Lung Cancer Screening in Greece: A Modelling Study to Estimate the Impact on Lung Cancer Life Years
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Verification Study
2.2. Modelling Study Objectives
2.3. Modelling Study Methods
2.4. Modelling Study Data Inputs
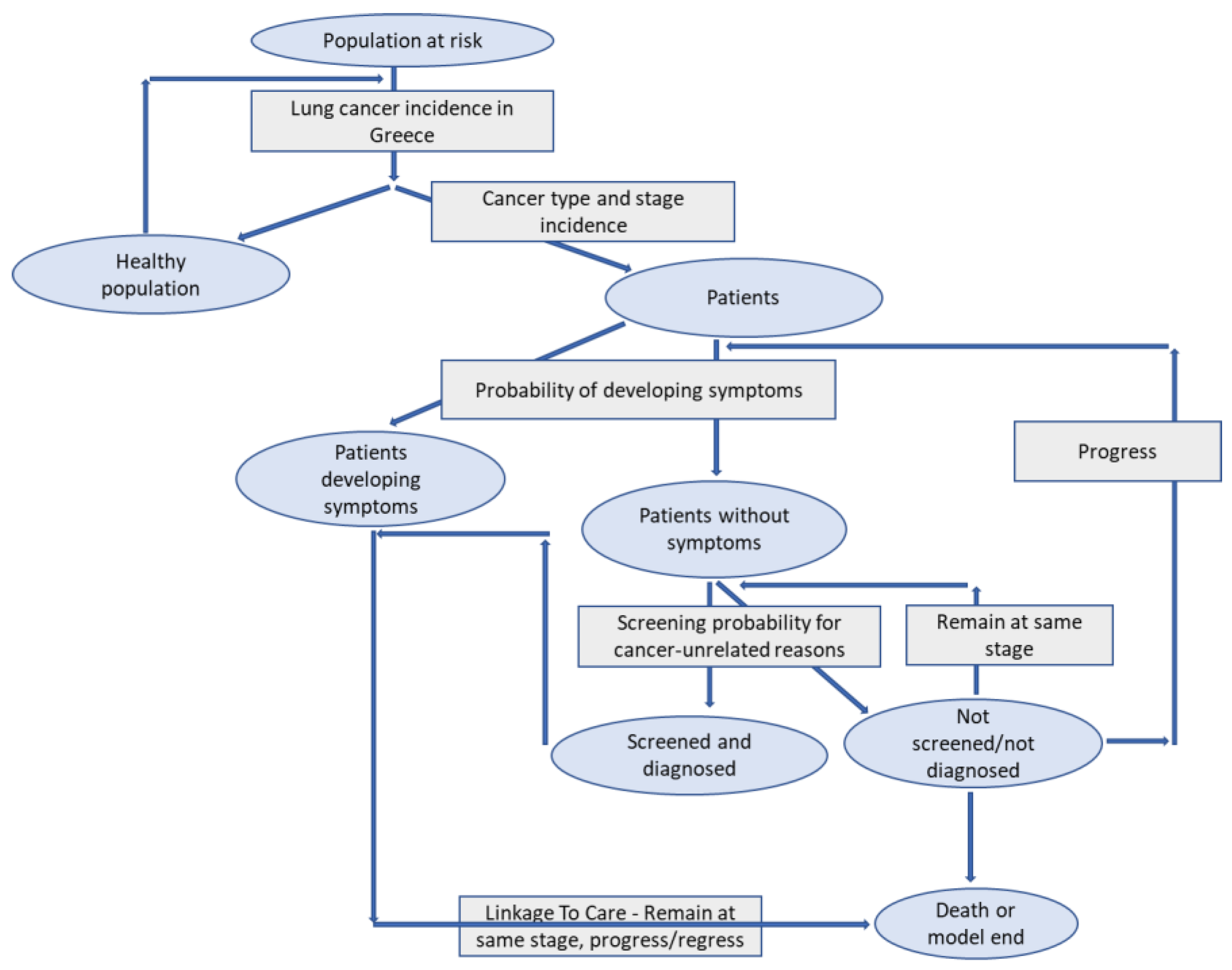
2.5. Modelling Study Schema
2.6. Model Transitions’ Structure
3. Results
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Veronesi, G.; Baldwin, D.R.; Henschke, C.I.; Ghislandi, S.; Iavicoli, S.; Oudkerk, M.; De Koning, H.J.; Shemesh, J.; Field, J.K.; Zulueta, J.J.; et al. Recommendations for Implementing Lung Cancer Screening with Low-Dose Computed Tomography in Europe. Cancers 2020, 12, 1672. [Google Scholar] [CrossRef] [PubMed]
- Haralsingh, A.; West, M. Tissue yields for epidermal growth factor receptor analysis in non-small cell lung cancer patients in Trinidad and Tobago. Cureus 2021, 13, e12531. [Google Scholar] [CrossRef] [PubMed]
- Thandra, K.C.; Barsouk, A.; Saginala, K.; Aluru, J.S.; Barsouk, A. Epidemiology of lung cancer. Contemp. Oncol./Współczesna Onkol. 2021, 25, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Greece-Global Cancer Observatory. Available online: https://gco.iarc.fr/today/data/factsheets/populations/300-greece-fact-sheets.pdf (accessed on 21 July 2022).
