N6-Methyladenosine Modification of CIRCKRT17 Initiated by METTL3 Promotes Osimertinib Resistance of Lung Adenocarcinoma by EIF4A3 to Enhance YAP1 Stability
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Tissue Specimens
2.2. Cell Culture
2.3. Generation of Osimertinib-Resistant LUAD Cell Lines
2.4. Plasmid Construction and Transfection
2.5. Fluorescence In Situ Hybridization (FISH)
2.6. Treatment with Actinomycin D and RNase R
2.7. Calculation of IC50 Value Using MTT Assay
2.8. Colony Formation Assay
2.9. EdU Immunofluorescence Staining
2.10. Flow Cytometry Analysis of Cell Apoptosis
2.11. Immunofluorescence Analysis
2.12. RNA Immunoprecipitation (RIP)
2.13. RNA Pull-Down
2.14. MeRIP
2.15. Quantitative Real-Time PCR (qRT-PCR)
2.16. Western Blot Analysis
2.17. Xenograft Tumor Growth Assay
2.18. Immunohistochemistry and TUNEL Staining
2.19. Statistical Analysis
3. Results
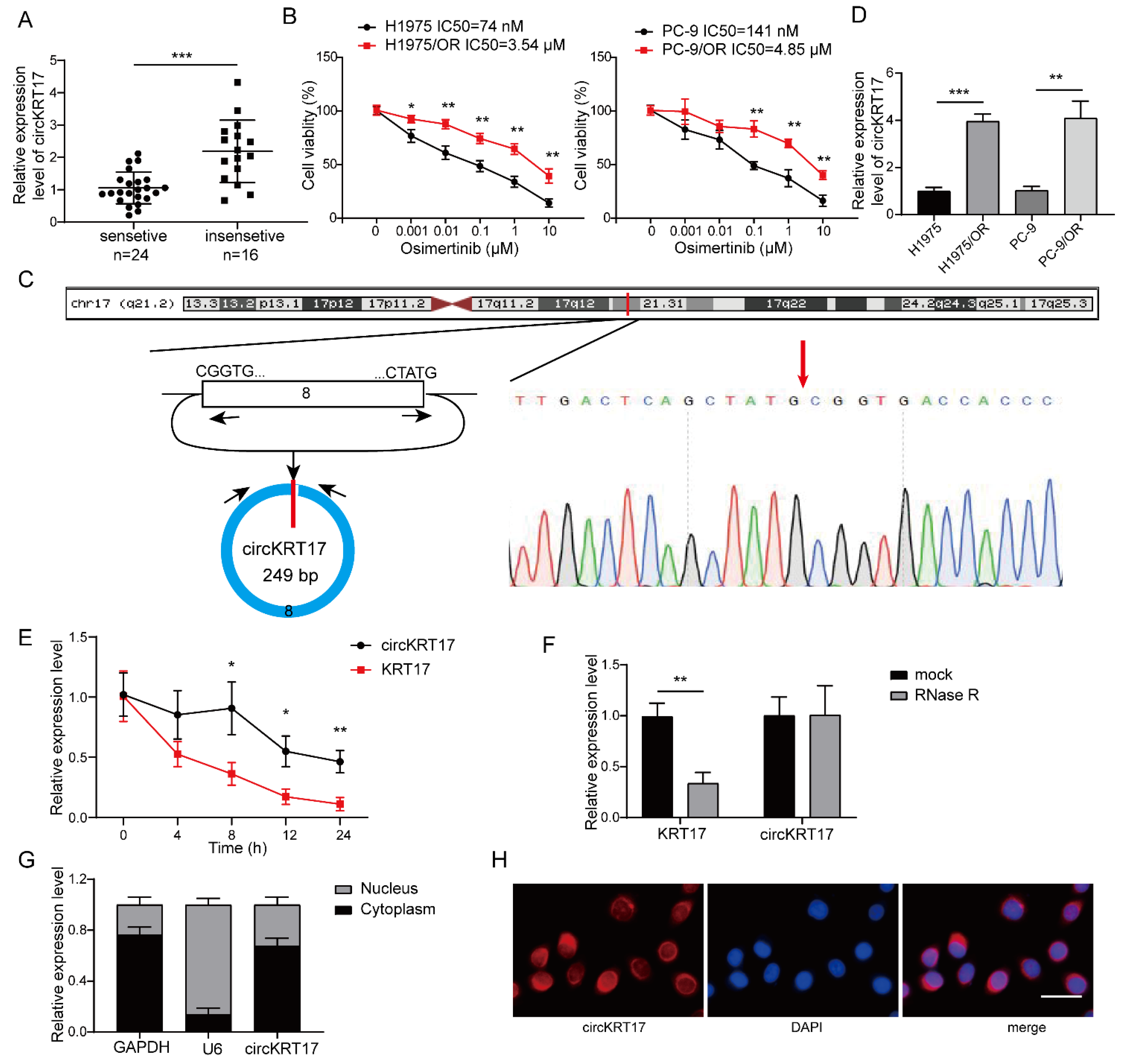
3.1. CircKRT17 Was Elevated in Osimertinib-Resistant LUAD
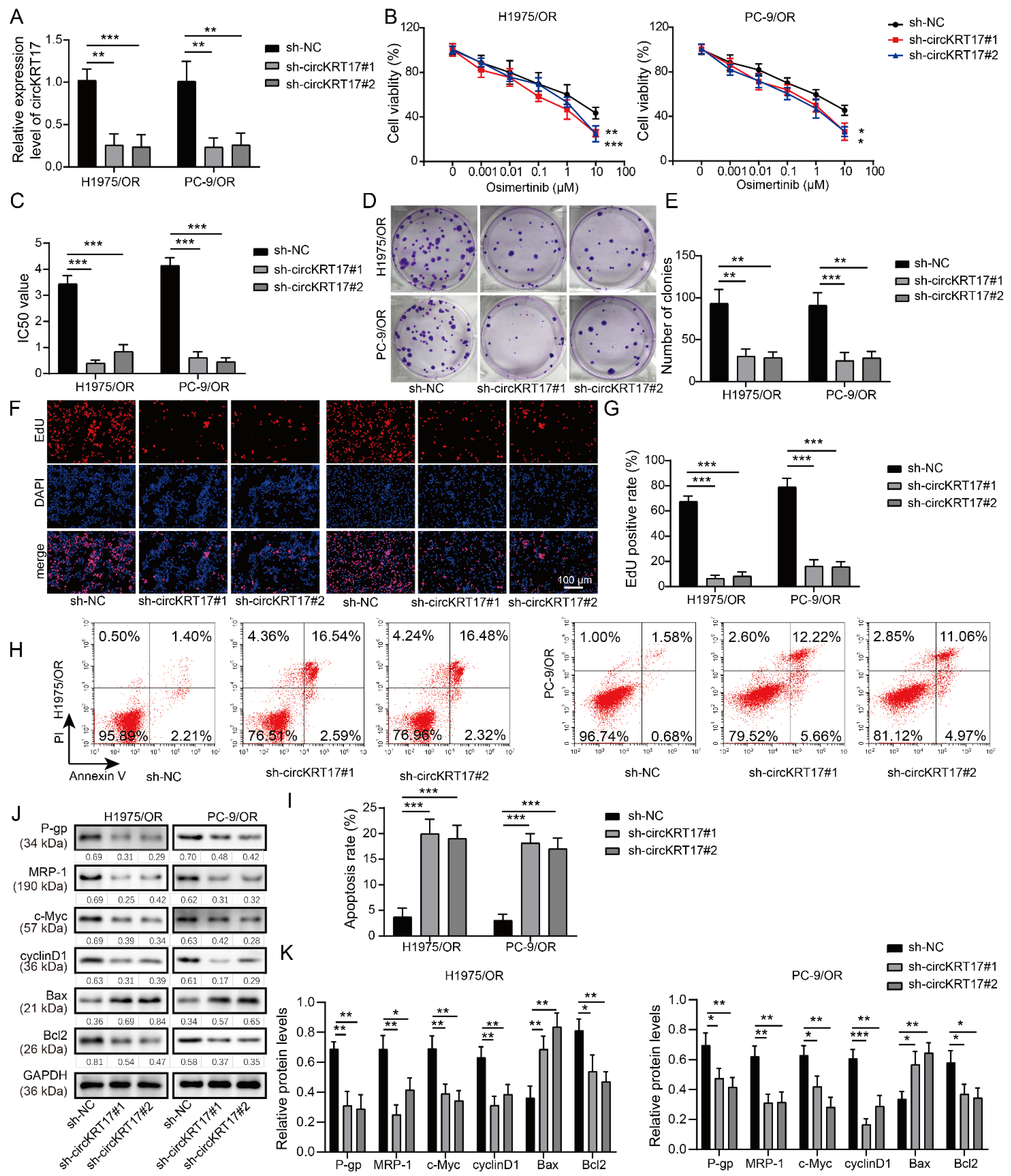
3.2. Knockdown of CIRCKRT17 Increased the Sensitivity of Osimertinib-Resistant LUAD Cells to Osimertinib
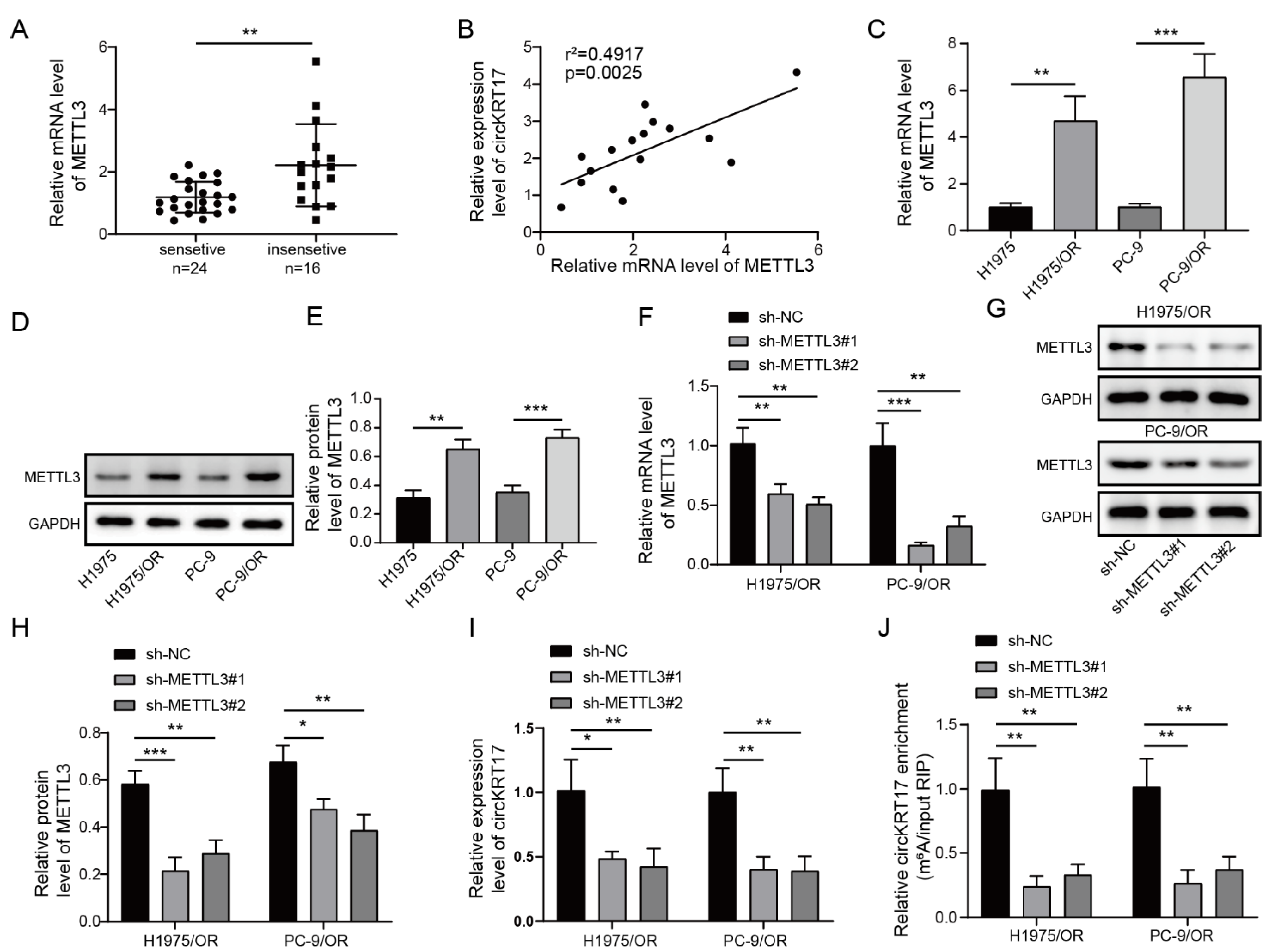
3.3. Elevated METTL3 in LUAD Regulated CIRCKRT17 Expression by m6A Modification
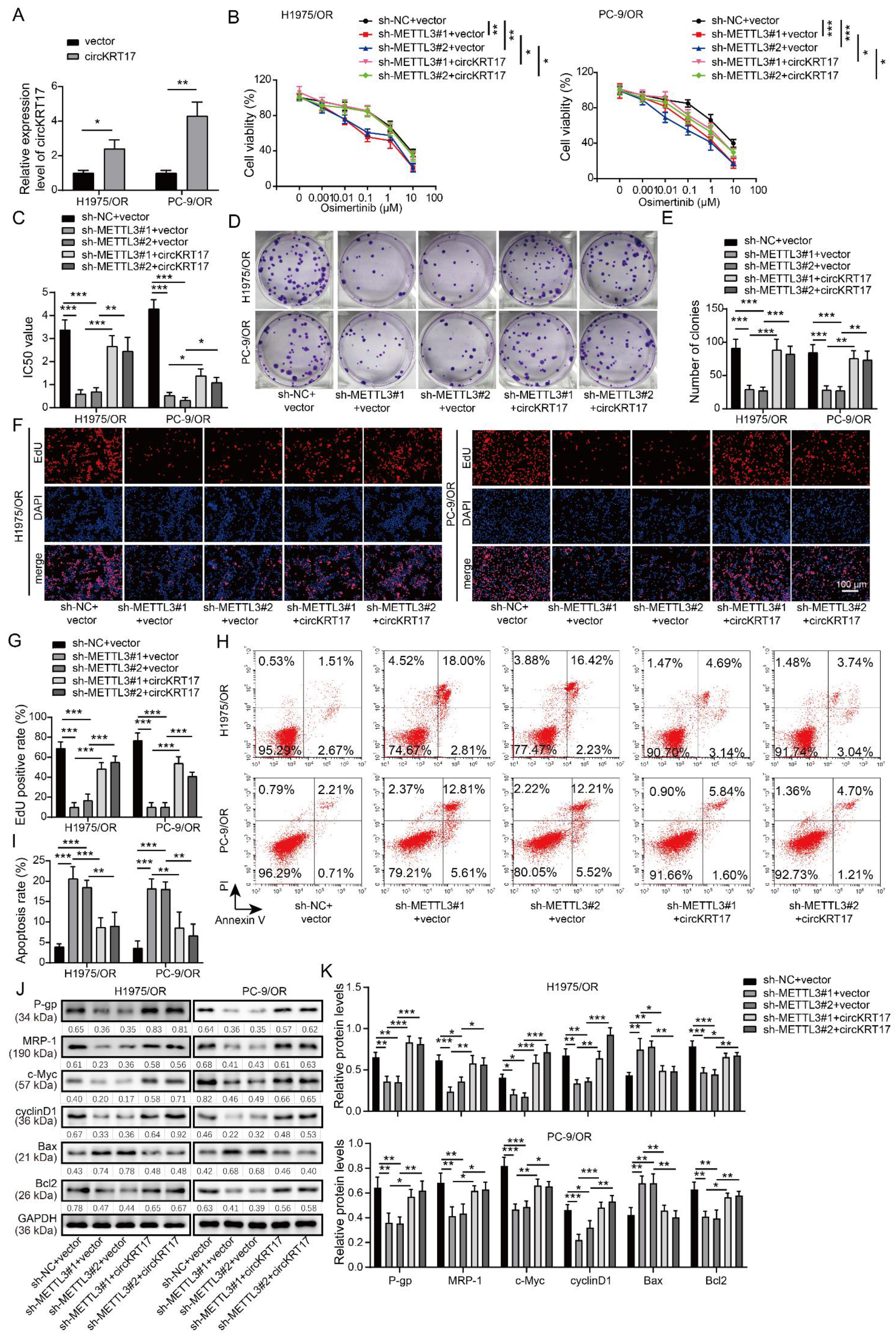
3.4. Knockdown of METTL3 Increased the Sensitivity of Osimertinib-Resistant LUAD Cells to Osimertinib by Inhibiting CIRCKRT17
3.5. CIRCKRT17 Promoted the Expression and Nuclear Transportation of YAP1
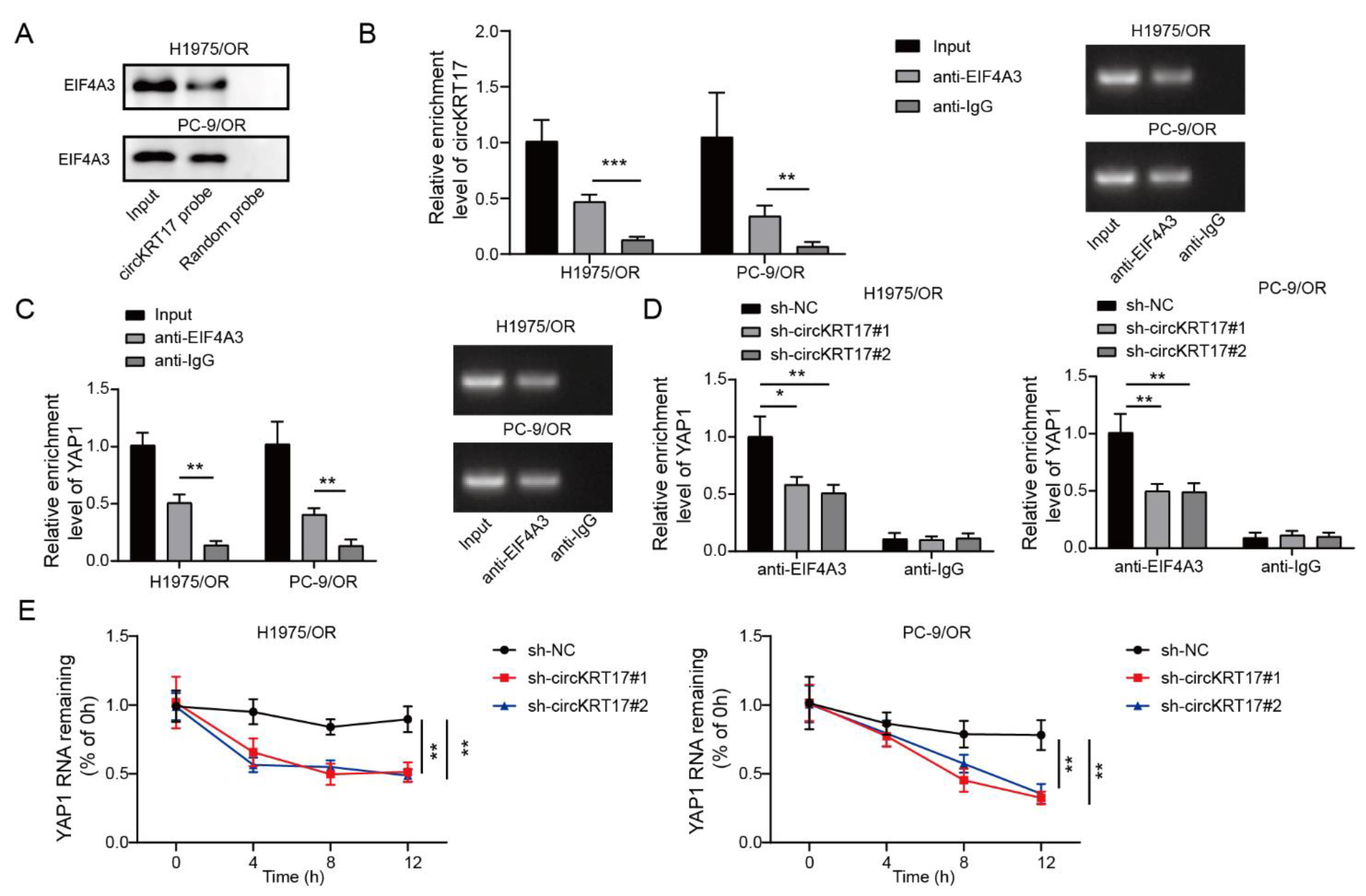
3.6. CIRCKRT17 Enhanced the Stability of YAP1 mRNA by Recruiting EIF4A3
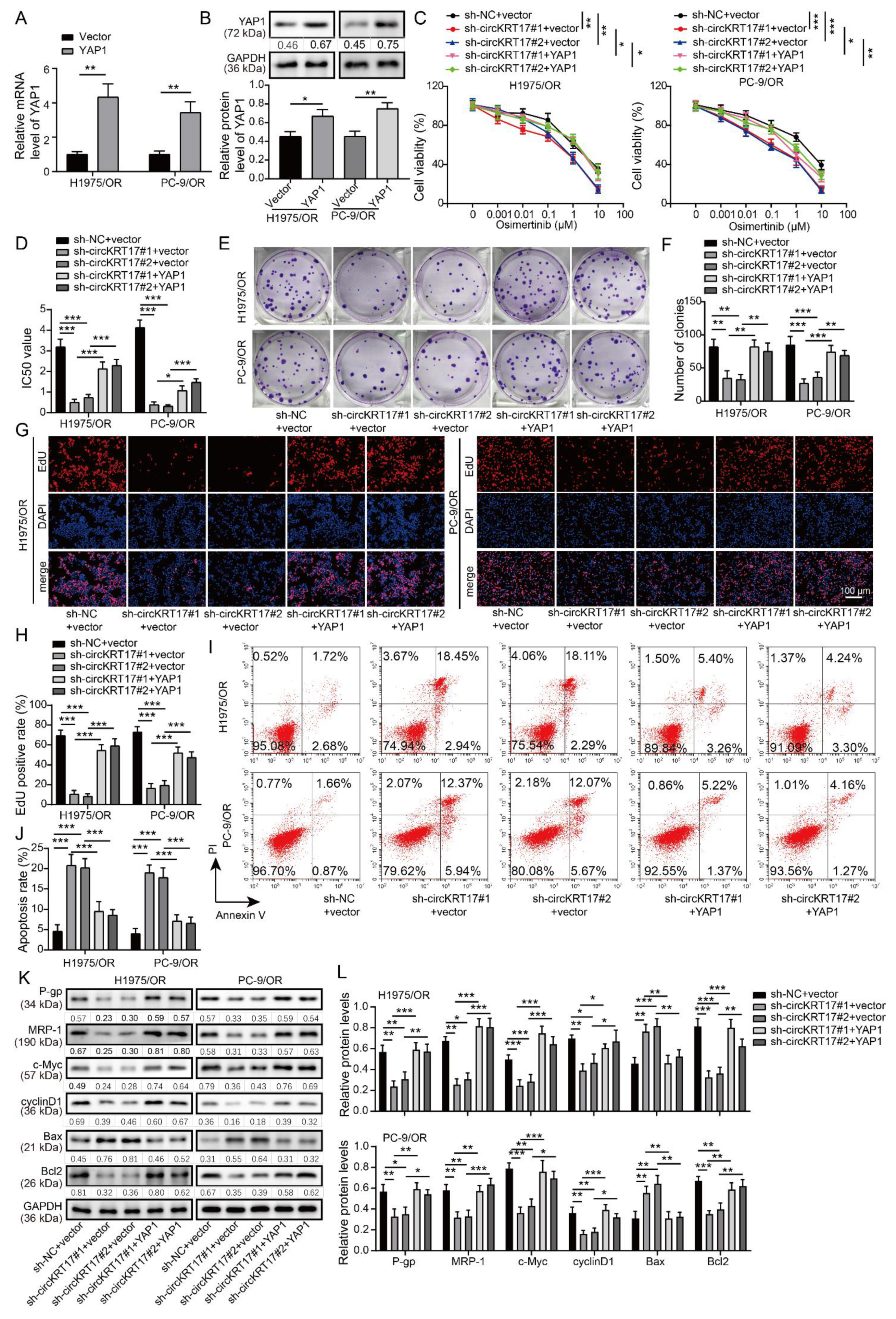
3.7. YAP1 Overexpression Reversed the Promotive Effects of CIRCKRT17 Knockdown on the Sensitivity of Osimertinib-Resistant LUAD Cells to Osimertinib
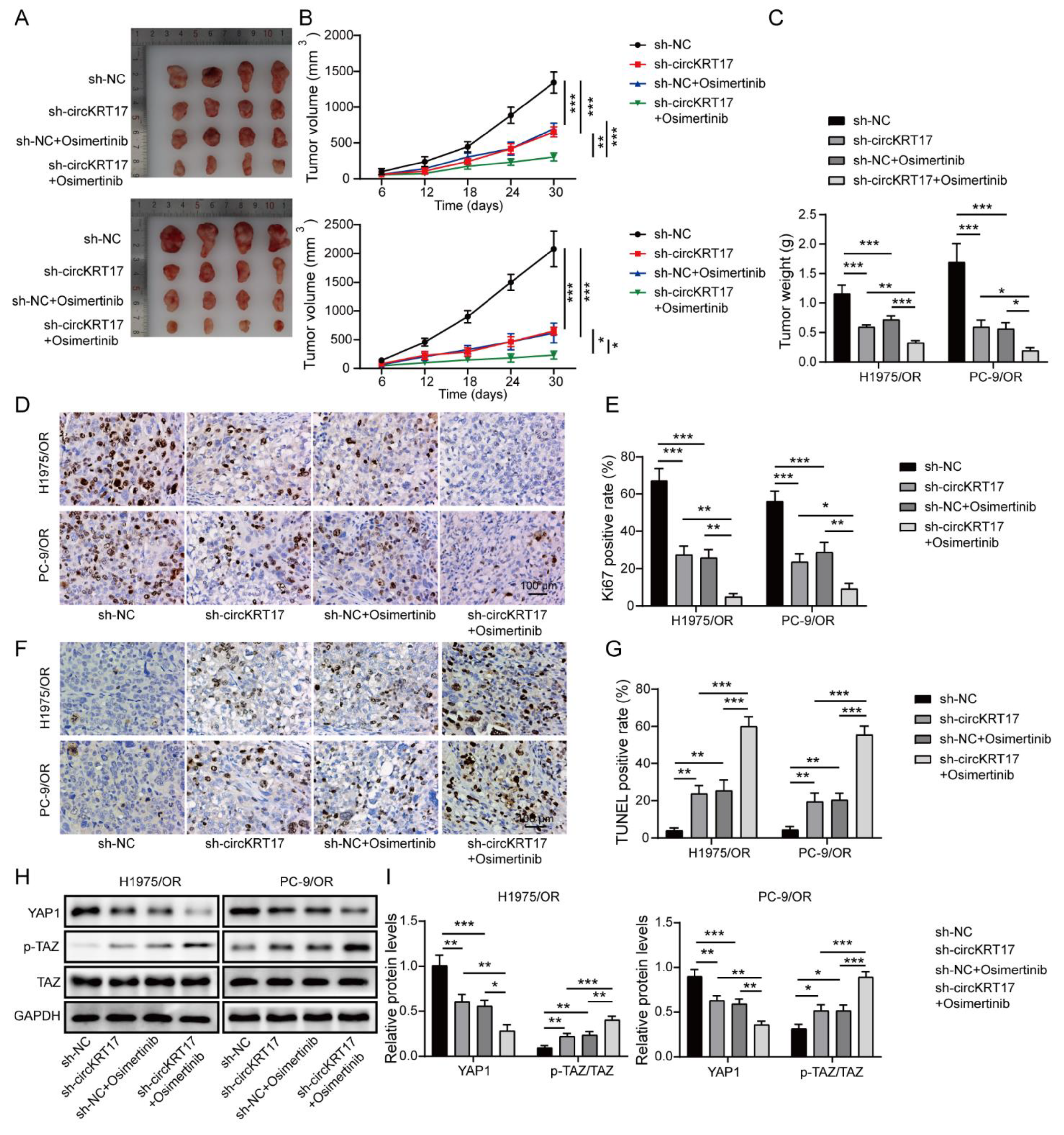
3.8. Knockdown of CIRCKRT17 Enhanced the Suppressive Effects of Osimertinib on Tumor Growth In Vivo by Inhibiting YAP1 Signaling
