Immune Cell Density Evaluation Improves the Prognostic Values of Staging and p16 in Oropharyngeal Cancer
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Clinical Characteristics
2.2. DNA Extraction
2.3. Detection of HPV by Polymerase Chain Reaction (PCR) Amplification
2.4. Real-Time PCR Amplification of HPV Type-Specific DNA
2.5. p16 Immunohistochemistry
2.6. Evaluation of Immune Cell Recruitment by Immunohistochemistry
2.7. Statistical Analyses
3. Results
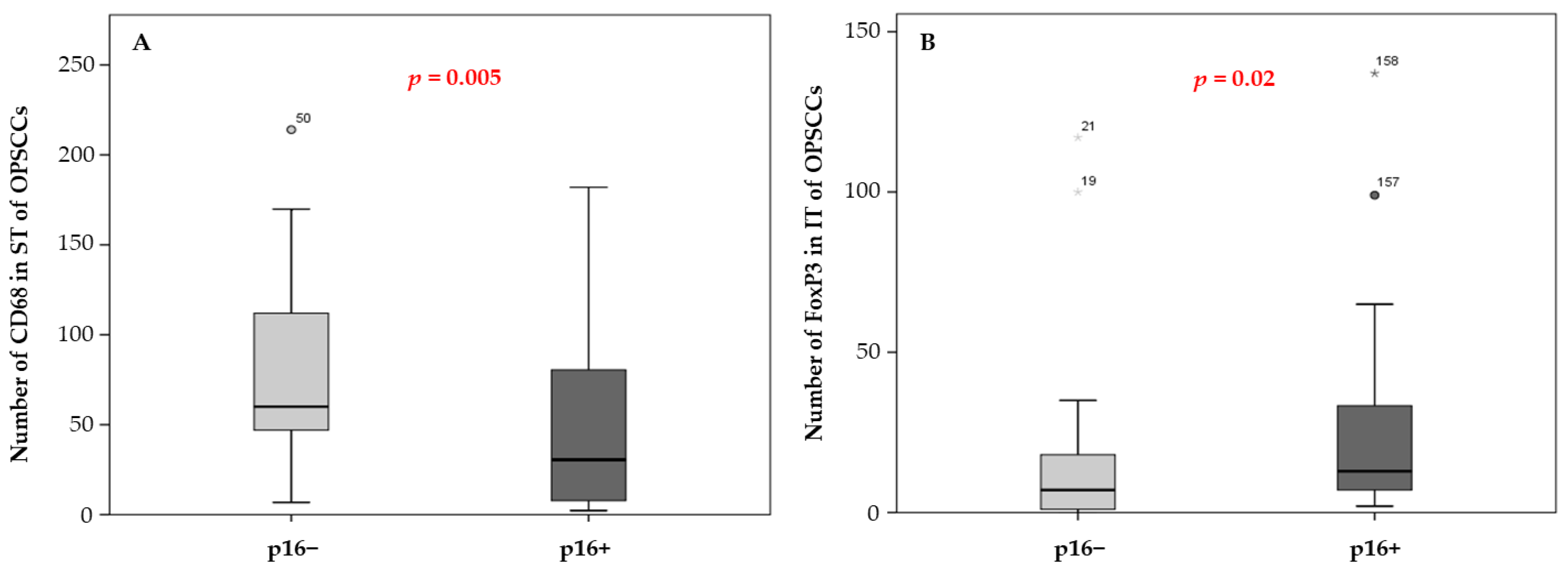
3.1. Immune Cell Density and Patient Survival
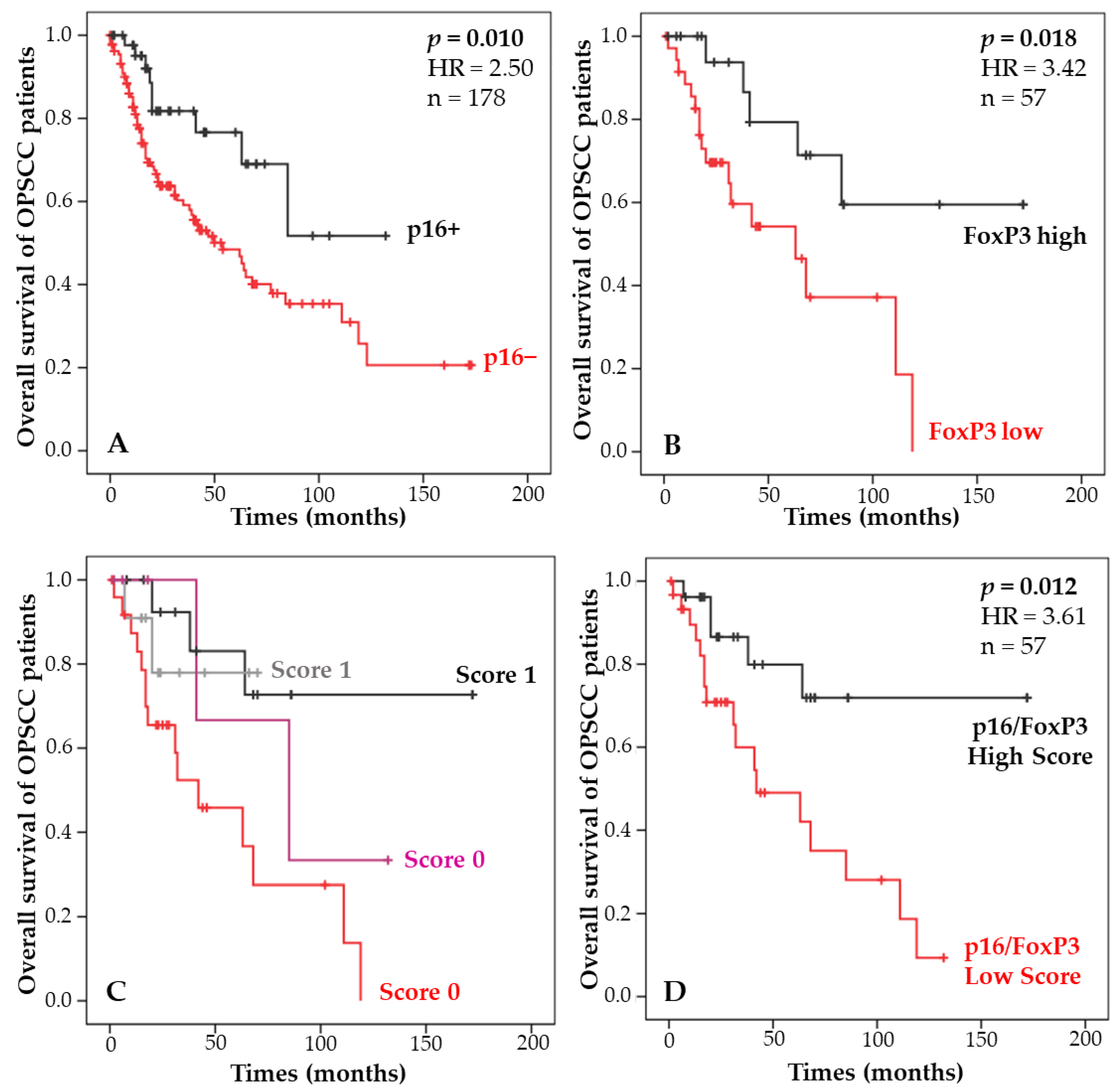
3.2. Combination of p16 Status and Regulatory T-Lymphocyte Density and Correlation with Patient Survival
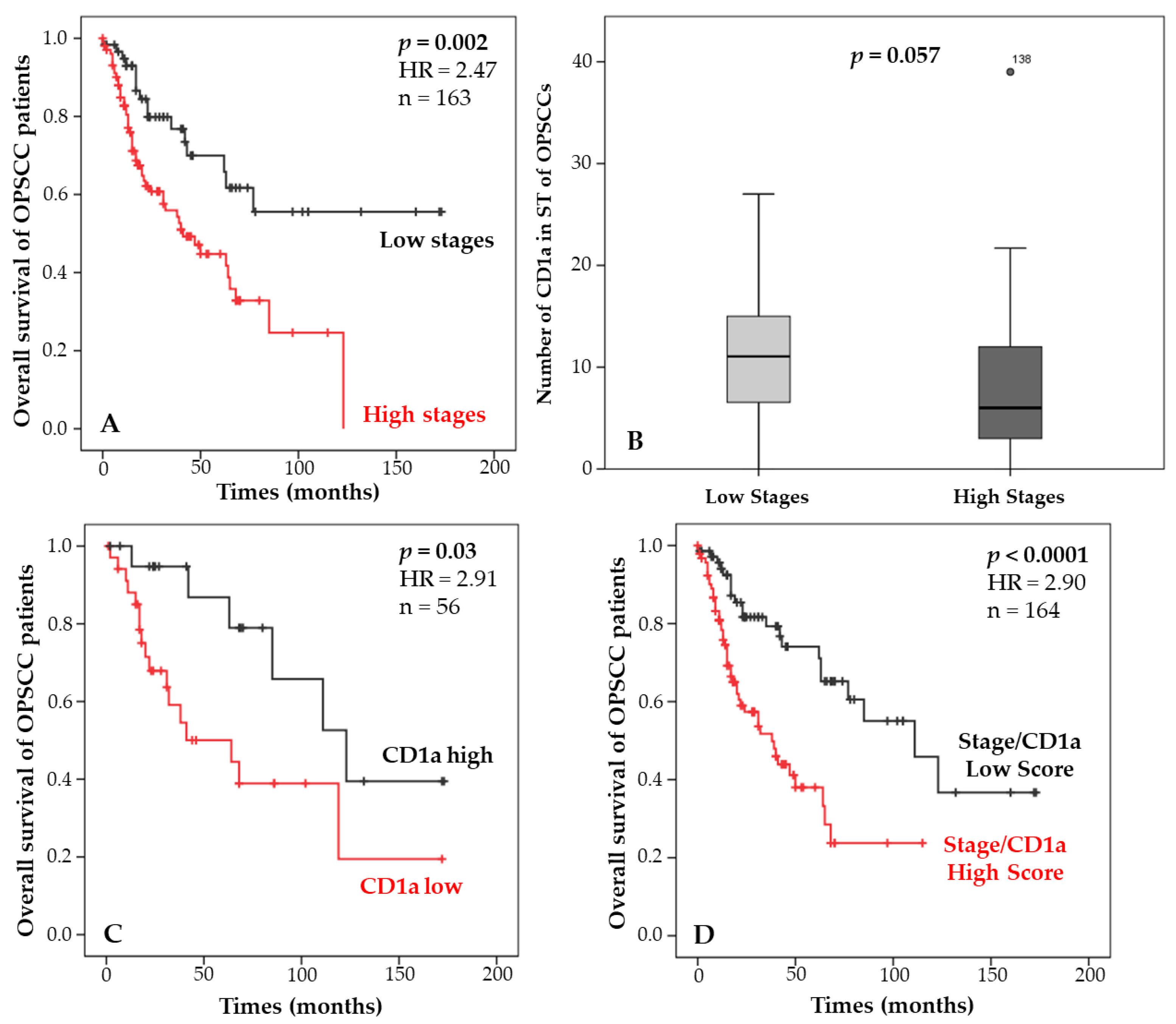
3.3. Combination of Staging and Langerhans Cell Density and Correlation with Patient Survival
3.4. Development of a Model Improving the Prediction of Overall Survival in OPSCC Patients
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Oguejiofor, K.; Hall, J.; Slater, C.; Betts, G.; Hall, G.; Slevin, N.; Dovedi, S.; Stern, P.L.; West, C.M.L. Stromal Infiltration of CD8 T Cells Is Associated with Improved Clinical Outcome in HPV-Positive Oropharyngeal Squamous Carcinoma. Br. J. Cancer 2015, 113, 886–893. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, B.; Huang, S.H.; Su, J.; Garden, A.S.; Sturgis, E.M.; Dahlstrom, K.; Lee, N.; Riaz, N.; Pei, X.; Koyfman, S.A.; et al. Development and Validation of a Staging System for HPV-Related Oropharyngeal Cancer by the International Collaboration on Oropharyngeal Cancer Network for Staging (ICON-S): A Multicentre Cohort Study. Lancet Oncol. 2016, 17, 440–451. [Google Scholar] [CrossRef]
- Taberna, M.; Mena, M.; Pavón, M.A.; Alemany, L.; Gillison, M.L.; Mesía, R. Human Papillomavirus-Related Oropharyngeal Cancer. Ann. Oncol. 2017, 28, 2386–2398. [Google Scholar] [CrossRef] [PubMed]
- Balermpas, P.; Rödel, F.; Rödel, C.; Krause, M.; Linge, A.; Lohaus, F.; Baumann, M.; Tinhofer, I.; Budach, V.; Gkika, E.; et al. CD8+ Tumour-Infiltrating Lymphocytes in Relation to HPV Status and Clinical Outcome in Patients with Head and Neck Cancer after Postoperative Chemoradiotherapy: A Multicentre Study of the German Cancer Consortium Radiation Oncology Group (DKTK-ROG). Int. J. Cancer 2016, 138, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Donnem, T.; Hald, S.M.; Paulsen, E.-E.; Richardsen, E.; Al-Saad, S.; Kilvaer, T.K.; Brustugun, O.T.; Helland, A.; Lund-Iversen, M.; Poehl, M.; et al. Stromal CD8+ T-Cell Density—A Promising Supplement to TNM Staging in Non-Small Cell Lung Cancer. Clin. Cancer Res. 2015, 21, 2635–2643. [Google Scholar] [CrossRef]
