Nanoparticle Enhancement of Natural Killer (NK) Cell-Based Immunotherapy
Abstract
Simple Summary
Abstract
1. Introduction
1.1. Natural Killer Cells
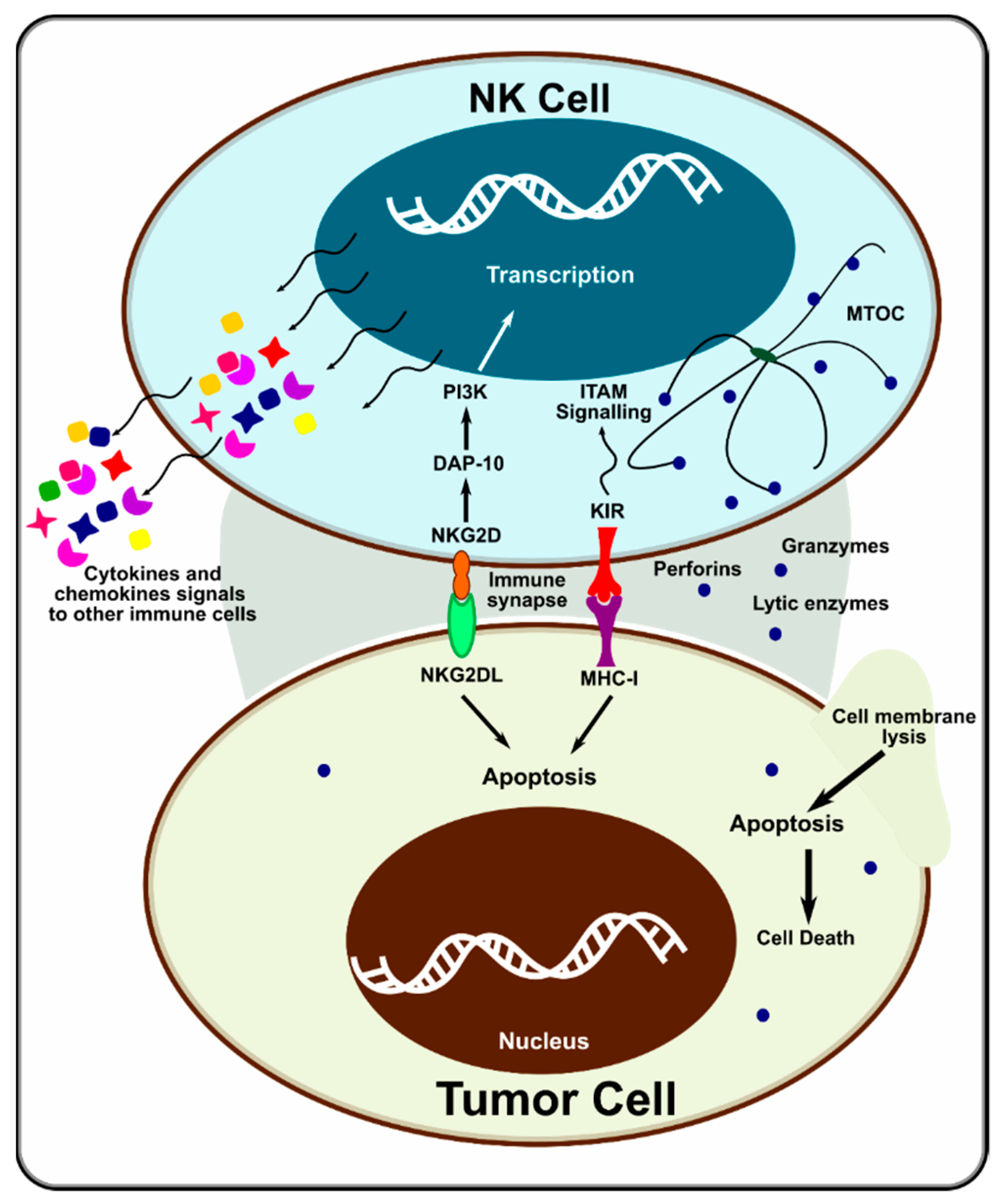
1.2. Potential NK Cell Activation Targets
2. Application of Nanoparticles in NK Cell-Based Immunotherapy
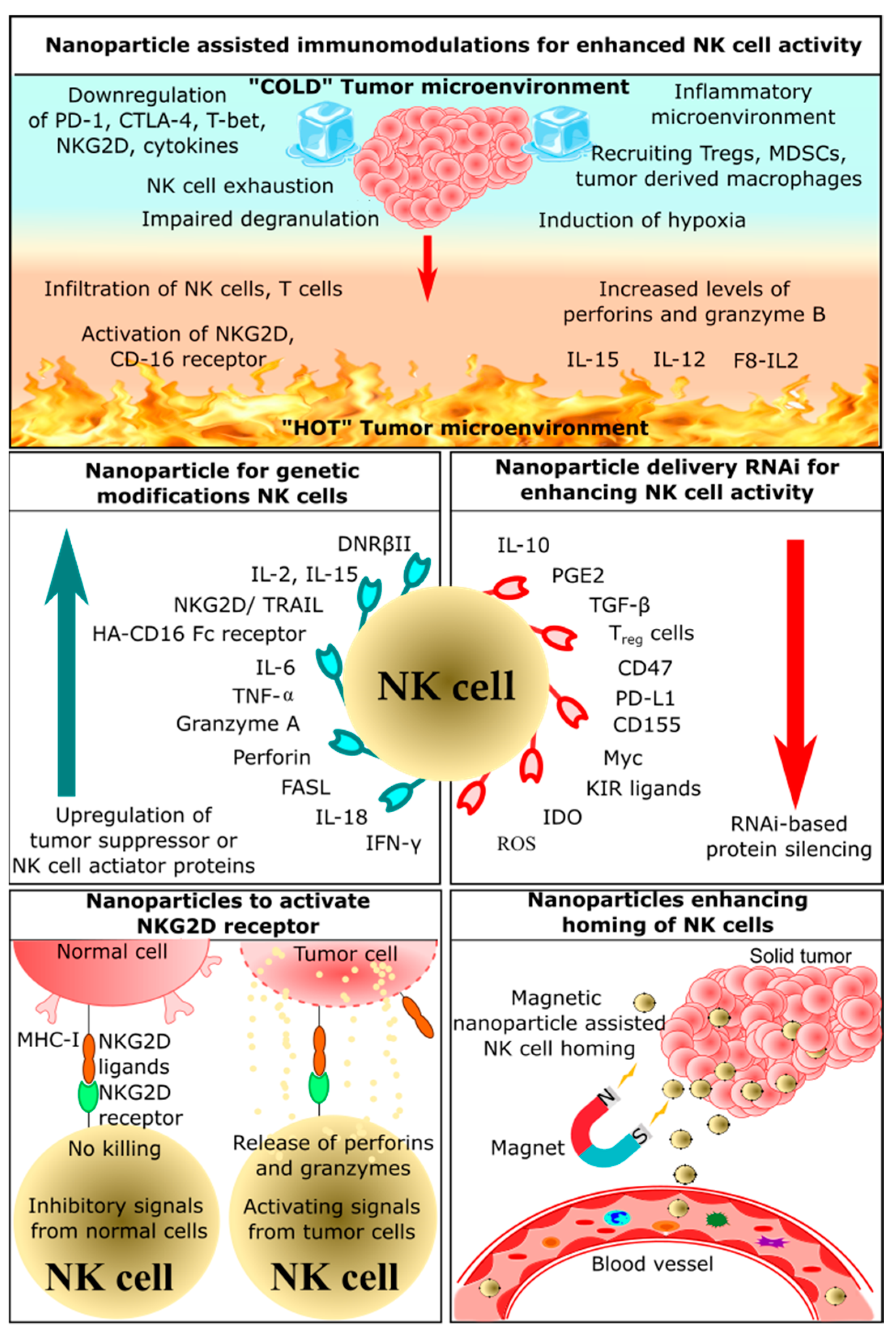
2.1. Nanoparticle-Assisted Immunomodulation for Enhanced NK Cell Activity
2.2. Nanoparticles Enhancing Homing of NK Cells
2.3. Nanoparticles Delivering RNAi for Enhancing NK Cells Activity
2.4. Nanoparticles for Genetic Modification of NK Cells
| Sr No. | Nanoparticles | Target NK Cell | Ligand | Mechanism of Activation | Effect on Tumor Cells | Ref. |
|---|---|---|---|---|---|---|
| 1. | Chitosan nanoparticles comprised of extracellular NKG2D gene domains and IL-21 gene | - | - |
|
| [71] |
| 2. | Chitosan nanoparticles comprised of extracellular NKG2D gene domains and IL-15 gene | - | - |
|
| [148] |
| 3. | Dendrimer-entrapped gold nanoparticles containing human ferritin heavy chain (hFTH1) gene | - | - |
|
| [149] |
| 4. | Magnetic nanoparticle coated with polydopamine containing plasmid DNA for targeting EGFR chimeric antigen receptor | EGFR | EGFR targeting CAR-NKs |
|
| [151] |
2.5. Nanoparticles Activating NKG2D Receptor
3. Limitations of Nanotechnology-Based NK Cell Therapy
4. Future Prospects
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Parkin, J.; Cohen, B. An overview of the immune system. Lancet 2001, 357, 1777–1789. [Google Scholar] [CrossRef]
- Delves, P.J.; Roitt, I.M. The Immune System. N. Engl. J. Med. 2000, 343, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Dunn, G.P.; Old, L.J.; Schreiber, R.D. The Immunobiology of Cancer Immunosurveillance and Immunoediting. Immunity 2004, 21, 137–148. [Google Scholar] [CrossRef] [PubMed]
- Garg, A.D.; Dudek, A.M.; Agostinis, P. Cancer immunogenicity, danger signals, and DAMPs: What, when, and how? BioFactors 2013, 39, 355–367. [Google Scholar] [CrossRef]
- Hobohm, U.; Stanford, J.L.; Grange, J.M. Pathogen-Associated Molecular Pattern in Cancer Immunotherapy. Crit. Rev. Immunol. 2008, 28, 95–107. [Google Scholar] [CrossRef]
- Locy, H.; DE Mey, S.L.; De Mey, W.; De Ridder, M.; Thielemans, K.; Maenhout, S.K. Immunomodulation of the Tumor Microenvironment: Turn Foe into Friend. Front. Immunol. 2018, 9, 2909. [Google Scholar] [CrossRef]
- Kawasaki, T.; Kawai, T. Toll-Like Receptor Signaling Pathways. Front. Immunol. 2014, 5, 461. [Google Scholar] [CrossRef]
- Chalifour, A.; Jeannin, P.; Gauchat, J.-F.; Blaecke, A.; Malissard, M.; N’Guyen, T.; Thieblemont, N.; Delneste, Y. Direct bacterial protein PAMP recognition by human NK cells involves TLRs and triggers α-defensin production. Blood 2004, 104, 1778–1783. [Google Scholar] [CrossRef]
- Bianchi, M.E. DAMPs, PAMPs and alarmins: All we need to know about danger. J. Leukoc. Biol. 2006, 81, 1–5. [Google Scholar] [CrossRef]
- Vinay, D.S.; Ryan, E.P.; Pawelec, G.; Talib, W.H.; Stagg, J.; Elkord, E.; Lichtor, T.; Decker, W.K.; Whelan, R.L.; Kumara, H.M.C.S.; et al. Immune evasion in cancer: Mechanistic basis and therapeutic strategies. Semin. Cancer Biol. 2015, 35, S185–S198. [Google Scholar] [CrossRef]
- Li, Y.; Sun, R. Tumor immunotherapy: New aspects of natural killer cells. Chin. J. Cancer Res. 2018, 30, 173–196. [Google Scholar] [CrossRef] [PubMed]
- Harjunpää, H.; Asens, M.L.; Guenther, C.; Fagerholm, S.C. Cell Adhesion Molecules and Their Roles and Regulation in the Immune and Tumor Microenvironment. Front. Immunol. 2019, 10, 1078. [Google Scholar] [CrossRef] [PubMed]
- Petty, A.J.; Yang, Y. Tumor-associated macrophages: Implications in cancer immunotherapy. Immunotherapy 2017, 9, 289–302. [Google Scholar] [CrossRef] [PubMed]
- Pu, Y.; Ji, Q. Tumor-Associated Macrophages Regulate PD-1/PD-L1 Immunosuppression. Front. Immunol. 2022, 13, 874589. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, S.; Zhang, B.; Qiao, L.; Zhang, Y. T Cell Dysfunction and Exhaustion in Cancer. Front. Cell Dev. Biol. 2020, 8, 17. [Google Scholar] [CrossRef] [PubMed]
- Solinas, G.; Germano, G.; Mantovani, A.; Allavena, P. Tumor-associated macrophages (TAM) as major players of the cancer-related inflammation. J. Leukoc. Biol. 2009, 86, 1065–1073. [Google Scholar] [CrossRef]
- Yang, H.-L.; Zhou, W.-J.; Chang, K.-K.; Mei, J.; Huang, L.-Q.; Wang, M.-Y.; Meng, Y.; Ha, S.-Y.; Li, D.-J.; Li, M.-Q. The crosstalk between endometrial stromal cells and macrophages impairs cytotoxicity of NK cells in endometriosis by secreting IL-10 and TGF-β. Reproduction 2017, 154, 815–825. [Google Scholar] [CrossRef]
- Mellor, A.L.; Keskin, D.B.; Johnson, T.; Chandler, P.; Munn, D. Cells Expressing Indoleamine 2,3-Dioxygenase Inhibit T Cell Responses. J. Immunol. 2002, 168, 3771–3776. [Google Scholar] [CrossRef]
- Zou, W.; Chen, L. Inhibitory B7-family molecules in the tumour microenvironment. Nat. Rev. Immunol. 2008, 8, 467–477. [Google Scholar] [CrossRef]
