Feasibility Study on the Radiation Dose by Radioactive Magnetic Core-Shell Nanoparticles for Open-Source Brachytherapy
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Simulation Setup
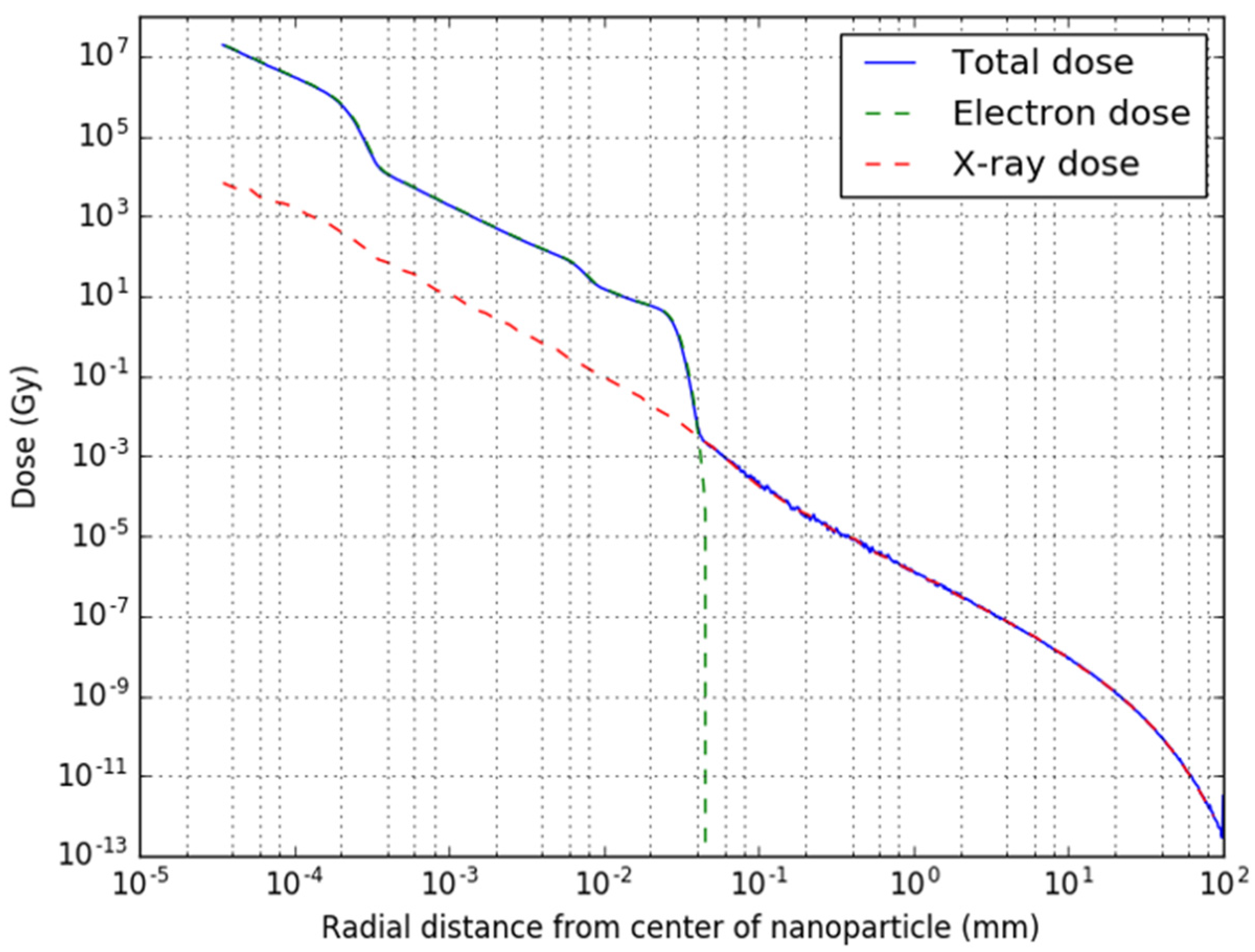
2.2. Single Nanoparticle Simulations
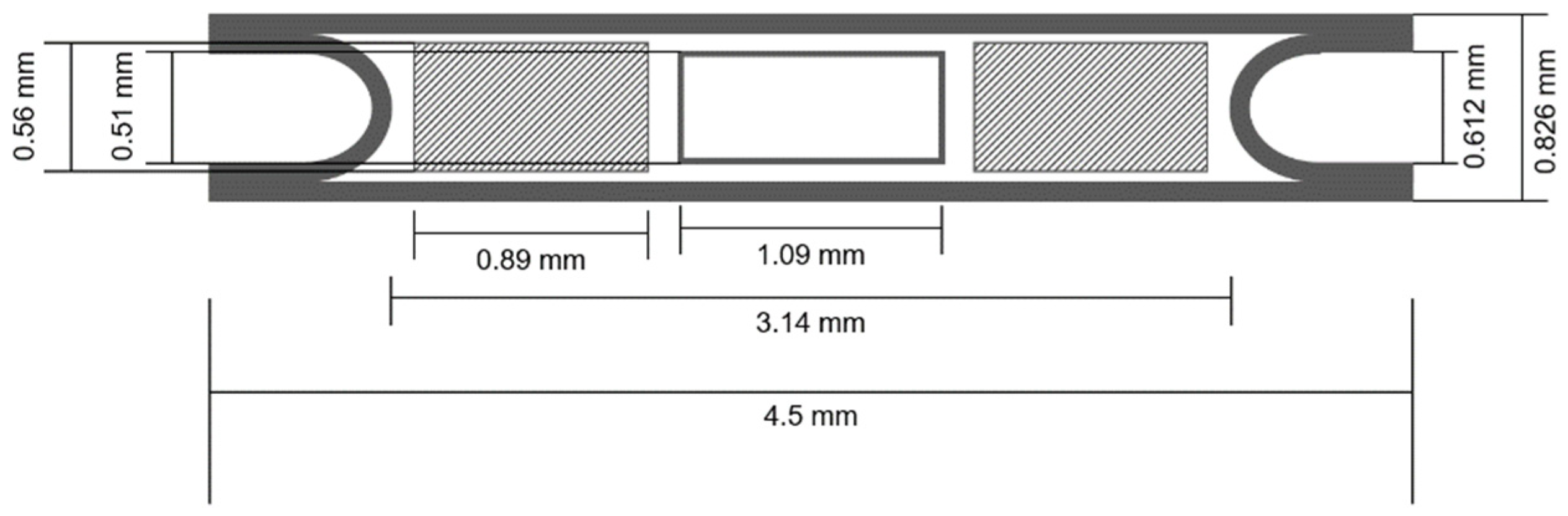
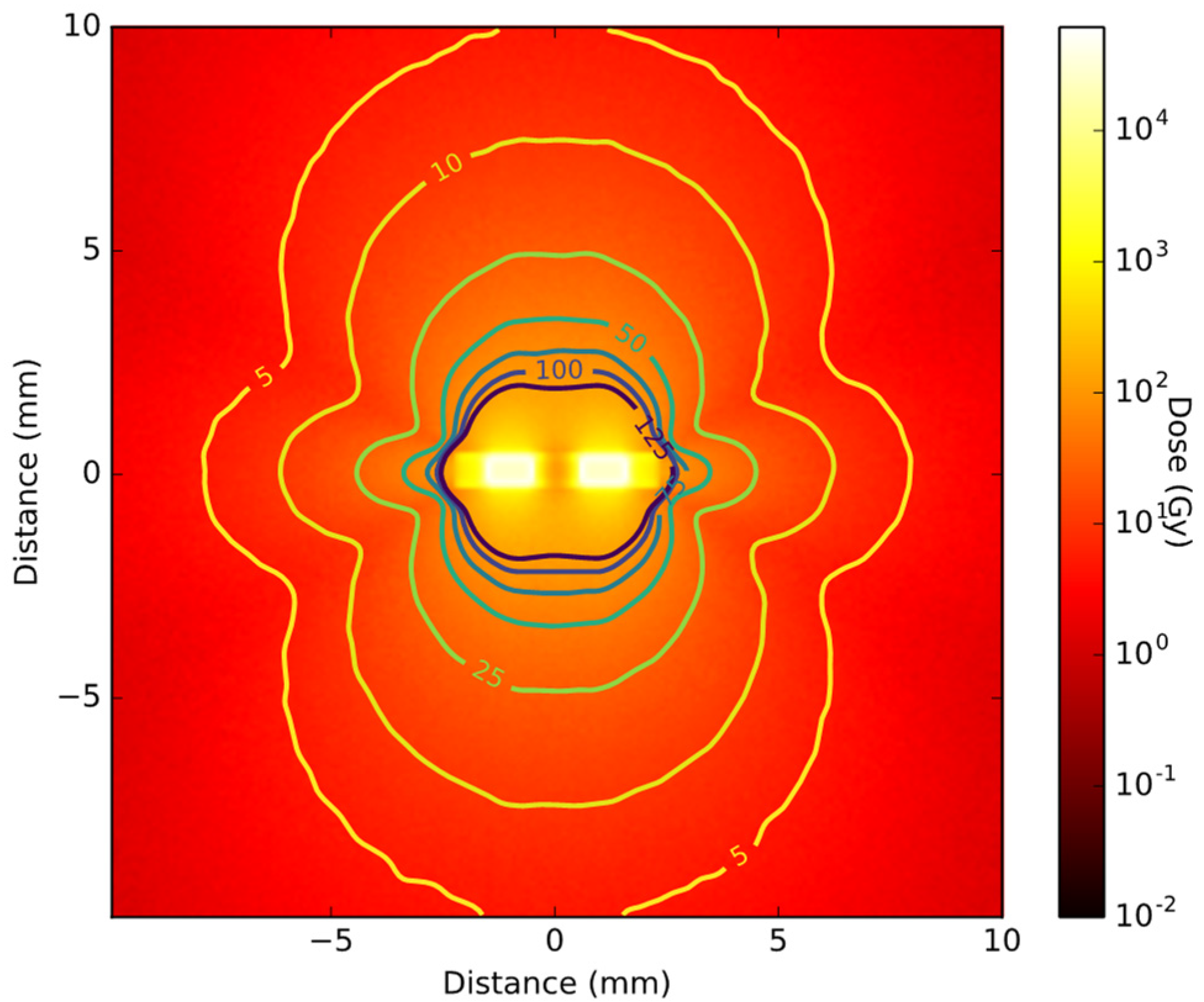
2.3. Brachytherapy Seed Simulations
3. Results
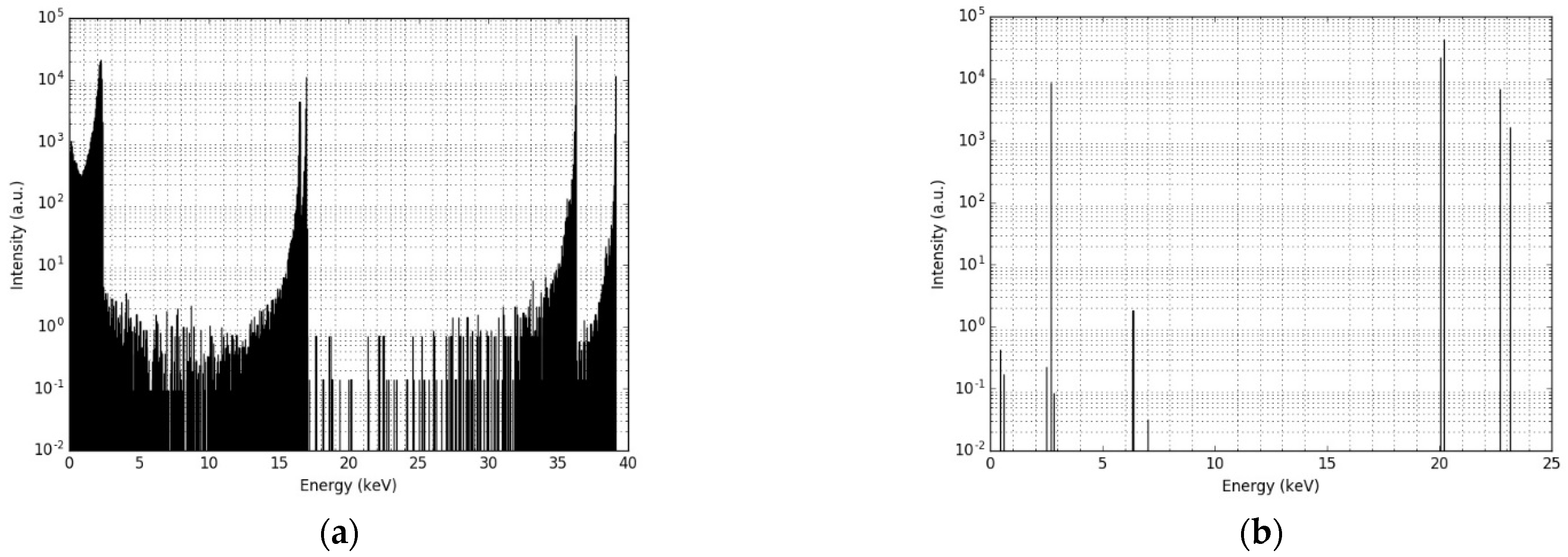
3.1. Single Nanoparticle Energy Spectra
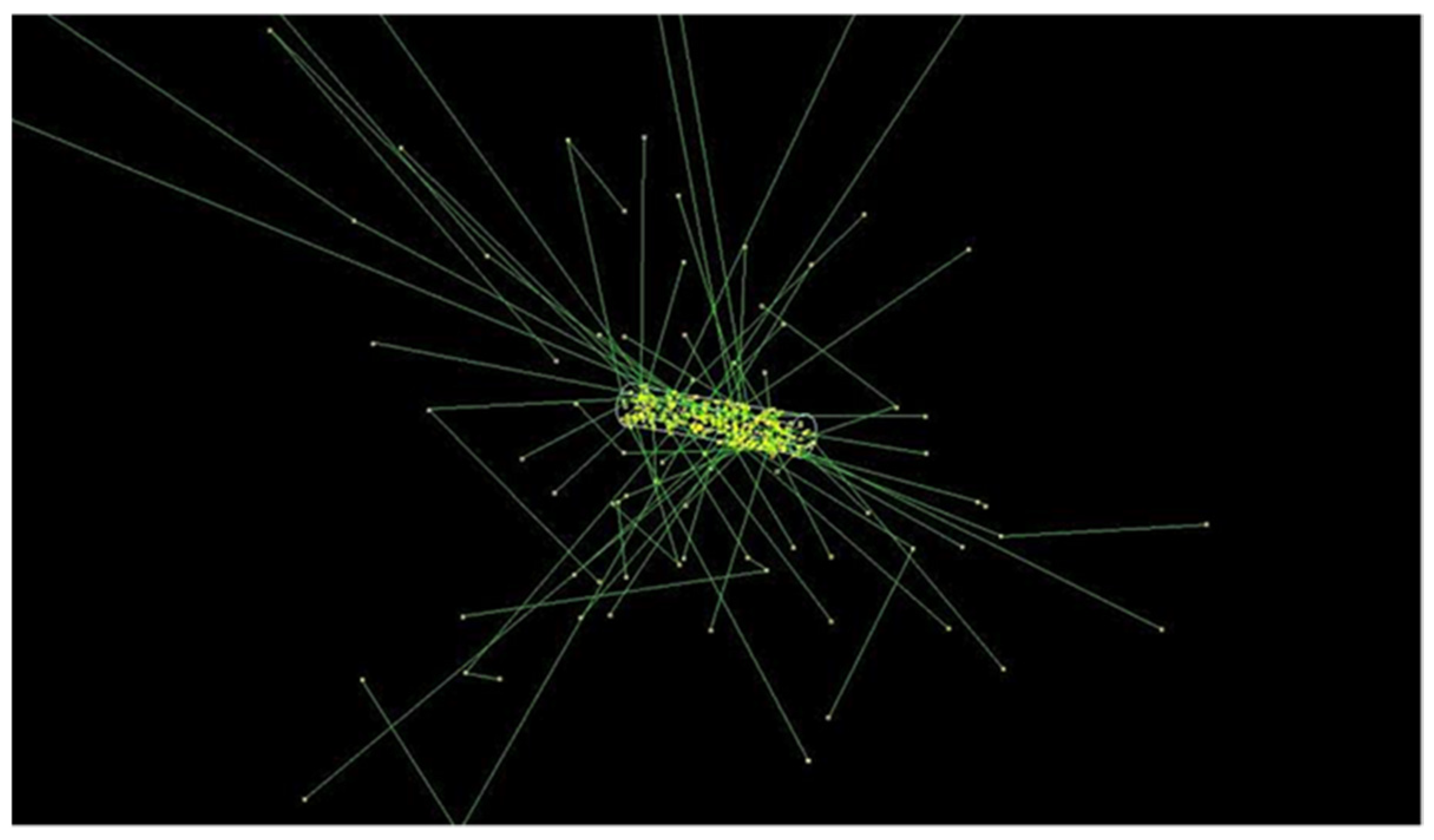
3.2. Radioactive Magnetic Nanoparticle (RMNP) Seed
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Breast Cancer. Available online: https://www.who.int/cancer/prevention/diagnosis-screening/breast-cancer/en/ (accessed on 22 October 2020).
- SEER. Female Breast Cancer Statistics. Available online: https://seer.cancer.gov/statfacts/html/breast.html (accessed on 21 October 2020).
- EBCTCG; McGale, P.; Taylor, C.; Correa, C.; Cutter, D.; Duane, F.; Ewertz, M.; Gray, R.; Mannu, G.; Peto, R.; et al. Effect of radiotherapy after mastectomy and axillary surgery on 10-year recurrence and 20-year breast cancer mortality: Meta-analysis of individual patient data for 8135 women in 22 randomised trials. Lancet 2014, 383, 2127–2135. [Google Scholar] [CrossRef] [PubMed]
- Brown, L.C.; Mutter, R.W.; Halyard, M.Y. Benefits, risks, and safety of external beam radiation therapy for breast cancer. Int. J. Womens Health 2015, 7, 449–458. [Google Scholar] [CrossRef] [PubMed]
