2-Positive Advanced Breast Cancer: A Review of the Anatolian Society of Medical Oncology (ASMO) Experience. Onkologie 2012, 35, 740–745. [Google Scholar] [CrossRef] [PubMed]
- Bachelot, T.; Romieu, G.; Campone, M.; Diéras, V.; Cropet, C.; Dalenc, F.; Jimenez, M.; Le Rhun, E.; Pierga, J.-Y.; Gonçalves, A.; et al. Lapatinib plus capecitabine in patients with previously untreated brain metastases from HER2-positive metastatic breast cancer (LANDSCAPE): A single-group phase 2 study. Lancet Oncol. 2013, 14, 64–71. [Google Scholar] [CrossRef]
- Ro, J.; Park, S.; Kim, S.B.; Kim, T.Y.; Im, Y.H.; Rha, S.Y.; Chung, J.S.; Moon, H.; Santillana, S. Clinical outcomes of HER2-positive metastatic breast cancer patients with brain metastasis treated with lapatinib and capecitabine: An open-label expanded access study in Korea. BMC Cancer 2012, 12, 322. [Google Scholar] [CrossRef]
- Dubianski, R.; Brewczynska, E.; Lemanska, I.; Szombara, E.; Nowecki, Z.I. Lapatinib and Capecitabine in the Treatment of Her2-Positive Breast Cancer with Brain Metastases. Ann. Oncol. 2014, 25, iv131. [Google Scholar] [CrossRef]
- Shawky, H.; Tawfik, H. All-oral combination of lapatinib and capecitabine in patients with brain metastases from HER2-positive breast cancer – A phase II study. J. Egypt. Natl. Cancer Inst. 2014, 26, 187–194. [Google Scholar] [CrossRef]
- Kaplan, M.A.; Isikdogan, A.; Koca, D.; Kucukoner, M.; Gumusay, O.; Yildiz, R.; Dayan, A.; Demir, L.; Geredeli, C.; Kocer, M.; et al. Clinical outcomes in patients who received lapatinib plus capecitabine combination therapy for HER2-positive breast cancer with brain metastasis and a comparison of survival with those who received trastuzumab-based therapy: A study by the Anatolian Society of Medical Oncology. Breast Cancer 2014, 21, 677–683. [Google Scholar] [CrossRef]
- Gui, X.; Li, H.; Yan, Y.; Zhang, R. Efficacy of lapatinib combined with capecitabine in patients with HER2-positive metastatic breast cancer in a real-world study. Oncol. Lett. 2020, 20, 656–664. [Google Scholar] [CrossRef]
- Seligmann, J.F.; Wright-Hughes, A.; Pottinger, A.; Velikova, G.; Oughton, J.B.; Murden, G.; Rizwanullah, M.; Price, C.; Passant, H.; Heudtlass, P.; et al. Lapatinib plus Capecitabine versus Trastuzumab plus Capecitabine in the Treatment of Human Epidermal Growth Factor Receptor 2-positive Metastatic Breast Cancer with Central Nervous System Metastases for Patients Currently or Previously Treated with Trastuzumab (LANTERN): A Phase II Randomised Trial. Clin. Oncol. 2020, 32, 656–664. [Google Scholar] [CrossRef]
- Yang, H.; Wang, W. Comparison of pyrotinib or lapatinib with chemotherapy for patients with HER2 positive breast cancer after first-line treatment failure: A retrospective study. Am. J. Transl. Res. 2021, 13, 10863–10870. [Google Scholar]