- WHO. WHO Global Report on Trends in Prevalence of Tobacco Smoking 2000–2025. Second Edition. Available online: https://apps.who.int/iris/bitstream/handle/10665/272694/9789241514170-eng.pdf?ua=1 (accessed on 21 July 2022).
- de Koning, H.J.; van der Aalst, C.M.; de Jong, P.A.; Scholten, E.T.; Nackaerts, K.; Heuvelmans, M.A.; Lammers, J.J.; Weenink, C.; Yousaf-Khan, U.; Horeweg, N.; et al. Reduced lung-cancer mortality with volume CT screening in a randomized trial. N. Engl. J. Med. 2020, 382, 503–513. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.L.; Wang, S.Y.; Lu, W.C.; Chang, Y.H.; Su, J.; Lu, Y.T. Effects of low-dose computed tomography on lung cancer screening: A systematic review, meta-analysis, and trial sequential analysis. BMC Pulm. Med. 2021, 19, 126. [Google Scholar]
- Snowsill, T.M.; Yang, H.; Griffin, E.; Long, H.L.; Varley-Campbell, J.; Coelho, H.; Robinson, S.; Hyde, C. Low-dose computed tomography for lung cancer screening in high risk populations: A systematic review and economic evaluation. Health Technol. Assess. 2018, 22, 1–276. [Google Scholar] [CrossRef] [PubMed]
- Field, J.K.; Vulkan, D.; Davies, M.P.; Baldwin, D.R.; Brain, K.E.; Devaraj, A.; Eisen, T.; Gosney, J.; Green, B.A.; Holemans, J.A.; et al. Lung cancer mortality reduction by LDCT screening: UKLS randomised trial results and international meta-analysis. Lancet Reg. Health-Eur. 2021, 10, 100179. [Google Scholar]
- Delorme, S.; Kaaks, R. Lung Cancer Screening by Low-Dose Computed Tomography: Part 2–Key Elements for Programmatic Implementation of Lung Cancer Screening. In RöFo-Fortschritte auf dem Gebiet der Röntgenstrahlen und der bildgebenden Verfahren; Georg Thieme Verlag KG: New York, NY, USA, 2021; Volume 193, pp. 644–651. [Google Scholar]
- USPSTF, US Preventive Services Task Force. Recommendations on Lung Cancer Screening. Eligibility Criteria. Available online: https://www.uspreventiveservicestaskforce.org/uspstf/recommendation/lung-cancer-screening (accessed on 21 July 2022).
- Du, Y.; Sidorenkov, G.; Heuvelmans, M.A.; Groen, H.J.; Vermeulen, K.M.; Greuter, M.J.; de Bock, G.H. Cost-effectiveness of lung cancer screening with low-dose computed tomography in heavy smokers: A microsimulation modelling study. Eur. J. Cancer 2020, 135, 121–129. [Google Scholar] [CrossRef]
- Meza, R.; Jeon, J.; Toumazis, I.; Ten Haaf, K.; Cao, P.; Bastani, M.; Han, S.S.; Blom, E.F.; Jonas, D.E.; Feuer, E.J.; et al. Evaluation of the benefits and harms of lung cancer screening with low-dose computed tomography: Modeling study for the US Preventive Services Task Force. JAMA 2021, 325, 988–997. [Google Scholar] [CrossRef]
- The Health Policy Partnership. Lung Cancer Screening Building Resilience and Sustainability of Healthcare Systems. 2021. Available online: https://www.healthpolicypartnership.com/app/uploads/Lung-cancer-screening-Building-resilience-and-sustainability-of-healthcare-systems.pdf (accessed on 20 October 2022).