- Hou, D.-Y.; Muller, A.J.; Sharma, M.D.; DuHadaway, J.; Banerjee, T.; Johnson, M.; Mellor, A.L.; Prendergast, G.C.; Munn, D.H. Inhibition of Indoleamine 2,3-Dioxygenase in Dendritic Cells by Stereoisomers of 1-Methyl-Tryptophan Correlates with Antitumor Responses. Cancer Res. 2007, 67, 792–801. [Google Scholar] [CrossRef]
- Rodríguez, P.C.; Ochoa, A.C. Arginine regulation by myeloid derived suppressor cells and tolerance in cancer: Mechanisms and therapeutic perspectives. Immunol. Rev. 2008, 222, 180–191. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, P.C.; Ernstoff, M.S.; Hernandez, C.; Atkins, M.; Zabaleta, J.; Sierra, R.; Ochoa, A.C. Arginase I–Producing Myeloid-Derived Suppressor Cells in Renal Cell Carcinoma Are a Subpopulation of Activated Granulocytes. Cancer Res. 2009, 69, 1553–1560. [Google Scholar] [CrossRef] [PubMed]
- Dunn, G.P.; Old, L.J.; Schreiber, R.D. The Three Es of Cancer Immunoediting. Annu. Rev. Immunol. 2004, 22, 329–360. [Google Scholar] [CrossRef] [PubMed]
- Mittal, D.; Gubin, M.M.; Schreiber, R.D.; Smyth, M.J. New insights into cancer immunoediting and its three component phases—Elimination, equilibrium and escape. Curr. Opin. Immunol. 2014, 27, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Gubin, M.M.; Vesely, M.D. Cancer Immunoediting in the Era of Immuno-oncology. Clin. Cancer Res. 2022, 28, 3917–3928. [Google Scholar] [CrossRef] [PubMed]
- Gameiro, S.R.; Jammeh, M.L.; Wattenberg, M.M.; Tsang, K.Y.; Ferrone, S.; Hodge, J.W. Radiation-induced immunogenic modulation of tumor enhances antigen processing and calreticulin exposure, resulting in enhanced T-cell killing. Oncotarget 2014, 5, 403–416. [Google Scholar] [CrossRef]
- Fucikova, J.; Kline, J.P.; Galluzzi, L.; Spisek, R. Calreticulin arms NK cells against leukemia. OncoImmunology 2019, 9, 1671763. [Google Scholar] [CrossRef]
- Porcellini, S.; Traggiai, E.; Schenk, U.; Ferrera, D.; Matteoli, M.; Lanzavecchia, A.; Michalak, M.; Grassi, F. Regulation of peripheral T cell activation by calreticulin. J. Exp. Med. 2006, 203, 461–471. [Google Scholar] [CrossRef]
- Zitvogel, L.; Kepp, O.; Senovilla, L.; Menger, L.; Chaput, N.; Kroemer, G. Immunogenic Tumor Cell Death for Optimal Anticancer Therapy: The Calreticulin Exposure Pathway. Clin. Cancer Res. 2010, 16, 3100–3104. [Google Scholar] [CrossRef]
- Palucka, K.; Banchereau, J. Cancer immunotherapy via dendritic cells. Nat. Cancer 2012, 12, 265–277. [Google Scholar] [CrossRef]
- O’Donnell, J.S.; Teng, M.W.L.; Smyth, M.J. Cancer immunoediting and resistance to T cell-based immunotherapy. Nat. Rev. Clin. Oncol. 2019, 16, 151–167. [Google Scholar] [CrossRef] [PubMed]
- Romagnani, P.; Lasagni, L.; Annunziato, F.; Serio, M.; Romagnani, S. CXC chemokines: The regulatory link between inflammation and angiogenesis. Trends Immunol. 2004, 25, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Yanofsky, V.R.; Mitsui, H.; Felsen, D.; Carucci, J.A. Understanding Dendritic Cells and Their Role in Cutaneous Carcinoma and Cancer Immunotherapy. Clin. Dev. Immunol. 2013, 2013, 624123. [Google Scholar] [CrossRef] [PubMed]
- Conlon, K.C.; Lugli, E.; Welles, H.C.; Rosenberg, S.A.; Fojo, A.T.; Morris, J.C.; Fleisher, T.A.; Dubois, S.P.; Perera, L.P.; Stewart, D.M.; et al. Redistribution, Hyperproliferation, Activation of Natural Killer Cells and CD8 T Cells, and Cytokine Production During First-in-Human Clinical Trial of Recombinant Human Interleukin-15 in Patients with Cancer. J. Clin. Oncol. 2015, 33, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Romano, E.; Rossi, M.; Ratzinger, G.; De Cos, M.-A.; Chung, D.J.; Panageas, K.S.; Wolchock, J.D.; Houghton, A.N.; Chapman, P.B.; Heller, G.; et al. Peptide-Loaded Langerhans Cells, Despite Increased IL15 Secretion and T-Cell Activation In Vitro, Elicit Antitumor T-Cell Responses Comparable to Peptide-Loaded Monocyte-Derived Dendritic Cells In Vivo. Clin. Cancer Res. 2011, 17, 1984–1997. [Google Scholar] [CrossRef]
- Padoan, A.; Plebani, M.; Basso, D. Inflammation and Pancreatic Cancer: Focus on Metabolism, Cytokines, and Immunity. Int. J. Mol. Sci. 2019, 20, 676. [Google Scholar] [CrossRef]
- Olson, B.M.; McNeel, D. Antigen loss and tumor-mediated immunosuppression facilitate tumor recurrence. Expert Rev. Vaccines 2012, 11, 1315–1317. [Google Scholar] [CrossRef]
- Saleh, R.; Elkord, E. Acquired resistance to cancer immunotherapy: Role of tumor-mediated immunosuppression. Semin. Cancer Biol. 2019, 65, 13–27. [Google Scholar] [CrossRef]
- Pardali, K.; Moustakas, A. Actions of TGF-β as tumor suppressor and pro-metastatic factor in human cancer. Biochim. Biophys. Acta Rev. Cancer. 2007, 1775, 21–62. [Google Scholar] [CrossRef]
- Wojtowicz-Praga, S. Reversal of Tumor-Induced Immunosuppression: A New Approach to Cancer Therapy. J. Immunother. 1997, 20, 165–177. [Google Scholar] [CrossRef]
- Gold, L.I. The role for transforming growth factor-beta (TGF-beta) in human cancer. Crit. Rev. Oncog. 1999, 10, 303–360. [Google Scholar] [PubMed]
- Mittal, S.K.; Cho, K.-J.; Ishido, S.; Roche, P.A. Interleukin 10 (IL-10)-mediated Immunosuppression. J. Biol. Chem. 2015, 290, 27158–27167. [Google Scholar] [CrossRef] [PubMed]