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Peng, W.; Wang, J.; Shan, B.; Peng, Z.; Dong, Y.; Shi, W.; He, D.; Cheng, Y.; Zhao, W.; Zhang, C.; et al. Diagnostic and Prognostic Potential of Circulating Long Non-Coding RNAs in Non Small Cell Lung Cancer. Cell. Physiol. Biochem. 2018, 49, 816–827. [Google Scholar] [CrossRef]
- Testa, U.; Castelli, G.; Pelosi, E. Lung Cancers: Molecular Characterization, Clonal Heterogeneity and Evolution, and Cancer Stem Cells. Cancers 2018, 10, 248. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.G.; Shih, J.Y. Management of acquired resistance to EGFR TKI-targeted therapy in advanced non-small cell lung cancer. Mol. Cancer 2018, 17, 38. [Google Scholar] [CrossRef]
- Passaro, A.; Mok, T.; Peters, S.; Popat, S.; Ahn, M.J.; de Marinis, F. Recent Advances on the Role of EGFR Tyrosine Kinase Inhibitors in the Management of NSCLC With Uncommon, Non Exon 20 Insertions, EGFR Mutations. J. Thorac. Oncol. 2021, 16, 764–773. [Google Scholar] [CrossRef]
- Shah, R.; Lester, J.F. Tyrosine Kinase Inhibitors for the Treatment of EGFR Mutation-Positive Non-Small-Cell Lung Cancer: A Clash of the Generations. Clin. Lung Cancer 2020, 21, e216–e228. [Google Scholar] [CrossRef]
- Leonetti, A.; Sharma, S.; Minari, R.; Perego, P.; Giovannetti, E.; Tiseo, M. Resistance mechanisms to osimertinib in EGFR-mutated non-small cell lung cancer. Br. J. Cancer 2019, 121, 725–737. [Google Scholar] [CrossRef]
- Mu, Y.; Hao, X.; Xing, P.; Hu, X.; Wang, Y.; Li, T.; Zhang, J.; Xu, Z.; Li, J. Acquired resistance to osimertinib in patients with non-small-cell lung cancer: Mechanisms and clinical outcomes. J. Cancer Res. Clin. Oncol. 2020, 146, 2427–2433. [Google Scholar] [CrossRef] [PubMed]
- Qu, S.; Yang, X.; Li, X.; Wang, J.; Gao, Y.; Shang, R.; Sun, W.; Dou, K.; Li, H. Circular RNA: A new star of noncoding RNAs. Cancer Lett. 2015, 365, 141–148. [Google Scholar] [CrossRef]
- Memczak, S.; Jens, M.; Elefsinioti, A.; Torti, F.; Krueger, J.; Rybak, A.; Maier, L.; Mackowiak, S.D.; Gregersen, L.H.; Munschauer, M.; et al. Circular RNAs are a large class of animal RNAs with regulatory potency. Nature 2013, 495, 333–338. [Google Scholar] [CrossRef]