- Bethune, W.A. Partial breast irradiation for early breast cancer. J. Natl. Med. Assoc. 1991, 83, 768, 800, 808. [Google Scholar] [PubMed]
- Pawlik, T.M.; Buchholz, T.A.; Kuerer, H.M. The biologic rationale for and emerging role of accelerated partial breast irradiation for breast cancer. J. Am. Coll. Surg. 2004, 199, 479–492. [Google Scholar] [CrossRef] [PubMed]
- Vicini, F.A.; Chen, P.; Wallace, M.; Mitchell, C.; Hasan, Y.; Grills, I.; Kestin, L.; Schell, S.; Goldstein, N.S.; Kunzman, J.; et al. Interim cosmetic results and toxicity using 3d conformal external beam radiotherapy to deliver accelerated partial breast irradiation in patients with early-stage breast cancer treated with breast-conserving therapy. Int. J. Radiat. Oncol. Biol. Phys. 2007, 69, 1124–1130. [Google Scholar] [CrossRef]
- Haussmann, J.; Budach, W.; Strnad, V.; Corradini, S.; Krug, D.; Schmidt, L.; Tamaskovics, B.; Bolke, E.; Simiantonakis, I.; Kammers, K.; et al. Comparing Local and Systemic Control between Partial- and Whole-Breast Radiotherapy in Low-Risk Breast Cancer-A Meta-Analysis of Randomized Trials. Cancers 2021, 13, 2967. [Google Scholar] [CrossRef]
- Schafer, R.; Strnad, V.; Polgar, C.; Uter, W.; Hildebrandt, G.; Ott, O.J.; Kauer-Dorner, D.; Knauerhase, H.; Major, T.; Lyczek, J.; et al. Quality-of-life results for accelerated partial breast irradiation with interstitial brachytherapy versus whole-breast irradiation in early breast cancer after breast-conserving surgery (GEC-ESTRO): 5-year results of a randomised, phase 3 trial. Lancet Oncol. 2018, 19, 834–844. [Google Scholar] [CrossRef]