- Gabrielson, A.; Wu, Y.; Wang, H.; Jiang, J.; Kallakury, B.; Gatalica, Z.; Reddy, S.; Kleiner, D.; Fishbein, T.; Johnson, L.; et al. Intratumoral CD3 and CD8 T-Cell Densities Associated with Relapse-Free Survival in HCC. Cancer Immunol. Res. 2016, 4, 419–430. [Google Scholar] [CrossRef]
- Ou, D.; Adam, J.; Garberis, I.; Blanchard, P.; Nguyen, F.; Levy, A.; Casiraghi, O.; Gorphe, P.; Breuskin, I.; Janot, F.; et al. Clinical Relevance of Tumor Infiltrating Lymphocytes, PD-L1 Expression and Correlation with HPV/P16 in Head and Neck Cancer Treated with Bio- or Chemo-Radiotherapy. Oncoimmunology 2017, 6, e1341030. [Google Scholar] [CrossRef]
- Wansom, D.; Light, E.; Thomas, D.; Worden, F.; Prince, M.; Urba, S.; Chepeha, D.; Kumar, B.; Cordell, K.; Eisbruch, A.; et al. Infiltrating Lymphocytes and Human Papillomavirus-16—Associated Oropharyngeal Cancer. Laryngoscope 2012, 122, 121–127. [Google Scholar] [CrossRef]
- Näsman, A.; Romanitan, M.; Nordfors, C.; Grün, N.; Johansson, H.; Hammarstedt, L.; Marklund, L.; Munck-Wikland, E.; Dalianis, T.; Ramqvist, T. Tumor Infiltrating CD8+ and Foxp3+ Lymphocytes Correlate to Clinical Outcome and Human Papillomavirus (HPV) Status in Tonsillar Cancer. PLoS ONE 2012, 7, e38711. [Google Scholar] [CrossRef]
- Nordfors, C.; Grün, N.; Tertipis, N.; Ährlund-Richter, A.; Haeggblom, L.; Sivars, L.; Du, J.; Nyberg, T.; Marklund, L.; Munck-Wikland, E.; et al. CD8+ and CD4+ Tumour Infiltrating Lymphocytes in Relation to Human Papillomavirus Status and Clinical Outcome in Tonsillar and Base of Tongue Squamous Cell Carcinoma. Eur. J. Cancer 2013, 49, 2522–2530. [Google Scholar] [CrossRef]
- Sakaguchi, S.; Yamaguchi, T.; Nomura, T.; Ono, M. Regulatory T Cells and Immune Tolerance. Cell 2008, 133, 775–787. [Google Scholar] [CrossRef]
- Vignali, D.A.A.; Collison, L.W.; Workman, C.J. How Regulatory T Cells Work. Nat. Rev. Immunol. 2008, 8, 523–532. [Google Scholar] [CrossRef]
- Weller, P.; Bankfalvi, A.; Gu, X.; Dominas, N.; Lehnerdt, G.F.; Zeidler, R.; Lang, S.; Brandau, S.; Dumitru, C.A. The Role of Tumour FoxP3 as Prognostic Marker in Different Subtypes of Head and Neck Cancer. Eur. J. Cancer 2014, 50, 1291–1300. [Google Scholar] [CrossRef]