- Xi, Y.; Fowdur, M.; Liu, Y.; Wu, H.; He, M.; Zhao, J. Differential expression and bioinformatics analysis of circRNA in osteosarcoma. Biosci. Rep. 2019, 39, BSR20181514. [Google Scholar] [CrossRef]
- Kristensen, L.S.; Andersen, M.S.; Stagsted, L.V.W.; Ebbesen, K.K.; Hansen, T.B.; Kjems, J. The biogenesis, biology and characterization of circular RNAs. Nat. Rev. Genet 2019, 20, 675–691. [Google Scholar] [CrossRef] [PubMed]
- Cui, C.; Yang, J.; Li, X.; Liu, D.; Fu, L.; Wang, X. Functions and mechanisms of circular RNAs in cancer radiotherapy and chemotherapy resistance. Mol. Cancer 2020, 19, 58. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yang, Y.; Wang, Z.; Fu, X.; Chu, X.M.; Li, Y.; Wang, Q.; He, X.; Li, M.; Wang, K.; et al. Insights into the regulatory role of circRNA in angiogenesis and clinical implications. Atherosclerosis 2020, 298, 14–26. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Zhu, L.; Lu, C.; Wang, C.; Wang, H.; Jin, H.; Ma, X.; Cheng, Z.; Yu, C.; Wang, S.; et al. circNDUFB2 inhibits non-small cell lung cancer progression via destabilizing IGF2BPs and activating anti-tumor immunity. Nat. Commun. 2021, 12, 295. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Tan, S.; Li, J.; Liu, W.R.; Peng, Y.; Li, W. CircRNAs in lung cancer - Biogenesis, function and clinical implication. Cancer Lett. 2020, 492, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Luo, J.; Gu, Y.; Huang, J.; Luo, Q.; Yang, Y. Comprehensive analysis of circular RNA profiling in AZD9291-resistant non-small cell lung cancer cell lines. Thorac. Cancer 2019, 10, 930–941. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Li, H.; Wu, A.; Peng, Y.; Shu, G.; Yin, G. Functions of N6-methyladenosine and its role in cancer. Mol. Cancer 2019, 18, 176. [Google Scholar] [CrossRef]
- Wang, X.; Lu, Z.; Gomez, A.; Hon, G.C.; Yue, Y.; Han, D.; Fu, Y.; Parisien, M.; Dai, Q.; Jia, G.; et al. N6-methyladenosine-dependent regulation of messenger RNA stability. Nature 2014, 505, 117–120. [Google Scholar] [CrossRef]
