Validation of the DNA Methylation Landscape of TFF1/TFF2 in Gastric Cancer
Abstract
Simple Summary
Abstract
1. Introduction
2. Methods and Materials
2.1. Cell Culture
2.2. Data Sources
2.3. Data Processing of DEGs
2.4. GO and KEGG Pathway Analysis of DEGs
2.5. PPI Network Construction
2.6. RNA Isolation and Quantitative Real-Time PCR (qRT- PCR)
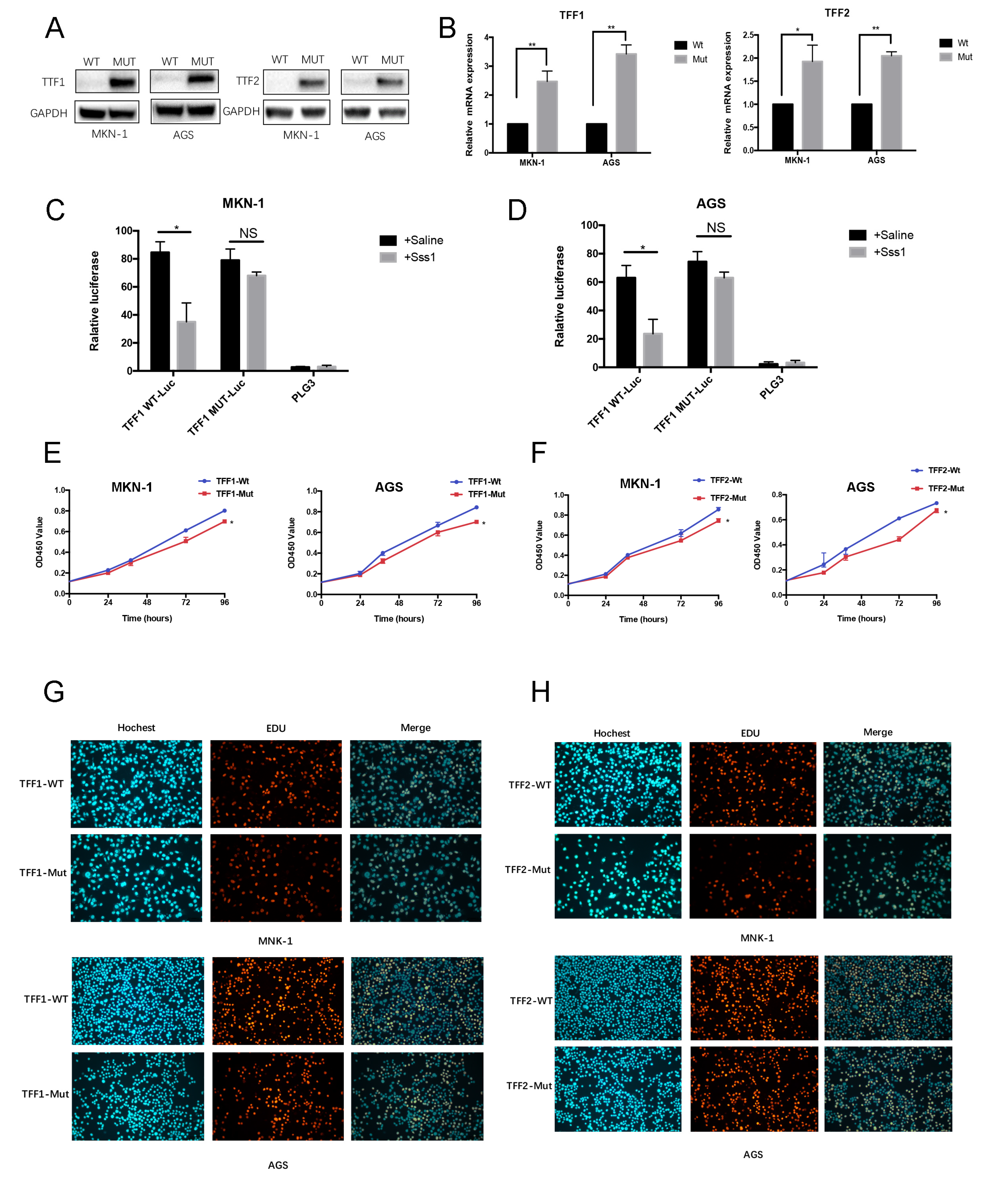
2.7. Construction of the Mutation Vectors for TFF1/TTF2 DNA Methylation Sites
2.8. Double Luciferase Report Assay
2.9. Cell Viability Assay and Ethynyl Deoxyuridine (EdU) Assay
2.10. Statistical Analysis
3. Result
3.1. Identification of the Differential Expression Genes in Gastric Cancer
3.2. Downregulation of TFFs Indicated the Poor Prognosis in Gastric Cancer Patients
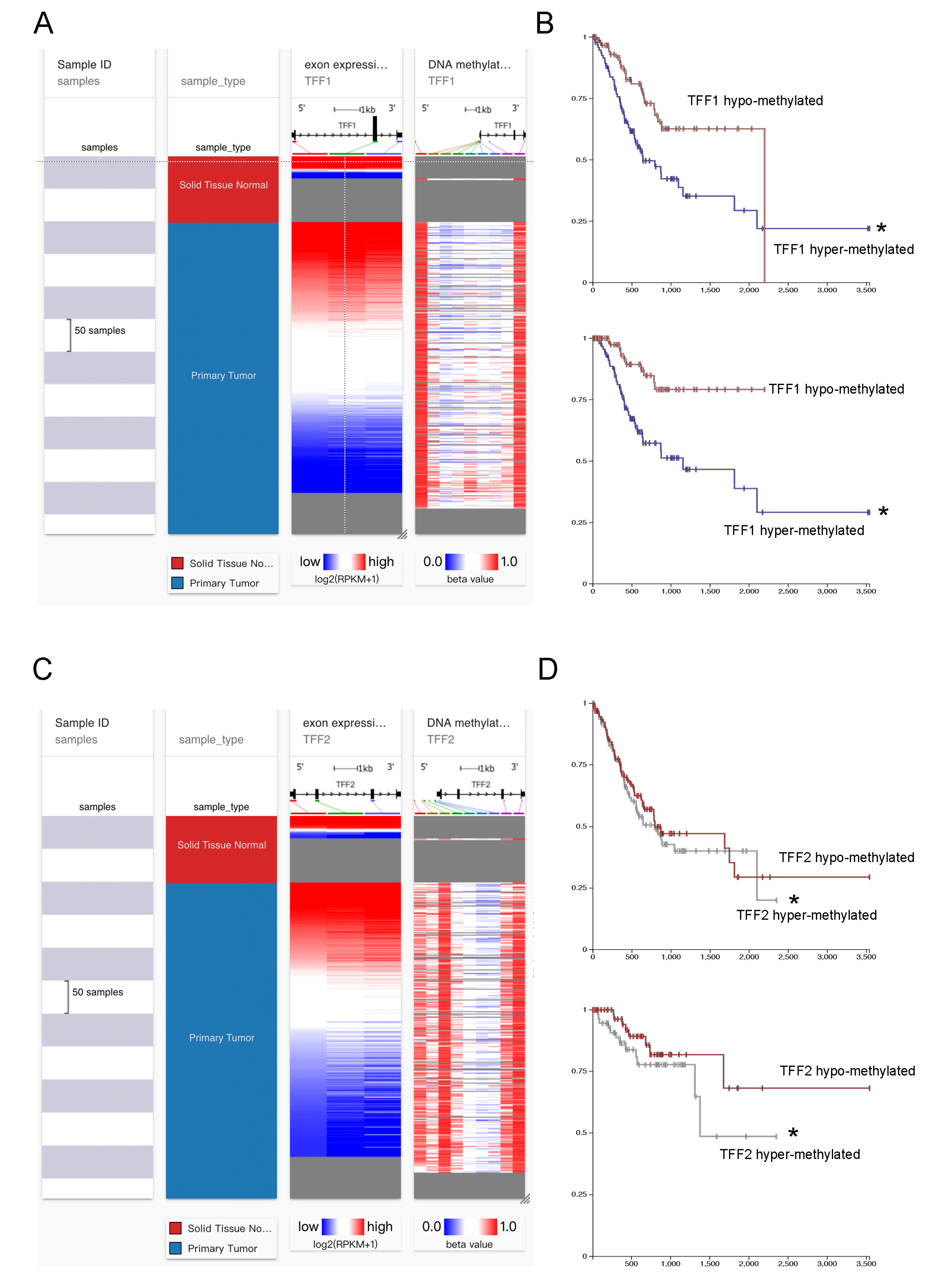
3.3. TFFs Could Be Modified by DNA Methylation in Gastric Cancer
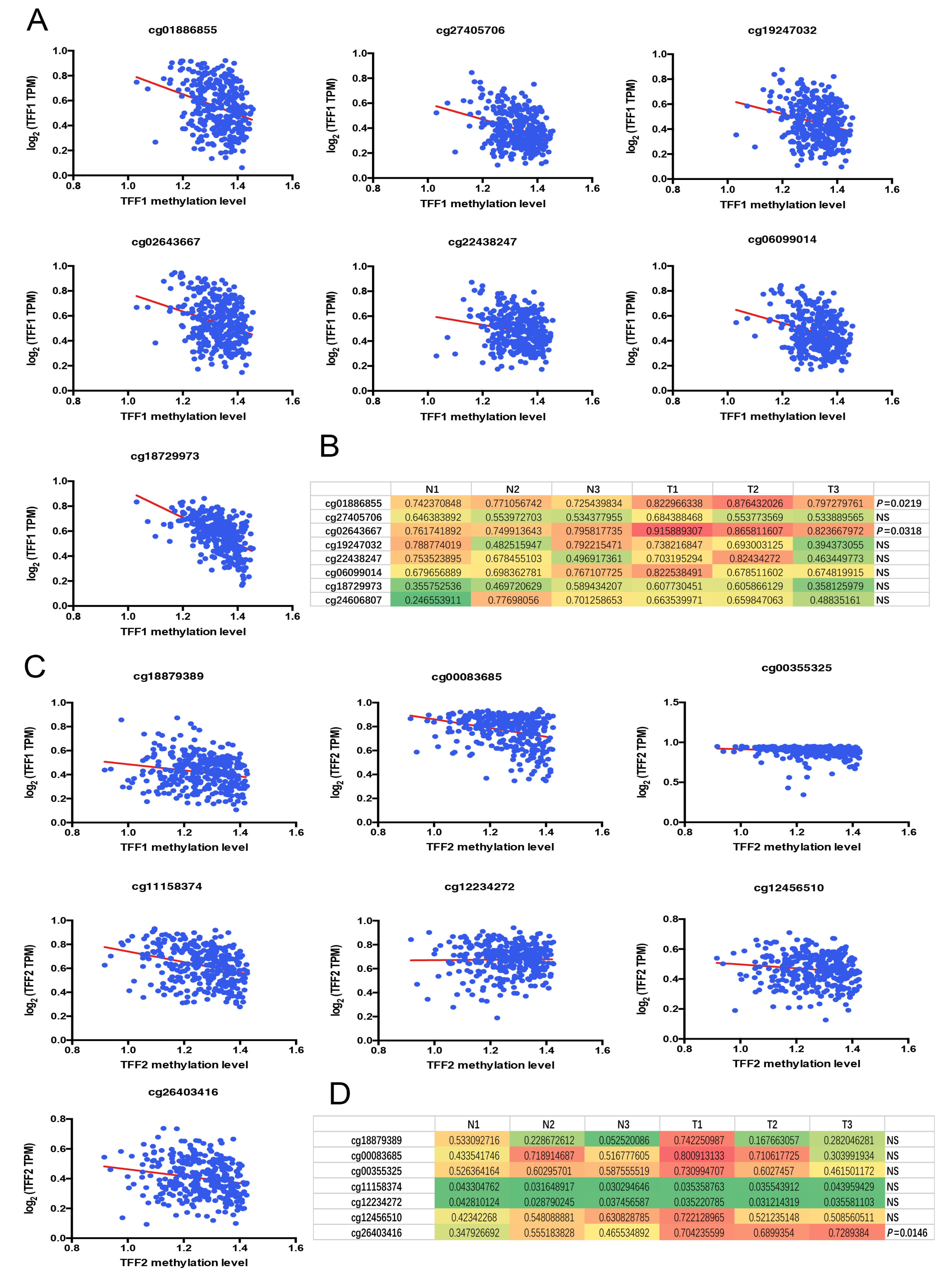
3.4. Identification of the Specific CpG Island Site for TFF1 and TFF2
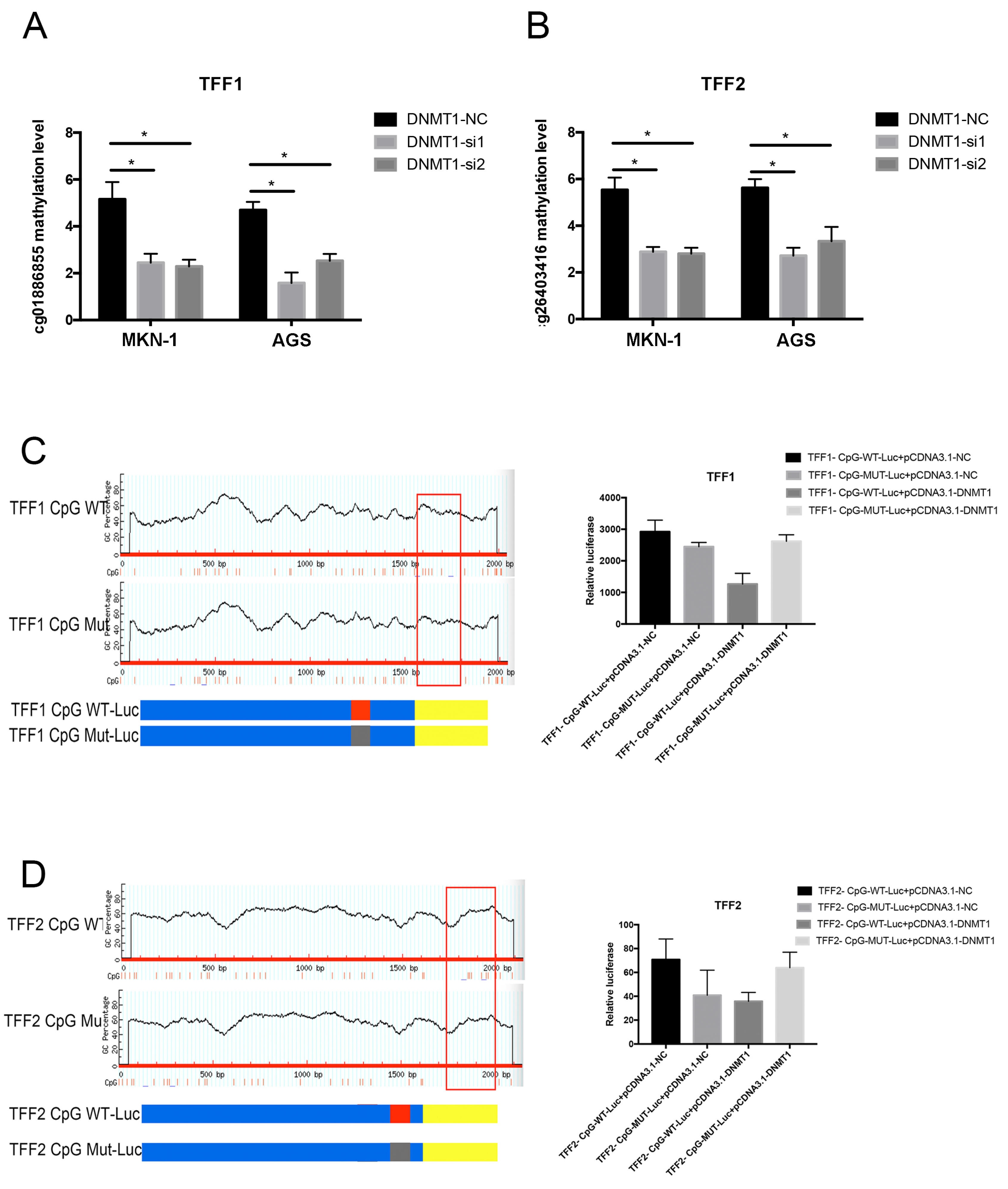
3.5. DNMT1 Regulated the TFFs DNA Methylation in Gastric Cancer
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhou, J.; Ma, X.; Bi, F.; Liu, M. Clinical significance of circulating tumor cells in gastric cancer patients. Oncotarget 2017, 8, 25713–25720. [Google Scholar] [CrossRef]