- Polgar, C.; Major, T.; Takacsi-Nagy, Z.; Fodor, J. Breast-Conserving Surgery Followed by Partial or Whole Breast Irradiation: Twenty-Year Results of a Phase 3 Clinical Study. Int. J. Radiat. Oncol. Biol. Phys. 2021, 109, 998–1006. [Google Scholar] [CrossRef]
- Deng, X.; Wu, H.; Gao, F.; Su, Y.; Li, Q.; Liu, S.; Cai, J. Brachytherapy in the treatment of breast cancer. Int. J. Clin. Oncol. 2017, 22, 641–650. [Google Scholar] [CrossRef]
- Polgár, C.; Major, T.; Fodor, J.; Sulyok, Z.; Somogyi, A.; Lövey, K.; Németh, G.; Kásler, M. Accelerated partial-breast irradiation using high-dose-rate interstitial brachytherapy: 12-year update of a prospective clinical study. Radiother. Oncol. 2010, 94, 274–279. [Google Scholar] [CrossRef]
- Strnad, V.; Hildebrandt, G.; Potter, R.; Hammer, J.; Hindemith, M.; Resch, A.; Spiegl, K.; Lotter, M.; Uter, W.; Bani, M.; et al. Accelerated Partial Breast Irradiation: 5-Year Results of the German-Austrian Multicenter Phase Ii Trial Using Interstitial Multicatheter Brachytherapy Alone after Breast-Conserving Surgery. Int. J. Radiat. Oncol. Biol. Phys. 2011, 80, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Yeo, S.G.; Kim, J.; Kwak, G.H.; Kim, J.Y.; Park, K.; Kim, E.S.; Han, S. Accelerated partial breast irradiation using multicatheter brachytherapy for select early-stage breast cancer: Local control and toxicity. Radiat. Oncol. 