- Chen, X.Y.; Zhang, J.; Zhu, J.S. The role of m(6)A RNA methylation in human cancer. Mol. Cancer 2019, 18, 103. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, X.; Liu, L.; Li, J.; Hu, Q.; Sun, R. Expression and Prognostic Significance of m6A-Related Genes in Lung Adenocarcinoma. Med. Sci. Monit. 2020, 26, e919644. [Google Scholar] [CrossRef]
- Jin, D.; Guo, J.; Wu, Y.; Du, J.; Yang, L.; Wang, X.; Di, W.; Hu, B.; An, J.; Kong, L.; et al. m(6)A mRNA methylation initiated by METTL3 directly promotes YAP translation and increases YAP activity by regulating the MALAT1-miR-1914-3p-YAP axis to induce NSCLC drug resistance and metastasis. J. Hematol. Oncol. 2019, 12, 135. [Google Scholar] [CrossRef] [PubMed]
- Ajani, J.A.; Xu, Y.; Huo, L.; Wang, R.; Li, Y.; Wang, Y.; Pizzi, M.P.; Scott, A.; Harada, K.; Ma, L.; et al. YAP1 mediates gastric adenocarcinoma peritoneal metastases that are attenuated by YAP1 inhibition. Gut 2021, 70, 55–66. [Google Scholar] [CrossRef]
- Zhang, L.; Song, X.; Li, X.; Wu, C.; Jiang, J. Yes-Associated Protein 1 as a Novel Prognostic Biomarker for Gastrointestinal Cancer: A Meta-Analysis. Biomed Res. Int. 2018, 2018, 4039173. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Wu, G.; Zhang, D.; Liu, J.; Ran, R. microRNA625 targets Yesassociated protein 1 to suppress cell proliferation and invasion of osteosarcoma. Mol. Med. Rep. 2018, 17, 2005–2011. [Google Scholar] [CrossRef] [PubMed]
- Gridelli, C.; De Marinis, F.; Di Maio, M.; Cortinovis, D.; Cappuzzo, F.; Mok, T. Gefitinib as first-line treatment for patients with advanced non-small-cell lung cancer with activating epidermal growth factor receptor mutation: Review of the evidence. Lung Cancer 2011, 71, 249–257. [Google Scholar] [CrossRef]
- Ujiie, D.; Okayama, H.; Saito, K.; Ashizawa, M.; Thar Min, A.K.; Endo, E.; Kase, K.; Yamada, L.; Kikuchi, T.; Hanayama, H.; et al. KRT17 as a prognostic biomarker for stage II colorectal cancer. Carcinogenesis 2020, 41, 591–599. [Google Scholar] [CrossRef]