- Shang, B.; Liu, Y.; Jiang, S.; Liu, Y. Prognostic Value of Tumor-Infiltrating FoxP3+ Regulatory T Cells in Cancers: A Systematic Review and Meta-Analysis. Sci. Rep. 2015, 5, 15179. [Google Scholar] [CrossRef]
- Kindt, N.; Descamps, G.; Seminerio, I.; Bellier, J.; Lechien, J.R.; Mat, Q.; Pottier, C.; Delvenne, P.; Journé, F.; Saussez, S. High Stromal Foxp3-Positive T Cell Number Combined to Tumor Stage Improved Prognosis in Head and Neck Squamous Cell Carcinoma. Oral Oncol. 2017, 67, 183–191. [Google Scholar] [CrossRef]
- Seminerio, I.; Descamps, G.; Dupont, S.; de Marrez, L.; Laigle, J.-A.; Lechien, J.R.; Kindt, N.; Journe, F.; Saussez, S. Infiltration of FoxP3+ Regulatory T Cells Is a Strong and Independent Prognostic Factor in Head and Neck Squamous Cell Carcinoma. Cancers 2019, 11, 227. [Google Scholar] [CrossRef]
- Evrard, D.; Szturz, P.; Tijeras-Raballand, A.; Astorgues-Xerri, L.; Abitbol, C.; Paradis, V.; Raymond, E.; Albert, S.; Barry, B.; Faivre, S. Macrophages in the Microenvironment of Head and Neck Cancer: Potential Targets for Cancer Therapy. Oral Oncol. 2019, 88, 29–38. [Google Scholar] [CrossRef]
- Lechien, J.R.; Descamps, G.; Seminerio, I.; Furgiuele, S.; Dequanter, D.; Mouawad, F.; Badoual, C.; Journe, F.; Saussez, S. HPV Involvement in the Tumor Microenvironment and Immune Treatment in Head and Neck Squamous Cell Carcinomas. Cancers 2020, 12, 1060. [Google Scholar] [CrossRef]
- Deng, R.; Lu, J.; Liu, X.; Peng, X.-H.; Wang, J.; Li, X.-P. PD-L1 Expression Is Highly Associated with Tumor-Associated Macrophage Infiltration in Nasopharyngeal Carcinoma. Cancer Manag. Res. 2020, 12, 11585–11596. [Google Scholar] [CrossRef]
- Snietura, M.; Brewczynski, A.; Kopec, A.; Rutkowski, T. Infiltrates of M2-Like Tumour-Associated Macrophages Are Adverse Prognostic Factor in Patients with Human Papillomavirus-Negative but Not in Human Papillomavirus-Positive Oropharyngeal Squamous Cell Carcinoma. Pathobiology 2020, 87, 75–86. [Google Scholar] [CrossRef]
- Troiano, G.; Caponio, V.C.A.; Adipietro, I.; Tepedino, M.; Santoro, R.; Laino, L.; Lo Russo, L.; Cirillo, N.; Lo Muzio, L. Prognostic Significance of CD68+ and CD163+ Tumor Associated Macrophages in Head and Neck Squamous Cell Carcinoma: A Systematic Review and Meta-Analysis. Oral Oncol. 2019, 93, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Costa, N.L.; Valadares, M.C.; Souza, P.P.C.; Mendonça, E.F.; Oliveira, J.C.; Silva, T.A.; Batista, A.C. Tumor-Associated Macrophages and the Profile of Inflammatory Cytokines in Oral Squamous Cell Carcinoma. Oral Oncol. 2013, 49, 216–223. [Google Scholar] [CrossRef] [PubMed]