- Lamichhane, P.; Karyampudi, L.; Shreeder, B.; Krempski, J.; Bahr, D.; Daum, J.; Kalli, K.R.; Goode, E.L.; Block, M.S.; Cannon, M.J.; et al. IL10 Release upon PD-1 Blockade Sustains Immunosuppression in Ovarian Cancer. Cancer Res. 2017, 77, 6667–6678. [Google Scholar] [CrossRef] [PubMed]
- Frumento, G.; Piazza, T.; Di Carlo, E.; Ferrini, S. Targeting Tumor-Related Immunosuppression for Cancer Immunotherapy. Endocr. Metab. Immune Disord. Drug Targets 2006, 6, 223–237. [Google Scholar] [CrossRef]
- Lapeyre-Prost, A.; Terme, M.; Pernot, S.; Pointet, A.-L.; Voron, T.; Tartour, E.; Taieb, J. Immunomodulatory Activity of VEGF in Cancer. Int. Rev. Cell Mol. Biol. 2017, 330, 295–342. [Google Scholar] [CrossRef]
- Johnson, B.F.; Clay, T.M.; Hobeika, A.C.; Lyerly, H.; Morse, M.A. Vascular endothelial growth factor and immunosuppression in cancer: Current knowledge and potential for new therapy. Expert Opin. Biol. Ther. 2007, 7, 449–460. [Google Scholar] [CrossRef]
- Li, Y.-L.; Zhao, H.; Ren, X.-B. Relationship of VEGF/VEGFR with immune and cancer cells: Staggering or forward? Cancer Biol. Med. 2016, 13, 206–214. [Google Scholar] [CrossRef]
- Bu, L.; Yu, G.; Wu, L.; Mao, L.; Deng, W.; Liu, J.; Kulkarni, A.; Zhang, W.; Zhang, L.; Sun, Z. STAT3 Induces Immunosuppression by Upregulating PD-1/PD-L1 in HNSCC. J. Dent. Res. 2017, 96, 1027–1034. [Google Scholar] [CrossRef]
- Lee, H.; Pal, S.K.; Reckamp, K.; Figlin, R.A.; Yu, H. STAT3: A Target to Enhance Antitumor Immune Response. Curr. Top Microbiol. Immunol. 2010, 344, 41–59. [Google Scholar] [CrossRef]
- Roychoudhuri, R.; Eil, R.L.; Clever, D.; Klebanoff, C.A.; Sukumar, M.; Grant, F.M.; Yu, Z.; Mehta, G.; Liu, H.; Jin, P.; et al. The transcription factor BACH2 promotes tumor immunosuppression. J. Clin. Investig. 2016, 126, 599–604. [Google Scholar] [CrossRef]
- Grant, F.M.; Yang, J.; Nasrallah, R.; Clarke, J.; Sadiyah, F.; Whiteside, S.K.; Imianowski, C.J.; Kuo, P.; Vardaka, P.; Todorov, T.; et al. BACH2 drives quiescence and maintenance of resting Treg cells to promote homeostasis and cancer immunosuppression. J. Exp. Med. 2020, 217, e20190711. [Google Scholar] [CrossRef] [PubMed]
- Ishida, Y.; Agata, Y.; Shibahara, K.; Honjo, T. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J. 1992, 11, 3887–3895. [Google Scholar] [CrossRef] [PubMed]
- Leach, D.R.; Krummel, M.F.; Allison, J.P. Enhancement of Antitumor Immunity by CTLA-4 Blockade. Science 1996, 271, 1734–1736. [Google Scholar] [CrossRef]
- Hodi, F.S.; O’Day, S.J.; McDermott, D.F.; Weber, R.W.; Sosman, J.A.; Haanen, J.B.; Gonzalez, R.; Robert, C.; Schadendorf, D.; Hassel, J.C.; et al. Improved Survival with Ipilimumab in Patients with Metastatic Melanoma. N. Engl. J. Med. 2010, 363, 711–723. [Google Scholar] [CrossRef] [PubMed]
- Fellner, C. Ipilimumab (yervoy) prolongs survival in advanced melanoma: Serious side effects and a hefty price tag may limit its use. Pharm. Ther. 2012, 37, 503–530. [Google Scholar]
- Haslam, A.; Prasad, V. Estimation of the Percentage of US Patients with Cancer Who Are Eligible for and Respond to Checkpoint Inhibitor Immunotherapy Drugs. JAMA Netw. Open 2019, 2, e192535. [Google Scholar] [CrossRef]
- Freud, A.G.; Yu, J.; Caligiuri, M.A. Human natural killer cell development in secondary lymphoid tissues. Semin. Immunol. 2014, 26, 132–137. [Google Scholar] [CrossRef]
- Töpfer, K.; Kempe, S.; Müller, N.; Schmitz, M.; Bachmann, M.; Cartellieri, M.; Schackert, G.; Temme, A. Tumor Evasion from T Cell Surveillance. J. Biomed. Biotechnol. 2011, 2011, 918471. [Google Scholar] [CrossRef]
- Vivier, E.; Tomasello, E.; Baratin, M.; Walzer, T.; Ugolini, S. Functions of natural killer cells. Nat. Immunol. 2008, 9, 503–510. [Google Scholar] [CrossRef]
- Yawata, M.; Yawata, N.; Draghi, M.; Partheniou, F.; Little, A.-M.; Parham, P. MHC class I–specific inhibitory receptors and their ligands structure diverse human NK-cell repertoires toward a balance of missing self-response. Blood 2008, 112, 2369–2380. [Google Scholar] [CrossRef]
- Long, E.O. Tumor cell recognition by natural killer cells. Semin. Cancer Biol. 2002, 12, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Molfetta, R.; Quatrini, L.; Santoni, A.; Paolini, R. Regulation of NKG2D-Dependent NK Cell Functions: The Yin and the Yang of Receptor Endocytosis. Int. J. Mol. Sci. 2017, 18, 1677. [Google Scholar] [CrossRef] [PubMed]
- Pauken, K.E.; Wherry, E.J. Overcoming T cell exhaustion in infection and cancer. Trends Immunol. 2015, 36, 265–276. [Google Scholar] [CrossRef] [PubMed]
- Yi, J.S.; Cox, M.A.; Zajac, A.J. T-Cell Exhaustion: Characteristics, Causes and Conversion. Immunology 2010, 129, 474–481. [Google Scholar] [CrossRef] [PubMed]
- Brudno, J.N.; Kochenderfer, J.N. Toxicities of chimeric antigen receptor T cells: Recognition and management. Blood 2016, 127, 3321–3330. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Zou, X.; Zheng, S.; Tang, H.; Zhang, L.; Liu, P.; Xie, X. Efficacy and predictive factors of immune checkpoint inhibitors in metastatic breast cancer: A systematic review and meta-analysis. Ther. Adv. Med. Oncol. 2020, 12, 175883592094092. [Google Scholar] [CrossRef]
