Global Association of COVID-19 Pandemic Measures with Cancer Treatment: A Systematic Review and Meta-Analysis
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy and Selection Criteria
2.2. Data Collection and Quality Assessment
2.3. Statistical Analyses
3. Results
3.1. Treatments Overall
3.2. Surgical Treatments
3.3. Medical Treatments
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, Y.; Wang, X.; Wang, W. The Impact of COVID-19 on Cancer. Infect. Drug Resist. 2021, 14, 3809–3816. [Google Scholar] [CrossRef] [PubMed]
- Di Felice, G.; Visci, G.; Teglia, F.; Angelini, M.; Boffetta, P. Effect of cancer on outcome of COVID-19 patients: A systematic review and meta-analysis of studies of unvaccinated patients. Elife 2022, 11, e74634. [Google Scholar] [CrossRef] [PubMed]
- Gundavda, M.K.; Gundavda, K.K. Cancer or COVID-19? A Review of Guidelines for Safe Cancer Care in the Wake of the Pandemic. SN Compr. Clin. Med. 2020, 2, 2691–2701. [Google Scholar] [CrossRef] [PubMed]
- Patt, D.; Gordan, L.; Diaz, M.; Okon, T.; Grady, L.; Harmison, M.; Markward, N.; Sullivan, M.; Peng, J.; Zhou, A. Impact of COVID-19 on Cancer Care: How the Pandemic Is Delaying Cancer Diagnosis and Treatment for American Seniors. JCO Clin. Cancer Inform. 2020, 4, 1059–1071. [Google Scholar] [CrossRef]
- Morris, E.J.A.; Goldacre, R.; Spata, E.; Mafham, M.; Finan, P.J.; Shelton, J.; Richards, M.; Spencer, K.; Emberson, J.; Hollings, S.; et al. Impact of the COVID-19 pandemic on the detection and management of colorectal cancer in England: A population-based study. Lancet Gastroenterol. Hepatol. 2021, 6, 199–208. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Teglia, F.; Angelini, M.; Astolfi, L.; Casolari, G.; Boffetta, P. Global Association of COVID-19 Pandemic Measures with Cancer Screening: A Systematic Review and Meta-analysis. JAMA Oncol. 2022, 8, 1287–1293. [Google Scholar] [CrossRef]
- Oxford Centre for Triple Value Healthcare. Critical Appraisal Skills Programme (CASP). Available online: https://casp-uk.b-cdn.net/wp-content/uploads/2018/03/CASP-Qualitative-Checklist-2018_fillable_form.pdf (accessed on 3 March 2022).
- Egger, M.; Davey Smith, G.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef]
- Akhtar, N.; Rajan, S.; Chakrabarti, D.; Kumar, V.; Gupta, S.; Misra, S.; Ms, A.C.; Azhar, T.; Parveen, S.; Qayoom, S.; et al. Continuing cancer surgery through the first six months of the COVID-19 pandemic at an academic university hospital in India: A lower-middle-income country experience. J. Surg. Oncol. 2021, 123, 1177–1187. [Google Scholar] [CrossRef]
- Lee, J.; Holden, L.; Fung, K.; Danjoux, C.; Chow, E.; Gillies, C. Impact of severe acute respiratory syndrome on patient access to palliative radiation therapy. Support. Cancer Ther. 2005, 2, 109–113. [Google Scholar] [CrossRef]
- Tartarone, A.; Lerose, R. COVID-19 and cancer care: What do international guidelines say? Med. Oncol. 2020, 37, 80. [Google Scholar] [CrossRef] [PubMed]
- Beretta, D.G.; Cinieri, D.S.; Blasi, D.L.; Cipomo, P.; Aglietta, M.; Comu, P. Rischio Infettivo da Coronavirus COVID-19: Indicazioni per L’oncologia. Available online: https://www.aiom.it/wp-content/uploads/2020/03/20200313_COVID-19_indicazioni_AIOM-CIPOMO-COMU.pdf (accessed on 13 March 2020).