2010, 5, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Pignol, J.-P.; Keller, B.; Rakovitch, E.; Sankreacha, R.; Easton, H.; Que, W. First report of a permanent breast 103Pd seed implant as adjuvant radiation treatment for early-stage breast cancer. Int. J. Radiat. Oncol. Biol. Phys. 2006, 64, 176–181. [Google Scholar] [CrossRef]
- Pignol, J.-P.; Crook, J. Breast Brachytherapy: Permanent Breast Seed Implants–How and Why? In Brachytherapy; Springer: Berlin/Heidelberg, Germany, 2016; pp. 185–196. [Google Scholar]
- Pignol, J.-P.; Keller, B.M. Permanent breast seed implants. In Accelerated Partial Breast Irradiation; Springer: Berlin/Heidelberg, Germany, 2009; pp. 263–276. [Google Scholar]
- Chu, K.F.; Dupuy, D.E. Thermal ablation of tumours: Biological mechanisms and advances in therapy. Nat. Rev. Cancer 2014, 14, 199–208. [Google Scholar] [CrossRef]
- Henriques, F.C., Jr. Studies of thermal injury; the predictability and the significance of thermally induced rate processes leading to irreversible epidermal injury. Arch. Pathol. 1947, 43, 489–502. [Google Scholar]
- Sapareto, S.A.; Dewey, W.C. Thermal dose determination in cancer therapy. Int. J. Radiat. Oncol. Biol. Phys. 1984, 10, 787–800. [Google Scholar] [CrossRef]
- Brace, C. Thermal Tumor Ablation in Clinical Use. IEEE Pulse 2011, 2, 28–38. [Google Scholar] [CrossRef]
- Nikfarjam, M.; Muralidharan, V.; Christophi, C. Mechanisms of focal heat destruction of liver tumors. J. Surg. Res. 2005, 127, 208–223. [Google Scholar] [CrossRef]
