Longitudinal Survival Outcomes in Allogeneic Stem Cell Transplantation: An Institutional Experience
Abstract
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Patients and Study Design
2.2. Endpoints
2.3. Statistical Analysis
3. Results
3.1. Patient and Disease Characteristics
3.2. Transplant Characteristics
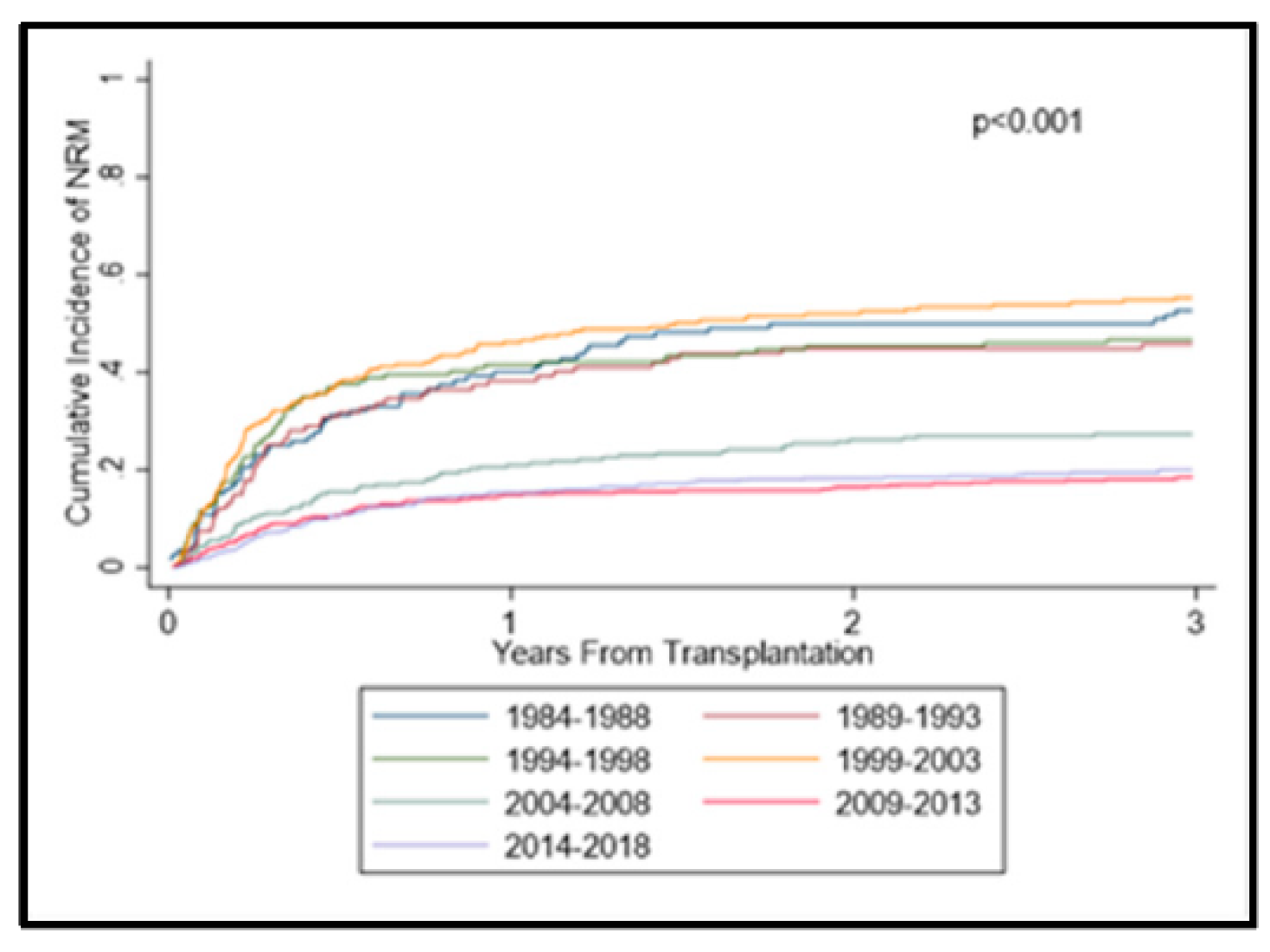
3.3. Acute Graft-versus-Host Disease and Non-Relapse Mortality
3.4. Relapse
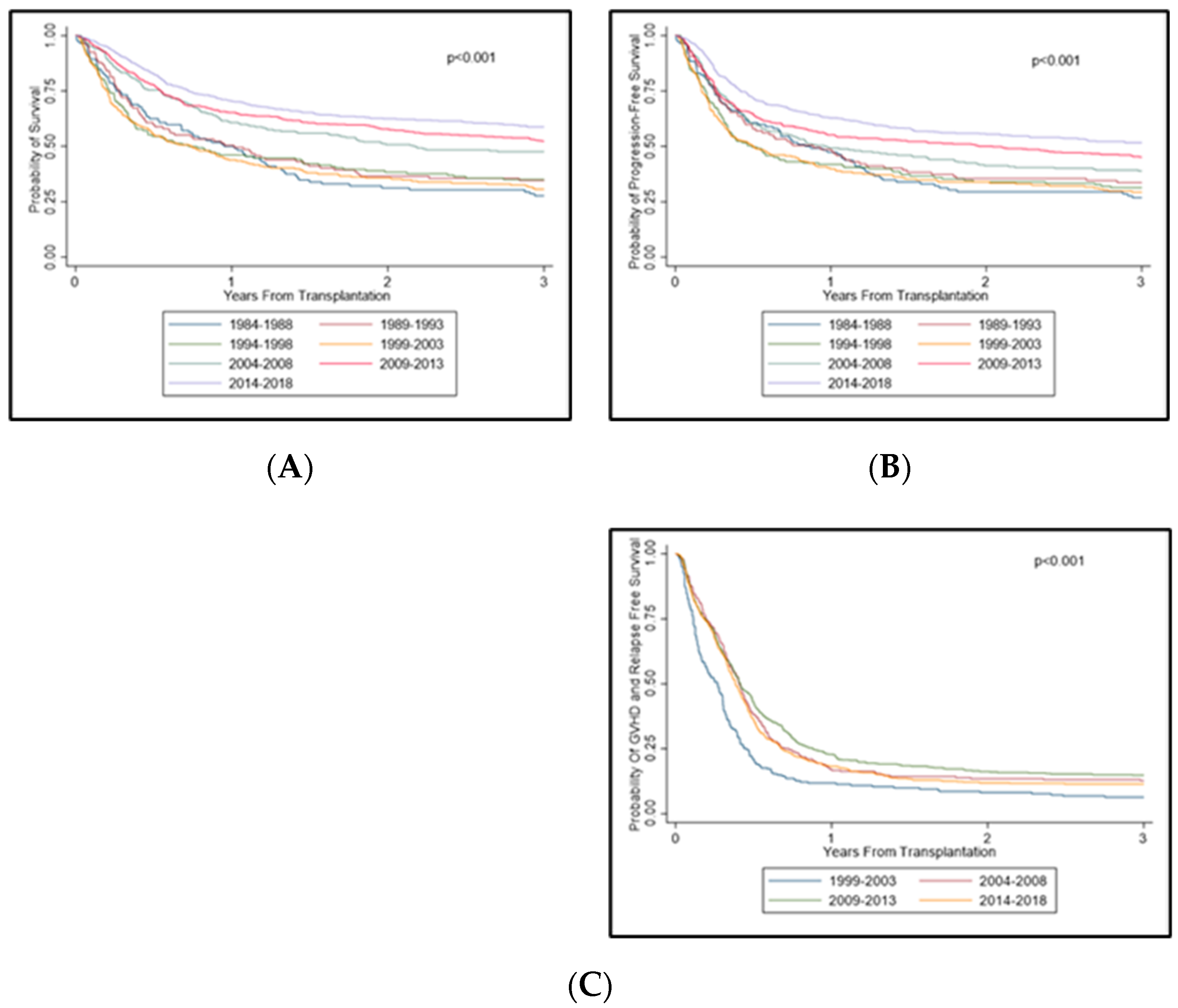
3.5. Survival Trends across Time Periods
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Gratwohl, A.; Pasquini, M.C.; Aljurf, M.; Atsuta, Y.; Baldomero, H.; Foeken, L.; Gratwohl, M.; Bouzas, L.F.; Confer, D.; Frauendorfer, K.; et al. One million haemopoietic stem-cell transplants: A retrospective observational study. Lancet Haematol. 2015, 2, e91–e100. [Google Scholar] [CrossRef]
- Marmont, A.M.; Horowitz, M.M.; Gale, R.P.; Sobocinski, K.; Ash, R.C.; van Bekkum, D.W.; Champlin, R.E.; Dicke, K.A.; Goldman, J.M.; Good, R.A. T-cell depletion of HLA-identical transplants in leukemia. Blood 1991, 78, 2120–2130. [Google Scholar] [CrossRef] [PubMed]
- Butcher, B.W.; Collins, R.H. The graft-versus-lymphoma effect: Clinical review and future opportunities. Bone Marrow Transplant. 2005, 36, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Donato, M.L.; Siegel, D.S.; Vesole, D.H.; McKiernan, P.; Nyirenda, T.; Pecora, A.L.; Baker, M.; Goldberg, S.L.; Mato, A.; Goy, A.; et al. The Graft-Versus-Myeloma Effect: Chronic Graft-Versus-Host Disease but Not Acute Graft-Versus-Host Disease Prolongs Survival in Patients with Multiple Myeloma Receiving Allogeneic Transplantation. Biol. Blood Marrow Transplant. 2014, 20, 1211–1216. [Google Scholar] [CrossRef] [PubMed]
- Thomas, E.D.; Buckner, C.D.; Banaji, M.; Clift, R.A.; Fefer, A.; Flournoy, N.; Goodell, B.W.; Hickman, R.O.; Lerner, K.G.; Neiman, P.E.; et al. One hundred patients with acute leukemia treated by chemotherapy, total body irradiation, and allogeneic marrow transplantation. Blood 1977, 49, 511–533. [Google Scholar] [CrossRef]
- Hingorani, S.R.; Guthrie, K.; Batchelder, A.; Schoch, G.; Aboulhosn, N.; Manchion, J.; Mcdonald, G.B. Acute renal failure after myeloablative hematopoietic cell transplant: Incidence and risk factors. Kidney Int. 2005, 67, 272–277. [Google Scholar] [CrossRef]
- Patriarca, F.; Skert, C.; Sperotto, A.; Damiani, D.; Cerno, M.; Geromin, A.; Zaja, F.; Stocchi, R.; Prosdocimo, S.; Fili’, C.; et al. Incidence, outcome, and risk factors of late-onset noninfectious pulmonary complications after unrelated donor stem cell transplantation. Bone Marrow Transplant. 2004, 33, 751–758. [Google Scholar] [CrossRef][Green Version]
- Issa, H.; Sharma, N.; Zhao, Q.; Ruppert, A.S.; Elder, P.; Benson, D.M.; Penza, S.; Vasu, S.; William, B.; Jaglowski, S.; et al. Comparison of Two Doses of Antithymocyte Globulin in Reduced-Intensity Conditioning Allogeneic Hematopoietic Stem Cell Transplantation. Biol. Blood Marrow Transplant. 2019, 25, 1993–2001. [Google Scholar] [CrossRef]