- Hale, T.; Angrist, N.; Goldszmidt, R.; Kira, B.; Petherick, A.; Phillips, T.; Webster, S.; Cameron-Blake, E.; Hallas, L.; Majumdar, S.; et al. A global panel database of pandemic policies (Oxford COVID-19 Government Response Tracker). Nat. Hum. Behav. 2021, 5, 529–538. [Google Scholar] [CrossRef] [PubMed]
- ESMO Guidelines. Cancer Patient Management during the COVID-19 Pandemic. Available online: https://www.esmo.org/guidelines/cancer-patient-management-during-the-covid-19-pandemic (accessed on 1 May 2020).
- ASCO. COVID-19 Patient Care Information. 2020. Available online: https://www.asco.org/covid-resources/patient-care-info (accessed on 1 May 2020).
- Baumann, B.C.; MacArthur, K.M.; Brewer, J.D.; Mendenhall, W.M.; Barker, C.A.; Etzkorn, J.R.; Jellinek, N.J.; Scott, J.F.; Gay, H.A.; Baumann, J.C.; et al. Management of primary skin cancer during a pandemic: Multidisciplinary recommendations. Cancer 2020, 126, 3900–3906. [Google Scholar] [CrossRef]
- Andrew, T.W.; Alrawi, M.; Lovat, P. Reduction in skin cancer diagnoses in the UK during the COVID-19 pandemic. Clin. Exp. Dermatol. 2021, 46, 145–146. [Google Scholar] [CrossRef] [PubMed]
- Gazivoda, V.; Greenbaum, A.; Roshal, J.; Lee, J.; Reddy, L.; Rehman, S.; Kangas-Dick, A.; Gregory, S.; Kowzun, M.; Stephenson, R.; et al. Assessing the immediate impact of COVID-19 on surgical oncology practice: Experience from an NCI-designated Comprehensive Cancer Center in the Northeastern United States. J. Surg. Oncol. 2021, 124, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Mattiuzzi, C.; Lippi, G. Current Cancer Epidemiology. J. Epidemiol. Glob. Health 2019, 9, 217–222. [Google Scholar] [CrossRef]
- Singh, A.P.; Berman, A.T.; Marmarelis, M.E.; Haas, A.R.; Feigenberg, S.J.; Braun, J.; Kangas-Dick, A.; Gregory, S.; Kowzun, M.; Stephenson, R.; et al. Management of Lung Cancer During the COVID-19 Pandemic. JCO Oncol. Pract. 2020, 16, 579–586. [Google Scholar] [CrossRef]
- Price, P.; Barney, S.E. Initiation of the Global Coalition for Radiotherapy during the COVID-19 pandemic. Lancet Oncol. 2020, 21, 752–753. [Google Scholar] [CrossRef]
- Powis, M.; Milley-Daigle, C.; Hack, S.; Alibhai, S.; Singh, S.; Krzyzanowska, M.K. Impact of the early phase of the COVID pandemic on cancer treatment delivery and the quality of cancer care: A scoping review and conceptual model. Int. J. Qual. Health Care 2021, 33, mzab088. [Google Scholar] [CrossRef]
- Dowdy, S.; Nickles Fader, A. Society of Gynecologic Oncology. Surgical Considerations for Gynecologic Oncologists during the COVID-19 Pandemic. 2020. Available online: https://www.sgo.org/resources/surgical-considerations-for-gynecologic-oncologists-during-the-covid-19-pandemic/ (accessed on 21 July 2022).
- Francis, N.; Dort, J.; Cho, E.; Feldman, L.; Keller, D.; Lim, R.; Mikami, D.; Phillips, E.; Spaniolas, K.; Tsuda, S.; et al. SAGES and EAES recommendations for minimally invasive surgery during COVID-19 pandemic. Surg. Endosc. 2020, 34, 2327–2331. [Google Scholar] [CrossRef]
- COVID-19: Guidance for Triage of Non-Emergent Surgical Procedures; American College of Surgeons: Chicago, IL, USA, 2020.