- Kok, H.P.; Cressman, E.N.K.; Ceelen, W.; Brace, C.L.; Ivkov, R.; Grull, H.; Ter Haar, G.; Wust, P.; Crezee, J. Heating technology for malignant tumors: A review. Int. J. Hyperth. 2020, 37, 711–741. [Google Scholar] [CrossRef]
- Zhao, Z.; Wu, F. Minimally-invasive thermal ablation of early-stage breast cancer: A systemic review. Eur. J. Surg. Oncol. 2010, 36, 1149–1155. [Google Scholar] [CrossRef]
- Laprise-Pelletier, M.; Simao, T.; Fortin, M.A. Gold Nanoparticles in Radiotherapy and Recent Progress in Nanobrachytherapy. Adv. Healthc. Mater. 2018, 7, e1701460. [Google Scholar] [CrossRef] [PubMed]
- de Souza, C.D.; Nogueira, B.R.; Zeituni, C.A.; Rostelato, M.E.C.M. Radioactive nanoparticles and their biomedical application in nanobrachytherapy. In Nanoparticle Therapeutics; Elsevier: Amsterdam, The Netherlands, 2022; pp. 529–560. [Google Scholar]
- Hahn, M.B.; Zutta Villate, J.M. Radioactive gold nanoparticles for cancer treatment: Size and cluster dependent damage studied by Geant4 Monte-Carlo simulations. Top. Issue Dyn. Syst. Nanoscale 2019, 73, 95. [Google Scholar]
- Hahn, M.B.; Zutta Villate, J.M. Combined cell and nanoparticle models for TOPAS to study radiation dose enhancement in cell organelles. Sci. Rep. 2021, 11, 6721. [Google Scholar] [CrossRef] [PubMed]