- Hu, H.; Xu, D.H.; Huang, X.X.; Zhu, C.C.; Xu, J.; Zhang, Z.Z.; Zhao, G. Keratin17 Promotes Tumor Growth and is Associated with Poor Prognosis in Gastric Cancer. J. Cancer 2018, 9, 346–357. [Google Scholar] [CrossRef]
- Wu, J.; Xu, H.; Ji, H.; Zhai, B.; Zhu, J.; Gao, M.; Zhu, H.; Wang, X. Low Expression of Keratin17 is Related to Poor Prognosis in Bladder Cancer. Onco Targets Ther. 2021, 14, 577–587. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, M.Q.; Lei, L.; Fei, L.R.; Zheng, Y.W.; Huang, W.J.; Li, Z.H.; Liu, C.C.; Xu, H.T. Overexpression of KRT17 promotes proliferation and invasion of non-small cell lung cancer and indicates poor prognosis. Cancer Manag. Res. 2019, 11, 7485–7497. [Google Scholar] [CrossRef]
- Liu, J.; Liu, L.; Cao, L.; Wen, Q. Keratin 17 Promotes Lung Adenocarcinoma Progression by Enhancing Cell Proliferation and Invasion. Med. Sci. Monit. 2018, 24, 4782–4790. [Google Scholar] [CrossRef]
- Huang, Q.; Guo, H.; Wang, S.; Ma, Y.; Chen, H.; Li, H.; Li, J.; Li, X.; Yang, F.; Qiu, M.; et al. A novel circular RNA, circXPO1, promotes lung adenocarcinoma progression by interacting with IGF2BP1. Cell Death Dis. 2020, 11, 1031. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Wang, H.; Chen, B.; Mao, Q.; Xia, W.; Zhang, T.; Song, X.; Zhang, Z.; Xu, L.; Dong, G.; et al. circDCUN1D4 suppresses tumor metastasis and glycolysis in lung adenocarcinoma by stabilizing TXNIP expression. Mol. Ther. Nucleic Acids 2021, 23, 355–368. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, S.; Chen, Z.; He, Z.; Xu, Y.; Li, Z. CircRNA-ENO1 promoted glycolysis and tumor progression in lung adenocarcinoma through upregulating its host gene ENO1. Cell Death Dis. 2019, 10, 885. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Jiang, Z.; Xiao, P.; Li, X.; Chen, Y.; Tang, H.; Chai, Y.; Liu, Y.; Zhu, Z.; Xie, Q.; et al. Hsa_circ_0005576 promotes osimertinib resistance through the miR-512-5p/IGF1R axis in lung adenocarcinoma cells. Cancer Sci. 2022, 113, 79–90. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Lv, J.; Yu, H.; Han, J.; Yang, X.; Feng, D.; Wu, Q.; Yuan, B.; Lu, Q.; Yang, H. Mechanism of RNA modification N6-methyladenosine in human cancer. Mol. Cancer 2020, 19, 104. [Google Scholar] [CrossRef]