- Venerito, M.; Link, A.; Rokkas, T.; Malfertheiner, P. Gastric cancer-clinical and epidemiological aspects. Helicobacter 2016, 21 (Suppl. S1), 39–44. [Google Scholar] [CrossRef]
- Song, Z.; Wu, Y.; Yang, J.; Yang, D.; Fang, X. Progress in the treatment of advanced gastric cancer. Tumor Biol. 2017, 39, 1010428317714626. [Google Scholar] [CrossRef]
- Sano, T. Gastric cancer: Asia and the world. Gastric Cancer 2017, 20, 1–2. [Google Scholar] [CrossRef]
- Petryszyn, P.; Chapelle, N.; Matysiak-Budnik, T. Gastric cancer: Where are we heading? Dig. Dis. 2020, 38, 280–285. [Google Scholar] [CrossRef]
- O’Connor, K.G. Gastric cancer. Semin. Oncol. Nurs. 1999, 15, 26–35. [Google Scholar] [CrossRef]
- Hamashima, C. Current issues and future perspectives of gastric cancer screening. World J. Gastroenterol. 2014, 20, 13767–13774. [Google Scholar] [CrossRef]
- Guggenheim, D.E.; Shah, M.A. Gastric cancer epidemiology and risk factors. J. Surg. Oncol. 2013, 107, 230–236. [Google Scholar] [CrossRef]
- Griniatsos, J.; Trafalis, D. Differences in gastric cancer surgery outcome between East and West: Differences in surgery or different diseases? J. Buon 2018, 23, 1210–1215. [Google Scholar]
- Fu, D.G. Epigenetic alterations in gastric cancer (review). Mol. Med. Rep. 2015, 12, 3223–3230. [Google Scholar] [CrossRef]