- Yan, M.; Bian, L.; Hu, X.; Zhang, Q.; Ouyang, Q.; Feng, J.; Yin, Y.; Sun, T.; Tong, Z.; Wang, X.; et al. Pyrotinib plus capecitabine for human epidermal factor receptor 2-positive metastatic breast cancer after trastuzumab and taxanes (PHENIX): A randomized, dou-ble-blind, placebo-controlled phase 3 study. Transl. Breast Cancer Res. 2020, 1, 13. [Google Scholar] [CrossRef]
- Lin, Y.; Lin, M.; Zhang, J.; Wang, B.; Tao, Z.; Du, Y.; Zhang, S.; Cao, J.; Wang, L.; Hu, X. Real-World Data of Pyrotinib-Based Therapy in Metastatic HER2-Positive Breast Cancer: Promising Efficacy in Lapatinib-Treated Patients and in Brain Metastasis. Cancer Res. Treat. 2020, 52, 1059–1066. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Fu, C.; Li, S.; Chen, F.; Yang, Y.; Wang, C.; Qin, J.; Liu, S.; Zhang, R.; Wang, C.; et al. The efficacy and safety of pyrotinib in treating HER2-positive breast cancer patients with brain metastasis: A multicenter study. Cancer Med. 2021, 11, 735–742. [Google Scholar] [CrossRef]
- Zhang, L.; Wu, X.; Zhou, J.; Zhu, M.; Yu, H.; Zhang, Y.; Zhao, Y.; Han, Z.; Guo, Y.; Guan, X.; et al. Pyrotinib in the Treatment of Women With HER2-Positive Advanced Breast Cancer: A Multicenter, Prospective, Real-World Study. Front. Oncol. 2021, 11. [Google Scholar] [CrossRef] [PubMed]
- Anwar, M.; Chen, Q.; Ouyang, D.; Wang, S.; Xie, N.; Ouyang, Q.; Fan, P.; Qian, L.; Chen, G.; Zhou, E.; et al. Pyrotinib Treatment in Patients With HER2-positive Metastatic Breast Cancer and Brain Metastasis: Exploratory Final Analysis of Real-World, Multicenter Data. Clin. Cancer Res. 2021, 27, 4634–4641. [Google Scholar] [CrossRef]
- Li, C.; Bian, X.; Liu, Z.; Wang, X.; Song, X.; Zhao, W.; Liu, Y.; Yu, Z. Effectiveness and safety of pyrotinib-based therapy in patients with HER2-positive metastatic breast cancer: A real-world retrospective study. Cancer Med. 2021, 10, 8352–8364. [Google Scholar] [CrossRef]
- Li, Y.; Qiu, Y.; Li, H.; Luo, T.; Li, W.; Wang, H.; Shao, B.; Wang, B.; Ge, R. Pyrotinib Combined With Vinorelbine in HER2-Positive Metastatic Breast Cancer: A Multicenter Retrospective Study. Front. Oncol. 2021, 11. [Google Scholar] [CrossRef]
- Yan, M.; Ouyang, Q.; Sun, T.; Niu, L.; Yang, J.; Li, L.; Song, Y.; Hao, C.; Chen, Z.; Orlandi, A.; et al. Pyrotinib plus capecitabine for patients with human epidermal growth factor receptor 2-positive breast cancer and brain metastases (PERMEATE): A multicentre, single-arm, two-cohort, phase 2 trial. Lancet Oncol. 2022, 23, 353–361. [Google Scholar] [CrossRef]
- Lewis Phillips, G.D.; Nishimura, M.C.; Lacap, J.A.; Kharbanda, S.; Mai, E.; Tien, J.; Malesky, K.; Williams, S.P.; Marik, J.; Phillips, H.S. Trastuzumab uptake and its relation to efficacy in an animal model of HER2-positive breast cancer brain metastasis. Breast Cancer Res. Treat. 2017, 164, 581–591. [Google Scholar] [CrossRef]