- Chen, M.; Wong, C.M. The emerging roles of N6-methyladenosine (m6A) deregulation in liver carcinogenesis. Mol. Cancer 2020, 19, 44. [Google Scholar] [CrossRef]
- Muller, S.; Glass, M.; Singh, A.K.; Haase, J.; Bley, N.; Fuchs, T.; Lederer, M.; Dahl, A.; Huang, H.; Chen, J.; et al. IGF2BP1 promotes SRF-dependent transcription in cancer in a m6A- and miRNA-dependent manner. Nucleic Acids Res. 2019, 47, 375–390. [Google Scholar] [CrossRef]
- Zhang, C.; Huang, S.; Zhuang, H.; Ruan, S.; Zhou, Z.; Huang, K.; Ji, F.; Ma, Z.; Hou, B.; He, X. YTHDF2 promotes the liver cancer stem cell phenotype and cancer metastasis by regulating OCT4 expression via m6A RNA methylation. Oncogene 2020, 39, 4507–4518. [Google Scholar] [CrossRef]
- Ma, J.Z.; Yang, F.; Zhou, C.C.; Liu, F.; Yuan, J.H.; Wang, F.; Wang, T.T.; Xu, Q.G.; Zhou, W.P.; Sun, S.H. METTL14 suppresses the metastatic potential of hepatocellular carcinoma by modulating N(6) -methyladenosine-dependent primary MicroRNA processing. Hepatology 2017, 65, 529–543. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, C.; Ding, Q.; Zhao, Y.; Wang, Z.; Chen, J.; Jiang, Z.; Zhang, Y.; Xu, G.; Zhang, J.; et al. METTL3-mediated m(6)A modification of HDGF mRNA promotes gastric cancer progression and has prognostic significance. Gut 2020, 69, 1193–1205. [Google Scholar] [CrossRef]
- Wang, Q.; Guo, X.; Li, L.; Gao, Z.; Su, X.; Ji, M.; Liu, J. N(6)-methyladenosine METTL3 promotes cervical cancer tumorigenesis and Warburg effect through YTHDF1/HK2 modification. Cell Death Dis. 2020, 11, 911. [Google Scholar] [CrossRef]
- Ma, Z.; Li, Q.; Liu, P.; Dong, W.; Zuo, Y. METTL3 regulates m6A in endometrioid epithelial ovarian cancer independently of METTl14 and WTAP. Cell Biol. Int. 2020, 44, 2524–2531. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, S.; Zhao, T.; Dang, C. METTL3mediated m6A modification of Bcl2 mRNA promotes nonsmall cell lung cancer progression. Oncol. Rep. 2021, 46, 1–10. [Google Scholar] [CrossRef]