- ELSTAT, Hellenic Statistical Authority. Database. Available online: https://www.statistics.gr/el/statistics/-/publication/SPO18/- (accessed on 21 July 2022).
- NCCN. Guidelines for Treatment of Cancer by Type. NCCN Guidelines with Evidence Blocks—Non-Small Cell Lung Cancer Version 5.2022. 2022. Available online: https://www.nccn.org/guidelines/recently-published-guidelines (accessed on 20 October 2022).
- ESMO. Clinical Practice Guidelines: Lung and Chest Tumors. 2022. Available online: https://www.esmo.org/guidelines/guidelines-by-topic/lung-and-chest-tumours (accessed on 20 October 2022).
- EOPE. Therapeutic Protocols for Chemotherapy in Cancer Patients (Greek Guidelines). 2018. Available online: www.hesmo.gr/images/Θεραπευτικά_Πρωτόκολλα_ΕOΠΕ_7η_Έκδοση_Τελικό-fA-.pdf (accessed on 20 October 2022).
- Coughlin, J.M.; Zang, Y.; Terranella, S.; Alex, G.; Karush, J.; Geissen, N.; Chmielewski, G.W.; Arndt, A.T.; Liptay, M.J.; Zimmermann, L.J.; et al. Understanding barriers to lung cancer screening in primary care. J. Thorac. Dis. 2020, 12, 2536. [Google Scholar] [CrossRef]
- Rankin, N.M.; McWilliams, A.; Marshall, H.M. Lung cancer screening implementation: Complexities and priorities. Respirology 2020, 25, 5–23. [Google Scholar] [PubMed]
- Allen, C.G.; Cotter, M.M.; Smith, R.A.; Watson, L. Successes and challenges of implementing a lung cancer screening program in federally qualified health centers: A qualitative analysis using the Consolidated Framework for Implementation Research. Transl. Behav. Med. 2021, 11, 1088–1098. [Google Scholar] [CrossRef] [PubMed]
- Carter-Harris, L.; Gould, M.K. Multilevel barriers to the successful implementation of lung cancer screening: Why does it have to be so hard? Ann. Am. Thorac. Soc. 2017, 14, 1261–1265. [Google Scholar] [PubMed]
- Wait, S.; Alvarez-Rosete, A.; Osama, T.; Bancroft, D.; Cornelissen, R.; Marušić, A.; Garrido, P.; Adamek, M.; van Meerbeeck, J.; Snoeckx, A.; et al. Implementing lung cancer screening in Europe: Taking a systems approach. JTO Clin. Res. Rep. 2022, 3, 100329. [Google Scholar] [CrossRef]
- EUAPM. Revolutionizing Cancer Care All Together. Available online: https://www.euapm.eu/pdf/EAPM_revolutionising_lung_cancer_care_all_together_croatia_feb_2021.pdf (accessed on 21 July 2022).