- Garrido, F.; Aptsiauri, N.; Doorduijn, E.M.; Lora, A.M.G.; van Hall, T. The urgent need to recover MHC class I in cancers for effective immunotherapy. Curr. Opin. Immunol. 2016, 39, 44–51. [Google Scholar] [CrossRef]
- Yoon, S.R.; Kim, T.-D.; Choi, I. Understanding of molecular mechanisms in natural killer cell therapy. Exp. Mol. Med. 2015, 47, e141. [Google Scholar] [CrossRef]
- Cao, Y.; Wang, X.; Jin, T.; Tian, Y.; Dai, C.; Widarma, C.; Song, R.; Xu, F. Immune checkpoint molecules in natural killer cells as potential targets for cancer immunotherapy. Signal Transduct. Target. Ther. 2020, 5, 250. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, Y. Targeting NK Cell Checkpoint Receptors or Molecules for Cancer Immunotherapy. Front. Immunol. 2020, 11, 1295. [Google Scholar] [CrossRef]
- Tan, L.; Han, S.; Ding, S.; Xiao, W.; Ding, Y.; Qian, L.; Wang, C.; Gong, W. Chitosan nanoparticle-based delivery of fused NKG2D-IL-21 gene suppresses colon cancer growth in mice. Int. J. Nanomed. 2017, 12, 3095–3107. [Google Scholar] [CrossRef] [PubMed]
- Zhang, V.; Wu, N.; Lu, Y.; Davidson, D.; Colonna, M.; Veillette, A. DNAM-1 controls NK cell activation via an ITT-like motif. J. Exp. Med. 2015, 212, 2165–2182. [Google Scholar] [CrossRef] [PubMed]
- Konjević, G.; Vuletić, A.; Martinović, K.M.; Džodić, K.M.M.a.R. The Role of Activating and Inhibitory NK Cell Receptors in Antitumor Immune Response. In Natural Killer Cells; IntechOpen: London, UK, 2017. [Google Scholar] [CrossRef]
- Watzl, C. Chapter Five—How to Trigger a Killer: Modulation of Natural Killer Cell Reactivity on Many Levels. Adv. Immunol. 2014, 124, 137–170. [Google Scholar] [CrossRef] [PubMed]
- Van Acker, H.H.; Capsomidis, A.; Smits, E.L.; Van Tendeloo, V.F. CD56 in the Immune System: More Than a Marker for Cytotoxicity? Front. Immunol. 2017, 8, 892. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, A.; Cella, M.; Giurisato, E.; Shaw, A.S.; Colonna, M. Cutting Edge: CD96 (Tactile) Promotes NK Cell-Target Cell Adhesion by Interacting with the Poliovirus Receptor (CD155). J. Immunol. 2004, 172, 3994–3998. [Google Scholar] [CrossRef] [PubMed]
- Ng, Y.Y.; Tay, J.C.; Wang, S. CXCR1 Expression to Improve Anti-Cancer Efficacy of Intravenously Injected CAR-NK Cells in Mice with Peritoneal Xenografts. Mol. Ther. Oncolytics 2019, 16, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Rivas-Fuentes, S.; Salgado-Aguayo, A.; Arratia-Quijada, J.; Gorocica-Rosete, P. Regulation and biological functions of the CX3CL1-CX3CR1 axis and its relevance in solid cancer: A mini-review. J. Cancer 2021, 12, 571–583. [Google Scholar] [CrossRef]
- Abel, A.M.; Yang, C.; Thakar, M.S.; Malarkannan, S. Natural Killer Cells: Development, Maturation, and Clinical Utilization. Front. Immunol. 2018, 9, 1869. [Google Scholar] [CrossRef]
- Somanchi, S.S.; Somanchi, A.; Cooper, L.J.N.; Lee, D.A. Engineering lymph node homing of ex vivo–expanded human natural killer cells via trogocytosis of the chemokine receptor CCR7. Blood 2012, 119, 5164–5172. Available online: https://ashpublications.org/blood/article/119/22/5164/105509/Engineering-lymph-node-homing-of-ex-vivo-expanded (accessed on 27 September 2022). [CrossRef]
- Borrego, F.; Masilamani, M.; Kabat, J.; Sanni, T.B.; Coligan, J.E. The cell biology of the human natural killer cell CD94/NKG2A inhibitory receptor. Mol. Immunol. 2005, 42, 485–488. [Google Scholar] [CrossRef]
- Tang, R.; Rangachari, M.; Kuchroo, V.K. Tim-3: A co-receptor with diverse roles in T cell exhaustion and tolerance. Semin. Immunol. 2019, 42, 101302. [Google Scholar] [CrossRef] [PubMed]
- Stanietsky, N.; Simic, H.; Arapovic, J.; Toporik, A.; Levy, O.; Novik, A.; Levine, Z.; Beiman, M.; Dassa, L.; Achdout, H.; et al. The interaction of TIGIT with PVR and PVRL2 inhibits human NK cell cytotoxicity. Proc. Natl. Acad. Sci. USA 2009, 106, 17858–17863. [Google Scholar] [CrossRef] [PubMed]
- Pende, D.; Falco, M.; Vitale, M.; Cantoni, C.; Vitale, C.; Munari, E.; Bertaina, A.; Moretta, F.; Del Zotto, G.; Pietra, G.; et al. Killer Ig-Like Receptors (KIRs): Their Role in NK Cell Modulation and Developments Leading to Their Clinical Exploitation. Front. Immunol. 2019, 10, 1179. Available online: https://www.frontiersin.org/articles/10.3389/fimmu.2019.01179 (accessed on 27 September 2022). [CrossRef] [PubMed]
- Anderson, A.C.; Joller, N.; Kuchroo, V.K. Lag-3, Tim-3, and TIGIT: Co-inhibitory Receptors with Specialized Functions in Immune Regulation. Immunity 2016, 44, 989–1004. [Google Scholar] [CrossRef] [PubMed]
- Niu, C.; Li, M.; Zhu, S.; Chen, Y.; Zhou, L.; Xu, D.; Xu, J.; Li, Z.; Li, W.; Cui, J. PD-1-positive Natural Killer Cells have a weaker antitumor function than that of PD-1-negative Natural Killer Cells in Lung Cancer. Int. J. Med. Sci. 2020, 17, 1964–1973. [Google Scholar] [CrossRef] [PubMed]
- Press Release|CytoSen Therapeutics and KBI Biopharma Enter into Strategic Partnership to Manufacture NK Cells and Nanoparticles. Available online: https://www.kbibiopharma.com/news/cytosen-therapeutics-and-kbi-biopharma-enter-into-strategic-partnership-to-manufacture-nk-cells-and-nanoparticles (accessed on 30 October 2022).