- Efebera, Y.A.; Geyer, S.; Andritsos, L.; Vasu, S.; Jaglowski, S.; Bingman, A.; Blum, W.; Klisovic, R.; Hofmeister, C.C.; Benson, D.M.; et al. Atorvastatin for the Prophylaxis of Acute Graft-versus-Host Disease in Patients Undergoing HLA-Matched Related Donor Allogeneic Hematopoietic Stem Cell Transplantation (allo-HCT). Biol. Blood Marrow Transplant. 2015, 22, 71–79. [Google Scholar] [CrossRef]
- Salem, G.; Ruppert, A.S.; Elder, P.; Hofmeister, C.C.; Benson, D.M.; Penza, S.; Andritsos, L.; Klisovic, R.; Vasu, S.; Blum, W.; et al. Lower dose of antithymocyte globulin does not increase graft-versus-host disease in patients undergoing reduced-intensity conditioning allogeneic hematopoietic stem cell transplant. Leuk. Lymphoma 2014, 56, 1058–1065. [Google Scholar] [CrossRef]
- Chen, Y.-B.; Efebera, Y.A.; Johnston, L.; Ball, E.D.; Avigan, D.; Lekakis, L.J.; Bachier, C.R.; Martin, P.; Duramad, O.; Ishii, Y.; et al. Increased Foxp3 + Helios + Regulatory T Cells and Decreased Acute Graft-versus-Host Disease after Allogeneic Bone Marrow Transplantation in Patients Receiving Sirolimus and RGI-2001, an Activator of Invariant Natural Killer T Cells. Biol. Blood Marrow Transplant. 2017, 23, 625–634. [Google Scholar] [CrossRef] [PubMed]
- Gooley, T.A.; Chien, J.W.; Pergam, S.A.; Hingorani, S.; Sorror, M.L.; Boeckh, M.; Martin, P.J.; Sandmaier, B.M.; Marr, K.A.; Appelbaum, F.R.; et al. Reduced Mortality after Allogeneic Hematopoietic-Cell Transplantation. N. Engl. J. Med. 2010, 363, 2091–2101. [Google Scholar] [CrossRef] [PubMed]
- Remberger, M.; Ackefors, M.; Berglund, S.; Blennow, O.; Dahllöf, G.; Dlugosz, A.; Garming-Legert, K.; Gertow, J.; Gustafsson, B.; Hassan, M.; et al. Improved Survival after Allogeneic Hematopoietic Stem Cell Transplantation in Recent Years. A Single-Center Study. Biol. Blood Marrow Transplant. 2011, 17, 1688–1697. [Google Scholar] [CrossRef] [PubMed]
- Shouval, R.; Fein, J.A.; Labopin, M.; Kröger, N.; Duarte, R.F.; Bader, P.; Chabannon, C.; Kuball, J.; Basak, G.W.; Dufour, C.; et al. Outcomes of allogeneic haematopoietic stem cell transplantation from HLA-matched and alternative donors: A European Society for Blood and Marrow Transplantation registry retrospective analysis. Lancet Haematol. 2019, 6, e573–e584. [Google Scholar] [CrossRef]
- Penack, O.; Peczynski, C.; Mohty, M.; Yakoub-Agha, I.; Styczynski, J.; Montoto, S.; Duarte, R.F.; Kröger, N.; Schoemans, H.; Koenecke, C.; et al. How much has allogeneic stem cell transplant–related mortality improved since the 1980s? A retrospective analysis from the EBMT. Blood Adv. 2020, 4, 6283–6290. [Google Scholar] [CrossRef]
- McDonald, G.B.; Sandmaier, B.M.; Mielcarek, M.; Sorror, M.; Pergam, S.A.; Cheng, G.-S.; Hingorani, S.; Boeckh, M.; Flowers, M.D.; Lee, S.J.; et al. Survival, Nonrelapse Mortality, and Relapse-Related Mortality After Allogeneic Hematopoietic Cell Transplantation: Comparing 2003–2007 Versus 2013–2017 Cohorts. Ann. Intern. Med. 2020, 172, 229–239. [Google Scholar] [CrossRef]