- Pan, X.; Hong, X.; Li, S.; Meng, P.; Xiao, F. METTL3 promotes adriamycin resistance in MCF-7 breast cancer cells by accelerating pri-microRNA-221-3p maturation in a m6A-dependent manner. Exp. Mol. Med. 2021, 53, 91–102. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Niu, Y.; Wan, A.; Chen, D.; Liang, H.; Chen, X.; Sun, L.; Zhan, S.; Chen, L.; Cheng, C.; et al. RNA m(6) A methylation regulates sorafenib resistance in liver cancer through FOXO3-mediated autophagy. EMBO J. 2020, 39, e103181. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Li, Q.; Li, G.; Zhang, Q.; Zhuo, L.; Han, X.; Zhang, M.; Chen, X.; Pan, T.; Yan, L.; et al. The mechanism of m(6)A methyltransferase METTL3-mediated autophagy in reversing gefitinib resistance in NSCLC cells by beta-elemene. Cell Death Dis. 2020, 11, 969. [Google Scholar] [CrossRef] [PubMed]
- Dai, F.; Wu, Y.; Lu, Y.; An, C.; Zheng, X.; Dai, L.; Guo, Y.; Zhang, L.; Li, H.; Xu, W.; et al. Crosstalk between RNA m(6)A Modification and Non-coding RNA Contributes to Cancer Growth and Progression. Mol. Ther. Nucleic Acids 2020, 22, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Hou, C.; Chen, C.; Guo, Y.; Yuan, W.; Yin, D.; Liu, J.; Sun, Z. The role of N(6)-methyladenosine (m(6)A) modification in the regulation of circRNAs. Mol. Cancer 2020, 19, 105. [Google Scholar] [CrossRef]
- Hong, A.W.; Meng, Z.; Guan, K.L. The Hippo pathway in intestinal regeneration and disease. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 324–337. [Google Scholar] [CrossRef]
- Ni, X.; Tao, J.; Barbi, J.; Chen, Q.; Park, B.V.; Li, Z.; Zhang, N.; Lebid, A.; Ramaswamy, A.; Wei, P.; et al. YAP Is Essential for Treg-Mediated Suppression of Antitumor Immunity. Cancer Discov. 2018, 8, 1026–1043. [Google Scholar] [CrossRef]
- Panciera, T.; Azzolin, L.; Cordenonsi, M.; Piccolo, S. Mechanobiology of YAP and TAZ in physiology and disease. Nat. Rev. Mol. Cell Biol. 2017, 18, 758–770. [Google Scholar] [CrossRef]