- Klutstein, M.; Nejman, D.; Greenfield, R.; Cedar, H. DNA methylation in cancer and aging. Cancer Res. 2016, 76, 3446–3450. [Google Scholar] [CrossRef]
- Meng, H.; Cao, Y.; Qin, J.; Song, X.; Zhang, Q.; Shi, Y.; Cao, L. DNA methylation, its mediators and genome integrity. Int. J. Biol. Sci. 2015, 11, 604–617. [Google Scholar] [CrossRef]
- Dawson, M.A.; Kouzarides, T. Cancer epigenetics: From mechanism to therapy. Cell 2012, 150, 12–27. [Google Scholar] [CrossRef]
- Alizadeh, A.A.; Aranda, V.; Bardelli, A.; Blanpain, C.; Bock, C.; Borowski, C.; Caldas, C.; Califano, A.; Doherty, M.; Elsner, M.; et al. Toward understanding and exploiting tumor heterogeneity. Nat. Med. 2015, 21, 846–853. [Google Scholar] [CrossRef]
- Nebbioso, A.; Tambaro, F.P.; Dell’Aversana, C.; Altucci, L. Cancer epigenetics: Moving forward. PLoS Genet. 2018, 14, e1007362. [Google Scholar] [CrossRef]
- Köhler, F.; Rodríguez-Paredes, M. DNA methylation in epidermal differentiation, aging, and cancer. J. Investig. Dermatol. 2020, 140, 38–47. [Google Scholar] [CrossRef]
- Kuss-Duerkop, S.K.; Westrich, J.A.; Pyeon, D. DNA Tumor virus regulation of host dna methylation and its implications for immune evasion and oncogenesis. Viruses 2018, 10, 82. [Google Scholar] [CrossRef]