- Assaad, S.; Avrillon, V.; Fournier, M.L.; Mastroianni, B.; Russias, B.; Swalduz, A.; Cassier, P.; Eberst, L.; Steineur, M.-P.; Kazes, M.; et al. High mortality rate in cancer patients with symptoms of COVID-19 with or without detectable SARS-CoV-2 on RT-PCR. Eur. J. Cancer 2020, 135, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Clark, J.J.; Dwyer, D.; Pinwill, N.; Clark, P.; Johnson, P.; Hackshaw, A. The effect of clinical decision making for initiation of systemic anticancer treatments in response to the COVID-19 pandemic in England: A retrospective analysis. Lancet Oncol. 2021, 22, 66–73. [Google Scholar] [CrossRef]
- Ueda, M.; Martins, R.; Hendrie, P.C.; McDonnell, T.; Crews, J.R.; Wong, T.L.; McCreery, B.; Jagels, B.; Crane, A.; Byrd, D.R.; et al. Managing Cancer Care during the COVID-19 Pandemic: Agility and Collaboration toward a Common Goal. J. Natl. Compr. Cancer Netw. 2020, 18, 366–369. [Google Scholar] [CrossRef]
- Curigliano, G. The Treatment of Patients with Cancer and Containment of COVID-19: Experiences from Italy. ASCO Daily News. Available online: https://dailynews.ascopubs.org/do/10.1200/ADN.20.200068/full/ (accessed on 21 July 2022).
- BASO Guidance for Cancer Surgery British Association for Surgical Oncology, London. 2020. Available online: https://baso.org.uk/media/99217/baso_guidance_for_cancer_surgery_9th_april_2020_v7.pdf (accessed on 21 July 2022).
- Cadili, L.; DeGirolamo, K.; McKevitt, E.; Brown, C.J.; Prabhakar, C.; Pao, J.S.; Dingee, C.; Bazzarelli, A.; Warburton, R. COVID-19 and breast cancer at a Regional Breast Centre: Our flexible approach during the pandemic. Breast Cancer Res. Treat. 2021, 186, 519–525. [Google Scholar] [CrossRef]
- Canadian Anesthesiologists’ Society. COVID-19 Recommendations during Airway Manipulation. 2020. Available online: http://cas.ca/en/practice-resources/news/cas-articles/2020/covid-19-recommendations-during-airway-manipulation (accessed on 21 July 2022).