- Seminerio, I.; Kindt, N.; Descamps, G.; Bellier, J.; Lechien, J.R.; Mat, Q.; Pottier, C.; Journé, F.; Saussez, S. High Infiltration of CD68+ Macrophages Is Associated with Poor Prognoses of Head and Neck Squamous Cell Carcinoma Patients and Is Influenced by Human Papillomavirus. Oncotarget 2018, 9, 11046–11059. [Google Scholar] [CrossRef] [PubMed]
- Kindt, N.; Descamps, G.; Seminerio, I.; Bellier, J.; Lechien, J.R.; Pottier, C.; Larsimont, D.; Journé, F.; Delvenne, P.; Saussez, S. Langerhans Cell Number Is a Strong and Independent Prognostic Factor for Head and Neck Squamous Cell Carcinomas. Oral Oncol. 2016, 62, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, N.; Bellile, E.; Thomas, D.; McHugh, J.; Rozek, L.; Virani, S.; Peterson, L.; Carey, T.E.; Walline, H.; Moyer, J.; et al. Tumor Infiltrating Lymphocytes and Survival in Patients with Head and Neck Squamous Cell Carcinoma. Head Neck 2016, 38, 1074–1084. [Google Scholar] [CrossRef]
- Galon, J.; Costes, A.; Sanchez-Cabo, F.; Kirilovsky, A.; Mlecnik, B.; Lagorce-Pagès, C.; Tosolini, M.; Camus, M.; Berger, A.; Wind, P.; et al. Type, Density, and Location of Immune Cells Within Human Colorectal Tumors Predict Clinical Outcome. Science 2006, 313, 1960–1964. [Google Scholar] [CrossRef]
- Khoury, T.; Nagrale, V.; Opyrchal, M.; Peng, X.; Wang, D.; Yao, S. Prognostic Significance of Stromal Versus Intratumoral Infiltrating Lymphocytes in Different Subtypes of Breast Cancer Treated With Cytotoxic Neoadjuvant Chemotherapy. Appl. Immunohistochem. Mol. Morphol. 2018, 26, 523–532. [Google Scholar] [CrossRef]
- Pagès, F.; Mlecnik, B.; Marliot, F.; Bindea, G.; Ou, F.-S.; Bifulco, C.; Lugli, A.; Zlobec, I.; Rau, T.T.; Berger, M.D.; et al. International Validation of the Consensus Immunoscore for the Classification of Colon Cancer: A Prognostic and Accuracy Study. Lancet 2018, 391, 2128–2139. [Google Scholar] [CrossRef]
- Lanzi, A.; Pagès, F.; Lagorce-Pagès, C.; Galon, J. The Consensus Immunoscore: Toward a New Classification of Colorectal Cancer. Oncoimmunology 2020, 9, 1789032. [Google Scholar] [CrossRef]
- Furgiuele, S.; Descamps, G.; Lechien, J.R.; Dequanter, D.; Journe, F.; Saussez, S. Immunoscore Combining CD8, FoxP3, and CD68-Positive Cells Density and Distribution Predicts the Prognosis of Head and Neck Cancer Patients. Cells 2022, 11, 2050. [Google Scholar] [CrossRef]