- Nagler, A.; Ngoya, M.; Galimard, J.-E.; Labopin, M.; Bornhäuser, M.; Stelljes, M.; Finke, J.; Ganser, A.; Einsele, H.; Kröger, N.; et al. Longitudinal Outcome over Two Decades of Unrelated Allogeneic Stem Cell Transplantation for Relapsed/Refractory Acute Myeloid Leukemia: An ALWP/EBMT Analysis. Clin. Cancer Res. 2022, 28, 4258–4266. [Google Scholar] [CrossRef]
- Gragert, L.; Eapen, M.; Williams, E.; Freeman, J.; Spellman, S.; Baitty, R.; Hartzman, R.; Rizzo, J.D.; Horowitz, M.; Confer, D.; et al. HLA Match Likelihoods for Hematopoietic Stem-Cell Grafts in the U.S. Registry. N. Engl. J. Med. 2014, 371, 339–348. [Google Scholar] [CrossRef]
- Bacigalupo, A.; Ballen, K.; Rizzo, D.; Giralt, S.; Lazarus, H.; Ho, V.; Apperley, J.; Slavin, S.; Pasquini, M.; Sandmaier, B.M.; et al. Defining the Intensity of Conditioning Regimens: Working Definitions. Biol. Blood Marrow Transplant. 2009, 15, 1628–1633. [Google Scholar] [CrossRef]
- Holtan, S.G.; DeFor, T.E.; Lazaryan, A.; Bejanyan, N.; Arora, M.; Brunstein, C.G.; Blazar, B.R.; MacMillan, M.; Weisdorf, D.J. Composite end point of graft-versus-host disease-free, relapse-free survival after allogeneic hematopoietic cell transplantation. Blood 2015, 125, 1333–1338. [Google Scholar] [CrossRef]
- Glucksberg, H.; Storb, R.; Fefer, A.; Buckner, C.D.; Neiman, P.E.; Clift, R.A.; Lerner, K.G.; Thomas, E.D. Clinical manifestations of graft-versus-host disease in human recipients of marrow from HL-A-Matched sibling donors. Transplantation 1974, 18, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Shulman, H.M.; Sullivan, K.M.; Weiden, P.L.; McDonald, G.B.; Striker, G.E.; Sale, G.E.; Hackman, R.; Tsoi, M.-S.; Storb, R.; Thomas, E.D. Chronic graft-versus-host syndrome in man: A long-term clinicopathologic study of 20 seattle patients. Am. J. Med. 1980, 69, 204–217. [Google Scholar] [CrossRef]
- Sorror, M.L.; Maris, M.B.; Storb, R.; Baron, F.; Sandmaier, B.M.; Maloney, D.G.; Storer, B. Hematopoietic cell transplantation (HCT)-specific comorbidity index: A new tool for risk assessment before allogeneic HCT. Blood 2005, 106, 2912–2919. [Google Scholar] [CrossRef]
- Emadi, A.; Jones, R.J.; Brodsky, R.A. Cyclophosphamide and cancer: Golden anniversary. Nat. Rev. Clin. Oncol. 2009, 6, 638–647. [Google Scholar] [CrossRef] [PubMed]
- McDonald, G.B.; Slattery, J.T.; Bouvier, M.E.; Ren, S.; Batchelder, A.L.; Kalhorn, T.F.; Schoch, H.G.; Anasetti, C.; Gooley, T. Cyclophosphamide metabolism, liver toxicity, and mortality following hematopoietic stem cell transplantation. Blood 2003, 101, 2043–2048. [Google Scholar] [CrossRef]
- Ruutu, T.; Eriksson, B.; Remes, K.; Juvonen, E.; Volin, L.; Remberger, M.; Parkkali, T.; Hagglund, H.; Ringdén, O. Ursodeoxycholic acid for the prevention of hepatic complications in allogeneic stem cell transplantation. Blood 2002, 100, 1977–1983. [Google Scholar] [CrossRef] [PubMed]