- McPhail, S.; Elliss-Brookes, L.; Shelton, J.; Ives, A.; Greenslade, M.; Vernon, S.; A Morris, E.J.; Richards, M. Emergency presentation of cancer and short-term mortality. Br. J. Cancer 2013, 109, 2027–2034. [Google Scholar] [CrossRef] [PubMed]
- Lai, A.G.; Pasea, L.; Banerjee, A.; Hall, G.; Denaxas, S.; Chang, W.H.; Katsoulis, M.; Williams, B.; Pillay, D.; Noursadeghi, M.; et al. Estimated impact of the COVID-19 pandemic on cancer services and excess 1-year mortality in people with cancer and multimorbidity: Near real-time data on cancer care, cancer deaths and a population-based cohort study. BMJ Open 2020, 10, e043828. [Google Scholar] [CrossRef]
| Percent Difference, % (95% CI) | |||
|---|---|---|---|
| Characteristic | Overall Treatment | Surgical Treatment | Medical Treatment |
| Total | −18.7 (−24.1 to −13.3) | −33.9 (−39.9 to −27.9) | −12.6 (−20.4 to −4.8) |
| Period (2020) | |||
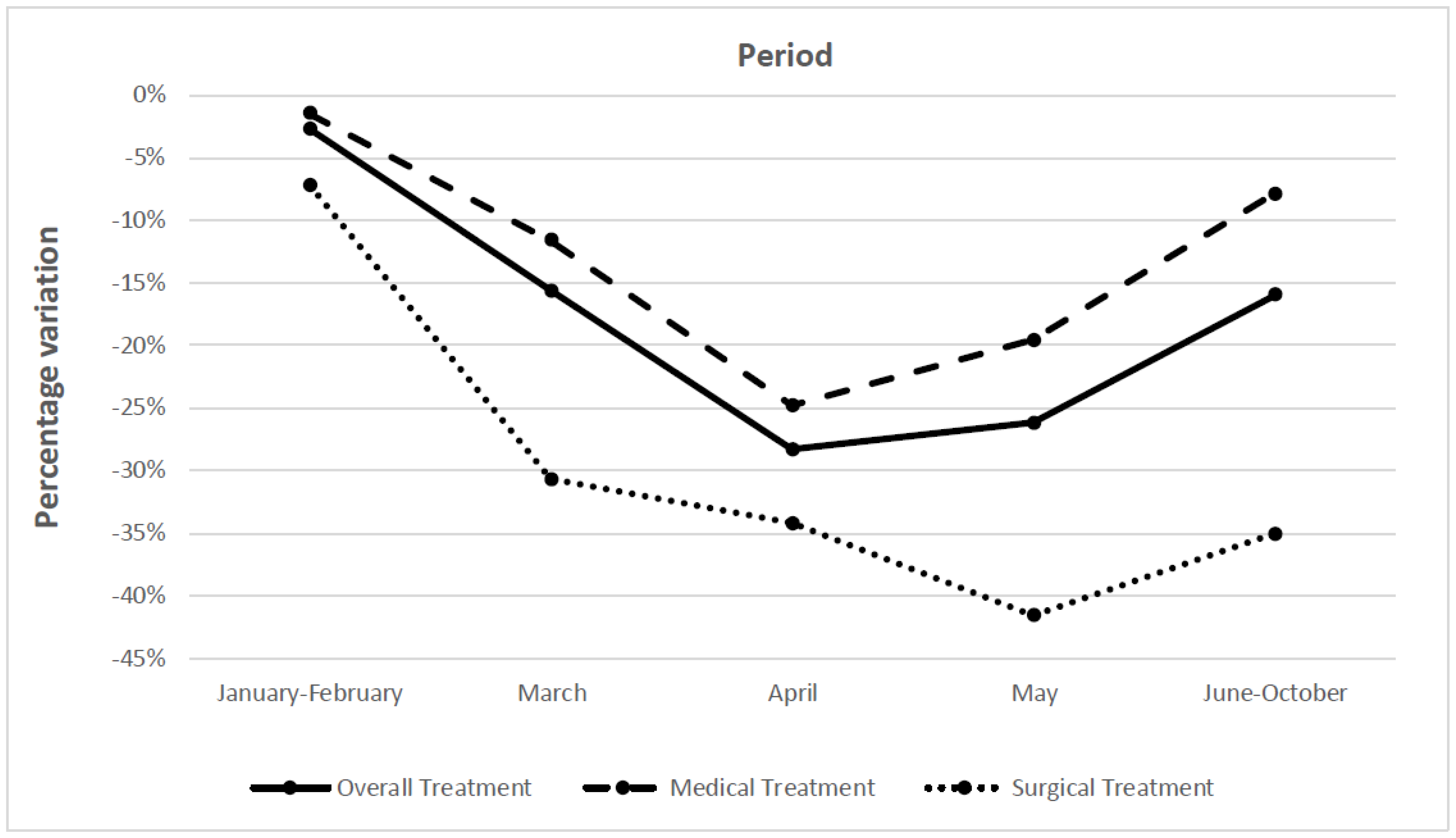
| January–February | −2.7 (−10.0 to 4.5) | −7.2 (−23.8 to 9.3) | −1.5 (−8.4 to 5.5) |
| March | −15.6 (−23.7 to −7.6) | −30.7 (−39.6 to −21.9) | −11.6 (−23.3 to 1.2) |
| April | −28.3 (−37.2 to −19.4) | −34.2 (−44.5 to −23.9) | −24.8 (−40.7 to −9.0) |
| May | −26.2 (−34.7 to −17.6) | −41.6 (−51.4 to −31.8) | −19.6 (−30.6 to −8.5) |
| June–October | −16.0 (27−9 to −4.1) | −35.1 (−51.6 to −18.6) | −7.9 (−23.6 to 7.8) |
| Geographic area | |||
| North America | −34.6 (−47.4 to −21.8) | −39.2 (−53.7 to −24.8) | −17.1 (−54.4 to 20.2) |
| Europe | −7.9 (−15.6 to −0.2) | −20.9 (−30.1 to −11.7) | −3.8 (−15.4 to 7.8) |
| Latin America | −20.3 (−31.2 to −9.4) | −38.3 (−54.8 to −21.7) | −18.2 (−30.4 to −6.1) |
| Asia | −42.1 (−49.6 to −34.7) | −45.8 (−52.1 to −39.6) | −36.7 (−59.4 to −13.9) |
| Study setting | |||
| Clinic-based | −21.5 (−31.3 to −11.7) | −38.1 (−47.5 to −28.7) | −17.2 (−33.0 to −1.5) |
| Population-based | −17.2 (−23.2 to −11.1) | −31.5 (−40.7 to −22.3) | −9.9 (−17.5 to −2.3) |
| Type of cancer | |||
| Breast | −18.0 (−29.4 to −6.5) | −26.8 (−51.3 to −2.2) | −4.6 (−17.6 to 8.3) |