- Rzyman, W.; Szurowska, E.; Adamek, M. Implementation of lung cancer screening at the national level: Polish example. Transl. Lung Cancer Res. 2019, 8 (Suppl. S1), S95. [Google Scholar]
- Van Meerbeeck, J.P.; O’Dowd, E.; Ward, B.; Van Schil, P.; Snoeckx, A. Lung Cancer Screening: New Perspective and Challenges in Europe. Cancers 2022, 14, 2343. [Google Scholar] [CrossRef]
- Ali, N.; Lifford, K.J.; Carter, B.; McRonald, F.; Yadegarfar, G.; Baldwin, D.R.; Weller, D.; Hansell, D.M.; Duffy, S.W.; Field, J.K.; et al. Barriers to uptake among high-risk individuals declining participation in lung cancer screening: A mixed methods analysis of the UK Lung Cancer Screening (UKLS) trial. BMJ Open 2015, 5, e008254. [Google Scholar]
- Katki, H.A.; Kovalchik, S.A.; Petito, L.C.; Cheung, L.C.; Jacobs, E.; Jemal, A.; Berg, C.D.; Chaturvedi, A.K. Implications of Nine Risk Prediction Models for Selecting Ever-Smokers for Computed Tomography Lung Cancer Screening. Ann. Intern. Med. 2018, 169, 10–19. [Google Scholar] [CrossRef]
- Hinde, S.; Crilly, T.; Balata, H.; Bartlett, R.; Crilly, J.; Barber, P.; Threlfall, A.; Tonge, J.; Booton, R.; Crosbie, P.A. The costeffectiveness of the Manchester ‘lung health checks’, a community-based lung cancer low-dose CT screening pilot. Lung Cancer 2018, 126, 119–124. [Google Scholar] [CrossRef]
- LeMense, G.P.; Waller, E.A.; Campbell, C.; Bowen, T. Development and outcomes of a comprehensive multidisciplinary incidental lung nodule and lung cancer screening program. BMC Pulm. Med. 2020, 20, 115. [Google Scholar] [CrossRef]
- Hardavella, G.; Desai, S. Development of a pulmonary nodule service & clinical pathway: A pragmatic approach addressing an unmet need. Eur. Respir. J. 2016, 48, 3079. [Google Scholar]
- Melton, N.; Lazar, J.F.; Moritz, T.A. A community-based pulmonary nodule clinic: Improving lung cancer stage at diagnosis. Cureus 2019, 11, 3. [Google Scholar]
- Yip, R.; Jirapatnakul, A.; Hu, M.; Chen, X.; Han, D.; Ma, T.; Zhu, Y.; Salvatore, M.M.; Margolies, L.R.; Yankelevitz, D.F.; et al. Added benefits of early detection of other diseases on low-dose CT screening. Transl. Lung Cancer Res. 2021, 10, 1141. [Google Scholar] [CrossRef] [PubMed]


| Parameter | Mean Value | Standard Deviation |
|---|---|---|
| Cancer cases (per model life cycle) | 1929.07 | 39.57 |
| Cancer-related deaths (per model life cycle) | 1371.21 | 34.68 |
| Lost years (per model life cycle) | 3042.84 | 90.98 |
| Parameter | Mean Value | Standard Deviation |
|---|---|---|
| Cancer cases (per model life cycle) | 1925.55 | 41.00 |
| Cancer-related deaths (per model life cycle) | 1031.89 | 31.54 |
| Lost years (per model life cycle) | 2098.85 | 77.37 |
| Parameter | Difference (%) | p-Value |
|---|---|---|
| Cancer-related deaths (per model life cycle) | −24.61% | <0.001 |
| Lost years (per model life cycle) | −30.90% | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Souliotis, K.; Golna, C.; Golnas, P.; Markakis, I.-A.; Linardou, H.; Sifaki-Pistolla, D.; Hatziandreou, E. Lung Cancer Screening in Greece: A Modelling Study to Estimate the Impact on Lung Cancer Life Years. Cancers 2022, 14, 5484. https://doi.org/10.3390/cancers14225484
Souliotis K, Golna C, Golnas P, Markakis I-A, Linardou H, Sifaki-Pistolla D, Hatziandreou E. Lung Cancer Screening in Greece: A Modelling Study to Estimate the Impact on Lung Cancer Life Years. Cancers. 2022; 14(22):5484. https://doi.org/10.3390/cancers14225484
Chicago/Turabian StyleSouliotis, Kyriakos, Christina Golna, Pavlos Golnas, Ioannis-Anestis Markakis, Helena Linardou, Dimitra Sifaki-Pistolla, and Evi Hatziandreou. 2022. "Lung Cancer Screening in Greece: A Modelling Study to Estimate the Impact on Lung Cancer Life Years" Cancers 14, no. 22: 5484. https://doi.org/10.3390/cancers14225484
APA StyleSouliotis, K., Golna, C., Golnas, P., Markakis, I.-A., Linardou, H., Sifaki-Pistolla, D., & Hatziandreou, E. (2022). Lung Cancer Screening in Greece: A Modelling Study to Estimate the Impact on Lung Cancer Life Years. Cancers, 14(22), 5484. https://doi.org/10.3390/cancers14225484