- Verma, S.; Miles, D.; Gianni, L.; Krop, I.E.; Welslau, M.; Baselga, J.; Pegram, M.; Oh, D.-Y.; Diéras, V.; Guardino, E.; et al. Trastuzumab Emtansine for HER2-Positive Advanced Breast Cancer. N. Engl. J. Med. 2012, 367, 1783–1791. [Google Scholar] [CrossRef]
- Doi, T.; Shitara, K.; Naito, Y.; Shimomura, A.; Fujiwara, Y.; Yonemori, K.; Shimizu, C.; Shimoi, T.; Kuboki, Y.; Matsubara, N.; et al. Safety, pharmacokinetics, and antitumour activity of trastuzumab deruxtecan (DS-8201), a HER2-targeting antibody–drug conjugate, in patients with advanced breast and gastric or gastro-oesophageal tumours: A phase 1 dose-escalation study. Lancet Oncol. 2017, 18, 1512–1522. [Google Scholar] [CrossRef]
- Geyer, C.E.; Forster, J.; Lindquist, D.; Chan, S.; Romieu, C.G.; Pienkowski, T.; Jagiello-Gruszfeld, A.; Crown, J.; Chan, A.; Kaufman, B.; et al. Lapatinib plus Capecitabine for HER2-Positive Advanced Breast Cancer. N. Engl. J. Med. 2006, 355, 2733–2743. [Google Scholar] [CrossRef] [PubMed]
- Awada, A.; Colomer, R.; Inoue, K.; Bondarenko, I.; Badwe, R.A.; Demetriou, G.; Lee, S.C.; Mehta, A.O.; Kim, S.B.; Bachelot, T.; et al. Neratinib Plus Paclitaxel vs. Trastuzumab Plus Paclitaxel in Previously Untreated Metastatic ERBB2-Positive Breast Cancer: The NEfERT-T Randomized Clinical Trial. JAMA Oncol. 2016, 2, 1557–1564. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Yan, M.; Ma, F.; Hu, X.; Feng, J.; Ouyang, Q.; Tong, Z.; Li, H.; Zhang, Q.; Sun, T.; et al. Pyrotinib plus capecitabine versus lapatinib plus capecitabine for the treatment of HER2-positive metastatic breast cancer (PHOEBE): A multicentre, open-label, randomised, controlled, phase 3 trial. Lancet Oncol. 2021, 22, 351–360. [Google Scholar] [CrossRef]
- Murthy, R.K.; Loi, S.; Okines, A.; Paplomata, E.; Hamilton, E.; Hurvitz, S.A.; Lin, N.U.; Borges, V.; Abramson, V.; Anders, C.; et al. Tucatinib, Trastuzumab, and Capecitabine for HER2-Positive Metastatic Breast Cancer. N. Engl. J. Med. 2020, 382, 597–609. [Google Scholar] [CrossRef]
- Gallardo, A.; Lerma, E.; Escuin, D.; Tibau, A.; Muñoz, J.; Ojeda, B.; Barnadas, A.; Adrover, E.; Sánchez-Tejada, L.; Giner, D.; et al. Increased signalling of EGFR and IGF1R, and deregulation of PTEN/PI3K/Akt pathway are related with trastuzumab resistance in HER2 breast carcinomas. Br. J. Cancer 2012, 106, 1367–1373. [Google Scholar] [CrossRef]
- Pivot, X.; Manikhas, A.; Żurawski, B.; Chmielowska, E.; Karaszewska, B.; Allerton, R.; Chan, S.; Fabi, A.; Bidoli, P.; Gori, S.; et al. CEREBEL (EGF111438): A Phase III, Randomized, Open-Label Study of Lapatinib Plus Capecitabine Versus Trastuzumab Plus Cape-citabine in Patients With Human Epidermal Growth Factor Receptor 2-Positive Metastatic Breast Cancer. J. Clin. Oncol. 2015, 33, 1564–1573. [Google Scholar] [CrossRef]