- Ni, X.F.; Xie, Q.Q.; Zhao, J.M.; Xu, Y.J.; Ji, M.; Hu, W.W.; Wu, J.; Wu, C.P. The hepatic microenvironment promotes lung adenocarcinoma cell proliferation, metastasis, and epithelial-mesenchymal transition via METTL3-mediated N6-methyladenosine modification of YAP1. Aging 2021, 13, 4357–4369. [Google Scholar] [CrossRef]
- Song, Y.; Sun, Y.; Lei, Y.; Yang, K.; Tang, R. YAP1 promotes multidrug resistance of small cell lung cancer by CD74-related signaling pathways. Cancer Med. 2020, 9, 259–268. [Google Scholar] [CrossRef]
- Chen, C.; Yuan, W.; Zhou, Q.; Shao, B.; Guo, Y.; Wang, W.; Yang, S.; Guo, Y.; Zhao, L.; Dang, Q.; et al. N6-methyladenosine-induced circ1662 promotes metastasis of colorectal cancer by accelerating YAP1 nuclear localization. Theranostics 2021, 11, 4298–4315. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ji, Y.; Zhao, Q.; Feng, W.; Peng, Y.; Hu, B.; Chen, Q. N6-Methyladenosine Modification of CIRCKRT17 Initiated by METTL3 Promotes Osimertinib Resistance of Lung Adenocarcinoma by EIF4A3 to Enhance YAP1 Stability. Cancers 2022, 14, 5582. https://doi.org/10.3390/cancers14225582
Ji Y, Zhao Q, Feng W, Peng Y, Hu B, Chen Q. N6-Methyladenosine Modification of CIRCKRT17 Initiated by METTL3 Promotes Osimertinib Resistance of Lung Adenocarcinoma by EIF4A3 to Enhance YAP1 Stability. Cancers. 2022; 14(22):5582. https://doi.org/10.3390/cancers14225582
Chicago/Turabian StyleJi, Ying, Qing Zhao, Wei Feng, Yue Peng, Bin Hu, and Qirui Chen. 2022. "N6-Methyladenosine Modification of CIRCKRT17 Initiated by METTL3 Promotes Osimertinib Resistance of Lung Adenocarcinoma by EIF4A3 to Enhance YAP1 Stability" Cancers 14, no. 22: 5582. https://doi.org/10.3390/cancers14225582
APA StyleJi, Y., Zhao, Q., Feng, W., Peng, Y., Hu, B., & Chen, Q. (2022). N6-Methyladenosine Modification of CIRCKRT17 Initiated by METTL3 Promotes Osimertinib Resistance of Lung Adenocarcinoma by EIF4A3 to Enhance YAP1 Stability. Cancers, 14(22), 5582. https://doi.org/10.3390/cancers14225582