- Duray, A.; Descamps, G.; Decaestecker, C.; Sirtaine, N.; Gilles, A.; Khalifé, M.; Chantrain, G.; Depuydt, C.E.; Delvenne, P.; Saussez, S. Human Papillomavirus Predicts the Outcome Following Concomitant Chemoradiotherapy in Patients with Head and Neck Squamous Cell Carcinomas. Oncol. Rep. 2013, 30, 371–376. [Google Scholar] [CrossRef]
- Almangush, A.; Bello, I.O.; Heikkinen, I.; Hagström, J.; Haglund, C.; Kowalski, L.P.; Coletta, R.D.; Mäkitie, A.A.; Salo, T.; Leivo, I. Improving Risk Stratification of Early Oral Tongue Cancer with TNM-Immune (TNM-I) Staging System. Cancers 2021, 13, 3235. [Google Scholar] [CrossRef]
- El Sissy, C.; Kirilovsky, A.; Zeitoun, G.; Marliot, F.; Haicheur, N.; Lagorce-Pagès, C.; Galon, J.; Pagès, F. Therapeutic Implications of the Immunoscore in Patients with Colorectal Cancer. Cancers 2021, 13, 1281. [Google Scholar] [CrossRef]
- Salgado, R.; Denkert, C.; Demaria, S.; Sirtaine, N.; Klauschen, F.; Pruneri, G.; Wienert, S.; Van den Eynden, G.; Baehner, F.L.; Penault-Llorca, F.; et al. The Evaluation of Tumor-Infiltrating Lymphocytes (TILs) in Breast Cancer: Recommendations by an International TILs Working Group 2014. Ann. Oncol. 2015, 26, 259–271. [Google Scholar] [CrossRef]
- Paulsen, E.-E.; Kilvaer, T.; Khanehkenari, M.R.; Maurseth, R.J.; Al-Saad, S.; Hald, S.M.; Al-Shibli, K.; Andersen, S.; Richardsen, E.; Busund, L.-T.; et al. CD45RO(+) Memory T Lymphocytes—A Candidate Marker for TNM-Immunoscore in Squamous Non-Small Cell Lung Cancer. Neoplasia 2015, 17, 839–848. [Google Scholar] [CrossRef]
- Feng, W.; Li, Y.; Shen, L.; Zhang, Q.; Cai, X.-W.; Zhu, Z.-F.; Sun, M.-H.; Chen, H.-Q.; Fu, X.-L. Clinical Impact of the Tumor Immune Microenvironment in Completely Resected Stage IIIA(N2) Non-Small Cell Lung Cancer Based on an Immunoscore Approach. Ther. Adv. Med. Oncol. 2021, 13, 1758835920984975. [Google Scholar] [CrossRef]
- Zhang, X.-M.; Song, L.-J.; Shen, J.; Yue, H.; Han, Y.-Q.; Yang, C.-L.; Liu, S.-Y.; Deng, J.-W.; Jiang, Y.; Fu, G.-H.; et al. Prognostic and Predictive Values of Immune Infiltrate in Patients with Head and Neck Squamous Cell Carcinoma. Hum. Pathol. 2018, 82, 104–112. [Google Scholar] [CrossRef]
- Gillison, M.L.; Chaturvedi, A.K.; Anderson, W.F.; Fakhry, C. Epidemiology of Human Papillomavirus-Positive Head and Neck Squamous Cell Carcinoma. J. Clin. Oncol. 2015, 33, 3235–3242. [Google Scholar] [CrossRef]
- Modesto, A.; Graff Cailleaud, P.; Blanchard, P.; Boisselier, P.; Pointreau, Y. Challenges and limits of therapeutic de-escalation for papillomavirus-related oropharyngeal cancer. Cancer Radiother. 2022, 26, 921–924. [Google Scholar] [CrossRef]