- Ohashi, K.; Tanabe, J.; Watanabe, R.; Tanaka, T.; Sakamaki, H.; Maruta, A.; Okamoto, S.; Aotsuka, N.; Saito, K.; Nishimura, M.; et al. The Japanese multicenter open randomized trial of ursodeoxycholic acid prophylaxis for hepatic veno-occlusive disease after stem cell transplantation. Am. J. Hematol. 2000, 64, 32–38. [Google Scholar] [CrossRef]
- Essell, J.H.; Schroeder, M.T.; Harman, G.S.; Halvorson, R.; Lew, V.; Callander, N.; Snyder, M.; Lewis, S.K.; Allerton, J.P.; Thompson, J.M. Ursodiol Prophylaxis against Hepatic Complications of Allogeneic Bone Marrow Transplantation. Ann. Intern. Med. 1998, 128, 975–981. [Google Scholar] [CrossRef]
- Ottinger, H.; Beelen, D.; Scheulen, B.; Schaefer, U.; Grosse-Wilde, H. Improved immune reconstitution after allotransplantation of peripheral blood stem cells instead of bone marrow. Blood 1996, 88, 2775–2779. [Google Scholar] [CrossRef]
- Malard, F.; Huang, X.-J.; Sim, J.P.Y. Treatment and unmet needs in steroid-refractory acute graft-versus-host disease. Leukemia 2020, 34, 1229–1240. [Google Scholar] [CrossRef]
- Zeiser, R.; von Bubnoff, N.; Butler, J.; Mohty, M.; Niederwieser, D.; Or, R.; Szer, J.; Wagner, E.M.; Zuckerman, T.; Mahuzier, B.; et al. Ruxolitinib for Glucocorticoid-Refractory Acute Graft-versus-Host Disease. N. Engl. J. Med. 2020, 382, 1800–1810. [Google Scholar] [CrossRef] [PubMed]
- Yakoub-Agha, I.; Mesnil, F.; Kuentz, M.; Boiron, J.M.; Ifrah, N.; Milpied, N.; Chehata, S.; Esperou, H.; Vernant, J.-P.; Michallet, M.; et al. Allogeneic Marrow Stem-Cell Transplantation From Human Leukocyte Antigen–Identical Siblings Versus Human Leukocyte Antigen–Allelic–Matched Unrelated Donors (10/10) in Patients With Standard-Risk Hematologic Malignancy: A Prospective Study From the French Society of Bone Marrow Transplantation and Cell Therapy. J. Clin. Oncol. 2006, 24, 5695–5702. [Google Scholar] [CrossRef] [PubMed]
- Weisdorf, D.J.; Anasetti, C.; Antin, J.H.; Kernan, N.A.; Kollman, C.; Snyder, D.; Petersdorf, E.; Nelson, G.; McGlave, P. Allogeneic bone marrow transplantation for chronic myelogenous leukemia: Comparative analysis of unrelated versus matched sibling donor transplantation. Blood 2002, 99, 1971–1977. [Google Scholar] [CrossRef] [PubMed]


| All (n = 1943) | 1984–1988 (n = 112) | 1989–1993 (n = 107) | 1994–1998 (n = 154) | 1999–2003 (n = 221) | 2004–2008 (n = 252) | 2009–2013 (n = 498) | 2014–2018 (n = 599) | p-Value | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | % | x̄ /N | SD/% | x̄ /N | SD/% | Mean/N | x̄ /N | x̄ /N | SD/% | x̄ /N | SD/% | x̄ /N | SD/% | Mean/N | SD/% | ||
| Age at SCT, mean, SD | 48.1 | 13.9 | 31.1 | 8.5 | 35.6 | 9.5 | 42.2 | 9.7 | 44.6 | 11.7 | 48.7 | 12.7 | 50.5 | 13.2 | 54.0 | 13.1 | <0.001 |
| Age at SCT, median, range | 50.0 | 18–76 | 31.0 | 18–53 | 36.0 | 18–62 | 43.0 | 20–65 | 46.0 | 21–68 | 50.0 | 20–75 | 53.0 | 18–73 | 57.0 | 18–76 | |
| Donor age, mean, SD | 37.4 | 15.4 | 31.4 | 9.6 | 36.3 | 10.4 | 36.4 | 17.3 | 40.4 | 16.5 | 37.4 | 19.5 | 37.8 | 14.1 | 37.6 | 14.7 | <0.001 |
| Donor age, median, range | 36.0 | 0–81 | 31.0 | 5–61 | 36.0 | 8–64 | 39.0 | 0–70 | 42.0 | 0–78 | 39.0 | 0–81 | 36.0 | 18–73 | 33.0 | 14–79 | |