- Laprise-Pelletier, M.; Lagueux, J.; Cote, M.F.; LaGrange, T.; Fortin, M.A. Low-Dose Prostate Cancer Brachytherapy with Radioactive Palladium-Gold Nanoparticles. Adv. Healthc. Mater. 2017, 6, 1601120. [Google Scholar] [CrossRef]
- Gholami, Y.H.; Maschmeyer, R.; Kuncic, Z. Radio-enhancement effects by radiolabeled nanoparticles. Sci. Rep. 2019, 9, 14346. [Google Scholar] [CrossRef]
- Agostinelli, S.; Allison, J.; Amako, K.; Apostolakis, J.; Araujo, H.; Arce, P.; Asai, M.; Axen, D.; Banerjee, S.; Barrand, G.; et al. GEANT4-a simulation toolkit. Nucl Instrum. Meth. A 2003, 506, 250–303. [Google Scholar] [CrossRef]
- Allison, J.; Amako, K.; Apostolakis, J.; Araujo, H.; Dubois, P.A.; Asai, M.; Barrand, G.; Capra, R.; Chauvie, S.; Chytracek, R.; et al. Geant4 developments and applications. IEEE Trans. Nucl. Sci. 2006, 53, 270–278. [Google Scholar] [CrossRef]
- Allison, J.; Amako, K.; Apostolakis, J.; Arce, P.; Asai, M.; Aso, T.; Bagli, E.; Bagulya, A.; Banerjee, S.; Barrand, G.; et al. Recent developments in GEANT4. Nucl. Instrum. Meth. A 2016, 835, 186–225. [Google Scholar] [CrossRef]
- Arce, P.; Bolst, D.; Bordage, M.C.; Brown, J.M.C.; Cirrone, P.; Cortes-Giraldo, M.A.; Cutajar, D.; Cuttone, G.; Desorgher, L.; Dondero, P.; et al. Report on G4-Med, a Geant4 benchmarking system for medical physics applications developed by the Geant4 Medical Simulation Benchmarking Group. Med. Phys. 2021, 48, 19–56. [Google Scholar] [CrossRef]