- Saura, C.; Oliveira, M.; Feng, Y.-H.; Dai, M.-S.; Chen, S.-W.; Hurvitz, S.A.; Kim, S.-B.; Moy, B.; Delaloge, S.; Gradishar, W.; et al. Neratinib Plus Capecitabine Versus Lapatinib Plus Capecitabine in HER2-Positive Metastatic Breast Cancer Previously Treated With ≥ 2 HER2-Directed Regimens: Phase III NALA Trial. J. Clin. Oncol. 2020, 38, 3138–3149. [Google Scholar] [CrossRef]
- Braccini, A.L.; Azria, D.; Thezenas, S.; Romieu, G.; Ferrero, J.M.; Jacot, W. Prognostic factors of brain metastases from breast cancer: Impact of targeted therapies. Breast 2013, 22, 993–998. [Google Scholar] [CrossRef]

| Study | Phase | Patients with Baseline BM (n) | Intervention (n) | Control (n) | Line of Therapy | Previous Local Treatment for BM (%) | Extra CNS Disease (%) | Number of BM | mPFS (Months) | mOS (Months) | CNS ORR % |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Lu 2015 [30] | phase 2 | 23 | BEEP (Bevacizumab, Etoposide, Cisplatin) | Single arm | median 3 (Range 1–8) | 100% | 94.3% | 7.7 (95% CI 6.6–8.8) | 11.8 (95% CI 7.0–16.6) | 69.6% | |
| Cortes 2015-lux breast 3 [31] | phase 2, randomised | 38 | Afatinib + Vinorelbine (38) | Investigator choice (43) or Afatinib (40) | 1–2.31%; 3–4.68% | 83% | 41% | 59% > 3 | 2.8 | 8.6 | 8.0% |
| Freedman 2016 [32] | phase 2 | 40 | Neratinib | Single arm | 0–2.17%; 3–4.83% | 100% | 1.9 | 8.7 | 8.0% | ||
| Freedman 2019 [33] | phase 2 | 49 (2 cohorts combined) | Neratinib + Capecitabine | Single arm | 0.22%; 1.45%; ≥2 33% | 92% | 78% | 5.5 (Range 0.8–18.8) | 13.3 (Range 2.2–27.6) | 44.9% 18 of 37.4 of 12 | |
| Hurvitz 2021-NALA [34] | phase 3b (posthoc) | 51 | Neratinib + Capecitabine (51) | Lapatinib + Capecitabine (50) | 2.68%; ≥3.32% | 80% | 84% | 5.6 (95% CI 3.7–7.1) | 13.9 (95% CI 8.9–17.5) | 28.6% | |
| Swearingen 2018 [35] | phase 2 | 32 | Everolimus + Trastuzumab + Vinorelbine | Single arm | median 2 (Range 0–7) | 97% | 66% | 3.9 (95% CI 2.3–5.0) | 12.1 (95% CI 6.8–12.4) | 4.0% | |
| Hurvitz 2018 [36] | phase 2 | 19 | Everolimus + Lapatinib + Capecitabine | Single arm | median 2.5 (Range 0–11) | 63% | 42% | 6.2 | 24.2 | 28.0% | |
| Leone 2020 [37] | phase 2 | 21 | Cabozantinib + Trastuzumab | Single arm | median 3 (Range 1–7) | 81% | >48% | 4.1 (95% CI 2.8–6.2) | 13.8 (95% CI 8.2–NR) | 5.0% | |
| N.Lin 2020-HER2Climb [38] | phase 3 | 198 | Tucatinib + Trastuzumab + Capecitabine (198) | Trastuzumab + Capecitabine (93) | median 3 (Range 1–14) | 87% | 97% | 9.9 (95% CI 8.0–13.9) | 18.1 (95% CI, 15.5–NR) | 47.3% | |
| Modi 2021 DESTINY-Breast01 [39] | phase 2 | 24 | Fam-Trastuzumab deruxtecan | Single arm | median > 6 | median 5 | 18.1 (95% CI 6.7–18.1) | NR | 58.3% | ||
| Bartsch 2021-Tuxedo 1 [40] | phase 2 | 10 | Fam-Trastuzumab deruxtecan | Single arm | 70% > 2 | 60% | 83.3% | ||||
| Cortes 2022-Destiny breast-03 [41] | phase 3 | 62 | Fam-Trastuzumab deruxtecan (62) | Trastuzumab-emtansine (52) | 2.50%; 3 22%; >5.8% | 15.0 (95% CI 12.6–22.2) | 62.9% | ||||