| Genitourinary | −2.7 (−20.6 to 15.1) | −20.9 (−33.9 to −8.0) | 13.3 (−30.3 to 56.9) |
| Gastrointestinal | −14.4 (−24.6 to −4.2) | −21.6 (−31.1 to −12.0) | −2.6 (−31.9 to 26.6) |
| Lung | −5.2 (−15.6 to 5.1) | ⸺ | ⸺ |
| Colorectal | −23.0 (−34.5 to −11.5) | ⸺ | ⸺ |
| Prostate | −11.5 (−39.0 to 16.0) | ⸺ | ⸺ |
| Cervix | −24.6 (−37.5 to −11.6) | ⸺ | ⸺ |
| Skin cancer | −34.7 (−46.8 to −22.5) | −29.9 (−45.3 to −14.4) | −53.5 (−83.3 to −23.6) |
| Type of medical treatment | |||
| Systemic therapy | ⸺ | ⸺ | −18.5 (−28.7 to −8.2) |
| Radiotherapy | ⸺ | ⸺ | −6.6 (−22.2 to 8.9) |
| Percent Difference, % (95% CI) | ||||
|---|---|---|---|---|
| Cancer Treatments | North America (%, 95% CI) | Europe (%, 95% CI) | Asia (%, 95% CI) | Latin America (%, 95% CI) |
| Period | ||||
| January–February | ⸺ | −0.2 (−8.2 to +7.9) | ⸺ | ⸺ |
| March | −21.6 (−40.3 to −2.9) | −3.6 (−20.2 to 13.0) | −38.7 (−47.2 to −30.2) | −17.1 (−29.2 to −5.0) |
| April | −39.0 (−68.7 to −9.3) | −20.1 (−30.9 to −9.3) | −53.6 (−70.7 to −36.4) | −43.8 (−94.2 to +6.5) |
| May | −32.0 (−65.6 to 1.7) | −14.4 (−26.5 to −2.4) | −59.0 (−95.5 to −22.5) | ⸺ |
| June–October | −31.5 (−51.5 to −11.6) | −0.6 (−21.1 to 20.0) | ⸺ | ⸺ |
| Cancer site | ||||
| Gastrointestinal | −31.0 (−91.8 to 29.7) | −8.6 (−19.6 to 2.3) | −47.3 (−71.4 to −23.2) | ⸺ |
| Genitourinary | −27.7 (−45.5 to −9.8) | 4.9 (−18.6 to 28.5) | ⸺ | ⸺ |
| Skin | −34.9 (−52.0 to −17.7) | −39.9 (−75.9 to −3.9) | ⸺ | ⸺ |
| Breast | −35.0 (−94.6 to 24.6) | −13.2 (−21.0 to −5.5) | ⸺ | −12.9 (−24.0 to −1.8) |
| Study setting | ||||
| Clinic-based | −24.2 (−66.0 to +17.5) | −9.8 (−25.5 to 5.8) | −46.3 (−56.0 to −36.5) | −25.7 (−45.6 to −5.7) |
| Population-based | −37.2% (−51.5 to −22.9) | −7.0 (−14.8 to 0.9) | −39.2 (−59.8 to −18.6) | −16.0 (−25.2 to −6.8) |
| Coefficient (95% CI) | |||
|---|---|---|---|
| Characteristic | Overall Treatment | Surgical Treatment | Medical Treatment |
| Type of treatment | |||
| Medical treatment | 0 [Reference] | ⸺ | ⸺ |
| Surgical treatment | −27.1% (−43.1 to −11.1) | ⸺ | ⸺ |