- Dolen, Y.; Kreutz, M.; Gileadi, U.; Tel, J.; Vasaturo, A.; Van Dinther, E.A.W.; Van Hout-Kuijer, M.A.; Cerundolo, V.; Figdor, C.G. Co-delivery of PLGA encapsulated invariant NKT cell agonist with antigenic protein induce strong T cell-mediated antitumor immune responses. OncoImmunology 2015, 5, e1068493. [Google Scholar] [CrossRef]
- Burga, R.A.; Khan, D.H.; Agrawal, N.; Bollard, C.M.; Fernandes, R. Designing Magnetically Responsive Biohybrids Composed of Cord Blood-Derived Natural Killer Cells and Iron Oxide Nanoparticles. Bioconjug. Chem. 2019, 30, 552–560. [Google Scholar] [CrossRef]
- Nayyar, G.; Chu, Y.; Cairo, M.S. Overcoming Resistance to Natural Killer Cell Based Immunotherapies for Solid Tumors. Front. Oncol. 2019, 9, 51. [Google Scholar] [CrossRef]
- Jonjic, S. Manipulation of NKG2D ligands by cytomegaloviruses: Impact on innate and adaptive immune response. Front. Immunol. 2011, 2, 85. [Google Scholar] [CrossRef]
- Jindal, A.; Sarkar, S.; Alam, A. Nanomaterials-Mediated Immunomodulation for Cancer Therapeutics. Front. Chem. 2021, 9, 629635. [Google Scholar] [CrossRef]
- Mikelez-Alonso, I.; Magadán, S.; González-Fernández, F.; Borrego, F. Natural killer (NK) cell-based immunotherapies and the many faces of NK cell memory: A look into how nanoparticles enhance NK cell activity. Adv. Drug Deliv. Rev. 2021, 176, 113860. [Google Scholar] [CrossRef] [PubMed]
- Xia, F.; Hou, W.; Liu, Y.; Wang, W.; Han, Y.; Yang, M.; Zhi, X.; Li, C.; Qi, D.; Li, T.; et al. Cytokine induced killer cells-assisted delivery of chlorin e6 mediated self-assembled gold nanoclusters to tumors for imaging and immuno-photodynamic therapy. Biomaterials 2018, 170, 1–11. [Google Scholar] [CrossRef]
- Wang, G.; Hu, W.; Chen, H.; Shou, X.; Ye, T.; Xu, Y. Cocktail Strategy Based on NK Cell-Derived Exosomes and Their Biomimetic Nanoparticles for Dual Tumor Therapy. Cancers 2019, 11, 1560. [Google Scholar] [CrossRef] [PubMed]
- Koshy, S.T.; Cheung, A.S.; Gu, L.; Graveline, A.R.; Mooney, D.J. Liposomal Delivery Enhances Immune Activation by STING Agonists for Cancer Immunotherapy. Adv. Biosyst. 2017, 1, 1600013. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Fong, C.I.; Xu, M.; Han, B.-N.; Yuan, Z.; Zhao, Q. Nano-loaded natural killer cells as carriers of indocyanine green for synergetic cancer immunotherapy and phototherapy. J. Innov. Opt. Health Sci. 2019, 12, 19410025. [Google Scholar] [CrossRef]
- Pitchaimani, A.; Nguyen, T.D.T.; Aryal, S. Natural killer cell membrane infused biomimetic liposomes for targeted tumor therapy. Biomaterials 2018, 160, 124–137. [Google Scholar] [CrossRef]
- Liu, Z.; Jiang, W.; Nam, J.; Moon, J.J.; Kim, B.Y.S. Immunomodulating Nanomedicine for Cancer Therapy. Nano Lett. 2018, 18, 6655–6659. [Google Scholar] [CrossRef]
- Christian, D.A.; Hunter, C.A. Particle-mediated delivery of cytokines for immunotherapy. Immunotherapy 2012, 4, 425–441. [Google Scholar] [CrossRef]
- Park, J.; Wrzesinski, S.H.; Stern, E.; Look, M.; Criscione, J.M.; Ragheb, R.; Jay, S.; Demento, S.L.; Agawu, A.; Limon, P.L.; et al. Combination delivery of TGF-β inhibitor and IL-2 by nanoscale liposomal polymeric gels enhances tumour immunotherapy. Nat. Mater. 2012, 11, 895–905. [Google Scholar] [CrossRef]
- McHugh, M.D.; Park, J.; Uhrich, R.; Gao, W.; Horwitz, D.A.; Fahmy, T.M. Paracrine co-delivery of TGF-β and IL-2 using CD4-targeted nanoparticles for induction and maintenance of regulatory T cells. Biomaterials 2015, 59, 172–181. [Google Scholar] [CrossRef]
- Gao, S.; Li, T.; Guo, Y.; Sun, C.; Xianyu, B.; Xu, H. Selenium-Containing Nanoparticles Combine the NK Cells Mediated Immunotherapy with Radiotherapy and Chemotherapy. Adv. Mater. 2020, 32, e1907568. [Google Scholar] [CrossRef] [PubMed]
- Yazdi, M.H.; Mahdavi, M.; Setayesh, N.; Esfandyar, M.; Shahverdi, A.R. Selenium nanoparticle-enriched Lactobacillus brevis causes more efficient immune responses in vivo and reduces the liver metastasis in metastatic form of mouse breast cancer. DARU J. Pharm. Sci. 2013, 21, 33. [Google Scholar] [CrossRef] [PubMed]
- Ferro, C.; Florindo, H.F.; Santos, H.A. Selenium Nanoparticles for Biomedical Applications: From Development and Characterization to Therapeutics. Adv. Health Mater. 2021, 10, e2100598. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Wen, Y.; Liu, Y.; Tan, X.; Chen, X.; Zhu, X.; Wei, C.; Chen, L.; Wang, Z.; Liu, J.; et al. Hollow mesoporous ruthenium nanoparticles conjugated bispecific antibody for targeted anti-colorectal cancer response of combination therapy. Nanoscale 2019, 11, 9661–9678. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; He, L.; Li, X.; Xu, L.; Chen, T. Ruthenium complexes boost NK cell immunotherapy via sensitizing triple-negative breast cancer and shaping immuno-microenvironment. Biomaterials 2022, 281, 121371. [Google Scholar] [CrossRef]
- Li, T.; Pan, S.; Gao, S.; Xiang, W.; Sun, C.; Cao, W.; Xu, H. Diselenide–Pemetrexed Assemblies for Combined Cancer Immuno-, Radio-, and Chemotherapies. Angew. Chem. 2019, 132, 2722–2726. [Google Scholar] [CrossRef]
- Chen, H.; Liu, L.; Ma, A.; Yin, T.; Chen, Z.; Liang, R.; Qiu, Y.; Zheng, M.; Cai, L. Noninvasively immunogenic sonodynamic therapy with manganese protoporphyrin liposomes against triple-negative breast cancer. Biomaterials 2021, 269, 120639. [Google Scholar] [CrossRef]
- Liu, B.; Cao, W.; Cheng, E.A.J.; Fan, S.; Pan, S.; Wang, L.; Niu, J.; Pan, Y.; Liu, Y.; Sun, X.; et al. Human natural killer cells for targeting delivery of gold nanostars and bimodal imaging directed photothermal/photodynamic therapy and immunotherapy. Cancer Biol. Med. 2019, 16, 756–770. [Google Scholar] [CrossRef]
- Yang, X.; Lai, C.; Liu, A.; Hou, X.; Tang, Z.; Mo, F.; Yin, S.; Lu, X. Anti-Tumor Activity of Mannose-CpG-Oligodeoxynucleotides-Conjugated and Hepatoma Lysate-Loaded Nanoliposomes for Targeting Dendritic Cells In Vivo. J. Biomed. Nanotechnol. 2019, 15, 1018–1032. [Google Scholar] [CrossRef]
- Kim, H.; Sehgal, D.; Kucaba, T.A.; Ferguson, D.M.; Griffith, T.S.; Panyam, J. Acidic pH-responsive polymer nanoparticles as a TLR7/8 agonist delivery platform for cancer immunotherapy. Nanoscale 2018, 10, 20851–20862. [Google Scholar] [CrossRef]
- Chen, Y.-F.; Wang, Y.-H.; Lei, C.-S.; Changou, C.A.; Davis, M.E.; Yen, Y. Host immune response to anti-cancer camptothecin conjugated cyclodextrin-based polymers. J. Biomed. Sci. 2019, 26, 85. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-S.; Han, J.-H.; Choi, S.H.; Jung, H.-Y.; Park, J.D.; An, H.-J.; Kim, S.-E.; Kim, D.-H.; Doh, J.; Han, D.K.; et al. Cationic Nanoparticle-Mediated Activation of Natural Killer Cells for Effective Cancer Immunotherapy. ACS Appl. Mater. Interfaces 2020, 12, 56731–56740. [Google Scholar] [CrossRef] [PubMed]
- Loftus, C.; Saeed, M.; Davis, D.M.; Dunlop, I.E. Activation of Human Natural Killer Cells by Graphene Oxide-Templated Antibody Nanoclusters. Nano Lett. 2018, 18, 3282–3289. [Google Scholar] [CrossRef] [PubMed]