| Lin 2021-PATRICIA [42] | phase 2 | 39 | High dose Trastuzumab/Pertuzumab (+28% Other ) | Single arm | median 3 (Range 2–5) | 11.0% | |||||
| Bergen 2021 [43] | retrospective | 26 | Trastuzumab/Pertuzumab (60% + Chemo/Local Therapy) | Single arm | median 1 (Range 1–6) | 80% | 8.0 (Range 1.0–55.0) | 44.0 (range 2.0–61.0) | 92.9% | ||
| Gamucci 2019- RePer [44] | retrospective | 21 | Trastuzumab/Pertuzumab+ taxane | Single arm | Median 1 | 48% | 20 (95% CI 13–27) | 52.4% |
| Study | Phase | Patients with Baseline BM (n) | Intervention (n) | Control (n) | Line of Therapy | Previous Local Treatment for BM (%) | Extra CNS Disease (%) | Numberof BM | mPFS (Months) | mOS (Months) | CNS ORR % |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Krop 2015-Emilia [45] | phase 3b (posthoc) | 45 | Trastuzumab-emtansine (45) | Lapatinib + Capecitabine (50) | median 3 (Range 1–13) | 70% | 79% | 5.9 | 26.8 | ||
| Bartsch 2015 [46] | case series | 10 | Trastuzumab-emtansine | Single arm | 1.40%; 2.60% | 80% | 90% | 50% > 3 | 5.0 (95% CI 3.7–6.3) | 8.5 | 30.0% |
| Yardley 2015 [47] | open label, prospective | 26 | Trastuzumab-emtansine | Single arm | median 8 (Range 3–23) | 6.9 (95% CI 2.7–12.3) | 27.3% | ||||
| Mailliez 2016 [48] | retrospectief | 14 | Trastuzumab-emtansine | Single arm | median 2 (Range 0–7) | 2.4 (Range 2.0–9.4) | 9.1 (Range 3.7–24.8) | 28.6% | |||
| Jacot 2016 [49] | retrospectief | 39 | Trastuzumab-emtansine | Single arm | median 2 (Range 0–8) | 95% | 82% | median 2 (Range 1–11) | 6.1 (Range 5.2–18.3) | NR | 43.6% |
| Okines 2018 [50] | retrospectief | 16 | Trastuzumab-emtansine | Single arm | median 2 (Range 0–6) | 100% | 9.9 (95% CI 3.9–12.2) | 15.3 (95% CI 4.7–NR) | |||
| Fabi 2018 [51] | retrospectief | 87 | Trastuzumab-emtansine | Single arm | 1–2.51%; 3–4.49% | 100% | 25% > 3 | 7.0 (95% CI 5.4–8.6) | 14.0 (95% CI 12.2–15.8) | 25.3% | |
| Montemurro 2019- Kamilla [52] | phase 3b (posthoc) | 398 | Trastuzumab-emtansine | Single arm | 0–2.48%; 3–4.31%; ≥5.19% | 47% | 79% | 5.5 (95% CI 5.3–5.6) | 18.9 (95% CI 17.1–21.3) | 21.4% | |
| Bahceci 2021 [53] | retrospectief | 87 | Trastuzumab-emtansine | Single arm | 9.0 | 19 | |||||
| Cortes 2022-Destiny breast-03 [41] | phase 3b (posthoc) | 52 | Trastuzumab-emtansine (52) | Fam-Trastuzumab deruxtecan (62) | 2 | 5.7 (95% CI 2.9–7.1) | 34.0% |
| Study | Phase | Patients with Baseline BM (n) | Intervention (n) | Control (n) | Line of Therapy | Previous Local Treatment for BM (%) | Extra CNS Disease (%) | Number of BM | mPFS (Months) | mOS (Months) | CNS ORR % |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Lin 2008 [54] | phase 2 | 39 | Lapatinib | Single arm | 1–2.25%; ≥3.75% | 95% | >62% | 3.0 (95% CI 2.3–3.7) | 7 | 2.6% | |
| Lin 2009 [55] | phase 2 | 242 | Lapatinib | Single arm | 1–2.56%; 3–4.43%; ≥5.11% | 95% | 2.4 (95% CI 1.9–3.3) | 6.4 (95% CI 5.5–8.3) | 6.2% | ||