| Period (2020) | |||
| January–February | 0 [Reference] | 0 [Reference] | 0 [Reference] |
| March | 1.0% (−27.7 to 28.7) | −3.1% (−42.5 to 36.3) | 3.3% (−41.0 to 47.5) |
| April | −22.1% (−53.2 to 8.9) | −14.4% (−60.2 to 31.4) | −32.7% (−80.6 to 15.3) |
| May | −13.7% (−54.2 to 26.9) | −29.1% (−100.0 to 48.7) | −11.6% (−69.0 to 45.8) |
| June–October | −1.9 (−38.7 to 34.9) | −20.0% (−77.7 to 37.7) | 4.3% (−51.3 to 59.8) |
| Geographic area | |||
| North America | 0 [Reference] | 0 [Reference] | 0 [Reference] |
| Europe | 6.6 (−17.6 to 30.9) | 2.1% (−26.9 to 31.2) | 29.7% (−41.5 to 100.9) |
| Latin America | −17.5 (−46.4 to 11.4) | −22.1% (−57.8 to 13.7) | 3.5% (−70.1 to 77.1) |
| Asia | −19.5 (−48.0 to 9.0) | −20.0% (−53.0 to 13.0) | −4.8% (−83.0 to 73.4) |
| Study setting | |||
| Clinic-based | 0 [Reference] | 0 [Reference] | 0 [Reference] |
| Population-based | −2.3(−19.9 to 15.3) | 0.9% (−23.3 to 25.1) | −6.4% (−35.9 to 23.1) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teglia, F.; Angelini, M.; Casolari, G.; Astolfi, L.; Boffetta, P. Global Association of COVID-19 Pandemic Measures with Cancer Treatment: A Systematic Review and Meta-Analysis. Cancers 2022, 14, 5490. https://doi.org/10.3390/cancers14225490
Teglia F, Angelini M, Casolari G, Astolfi L, Boffetta P. Global Association of COVID-19 Pandemic Measures with Cancer Treatment: A Systematic Review and Meta-Analysis. Cancers. 2022; 14(22):5490. https://doi.org/10.3390/cancers14225490
Chicago/Turabian StyleTeglia, Federica, Marco Angelini, Giulia Casolari, Laura Astolfi, and Paolo Boffetta. 2022. "Global Association of COVID-19 Pandemic Measures with Cancer Treatment: A Systematic Review and Meta-Analysis" Cancers 14, no. 22: 5490. https://doi.org/10.3390/cancers14225490
APA StyleTeglia, F., Angelini, M., Casolari, G., Astolfi, L., & Boffetta, P. (2022). Global Association of COVID-19 Pandemic Measures with Cancer Treatment: A Systematic Review and Meta-Analysis. Cancers, 14(22), 5490. https://doi.org/10.3390/cancers14225490