| Gender, Patients | 0.20 | ||||||||||||||||
| Female | 785 | 40.4 | 42 | 37.5 | 48 | 44.9 | 61 | 39.6 | 82 | 37.1 | 86 | 34.1 | 215 | 43.2 | 251 | 41.9 | |
| Male | 1158 | 59.6 | 70 | 62.5 | 59 | 55.1 | 93 | 60.4 | 139 | 62.9 | 166 | 65.9 | 283 | 56.8 | 348 | 58.1 | |
| Gender, Donor | <0.001 | ||||||||||||||||
| Male | 1267 | 65.7 | 63 | 56.3 | 51 | 47.7 | 73 | 49.3 | 117 | 53.9 | 162 | 65.1 | 357 | 71.8 | 444 | 74.2 | |
| Female | 656 | 34.0 | 49 | 43.8 | 56 | 52.3 | 75 | 50.7 | 100 | 46.1 | 86 | 34.5 | 140 | 28.2 | 150 | 25.1 | |
| Mix of F and M | 5 | 0.3 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 0.4 | 0 | 0.0 | 4 | 0.7 | |
| Recipient-donor gender | <0.001 | ||||||||||||||||
| M–M | 782 | 40.7 | 47 | 42.0 | 31 | 29.0 | 49 | 33.1 | 81 | 37.3 | 110 | 44.4 | 207 | 41.6 | 257 | 43.3 | |
| M–F | 368 | 19.1 | 23 | 20.5 | 28 | 26.2 | 41 | 27.7 | 56 | 25.8 | 53 | 21.4 | 76 | 15.3 | 91 | 15.3 | |
| F–M | 485 | 25.2 | 16 | 14.3 | 20 | 18.7 | 24 | 16.2 | 36 | 16.6 | 52 | 21.0 | 150 | 30.2 | 187 | 31.5 | |
| F–F | 288 | 15.0 | 26 | 23.2 | 28 | 26.2 | 34 | 23.0 | 44 | 20.3 | 33 | 13.3 | 64 | 12.9 | 59 | 9.9 | |
| Race, patients | 0.13 | ||||||||||||||||
| Caucasian | 1830 | 94.3 | 111 | 99.1 | 105 | 98.1 | 143 | 92.9 | 212 | 95.9 | 240 | 95.2 | 465 | 93.4 | 554 | 93.0 | |
| African American | 89 | 4.6 | 1 | 0.9 | 2 | 1.9 | 11 | 7.1 | 8 | 3.6 | 9 | 3.6 | 25 | 5.0 | 33 | 5.5 | |
| Others | 21 | 1.1 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 0.5 | 3 | 1.2 | 8 | 1.6 | 9 | 1.5 | |
| Zip code | 0.02 | ||||||||||||||||
| Rural | 1231 | 63.4 | 74 | 66.1 | 68 | 63.6 | 99 | 64.3 | 139 | 62.9 | 178 | 70.6 | 307 | 61.6 | 366 | 61.1 | |
| Sub urban | 444 | 22.9 | 30 | 26.8 | 24 | 22.4 | 23 | 14.9 | 47 | 21.3 | 46 | 18.3 | 121 | 24.3 | 153 | 25.5 | |
| Urban | 268 | 13.8 | 8 | 7.1 | 15 | 14.0 | 32 | 20.8 | 35 | 15.8 | 28 | 11.1 | 70 | 14.1 | 80 | 13.4 | |
| Diagnosis | <0.001 | ||||||||||||||||
| AA | 37 | 1.9 | 5 | 4.5 | 2 | 1.9 | 6 | 3.9 | 5 | 2.3 | 1 | 0.4 | 11 | 2.2 | 7 | 1.2 | |
| ALL | 229 | 11.8 | 15 | 13.4 | 13 | 12.1 | 15 | 9.7 | 18 | 8.1 | 30 | 11.9 | 58 | 11.6 | 80 | 13.4 | |
| AML | 705 | 36.3 | 40 | 35.7 | 29 | 27.1 | 46 | 29.9 | 67 | 30.3 | 85 | 33.7 | 199 | 40.0 | 239 | 39.9 | |
| MM | 55 | 2.8 | 1 | 0.9 | 6 | 5.6 | 5 | 3.2 | 16 | 7.2 | 11 | 4.4 | 5 | 1.0 | 11 | 1.8 | |
| CML | 196 | 10.1 | 37 | 33.0 | 42 | 39.3 | 47 | 30.5 | 28 | 12.7 | 16 | 6.3 | 12 | 2.4 | 14 | 2.3 | |
| CLL | 85 | 4.4 | 0 | 0.0 | 0 | 0.0 | 4 | 2.6 | 9 | 4.1 | 28 | 11.1 | 28 | 5.6 | 16 | 2.7 | |
| HD | 70 | 3.6 | 4 | 3.6 | 3 | 2.8 | 4 | 2.6 | 12 | 5.4 | 16 | 6.3 | 14 | 2.8 | 17 | 2.8 | |
| NHL | 275 | 14.2 | 7 | 6.3 | 7 | 6.5 | 8 | 5.2 | 47 | 21.3 | 45 | 17.9 | 87 | 17.5 | 74 | 12.4 | |
| MDS | 195 | 10.0 | 2 | 1.8 | 5 | 4.7 | 16 | 10.4 | 15 | 6.8 | 12 | 4.8 | 57 | 11.4 | 88 | 14.7 | |
| MPD | 89 | 4.6 | 0 | 0.0 | 0 | 0.0 | 3 | 1.9 | 4 | 1.8 | 7 | 2.8 | 26 | 5.2 | 49 | 8.2 | |
| Others | 7 | 0.4 | 1 | 0.9 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 0.4 | 1 | 0.2 | 4 | 0.7 | |
| KPS | <0.001 | ||||||||||||||||