- Siegler, E.L.; Kim, Y.J.; Chen, X.; Siriwon, N.; Mac, J.; Rohrs, J.A.; Bryson, P.D.; Wang, P. Combination Cancer Therapy Using Chimeric Antigen Receptor-Engineered Natural Killer Cells as Drug Carriers. Mol. Ther. 2017, 25, 2607–2619. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Qi, M.; Ding, F.; Lv, Y.; Ma, J.; Zhu, Y. Tumour targetable and microenvironment-responsive nanoparticles simultaneously disrupt the PD-1/PD-L1 pathway and MAPK/ERK/JNK pathway for efficient treatment of colorectal cancer. J. Drug Target. 2020, 29, 454–465. [Google Scholar] [CrossRef]
- Lee, M.-H.; Liu, K.-H.; Thomas, J.L.; Chen, J.-R.; Lin, H.-Y. Immunotherapy of Hepatocellular Carcinoma with Magnetic PD-1 Peptide-Imprinted Polymer Nanocomposite and Natural Killer Cells. Biomolecules 2019, 9, 651. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, N.; Suh, H.; Irvine, D.J. Nanoparticle anchoring targets immune agonists to tumors enabling anti-cancer immunity without systemic toxicity. Nat. Commun. 2018, 9, 6. [Google Scholar] [CrossRef]
- Au, K.M.; Park, S.I.; Wang, A.Z. Trispecific natural killer cell nanoengagers for targeted chemoimmunotherapy. Sci. Adv. 2020, 6, eaba8564. [Google Scholar] [CrossRef]
- Chandrasekaran, S.; Chan, M.F.; Li, J.; King, M.R. Super natural killer cells that target metastases in the tumor draining lymph nodes. Biomaterials 2015, 77, 66–76. [Google Scholar] [CrossRef]
- Atukorale, P.U.; Raghunathan, S.P.; Raguveer, V.; Moon, T.J.; Zheng, C.; Bielecki, P.A.; Wiese, M.L.; Goldberg, A.L.; Covarrubias, G.; Hoimes, C.J.; et al. Nanoparticle Encapsulation of Synergistic Immune Agonists Enables Systemic Codelivery to Tumor Sites and IFNβ-Driven Antitumor Immunity. Cancer Res. 2019, 79, 5394–5406. [Google Scholar] [CrossRef]
- Murphy, D.A.; Cheng, H.; Yang, T.; Yan, X.; Adjei, I.M. Reversing Hypoxia with PLGA-Encapsulated Manganese Dioxide Nanoparticles Improves Natural Killer Cell Response to Tumor Spheroids. Mol. Pharm. 2021, 18, 2935–2946. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Sato, T.; Endo, R.; Sasaki, S.; Takahashi, N.; Sato, Y.; Hyodo, M.; Hayakawa, Y.; Harashima, H. STING agonist loaded lipid nanoparticles overcome anti-PD-1 resistance in melanoma lung metastasis via NK cell activation. J. Immunother. Cancer 2021, 9, e002852. [Google Scholar] [CrossRef] [PubMed]
- Pan, S.; Li, T.; Tan, Y.; Xu, H. Selenium-containing nanoparticles synergistically enhance Pemetrexed&NK cell-based chemoimmunotherapy. Biomaterials 2021, 280, 121321. [Google Scholar] [CrossRef]
- Wei, Z.; Yi, Y.; Luo, Z.; Gong, X.; Jiang, Y.; Hou, D.; Zhang, L.; Liu, Z.; Wang, M.; Wang, J.; et al. Selenopeptide Nanomedicine Activates Natural Killer Cells for Enhanced Tumor Chemoimmunotherapy. Adv. Mater. 2022, 34, 2108167. [Google Scholar] [CrossRef] [PubMed]
- Deng, G.; Sun, Z.; Li, S.; Peng, X.; Li, W.; Zhou, L.; Ma, Y.; Gong, P.; Cai, L. Cell-Membrane Immunotherapy Based on Natural Killer Cell Membrane Coated Nanoparticles for the Effective Inhibition of Primary and Abscopal Tumor Growth. ACS Nano 2018, 12, 12096–12108. [Google Scholar] [CrossRef]
- Kwak, M.; Erdag, G.; Leick, K.M.; Bekiranov, S.; Engelhard, V.H.; Slingluff, C.L. Associations of immune cell homing gene signatures and infiltrates of lymphocyte subsets in human melanomas: Discordance with CD163+ myeloid cell infiltrates. J. Transl. Med. 2021, 19, 371. [Google Scholar] [CrossRef]
- Zhang, S.-C.; Hu, Z.-Q.; Long, J.-H.; Zhu, G.-M.; Wang, Y.; Jia, Y.; Zhou, J.; Ouyang, Y.; Zeng, Z. Clinical Implications of Tumor-Infiltrating Immune Cells in Breast Cancer. J. Cancer 2019, 10, 6175–6184. [Google Scholar] [CrossRef]
- Tan, Z.; Xue, H.; Sun, Y.; Zhang, C.; Song, Y.; Qi, Y. The Role of Tumor Inflammatory Microenvironment in Lung Cancer. Front. Pharmacol. 2021, 12, 1168. [Google Scholar] [CrossRef]
- Zhang, S.; Yang, X.; Wang, L.; Zhang, C. Interplay between inflammatory tumor microenvironment and cancer stem cells (Review). Oncol. Lett. 2018, 16, 679–686. [Google Scholar] [CrossRef]
- Gun, S.Y.; Lee, S.W.L.; Sieow, J.L.; Wong, S.C. Targeting immune cells for cancer therapy. Redox Biol. 2019, 25, 101174. [Google Scholar] [CrossRef]
- Melero, I.; Rouzaut, A.; Motz, G.T.; Coukos, G. T-Cell and NK-Cell Infiltration into Solid Tumors: A Key Limiting Factor for Efficacious Cancer Immunotherapy. Cancer Discov. 2014, 4, 522–526. [Google Scholar] [CrossRef] [PubMed]
- Ran, G.H.; Lin, Y.Q.; Tian, L.; Zhang, T.; Yan, D.M.; Yu, J.H.; Deng, Y.C. Natural killer cell homing and trafficking in tissues and tumors: From biology to application. Signal Transduct. Target. Ther. 2022, 7, 205. [Google Scholar] [CrossRef] [PubMed]
- Cifaldi, L.; Doria, M.; Cotugno, N.; Zicari, S.; Cancrini, C.; Palma, P.; Rossi, P. DNAM-1 Activating Receptor and Its Ligands: How Do Viruses Affect the NK Cell-Mediated Immune Surveillance during the Various Phases of Infection? Int. J. Mol. Sci. 2019, 20, 3715. [Google Scholar] [CrossRef] [PubMed]
- Park, W.; Gordon, A.C.; Cho, S.; Huang, X.; Harris, K.R.; Larson, A.C.; Kim, D.-H. Immunomodulatory Magnetic Microspheres for Augmenting Tumor-Specific Infiltration of Natural Killer (NK) Cells. ACS Appl. Mater. Interfaces 2017, 9, 13819–13824. [Google Scholar] [CrossRef]
- Wu, L.; Zhang, F.; Wei, Z.; Li, X.; Zhao, H.; Lv, H.; Ge, R.; Ma, H.; Zhang, H.; Yang, B.; et al. Magnetic delivery of Fe3O4@polydopamine nanoparticle-loaded natural killer cells suggest a promising anticancer treatment. Biomater. Sci. 2018, 6, 2714–2725. [Google Scholar] [CrossRef]
- Shevtsov, M.; Stangl, S.; Nikolaev, B.; Yakovleva, L.; Marchenko, Y.; Tagaeva, R.; Sievert, W.; Pitkin, E.; Mazur, A.; Tolstoy, P.; et al. Granzyme B Functionalized Nanoparticles Targeting Membrane Hsp70-Positive Tumors for Multimodal Cancer Theranostics. Small 2019, 15, e1900205. [Google Scholar] [CrossRef]
- Gasparri, A.M.; Sacchi, A.; Basso, V.; Cortesi, F.; Freschi, M.; Rrapaj, E.; Bellone, M.; Casorati, G.; Dellabona, P.; Mondino, A.; et al. Boosting Interleukin-12 Antitumor Activity and Synergism with Immunotherapy by Targeted Delivery with isoDGR-Tagged Nanogold. Small 2019, 15, e1903462. [Google Scholar] [CrossRef]
- Xu, Q.; Guo, L.; Gu, X.; Zhang, B.; Hu, X.; Zhang, J.; Chen, J.; Wang, Y.; Chen, C.; Gao, B.; et al. Prevention of colorectal cancer liver metastasis by exploiting liver immunity via chitosan-TPP/nanoparticles formulated with IL-12. Biomaterials 2012, 33, 3909–3918. [Google Scholar] [CrossRef]
- Jiao, P.; Otto, M.; Geng, Q.; Li, C.; Li, F.; Butch, E.R.; Snyder, S.E.; Zhou, H.; Yan, B. Enhancing both CT imaging and natural killer cell-mediated cancer cell killing by a GD2-targeting nanoconstruct. J. Mater. Chem. B 2015, 4, 513–520. [Google Scholar] [CrossRef]
- Monty, M.A.; Islam, A.; Nan, X.; Tan, J.; Tuhin, I.J.; Tang, X.; Miao, M.; Wu, D.; Yu, L. Emerging role of RNA interference in immune cells engineering and its therapeutic synergism in immunotherapy. Br. J. Pharmacol. 2021, 178, 1741–1755. [Google Scholar] [CrossRef]