| Wang 2021 [56] | retrospective | 42 | Lapatinib | Single arm | 1.17.4%; 2.53.9%; 3.20.1%; ≥4.7.8% | 59% | 6.3 (95% CI 5.1–7.5) | 31.0% | |||
| Gavilá 2019 [57] | retrospective | 38 | Lapatinib + Trastuzumab | Single arm | 3 (2–4) | 3.8 | 15.2 | ||||
| Boccardo 2008 [58] | open label, prospective | 138 | Lapatinib + Capecitabine | Single arm | ≥2 100% | 18.1% | |||||
| Lin 2009 * [55] | phase 2 (expansion) | 50 | Lapatinib + Capecitabine | Single arm | 2 | 95% | 3.7 (95% CI 2.4–4.4) | NR | 20.0% | ||
| Sutherland 2010 [59] | open label, prospective | 34 | Lapatinib + Capecitabine | Single arm | mean 2.4 (Range 1–5) | 94% | 5.1 (95% CI 3.5–6.5) | NR | 20.6% | ||
| Metro 2011 [60] | retrospective | 30 | Lapatinib + Capecitabine | Single arm | median 2 (Range 1–5) | 87% | 97% | 40% > 3 | 5.1 (95% CI 2.6–7.5) | 11 (95% CI 4.3–17.6) | 31.8% |
| Lin 2011 [61] | phase 2, randomised | 13 | Lapatinib + Capecitabine (13) | Lapatinib + Topotecan (9) | >1 | 100% | 59% | NR | NR | 38.5% | |
| Cetin 2012 [62] | retrospective | 85 | Lapatinib + Capecitabine | Single arm | >3.74.1% | 100% | 96.5% | 7.0 (95% CI 5.0–10.0) | 13 (95% CI 9–17) | 27.1% | |
| Bachelot 2013-LANDSCAPE [63] | phase 2 | 44 | Lapatinib + Capecitabine | Single arm | 1–2.78%; 3–4.22% | 0% | 84% | median 3 (Range 1–25) | 5.5 (95% CI 4.3–6.0) | 17 (95% CI 13.7–24.9) | 56.8% |
| Ro 2012 [64] | open label, prospective | 58 | Lapatinib + Capecitabine | Single arm | >3.38% | 91% | 4.5 (95% CI 4.2–5.5) | 12.2 (9.9–14.5) | 17.0% | ||
| Dubianski 2014 [65] | retrospective | 19 | Lapatinib + Capecitabine | Single arm | 8.1 | ||||||
| Shawky 2014 [66] | phase 2 | 21 | Lapatinib + Capecitabine | Single arm | >2.100% | 76% | 91% | 57% > 3 | 5.5 (Range 1.1–22.0) | 11 | 33.3% |
| Krop 2015-Emilia [45] | phase 3b (posthoc) | 50 | Lapatinib + Capecitabine (50) | Trastuzumab-emtansine (45) | median 3 (Range 1–13) | 70% | 79% | 5.7 | 12.9 | ||
| Kaplan 2014 [67] | retrospective | 46 | Lapatinib + Capecitabine | Single arm | >2.48.9% | 96% | 86.5% | 48% > 3 | 19.1 | 36.9% | |
| Gui 2020 [68] | retrospective | 14 | Lapatinib + Capecitabine | Single arm | >3.82.6% | 100% | 8.4 (95% CI 2.2–14.7) | 35.7% | |||
| Seligmann 2020-LANTERN [69] | phase 2, randomised | 16 | Lapatinib + Capecitabine (16) | Trastuzumab + Capecitabine (14) | 100% | 70% | 6.2 (95% CI 3.6–7.1) | NR | 25.0% | ||
| Hurvitz 2021-NALA [34] | phase 3b (posthoc) | 50 | Lapatinib + Capecitabine (50) | Neratinib + Capecitabine (51) | 2.68%; ≥3.32% | 80% | 84% | 4.3 (95% CI 2.8–5.6) | 12.4 (95% CI 9.7–16.9) | 28.2% | |
| Yang 2021 [70] | retrospective | 25 | Lapatinib + Chemo (71%) Capecitabine) | Pyrotinib + Chemo (80% Capecitabine) | 3.5 |