| <90 | 540 | 30.8 | 9 | 81.8 | 11 | 34.4 | 66 | 43.4 | 65 | 30.7 | 60 | 24.1 | 153 | 30.7 | 176 | 29.4 | |
| >=90 | 1213 | 69.2 | 2 | 18.2 | 21 | 65.6 | 86 | 56.6 | 147 | 69.3 | 189 | 75.9 | 345 | 69.3 | 423 | 70.6 | |
| Tissue | <0.001 | ||||||||||||||||
| BM | 630 | 32.4 | 112 | 100.0 | 107 | 100.0 | 154 | 100.0 | 86 | 38.9 | 14 | 5.6 | 37 | 7.4 | 120 | 20.0 | |
| CB | 86 | 4.4 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 2 | 0.8 | 66 | 13.3 | 18 | 3.0 | |
| PB | 1227 | 63.2 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 135 | 61.1 | 236 | 93.7 | 395 | 79.3 | 461 | 77.0 | |
| Donor type | <0.001 | ||||||||||||||||
| Matched related | 908 | 46.7 | 107 | 95.5 | 82 | 76.6 | 102 | 66.2 | 147 | 66.5 | 138 | 54.8 | 164 | 32.9 | 168 | 28.0 | |
| Matched unrelated | 756 | 38.9 | 0 | 0.0 | 15 | 14.0 | 37 | 24.0 | 57 | 25.8 | 85 | 33.7 | 240 | 48.2 | 322 | 53.8 | |
| Mismatch related | 113 | 5.8 | 5 | 4.5 | 9 | 8.4 | 6 | 3.9 | 8 | 3.6 | 0 | 0.0 | 10 | 2.0 | 75 | 12.5 | |
| Mismatch unrelated | 166 | 8.5 | 0 | 0.0 | 1 | 0.9 | 9 | 5.8 | 9 | 4.1 | 29 | 11.5 | 84 | 16.9 | 34 | 5.7 | |
| Conditioning | <0.001 | ||||||||||||||||
| MA | 1068 | 55.0 | 112 | 100.0 | 107 | 100.0 | 154 | 100.0 | 185 | 83.7 | 113 | 44.8 | 126 | 25.3 | 271 | 45.2 | |
| RIC | 875 | 45.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 36 | 16.3 | 139 | 55.2 | 372 | 74.7 | 328 | 54.8 | |
| Comorbidity index, median, range | 2 | 0–12 | NA | NA | NA | NA | 1 | 0–6 | 3 | 0–12 | 2 | 0–10 | <0.001 | ||||
| 0–1 | 422 | 33.8 | NA | NA | NA | NA | 78 | 51.7 | 120 | 24.1 | 224 | 37.4 | |||||
| 2–3 | 461 | 36.9 | NA | NA | NA | NA | 60 | 39.7 | 189 | 38.0 | 212 | 35.4 | |||||
| 4–5 | 278 | 22.3 | NA | NA | NA | NA | 12 | 7.9 | 140 | 28.1 | 126 | 21.0 | |||||
| 5+ | 87 | 7.0 | NA | NA | NA | NA | 1 | 0.7 | 49 | 9.8 | 37 | 6.2 | |||||
| All (n = 1943) | 1984–1988 (n = 112) | 1989–1993 (n = 107) | 1994–1998 (n = 154) | 1999–2003 (n = 221) | 2004–2008 (n = 252) | 2009–2013 (n = 498) | 2014–2018 (n = 599) | p-Value | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ANC engraftment day, median, range | 16 | 2–120 | 14 | 10–31 | 17 | 9–42 | 20 | 3–120 | 16 | 8–28 | 15 | 8–23 | 16 | 2–45 | 16 | 6–43 | <0.001 |
| Platelet engraftment day, median, range | 19 | 8–758 | 16 | 9–65 | 23 | 10–94 | 31 | 10–230 | 25 | 8–112 | 19 | 10–387 | 17 | 8–242 | 18 | 8–758 | <0.001 |
| Post-transplant response | <0.001 | ||||||||||||||||
| CR | 1486 | 76.5 | 56 | 50.0 | 49 | 45.8 | 100 | 64.9 | 169 | 76.5 | 193 | 76.6 | 400 | 80.3 | 519 | 86.6 | |
| Less than CR | 164 | 8.4 | 20 | 17.9 | 5 | 4.7 | 8 | 5.2 | 12 | 5.4 | 26 | 10.3 | 40 | 8.0 | 53 | 8.8 | |
| Progression | 136 | 7.0 | 10 | 8.9 | 6 | 5.6 | 17 | 11.0 | 15 | 6.8 | 24 | 9.5 | 43 | 8.6 | 21 | 3.5 | |
| Not available | 157 | 8.1 | 26 | 23.2 | 47 | 43.9 | 29 | 18.8 | 25 | 11.3 | 9 | 3.6 | 15 | 3.0 | 6 | 1.0 | |
| Year of Transplant | aGVHD 2–4 | aGVHD 3–4 | cGVHD E + L | cGVHD E | |
|---|---|---|---|---|---|
| 1999–2003 | 1999–2003 | ||||