- Incerti, S.; Suerfu, B.; Xu, J.; Ivantchenko, V.; Mantero, A.; Brown, J.M.C.; Bernal, M.A.; Francis, Z.; Karamitros, M.; Tran, H.N. Simulation of Auger electron emission from nanometer-size gold targets using the Geant4 Monte Carlo simulation toolkit. Nucl. Instrum. Methods Phys. Res. Sect. B-Beam Interact. Mater. At. 2016, 372, 91–101. [Google Scholar] [CrossRef]
- Perl, J.; Shin, J.; Schumann, J.; Faddegon, B.; Paganetti, H. TOPAS: An innovative proton Monte Carlo platform for research and clinical applications. Med. Phys. 2012, 39, 6818–6837. [Google Scholar] [CrossRef] [PubMed]
- De Frenne, D. Nuclear data sheets for A = 103. Nucl. Data Sheets 2009, 110, 2081–2256. [Google Scholar] [CrossRef]
- Sommer, H.; Ebenau, M.; Spaan, B.; Eichmann, M. Monte Carlo simulation of ruthenium eye plaques with GEANT4: Influence of multiple scattering algorithms, the spectrum and the geometry on depth dose profiles. Phys. Med. Biol. 2017, 62, 1848. [Google Scholar] [CrossRef] [PubMed]
- Tange, O. Gnu parallel-the command-line power tool. USENIX Mag. 2011, 36, 42–47. [Google Scholar]
- Monroe, J.I.; Williamson, J.F. Monte Carlo-aided dosimetry of the Theragenics TheraSeed (R) Model 200 Pd-103 interstitial brachytherapy seed. Med. Phys. 2002, 29, 609–621. [Google Scholar] [CrossRef]
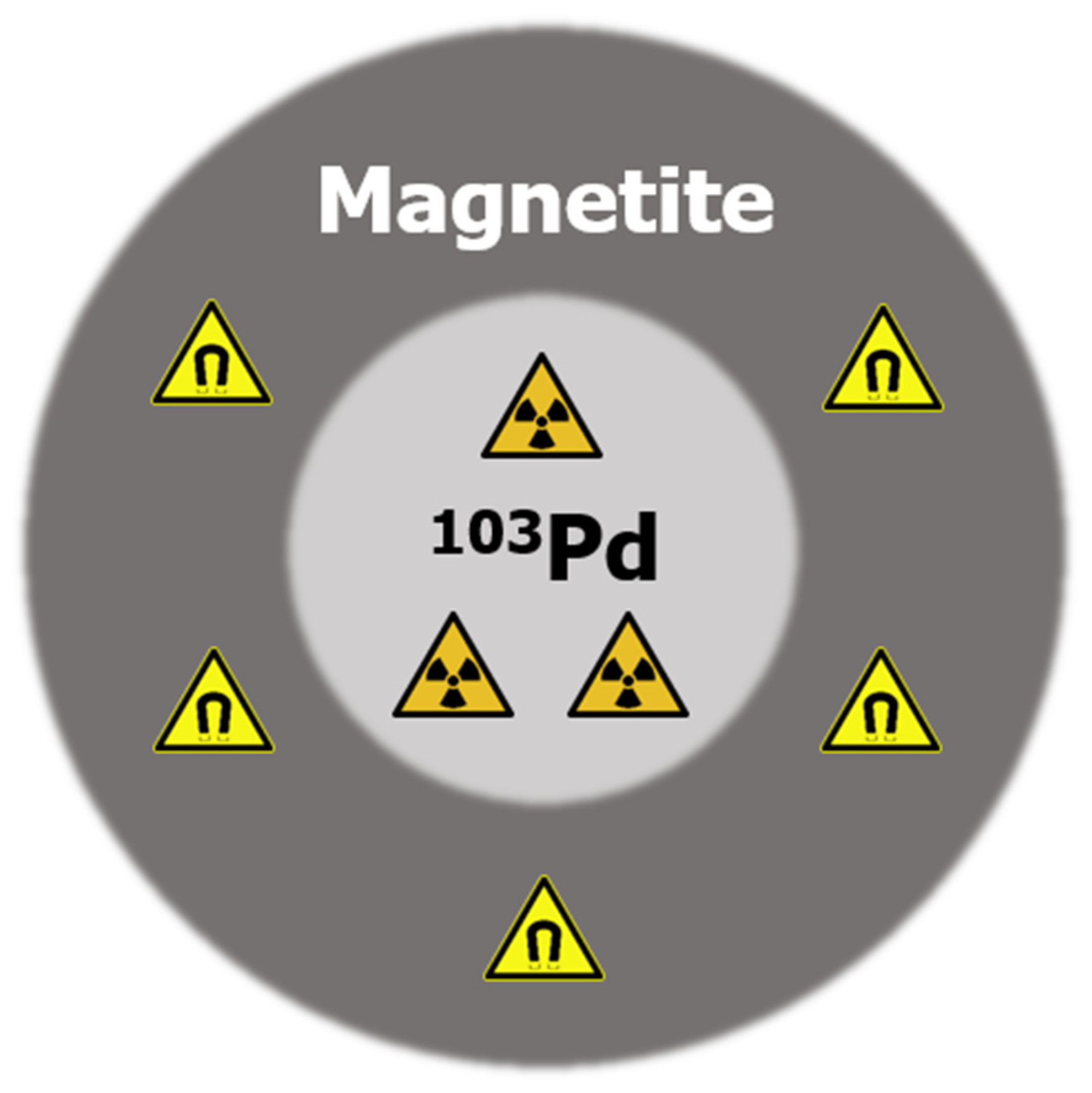
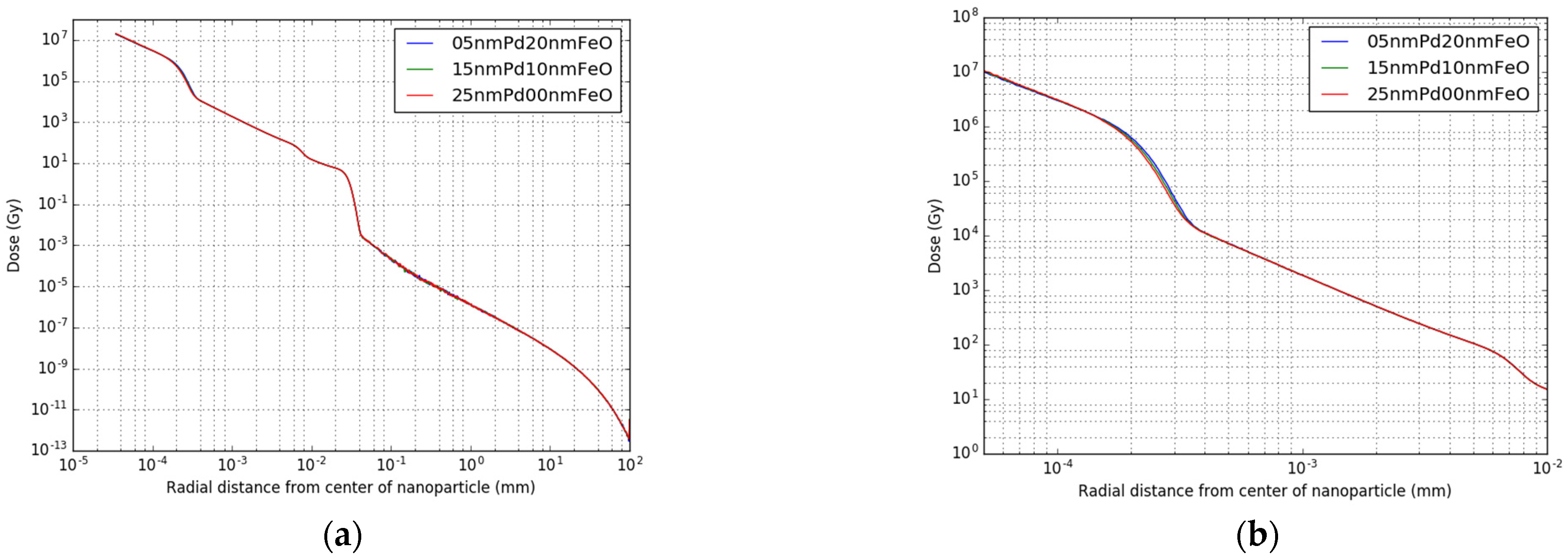

| 103Pd Core Radius (nm) | Iron Oxide Shell Thickness (nm) |
|---|---|
| 5 | 20 |
| 10 | 15 |
| 15 | 10 |
| 20 | 5 |
| 25 | 0 |
| X-ray Energy (keV) | Intensity (%) 1 | Electron Energy (keV) | Intensity (%) 1 |
|---|---|---|---|
| 2.7 | 8.7 | 2.4 | 168.0 |