- Li, J.; Li, Y.; Li, W.; Luo, H.; Xi, Y.; Dong, S.; Gao, M.; Xu, P.; Zhang, B.; Liang, Y.; et al. Guide positioning sequencing identifies aberrant DNA methylation patterns that alter cell identity and tumor-immune surveillance networks. Genome Res. 2019, 29, 270–280. [Google Scholar] [CrossRef]
- Ehrlich, M. DNA hypermethylation in disease: Mechanisms and clinical relevance. Epigenetics 2019, 14, 1141–1163. [Google Scholar] [CrossRef]
- Cook, G.A.; Familari, M.; Thim, L.; Giraud, A.S. The trefoil peptides TFF2 and TFF3 are expressed in rat lymphoid tissues and participate in the immune response. FEBS Lett. 1999, 456, 155–159. [Google Scholar] [CrossRef]
- Mihalj, M.; Bujak, M.; Butković, J.; Zubčić, Ž.; Tolušić Levak, M.; Čes, J.; Kopić, V.; Baus Lončar, M.; Mihalj, H. Differential expression of TFF1 and TFF3 in patients suffering from chronic rhinosinusitis with nasal polyposis. Int. J. Mol. Sci. 2019, 20, 5461. [Google Scholar] [CrossRef]
- Viby, N.E.; Nexø, E.; Kissow, H.; Andreassen, H.; Clementsen, P.; Thim, L.; Poulsen, S.S. Trefoil factors (TFFs) are increased in bronchioalveolar lavage fluid from patients with chronic obstructive lung disease (COPD). Peptides 2015, 63, 90–95. [Google Scholar] [CrossRef]