| Day 100 | 36 (30–43) | 21 (16–26) | Day 180 | 29 (23–35) | 21 (16–26) |
| Day 180 | 37 (31–43) | 22 (17–27) | Day 365 | 38 (31–44) | 27 (21–33) |
| 2004–2008 | 2004–2008 | ||||
| Day 100 | 27 (33–22) | 10 (7–14) | Day 180 | 22 (17–28) | 19 (14–24) |
| Day 180 | 31 (36–25) | 11 (7–15) | Day 365 | 40 (34–46) | 34 (28–40) |
| 2009–2013 | 2009–2013 | ||||
| Day 100 | 38 (42–33) | 11 (8–14) | Day 180 | 15 (12–18) | 14 (11–17) |
| Day 180 | 44 (49–40) | 13 (10–16) | Day 365 | 34 (29–38) | 31 (27–35) |
| 2014–2018 | 2014–2018 | ||||
| Day 100 | 52 (56–48) | 19 (16–22) | Day 180 | 29 (25–32) | 26 (23–30) |
| Day 180 | 55 (59–51) | 21 (18–24) | Day 365 | 48 (44–52) | 44 (40–48) |
| Univariable Modeling | HR | 95% CI | p | |
|---|---|---|---|---|
| Year of HCT | ||||
| 1984–1988 | 1.00 | |||
| 1989–1993 | 0.88 | 0.48 | 1.60 | 0.668 |
| 1994–1998 | 1.32 | 0.79 | 2.21 | 0.296 |
| 1999–2003 | 1.25 | 0.76 | 2.04 | 0.376 |
| 2004–2008 | 1.61 | 1.02 | 2.55 | 0.042 |
| 2009–2013 | 1.57 | 1.02 | 2.42 | 0.042 |
| 2014–2018 | 1.12 | 0.73 | 1.74 | 0.601 |
| Multivariable Modeling | ||||
| Year of HCT | ||||
| 1984–1988 | ||||
| 1989–1993 | 0.91 | 0.50 | 1.63 | 0.744 |
| 1994–1998 | 1.37 | 0.79 | 2.37 | 0.263 |
| 1999–2003 | 1.37 | 0.82 | 2.30 | 0.228 |
| 2004–2008 | 2.12 | 1.28 | 3.52 | 0.004 |
| 2009–2013 | 2.25 | 1.35 | 3.74 | 0.002 |
| 2014–2018 | 1.86 | 1.12 | 3.09 | 0.016 |
| Age at HCT, 10-year | 0.92 | 0.84 | 1.00 | 0.05 |
| recipient_donor_gender | ||||
| M–M | ||||
| M–F | 0.79 | 0.61 | 1.04 | 0.09 |
| F–M | 1.40 | 1.12 | 1.74 | 0.003 |
| F–F | 1.03 | 0.77 | 1.38 | 0.832 |
| Race, patients | ||||
| Caucasian | ||||
| non-Caucasian | 1.14 | 0.77 | 1.66 | 0.515 |
| >Zip code | ||||
| Rural | ||||
| Sub urban | 0.93 | 0.75 | 1.16 | 0.524 |
| Urban | 0.72 | 0.53 | 0.97 | 0.031 |
| Diagnosis | ||||
| Lymphoid | ||||
| Myeloid | 1.10 | 0.87 | 1.38 | 0.429 |
| Others | 1.50 | 1.02 | 2.22 | 0.04 |
| Donor type | ||||
| Matched-related | ||||
| Matched-unrelated | 0.73 | 0.60 | 0.90 | 0.004 |
| Mismatch-related | 0.86 | 0.58 | 1.28 | 0.47 |
| Mismatch-unrelated | 0.64 | 0.45 | 0.93 | 0.019 |
| Conditioning | ||||
| MA | ||||
| RIC | 1.58 | 1.23 | 2.03 | <0.001 |
| Remission status at transplant | ||||
| CR | ||||
| PR | 1.26 | 0.95 | 1.68 | 0.112 |
| <PR | 1.46 | 1.16 | 1.84 | 0.001 |
| Year of Transplant | OS | PFS | Relapse | NRM | GRFS |
|---|---|---|---|---|---|
| 1984–1988 | |||||
| Median, years | 1 (0.7–1.2) | 0.8 (0.6–1.2) | |||
| Year 1 | 50 (40–59) | 47 (38–56) | 13 (7–19) | 40 (49–31) | |
| Year 3 | 28 (20–36) | 27 (19–35) | 21 (14–28) | 53 (61–43) | |
| Year 5 | 25 (17–33) | 24 (17–32) | 22 (15–30) | 54 (62–44) | |
| Year 10 | 21 (14–28) | 20 (13–27) | 23 (16–31) | 58 (67–48) | |
| 1989–1993 | |||||
| Median, years | 1 (0.5–1.6) | 0.9 (0.4–1.3) | |||
| Year 1 | 50 (41–59) | 49 (39–58) | 11 (6–18) | 38 (29–47) | |
| Year 3 | 35 (26–44) | 34 (25–43) | 19 (12–27) | 46 (36–55) | |
| Year 5 | 28 (20–37) | 25 (17–34) | 21 (14–30) | 51 (42–60) | |
| Year 10 | 23 (16–32) | 20 (13–28) | 24 (17–33) | 55 (45–64) | |