- Adjei, I.M.; Jordan, J.; Tu, N.; Le Trinh, T.; Kandell, W.; Wei, S.; Sharma, B. Functional recovery of natural killer cell activity by nanoparticle-mediated delivery of transforming growth factor beta 2 small interfering RNA. J. Interdiscip. Nanomed. 2019, 4, 98–112. [Google Scholar] [CrossRef]
- Lian, S.; Xie, R.; Ye, Y.; Xie, X.; Li, S.; Lu, Y.; Li, B.; Cheng, Y.; Katanaev, V.; Jia, L. Simultaneous blocking of CD47 and PD-L1 increases innate and adaptive cancer immune responses and cytokine release. eBioMedicine 2019, 42, 281–295. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Luo, Y.-L.; Chen, Y.-F.; Lu, Z.-D.; Wang, Y.; Czarna, A.; Shen, S.; Xu, C.-F.; Wang, J. Dually regulating the proliferation and the immune microenvironment of melanoma via nanoparticle-delivered siRNA targeting onco-immunologic CD155. Biomater. Sci. 2020, 8, 6683–6694. [Google Scholar] [CrossRef] [PubMed]
- Neviani, P.; Wise, P.M.; Murtadha, M.; Liu, C.W.; Wu, C.-H.; Jong, A.Y.; Seeger, R.C.; Fabbri, M. Natural killer–derived exosomal miR-186 inhibits neuroblastoma growth and immune escape mechanisms. Cancer Res. 2019, 79, 1151–1164. [Google Scholar] [CrossRef]
- Islam, R.; Pupovac, A.; Evtimov, V.; Boyd, N.; Shu, R.; Boyd, R.; Trounson, A. Enhancing a Natural Killer: Modification of NK Cells for Cancer Immunotherapy. Cells 2021, 10, 1058. [Google Scholar] [CrossRef]
- Yan, C.; Jie, L.; Yongqi, W.; Weiming, X.; Juqun, X.; Yanbing, D.; Li, Q.; Xingyuan, P.; Mingchun, J.; Weijuan, G. Delivery of human NKG2D-IL-15 fusion gene by chitosan nanoparticles to enhance antitumor immunity. Biochem. Biophys. Res. Commun. 2015, 463, 336–343. [Google Scholar] [CrossRef]
- Zhuo, Y.; Chen, F.; Kong, L.; Li, T.; Lu, L.; Yang, J.; Yu, T.; Shi, X.; Li, K. Magnetic Resonance Imaging of the Human Ferritin Heavy Chain Reporter Gene Carried by Dendrimer-Entrapped Gold Nanoparticles. J. Biomed. Nanotechnol. 2019, 15, 518–530. [Google Scholar] [CrossRef]
- Meraz, I.M.; Majidi, M.; Cao, X.; Lin, H.; Li, L.; Wang, J.; Baladandayuthapani, V.; Rice, D.; Sepesi, B.; Ji, L.; et al. TUSC2 Immunogene Therapy Synergizes with Anti–PD-1 through Enhanced Proliferation and Infiltration of Natural Killer Cells in Syngeneic Kras-Mutant Mouse Lung Cancer Models. Cancer Immunol. Res. 2018, 6, 163–177. [Google Scholar] [CrossRef]
- Kim, K.-S.; Han, J.-H.; Park, J.-H.; Kim, H.-K.; Choi, S.H.; Kim, G.R.; Song, H.; An, H.J.; Han, D.K.; Park, W.; et al. Multifunctional nanoparticles for genetic engineering and bioimaging of natural killer (NK) cell therapeutics. Biomaterials 2019, 221, 119418. [Google Scholar] [CrossRef]
- Spear, P.; Wu, M.R.; Sentman, M.L.; Sentman, C.L. NKG2D ligands as therapeutic targets. Cancer Immun. 2013, 13, 8. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3700746/ (accessed on 4 October 2022).
- Liu, C.; Lai, H.; Chen, T. Boosting Natural Killer Cell-Based Cancer Immunotherapy with Selenocystine/Transforming Growth Factor-Beta Inhibitor-Encapsulated Nanoemulsion. ACS Nano 2020, 14, 11067–11082. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Xu, Y.; Wu, Q.; Hu, P.; Shi, J. Mild Magnetic Hyperthermia-Activated Innate Immunity for Liver Cancer Therapy. J. Am. Chem. Soc. 2021, 143, 8116–8128. [Google Scholar] [CrossRef] [PubMed]
- Viaud, S.; Terme, M.; Flament, C.; Taieb, J.; Andre, F.; Novault, S.; Escudier, B.; Robert, C.; Caillat-Zucman, S.; Tursz, T.; et al. Dendritic Cell-Derived Exosomes Promote Natural Killer Cell Activation and Proliferation: A Role for NKG2D Ligands and IL-15Rα. PLoS ONE 2009, 4, e4942. [Google Scholar] [CrossRef] [PubMed]
- Shoae-Hassani, A.; Hamidieh, A.A.; Behfar, M.; Mohseni, R.; Mortazavi-Tabatabaei, S.A.; Asgharzadeh, S. NK Cell–derived Exosomes from NK Cells Previously Exposed to Neuroblastoma Cells Augment the Antitumor Activity of Cytokine-activated NK Cells. J. Immunother. 2017, 40, 265–276. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Xu, M.; Luo, J.; Zhao, L.; Ye, G.; Shi, F.; Lv, C.; Chen, H.; Wang, Y.; Li, Y. Liver toxicity assessments in rats following sub-chronic oral exposure to copper nanoparticles. Environ. Sci. Eur. 2019, 31, 30. [Google Scholar] [CrossRef]
- Yao, Y.; Zang, Y.; Qu, J.; Tang, M.; Zhang, T. The Toxicity of Metallic Nanoparticles on Liver: The Subcellular Damages, Mechanisms, and Outcomes. Int. J. Nanomed. 2019, 14, 8787–8804. [Google Scholar] [CrossRef] [PubMed]
- Kermanizadeh, A.; Powell, L.G.; Stone, V. A review of hepatic nanotoxicology—Summation of recent findings and considerations for the next generation of study designs. J. Toxicol. Environ. Health Part B 2020, 23, 137–176. [Google Scholar] [CrossRef]
- Wang, P.; Lu, Y.-Q. Ferroptosis: A Critical Moderator in the Life Cycle of Immune Cells. Front. Immunol. 2022, 13, 877634. [Google Scholar] [CrossRef]
- Miller, J.S.; Soignier, Y.; Panoskaltsis-Mortari, A.; McNearney, S.A.; Yun, G.H.; Fautsch, S.K.; McKenna, D.; Le, C.; DeFor, T.E.; Burns, L.J.; et al. Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. Blood 2005, 105, 3051–3057. [Google Scholar] [CrossRef]
- Shin, M.H.; Kim, J.; Lim, S.A.; Kim, J.; Kim, S.-J.; Lee, K.-M. NK Cell-Based Immunotherapies in Cancer. Immune Netw. 2020, 20, e14. [Google Scholar] [CrossRef]
- Liu, S.; Galat, V.; Galat, Y.; Lee, Y.K.A.; Wainwright, D.; Wu, J. NK cell-based cancer immunotherapy: From basic biology to clinical development. J. Hematol. Oncol. 2021, 14, 7. [Google Scholar] [CrossRef] [PubMed]
- Yamanaka, S. Pluripotent Stem Cell-Based Cell Therapy—Promise and Challenges. Cell Stem Cell 2020, 27, 523–531. [Google Scholar] [CrossRef] [PubMed]
- Langenberg, K.; Kamphorst, J.; Bonhomme, C.; D’Amico, E.; Dublin, S.; Braam, S.; Reijerkerk, A. Controlled stirred tank bioreactors for large-scale manufacture of human iPSC models for cell therapy. Cytotherapy 2020, 22, S43. [Google Scholar] [CrossRef]
- Kang, T.; Huang, Y.; Zhu, Q.; Cheng, H.; Pei, Y.; Feng, J.; Xu, M.; Jiang, G.; Song, Q.; Jiang, T.; et al. Necroptotic cancer cells-mimicry nanovaccine boosts anti-tumor immunity with tailored immune-stimulatory modality. Biomaterials 2018, 164, 80–97. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-Y.; Noh, Y.-W.; Kang, T.H.; Kim, J.-E.; Kim, S.; Um, S.H.; Oh, D.-B.; Park, Y.-M.; Lim, Y.T. Synthetic vaccine nanoparticles target to lymph node triggering enhanced innate and adaptive antitumor immunity. Biomaterials 2017, 130, 56–66. [Google Scholar] [CrossRef]
- Gang, M.; Wong, P.; Berrien-Elliott, M.M.; Fehniger, T.A. Memory-like natural killer cells for cancer immunotherapy. Semin. Hematol. 2020, 57, 185–193. [Google Scholar] [CrossRef]
- Fehniger, T.A.; Cooper, M.A. Harnessing NK Cell Memory for Cancer Immunotherapy. Trends Immunol. 2016, 37, 877–888. [Google Scholar] [CrossRef]
- Giustarini, G.; Pavesi, A.; Adriani, G. Nanoparticle-Based Therapies for Turning Cold Tumors Hot: How to Treat an Immunosuppressive Tumor Microenvironment. Front. Bioeng. Biotechnol. 2021, 9, 458. [Google Scholar] [CrossRef]