- Popp, J.; Schicht, M.; Garreis, F.; Klinger, P.; Gelse, K.; Sesselmann, S.; Tsokos, M.; Etzold, S.; Stiller, D.; Claassen, H.; et al. Human synovia contains trefoil factor family (TFF) peptides 1–3 although synovial membrane only produces TFF3: Implications in osteoarthritis and rheumatoid arthritis. Int. J. Mol. Sci. 2019, 20, 6105. [Google Scholar] [CrossRef]
- Tomasetto, C.; Masson, R.; Linares, J.L.; Wendling, C.; Lefebvre, O.; Chenard, M.P.; Rio, M.C. pS2/TFF1 interacts directly with the VWFC cysteine-rich domains of mucins. Gastroenterology 2000, 118, 70–80. [Google Scholar] [CrossRef]
- Tosco, A.; Monti, M.C.; Fontanella, B.; Rio, M.C.; Gomez-Paloma, L.; Leone, A.; Marzullo, L. Copper-binding activity of trefoil factor 1 (TFF1): A new perspective in the study of the multifunctional roles of TFFs. Peptides 2007, 28, 1461–1469. [Google Scholar] [CrossRef]
- Aamann, L.; Vestergaard, E.M.; Grønbæk, H. Trefoil factors in inflammatory bowel disease. World J. Gastroenterol. 2014, 20, 3223–3230. [Google Scholar] [CrossRef]
- Leung, W.K.; Yu, J.; Chan, F.K.; To, K.F.; Chan, M.W.; Ebert, M.P.; Ng, E.K.; Chung, S.C.; Malfertheiner, P.; Sung, J.J. Expression of trefoil peptides (TFF1, TFF2, and TFF3) in gastric carcinomas, intestinal metaplasia, and non-neoplastic gastric tissues. J. Pathol. 2002, 197, 582–588. [Google Scholar] [CrossRef]