| 1994–1998 | |||||
| Median, years | 0.7 (0.4–1.5) | 0.5 (0.3–0.9) | |||
| Year 1 | 46 (38–54) | 42 (34–49) | 20 (14–26) | 42 (34–49) | |
| Year 3 | 35 (28–43) | 31 (24–39) | 25 (18–32) | 47 (39–54) | |
| Year 5 | 28 (22–36) | 25 (19–32) | 28 (21–35) | 51 (43–58) | |
| Year 10 | 23 (17–30) | 21 (15–28) | 28 (21–35) | 55 (47–63) | |
| 1999–2003 | |||||
| Median, years | 0.7 (0.5–1) | 0.5 (0.3–0.8) | 0.3 (0.2–0.3) | ||
| Year 1 | 44 (37–50) | 40 (33–46) | 19 (15–25) | 46 (39–53) | 12 (8–16) |
| Year 3 | 31 (25–37) | 29 (23–35) | 23 (18–29) | 55 (48–62) | 6 (4–10) |
| Year 5 | 28 (23–34) | 28 (22–34) | 24 (19–30) | 57 (50–63) | 6 (3–10) |
| Year 10 | 26 (20–32) | 25 (20–31) | 25 (19–31) | 58 (51–64) | 5 (3–9) |
| 2004–2008 | |||||
| Median, years | 2.2 (1.4–3.9) | 1 (0.7–1.8) | 0.4 (0.4–0.4) | ||
| Year 1 | 61 (54–66) | 49 (43–55) | 31 (26–37) | 21 (16–26) | 17 (12–22) |
| Year 3 | 48 (41–54) | 39 (33–45) | 35 (29–41) | 27 (22–33) | 13 (9–17) |
| Year 5 | 40 (34–46) | 33 (27–39) | 38 (32–44) | 31 (26–37) | 11 (8–15) |
| Year 10 | 34 (28–40) | 28 (23–34) | 40 (34–46) | 34 (28–40) | 10 (6–14) |
| 2009–2013 | |||||
| Median, years | 3.8 (2.6–5.9) | 1.9 (1–2.9) | 0.4 (0.4–0.5) | ||
| Year 1 | 65 (61–69) | 55 (51–59) | 30 (26–34) | 15 (12–18) | 23 (19–27) |
| Year 3 | 52 (48–57) | 45 (41–49) | 36 (32–40) | 19 (15–22) | 15 (12–18) |
| Year 5 | 47 (42–51) | 41 (36–45) | 38 (33–42) | 22 (19–26) | 14 (11–17) |
| 2014–2018 | |||||
| Median, years | NR (4.2-NR) | 3.7 (2.3-NR) | 0.4 (0.4–0.4) | ||
| Year 1 | 70 (67–74) | 63 (59–67) | 22 (19–25) | 15 (13–18) | 18 (15–21) |
| Year 3 | 59 (55–63) | 52 (47–56) | 29 (25–32) | 20 (17–23) | 11 (9–14) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, J.; Sigmund, A.M.; Zhao, Q.; Elder, P.; Benson, D.M.; Vasu, S.; Jaglowski, S.; Mims, A.; Choe, H.; Larkin, K.; et al. Longitudinal Survival Outcomes in Allogeneic Stem Cell Transplantation: An Institutional Experience. Cancers 2022, 14, 5587. https://doi.org/10.3390/cancers14225587
Jiang J, Sigmund AM, Zhao Q, Elder P, Benson DM, Vasu S, Jaglowski S, Mims A, Choe H, Larkin K, et al. Longitudinal Survival Outcomes in Allogeneic Stem Cell Transplantation: An Institutional Experience. Cancers. 2022; 14(22):5587. https://doi.org/10.3390/cancers14225587
Chicago/Turabian StyleJiang, Justin, Audrey M. Sigmund, Qiuhong Zhao, Patrick Elder, Don M. Benson, Sumithira Vasu, Samantha Jaglowski, Alice Mims, Hannah Choe, Karilyn Larkin, and et al. 2022. "Longitudinal Survival Outcomes in Allogeneic Stem Cell Transplantation: An Institutional Experience" Cancers 14, no. 22: 5587. https://doi.org/10.3390/cancers14225587
APA StyleJiang, J., Sigmund, A. M., Zhao, Q., Elder, P., Benson, D. M., Vasu, S., Jaglowski, S., Mims, A., Choe, H., Larkin, K., Brammer, J. E., Wall, S., Grieselhuber, N., Saad, A., Penza, S., Efebera, Y. A., & Sharma, N. (2022). Longitudinal Survival Outcomes in Allogeneic Stem Cell Transplantation: An Institutional Experience. Cancers, 14(22), 5587. https://doi.org/10.3390/cancers14225587