- Ljokjel, B.; Haave, H.; Lybak, S.; Vintermyr, O.K.; Helgeland, L.; Aarstad, H.J. Tumor Infiltration Levels of CD3, Foxp3 (+) Lymphocytes and CD68 Macrophages at Diagnosis Predict 5-Year Disease-Specific Survival in Patients with Oropharynx Squamous Cell Carcinoma. Cancers 2022, 14, 1508. [Google Scholar] [CrossRef]
- Saito, T.; Nishikawa, H.; Wada, H.; Nagano, Y.; Sugiyama, D.; Atarashi, K.; Maeda, Y.; Hamaguchi, M.; Ohkura, N.; Sato, E.; et al. Two FOXP3(+)CD4(+) T Cell Subpopulations Distinctly Control the Prognosis of Colorectal Cancers. Nat. Med. 2016, 22, 679–684. [Google Scholar] [CrossRef] [PubMed]
- Gama-Cuellar, A.G.; Francisco, A.L.N.; Scarini, J.F.; Mariano, F.V.; Kowalski, L.P.; Gondak, R. Decreased CD1a + and CD83 + Cells in Tonsillar Squamous Cell Carcinoma Regardless of HPV Status. J. Appl. Oral Sci. 2022, 30, e20210702. [Google Scholar] [CrossRef] [PubMed]
- Gomes, J.O.; de Vasconcelos Carvalho, M.; Fonseca, F.P.; Gondak, R.O.; Lopes, M.A.; Vargas, P.A. CD1a+ and CD83+ Langerhans Cells Are Reduced in Lower Lip Squamous Cell Carcinoma. J. Oral Pathol. Med. 2016, 45, 433–439. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.-C.; Fonseca, F.-P.; Almeida, O.-P.; Mariz, B.-A.; Lopes, M.-A.; Radhakrishnan, R.; Sharma, M.; Kowalski, L.-P.; Vargas, P.-A. CD1a+ and CD207+ Cells Are Reduced in Oral Submucous Fibrosis and Oral Squamous Cell Carcinoma. Med. Oral Patol. Oral Cir. Bucal. 2020, 25, e49–e55. [Google Scholar] [CrossRef]
- Karpathiou, G.; Casteillo, F.; Giroult, J.-B.; Forest, F.; Fournel, P.; Monaya, A.; Froudarakis, M.; Dumollard, J.M.; Prades, J.M.; Peoc’h, M. Prognostic Impact of Immune Microenvironment in Laryngeal and Pharyngeal Squamous Cell Carcinoma: Immune Cell Subtypes, Immuno-Suppressive Pathways and Clinicopathologic Characteristics. Oncotarget 2016, 8, 19310–19322. [Google Scholar] [CrossRef]
- Goldman, S.A.; Baker, E.; Weyant, R.J.; Clarke, M.R.; Myers, J.N.; Lotze, M.T. Peritumoral CD1a-Positive Dendritic Cells Are Associated with Improved Survival in Patients with Tongue Carcinoma. Arch. Otolaryngol. Head Neck Surg. 1998, 124, 641–646. [Google Scholar] [CrossRef]
- Minesaki, A.; Kai, K.; Kuratomi, Y.; Aishima, S. Infiltration of CD1a-Positive Dendritic Cells in Advanced Laryngeal Cancer Correlates with Unfavorable Outcomes Post-Laryngectomy. BMC Cancer 2021, 21, 973. [Google Scholar] [CrossRef]