| Study | Phase | Patients with Baseline BM (n) | Intervention (n) | Control (n) | Line of Therapy | Previous Local Treatment for BM (%) | Extra CNS Disease (%) | Number of BM | mPFS (Months) | mOS (Months) | CNS ORR % |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Yan 2020-Phenix [71] | phase 3 | 21 | Pyrotinib + Capecitabine (21) | Capecitabine (10) | 6.9 (95% CI 5.4–NR) | ||||||
| Y.Lin 2020 [72] | retrospective | 31 | Pyrotinib + Capecitabine (59%)/Other * (41%) | Single arm | 1–2.38% 3.22% ≥4.40% | 55% | 88,50% | 6.7 (Range 4.7–8.7) | 28.0% | ||
| Gao 2021 [73] | retrospective | 42 | Pyrotinib (+Other 59%) | Single arm | >1.93% | 82% | 90,00% | 17% >5 | 11.1 | 47.6% | |
| Zhang 2021 [74] | retrospective | 21 | Pyrotinib + Capecitabine (55%)/Other (38%)/Mono (7%) | Single arm | >1.88% | 16.6 (95% CI 13.7–24.1) | 45.5%, only 50% measurable disease | ||||
| Yang 2021 [70] | retrospective | 13 | Pyrotinib + Other (80% Capecitabine) (13) | Lapatinib+ Chemo (71% Capecitabine) (35) | 6.5 | ||||||
| Anwar 2021 [75] | retrospective | 39 (2 cohorts combined) | Pyrotinib + Capecitabine (64%)/Other (36%) | Single arm | >3.62% | 43% (of both cohorts) | 8.7 (95% CI 6.4–11.9) | 13.9 | 28.2% = 24% of 17.31% of 22 | ||
| C.Li 2021 [76] | retrospective | 53 | Pyrotinib + Capecitabine (35%)/ Other (63%)/ Mono (3%) | Single arm | 77% | 7.0 (Range 6.1–7.8) | 43.4% | ||||
| Y.Li 2021 [77] | retrospective | 23 | Pyrotinib + Vinorelbine | Single arm | 6.3 (Range 3.4–9.2) | ||||||
| Yan 2022—Permeate [78] | phase 2 | 78 | Pyrotinib + Capecitabine | Single arm | 76% | 66.7% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Werter, I.M.; Remmelzwaal, S.; Burchell, G.L.; de Gruijl, T.D.; Konings, I.R.; van der Vliet, H.J.; Menke-van der Houven van Oordt, C.W. Systemic Therapy for Patients with HER2-Positive Breast Cancer and Brain Metastases: A Systematic Review and Meta-Analysis. Cancers 2022, 14, 5612. https://doi.org/10.3390/cancers14225612
Werter IM, Remmelzwaal S, Burchell GL, de Gruijl TD, Konings IR, van der Vliet HJ, Menke-van der Houven van Oordt CW. Systemic Therapy for Patients with HER2-Positive Breast Cancer and Brain Metastases: A Systematic Review and Meta-Analysis. Cancers. 2022; 14(22):5612. https://doi.org/10.3390/cancers14225612
Chicago/Turabian StyleWerter, Inge M., Sharon Remmelzwaal, George L. Burchell, Tanja D. de Gruijl, Inge R. Konings, Hans J. van der Vliet, and C. Willemien Menke-van der Houven van Oordt. 2022. "Systemic Therapy for Patients with HER2-Positive Breast Cancer and Brain Metastases: A Systematic Review and Meta-Analysis" Cancers 14, no. 22: 5612. https://doi.org/10.3390/cancers14225612
APA StyleWerter, I. M., Remmelzwaal, S., Burchell, G. L., de Gruijl, T. D., Konings, I. R., van der Vliet, H. J., & Menke-van der Houven van Oordt, C. W. (2022). Systemic Therapy for Patients with HER2-Positive Breast Cancer and Brain Metastases: A Systematic Review and Meta-Analysis. Cancers, 14(22), 5612. https://doi.org/10.3390/cancers14225612