- Hu, W.; Wang, G.; Huang, D.; Sui, M.; Xu, Y. Cancer Immunotherapy Based on Natural Killer Cells: Current Progress and New Opportunities. Front. Immunol. 2019, 10, 1205. [Google Scholar] [CrossRef]


| Sr No. | Type of Receptors | Potential Targets | Ligands | Function | Ref. |
|---|---|---|---|---|---|
| 1. | Activating receptors | NKG2D |
| Activation, proliferation, and expansion of the immune cells | [71] |
| 2. | DNAM-1 |
| Enhances the cytotoxic activity | [72] | |
| 3. | NCR |
| Could stimulate and inhibit NK cell activity based on the ligand | [73] | |
| 4. | CD16 |
| Promotes cytokine production and enhances cytotoxicity | [74] | |
| 5. | NCAM/ CD56 | - | Enhances cytokine production, IFN- γ production and enhances NK cell proliferation | [75] | |
| 6. | CD96 |
| Enhances recognition of tumor cells, increases expression of cytotoxic granules | [76] | |
| 7. | CXCR1 |
| Enhances homing of immune cells | [77] | |
| 8. | CX3CR1 |
| Enhances homing of immune cells | [78] | |
| 9. | CD11 | - | Maturation biomarker for NK cells | [79] | |
| 10. | CCR7 |
| Facilitates homing of NK cells to the lymph nodes | [80] | |
| 11. | Inhibitory receptors | CD94/ NKG2A |
| Inhibits cytotoxic ability | [81] |
| 12. | TIM-3 |
| NK cell dysfunction and induces NK cell exhaustion | [82] | |
| 13. | TIGIT |
| Inhibits cytotoxic ability | [83] | |
| 14. | KIRs |
| Inhibits cytokine release | [84] | |
| 15. | LAG-3 |
| Inhibits immune cell activation and cytokine production | [85] | |
| 16. | PD-1 |
| Functional defects in NK cells, T cells | [86] |
| Sr No. | Nanoparticles | Target NK Cell | Ligand | Mechanism of Activation | Effect on Tumor Cells | Ref. |
|---|---|---|---|---|---|---|
| 1. | RGD peptide-tagged polyethylene glycol loaded with doxorubicin and diselenium nanoparticle | - | - |
|
| [103] |
| 2. | Lactobacillus brevis enriched selenium nanoparticle |
|
| [104] | ||
| 3. | Pemetrexed and cytosine-containing diselenide-loaded nanoparticles | - | - |
|
| [108] |
| 4. | Folate-containing liposomes, manganese protoporphyrin | Folate receptor | Folate |
|
| [109] |
| 5. | Calcium carbonate-coated gold nanostars with chlorine e6 photosensitizer | - | - |
|
| [110] |
| 6. | Lipid nanoparticles conjugated to CpG oligonucleotides, mannose, and H22 hepatoma lysate | - | - |
|
| [111] |
| 7. | PLGA nanoparticles encapsulated TLR7/8 agonist | TLR 7/8 receptor | TLR7/8 agonist |
|
| [112] |
| 8. | TGF-β and IL-2 loaded nanolipogels | TGF-β receptor | TGF-β |
| - | [101] |
| 9. | Camptothecin-loaded cyclodextrin-based polymers | - | - |
|
| [113] |
| 10. | Magnetic nanoparticles | - | - |
|
| [114] |
| 11. | Graphene oxide nanoclusters loaded with anti-CD16 | CD16 | Anti-CD16 antibody |
| - | [115] |
| 12. | CAR-NK cell loaded with paclitaxel | CD16, HER-2 receptor | Anti-HER-1 antibody, Anti-CD16 antibody |
|
| [116] |
| 13. | PDMAEMA-PTPN6 conjugated atezolizumab | PD-L1 | Atezolizumab |
|
| [117] |
| 14. | Magnetic nanoparticle conjugated with a peptide derived from PD-1 | - | - |
|
| [118] |
| 15. | Immunoliposomes loaded IL-2 and anti-CD137 | CD137 | Anti-CD137 antibody |
|
| [119] |
| 16. | Mesoporous ruthenium nanoparticles conjugated bispecific antibodies (SS-Fc, anti-CD16, and anti-CEA) | CD16 and CEA | Anti-CD16 and anti-CEA |
|
| [106] |
| 17. | Trispecific (antibodies of α-CD16, α-4-1BB, and α-EFGR) nanoengagers | CD16, EGFR | α-CD16, α-4-1BB, and α-EFGR |
|
| [120] |
| 18. | TRAIL and anti-NK1.1 antibody decorated liposomes | NK1.1 receptor, death receptor | An anti-NK1.1 antibody, TRAIL |
|
| [121] |
| 19. | cdGMP and MPLA encapsulated nanoparticle | - | - |
|
| [122] |
| 20. | Manganese dioxide nanoparticles | - | - |
|
| [123] |
| 21. | Lipid nanoparticle-loaded STING agonist | STING receptor | STING agonist |
|
| [124] |
| 22. | Selenium containing nanoparticle loaded pemetrexed | - | - |
|
| [125] |
| 23. | Selenopeptide nanomedicine | - | - |
|
| [126] |
| 24. | 4,4′,4′′,4′′′-(porphine 5,10,15,20-tetrayl) tetrakis (benzoic acid) (TCPP)-loaded nanoparticle | - | - |
|
| [127] |
| Sr No. | Nanoparticles | Target NK Cell | Ligand | Mechanism of Activation | Effect on Tumor Cells | Ref. |
|---|---|---|---|---|---|---|
| 1. | Iron oxide nanoparticles on the surface of primary NK cells | - | - |
|
| [89] |
| 2. | Iron oxide nanoparticles labelled on NK cells | - | - |
|
| [137] |
| 3. | Magnetic PLGA microspheres containing recombinant IFN-γ and iron oxide nanocubes |
|
| [136] | ||
| 4. | Dextran-coated serine protease granzyme-B functionalized superparamagnetic iron oxide nanoparticles | mHsp70 | Granzyme B |
|
| [138] |
| 5. | IL-12 bound gold nanoparticle tagged with homing peptide | αvβ3-integrin receptors | isoAsp-Gly-Arg homing peptide |
|
| [139] |
| 6. | IL-12-loaded chitosan nanoparticles | - | - |
|
| [140] |
| 7. | Anti-GD2 antibody tagged gold nanoparticle | GD-2 receptor | Anti-GD2 antibody |
|
| [141] |
| Sr No. | Nanoparticles | Target NK Cell | Ligand | Mechanism of Activation | Effect on Tumor Cells | Ref. |
|---|---|---|---|---|---|---|
| 1. | Manganese dioxide nanoparticle containing siRNA-TGFBR2 | TGFBR2 silencing | siRNA |
|
| [143] |
| 2. | EpCAM targeted cationic liposomes containing si-CD47 and si-PD-L1 | EpCAM, CD47, PD-L1 | si-CD47, si-PD-L1 |
|
| [144] |
| 3. | Cationic lipid-assisted nanoparticles encapsulated with siCD155 | CD155 | si-CD155 |
|
| [145] |
| 4. | miRNA-loaded NK cell-derived exosomes | - | let-7a |
|
| [95] |
| 5. | miRNA-186 loaded NK cell-derived exosomes | - | miRNA-186 |
|
| [146] |
| Sr No. | Nanoparticles | Target NK Cell | Ligand | Mechanism of Activation | Effect on Tumor Cells | Ref. |
|---|---|---|---|---|---|---|
| 1. | Super magnetic nanoparticle Zn-CoFe2O4@Zn-MnFe2O4 | NKG2D, VEGFR | UL16-binding protein |
|
| [154] |
| 2. | Dendritic cell-derived exosomes | NKG2D | NKG2D ligands |
|
| [155] |
| 3. | Glioblastoma cells pre-exposed NK cell-derived exosomes | NKG2D | - |
|
| [156] |
| 4. | TGF-β inhibitor and selenocysteine-containing nanoemulsion | NKG2D | - |
|
| [153] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Murugan, D.; Murugesan, V.; Panchapakesan, B.; Rangasamy, L. Nanoparticle Enhancement of Natural Killer (NK) Cell-Based Immunotherapy. Cancers 2022, 14, 5438. https://doi.org/10.3390/cancers14215438
Murugan D, Murugesan V, Panchapakesan B, Rangasamy L. Nanoparticle Enhancement of Natural Killer (NK) Cell-Based Immunotherapy. Cancers. 2022; 14(21):5438. https://doi.org/10.3390/cancers14215438
Chicago/Turabian StyleMurugan, Dhanashree, Vasanth Murugesan, Balaji Panchapakesan, and Loganathan Rangasamy. 2022. "Nanoparticle Enhancement of Natural Killer (NK) Cell-Based Immunotherapy" Cancers 14, no. 21: 5438. https://doi.org/10.3390/cancers14215438
APA StyleMurugan, D., Murugesan, V., Panchapakesan, B., & Rangasamy, L. (2022). Nanoparticle Enhancement of Natural Killer (NK) Cell-Based Immunotherapy. Cancers, 14(21), 5438. https://doi.org/10.3390/cancers14215438