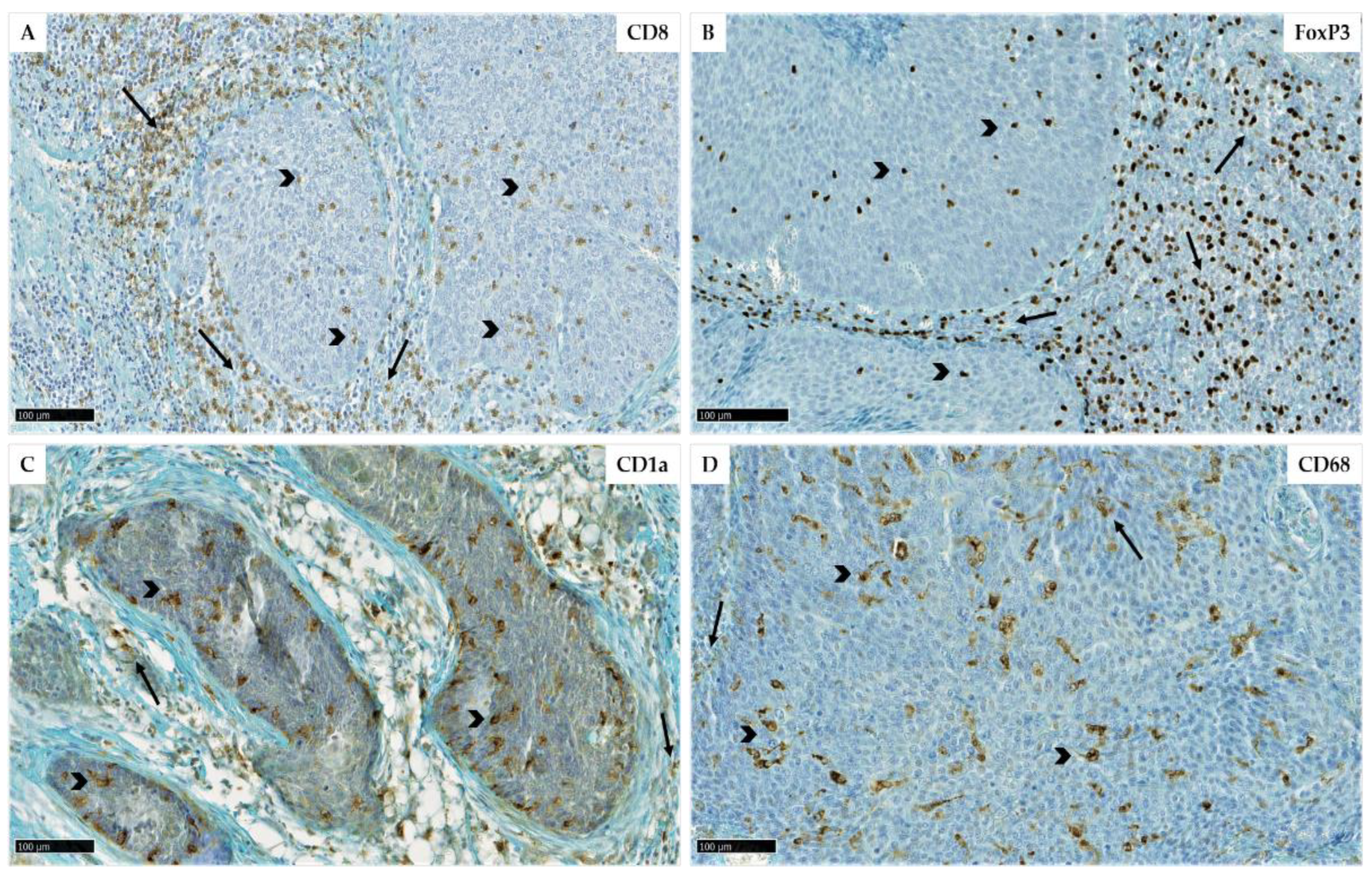
| Variables | Number of OPSCC Cases |
|---|---|
| n = 194 | |
| Age (years) | |
| Median (range) | 59 (24–89) |
| Recurrence (RFS) (months) | |
| Median (range) | 17 (1–188) |
| Yes | 75 |
| No | 106 |
| Unknown | 13 |
| Overall survival (OS) (months) | |
| Median (range) | 23 (1–173) |
| Alive | 104 |
| Dead | 76 |
| Unknown | 14 |
| Gender | |
| Male | 130 |
| Female | 64 |
| Tumor stage 8th | |
| I–II | 67 |
| III–IV | 107 |
| Unknown | 20 |
| Histological grade | |
| Undifferentiated | 50 |
| Poorly differentiated | 53 |
| Moderately differentiated | 9 |
| Well differentiated | 56 |
| Unknown | 26 |
| Risk factors | |
| Tobacco | |
| Smoker | 147 |
| Non-Smoker | 35 |
| Unknown | 12 |
| Alcohol | |
| Drinker | 132 |
| Non-Drinker | 50 |
| Unknown | 12 |
| HPV detection | |
| Positive | 27 |
| Negative | 49 |
| Unknown | 118 |
| p16 staining | |
| Positive | 52 |
| Negative | 142 |
| p16 status | |
| p16+ | 52 |
| p16+/HPV− | 0 |
| p16−/HPV+ | 9 |
| p16−/HPV− | 133 |
| Subgroups | Parameters | CD8 ST | CD8 IT | CD68 ST | CD68 IT | CD1a ST | CD1a IT | FoxP3 ST | FoxP3 IT |
|---|---|---|---|---|---|---|---|---|---|
| All data | n | 44 | 44 | 77 | 77 | 66 | 66 | 69 | 69 |
| Median | 90.6 | 14.4 | 55 | 16 | 8 | 55.5 | 92.5 | 12.6 | |
| Min-Max | 0–406.6 | 0–317.8 | 2.3–214 | 0–110 | 0–39 | 0–563 | 16–467 | 0–137 | |
| p16- tumors | n | 24 | 24 | 49 | 49 | 52 | 52 | 45 | 45 |
| Median | 90.6 | 13.35 | 60 | 14.1 | 8.35 | 53.5 | 88.2 | 7 | |
| Min-Max | 0–378.6 | 0–36.4 | 6.8–214 | 0–65 | 0–39 | 0–563 | 16–320 | 0–117 | |
| p16+ tumors | n | 20 | 20 | 28 | 28 | 14 | 14 | 24 | 24 |
| Median | 94.35 | 19.85 | 30.5 | 18 | 6 | 66 | 93.35 | 12.9 | |
| Min-Max | 3.3–406.6 | 0.2–317.8 | 2.3–182 | 0–110 | 0–27 | 0.8–180 | 17–467 | 2–137 | |
| Low stage patients | n | 21 | 21 | 35 | 35 | 24 | 24 | 29 | 29 |
| Median | 90.2 | 17.7 | 47 | 16 | 11.05 | 29.5 | 105 | 11 | |
| Min-Max | 3.3–406.6 | 0.2–317.8 | 2.3–182 | 0–110 | 0–27 | 0.3–199 | 16–467 | 0–137 | |
| High stage patients | n | 12 | 12 | 29 | 29 | 29 | 29 | 25 | 25 |
| Median | 106.45 | 13.65 | 58 | 22 | 6 | 56 | 84.5 | 13 | |
| Min-Max | 7.2–196.9 | 0.2–57.6 | 6.5–214 | 0–68.6 | 0–39 | 0.2–563 | 17–362 | 0–117 |
| Univariate Analysis | Overall Survival | |
|---|---|---|
| p Value | HR (95% CI) | |
| CD8 ST | 0.322 | 0.04 (0.0–25.7) |
| CD8 IT | 0.173 | 0.35 (0.8–1.6) |
| CD68 ST | 0.191 | 0.59 (0.3–1.3) |
| CD68 IT | 0.049 | 2.28 (1.0–5.2) |