| 20.0 | 22.4 | 16.5 | 9.5 |
| 20.2 | 42.5 | 17.0 | 18.2 |
| 22.7 | 6.9 | 36.3 | 71.2 |
| 23.2 | 1.6 | 39.1 | 14.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
van Oossanen, R.; Godart, J.; Brown, J.M.C.; Maier, A.; Pignol, J.-P.; Denkova, A.G.; Djanashvili, K.; van Rhoon, G.C. Feasibility Study on the Radiation Dose by Radioactive Magnetic Core-Shell Nanoparticles for Open-Source Brachytherapy. Cancers 2022, 14, 5497. https://doi.org/10.3390/cancers14225497
van Oossanen R, Godart J, Brown JMC, Maier A, Pignol J-P, Denkova AG, Djanashvili K, van Rhoon GC. Feasibility Study on the Radiation Dose by Radioactive Magnetic Core-Shell Nanoparticles for Open-Source Brachytherapy. Cancers. 2022; 14(22):5497. https://doi.org/10.3390/cancers14225497
Chicago/Turabian Stylevan Oossanen, Rogier, Jeremy Godart, Jeremy M. C. Brown, Alexandra Maier, Jean-Philippe Pignol, Antonia G. Denkova, Kristina Djanashvili, and Gerard C. van Rhoon. 2022. "Feasibility Study on the Radiation Dose by Radioactive Magnetic Core-Shell Nanoparticles for Open-Source Brachytherapy" Cancers 14, no. 22: 5497. https://doi.org/10.3390/cancers14225497
APA Stylevan Oossanen, R., Godart, J., Brown, J. M. C., Maier, A., Pignol, J.-P., Denkova, A. G., Djanashvili, K., & van Rhoon, G. C. (2022). Feasibility Study on the Radiation Dose by Radioactive Magnetic Core-Shell Nanoparticles for Open-Source Brachytherapy. Cancers, 14(22), 5497. https://doi.org/10.3390/cancers14225497