- Kulis, M.; Esteller, M. DNA methylation and cancer. Adv. Genet. 2010, 70, 27–56. [Google Scholar] [CrossRef]
- Choi, S.J.; Jung, S.W.; Huh, S.; Chung, Y.S.; Cho, H.; Kang, H. Alteration of DNA methylation in gastric cancer with chemotherapy. J. Microbiol. Biotechnol. 2017, 27, 1367–1378. [Google Scholar] [CrossRef]
- Voisin, S.; Eynon, N.; Yan, X.; Bishop, D.J. Exercise training and DNA methylation in humans. Acta Physiol. 2015, 213, 39–59. [Google Scholar] [CrossRef]
- Boddie, A.W., Jr.; McBride, C.M.; Balch, C.M. Gastric cancer. Am. J. Surg. 1989, 157, 595–606. [Google Scholar] [CrossRef]
- Soutto, M.; Chen, Z.; Katsha, A.M.; Romero-Gallo, J.; Krishna, U.S.; Piazuelo, M.B.; Washington, M.K.; Peek, R.M., Jr.; Belkhiri, A.; El-Rifai, W.M. Trefoil factor 1 expression suppresses helicobacter pylori-induced inflammation in gastric carcinogenesis. Cancer 2015, 121, 4348–4358. [Google Scholar] [CrossRef]
- Kuo, H.Y.; Chang, W.L.; Yeh, Y.C.; Tsai, Y.C.; Wu, C.T.; Cheng, H.C.; Yang, H.B.; Lu, C.C.; Sheu, B.S. Serum level of trefoil factor 2 can predict the extent of gastric spasmolytic polypeptide-expressing metaplasia in the H. pylori-infected gastric cancer relatives. Helicobacter 2017, 22, e12320. [Google Scholar] [CrossRef]