| FoxP3 ST | 0.441 | 0.71 (0.30–1.68) |
| FoxP3 IT | 0.018 | 3.42 (0.10–0.81) |
| CD1a ST | 0.029 | 2.91 (0.13–0.89) |
| CD1a IT | 0.183 | 0.54 (0.22–1.33) |
| Immune Cells | p Value versus p16 | p Value versus Staging |
|---|---|---|
| CD8 ST | 0.925 | 0.518 |
| CD8 IT | 0.071 | 0.868 |
| CD68 ST | 0.005 | 0.121 |
| CD68 IT | 0.155 | 0.761 |
| FoxP3 ST | 0.29 | 0.263 |
| FoxP3 IT | 0.022 | 0.627 |
| CD1a ST | 0.588 | 0.057 |
| CD1a IT | 0.451 | 0.335 |
| Curves | p16 | FoxP3 IT | Survival | Score |
|---|---|---|---|---|
| Red | Negative | Low | Poor − − | Low |
| Black | Negative | High | Good ++ | High |
| Gray | Positive | Low | Good + | High |
| Purple | Positive | High | Poor − | Low |
| Stage | Survival Stage | CD1a ST | Survival CD1a ST | Survival Using Combination | Score |
|---|---|---|---|---|---|
| Low | Good | Low | Poor | Good | Low |
| Low | Good | High | Good | Good | Low |
| High | Poor | Low | Poor | Poor | High |
| High | Poor | High | Good | Good | Low |
| Multivariate Analysis | Overall Survival | |
|---|---|---|
| p Value | HR (95% CI) | |
| p16 | 0.974 | 1.03 (0.19–5.50) |
| Staging | 0.052 | 3.20 (0.98–10.40) |
| CD1a ST | 0.376 | 1.70 (0.52–5.53) |
| FoxP3 IT | 0.057 | 3.15 (0.96–10.31) |
| Multivariate Analysis | Overall Survival | |
|---|---|---|
| p Value | HR (95% CI) | |
| p16/FoxP3 score | 0.038 | 3.24 (1.06–9.85) |
| Stage/CD1a score | 0.032 | 2.92 (1.09–7.77) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Descamps, G.; Furgiuele, S.; Mhaidly, N.; Journe, F.; Saussez, S. Immune Cell Density Evaluation Improves the Prognostic Values of Staging and p16 in Oropharyngeal Cancer. Cancers 2022, 14, 5560. https://doi.org/10.3390/cancers14225560
Descamps G, Furgiuele S, Mhaidly N, Journe F, Saussez S. Immune Cell Density Evaluation Improves the Prognostic Values of Staging and p16 in Oropharyngeal Cancer. Cancers. 2022; 14(22):5560. https://doi.org/10.3390/cancers14225560
Chicago/Turabian StyleDescamps, Géraldine, Sonia Furgiuele, Nour Mhaidly, Fabrice Journe, and Sven Saussez. 2022. "Immune Cell Density Evaluation Improves the Prognostic Values of Staging and p16 in Oropharyngeal Cancer" Cancers 14, no. 22: 5560. https://doi.org/10.3390/cancers14225560
APA StyleDescamps, G., Furgiuele, S., Mhaidly, N., Journe, F., & Saussez, S. (2022). Immune Cell Density Evaluation Improves the Prognostic Values of Staging and p16 in Oropharyngeal Cancer. Cancers, 14(22), 5560. https://doi.org/10.3390/cancers14225560