- Soutto, M.; Chen, Z.; Bhat, A.A.; Wang, L.; Zhu, S.; Gomaa, A.; Bates, A.; Bhat, N.S.; Peng, D.; Belkhiri, A.; et al. Activation of STAT3 signaling is mediated by TFF1 silencing in gastric neoplasia. Nat. Commun. 2019, 10, 3039. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qian, Z.; Jiang, Y.; Shou, C.; Yu, J.; Huang, D.; Xie, H.; Zhou, L.; Chen, D.; Zheng, S. Validation of the DNA Methylation Landscape of TFF1/TFF2 in Gastric Cancer. Cancers 2022, 14, 5474. https://doi.org/10.3390/cancers14225474
Qian Z, Jiang Y, Shou C, Yu J, Huang D, Xie H, Zhou L, Chen D, Zheng S. Validation of the DNA Methylation Landscape of TFF1/TFF2 in Gastric Cancer. Cancers. 2022; 14(22):5474. https://doi.org/10.3390/cancers14225474
Chicago/Turabian StyleQian, Ze, Yifan Jiang, Chunhui Shou, Jinghua Yu, Dongdong Huang, Haiyang Xie, Lin Zhou, Diyu Chen, and Shusen Zheng. 2022. "Validation of the DNA Methylation Landscape of TFF1/TFF2 in Gastric Cancer" Cancers 14, no. 22: 5474. https://doi.org/10.3390/cancers14225474
APA StyleQian, Z., Jiang, Y., Shou, C., Yu, J., Huang, D., Xie, H., Zhou, L., Chen, D., & Zheng, S. (2022). Validation of the DNA Methylation Landscape of TFF1/TFF2 in Gastric Cancer. Cancers, 14(22), 5474. https://doi.org/10.3390/cancers14225474




