Simple Summary
Heat shock proteins (HSPs) play an important role in cellular metabolism and therefore are highly expressed in malignant brain tumors. In the current review, the authors assessed the prognostic value of HSPs in neuro-oncology and the possibility of employing these proteins as a target to develop novel therapeutic approaches. Indeed, several preclinical studies indicate the therapeutic potential of small molecular inhibitors of HSPs for targeting brain tumors when being applied as a monotherapy or in combination with other treatment approaches.
Abstract
Heat shock proteins (HSPs) are conservative and ubiquitous proteins that are expressed both in prokaryotic and eukaryotic organisms and play an important role in cellular homeostasis, including the regulation of proteostasis, apoptosis, autophagy, maintenance of signal pathways, protection from various stresses (e.g., hypoxia, ionizing radiation, etc.). Therefore, HSPs are highly expressed in tumor cells, including malignant brain tumors, where they also associate with cancer cell invasion, metastasis, and resistance to radiochemotherapy. In the current review, we aimed to assess the diagnostic and prognostic values of HSPs expression in CNS malignancies as well as the novel treatment approaches to modulate the chaperone levels through the application of inhibitors (as monotherapy or in combination with other treatment modalities). Indeed, for several proteins (i.e., HSP10, HSPB1, DNAJC10, HSPA7, HSP90), a direct correlation between the protein level expression and poor overall survival prognosis for patients was demonstrated that provides a possibility to employ them as prognostic markers in neuro-oncology. Although small molecular inhibitors for HSPs, particularly for HSP27, HSP70, and HSP90 families, were studied in various solid and hematological malignancies demonstrating therapeutic potential, still their potential was not yet fully explored in CNS tumors. Some newly synthesized agents (e.g., HSP40/DNAJ inhibitors) have not yet been evaluated in GBM. Nevertheless, reported preclinical studies provide evidence and rationale for the application of HSPs inhibitors for targeting brain tumors.
Keywords:
heat shock proteins; small HSPs; Hsp27; Hsp40; Hsp70; Hsp90; inhibitors; glioblastoma; brain tumors; prognostic marker 1. Introduction
Malignant brain tumors, particularly multiforme glioblastoma (GBM), are a challenging diagnosis due to their deep location in the brain, aggressive behavior as well as dismal prognosis with a high mortality rate [1] and low quality of life [2] among the patients (recent CNS tumors classification reviewed in [3]). Generally, the treatment for malignant brain tumors includes surgical resection followed by radiotherapy, chemotherapy with temozolomide, and palliative care. However, the success rate of this treatment scheme remains low; the survival rate constitutes 14.6 months [4]. The addition of the Tumor-Treating Fields (TTF) to standard therapy, which could alter the GBM cell division, resulted in improved progression-free survival and overall survival (of 20.9 months) [5]. One of the promising treatment strategies could be based on the application of targeted therapies [6,7]. Apart from widely used key targets in dysregulated signaling pathways in GBM (e.g., TP53, tyrosine kinase receptors, PI3K/AKT/mTOR pathway, etc.), heat shock proteins (HSPs) represent a promising target for developing novel therapeutic approaches [8,9].
Heat shock proteins constitute a family of conserved proteins in both prokaryotic and eukaryotic organisms that play an important role in the regulation of polypeptides and protein folding/unfolding (i.e., proteostasis), regulation of apoptosis and autophagy, and protection of cells from various stresses (including hypoxia, heat shock, ionizing radiation, etc.) (Table 1) [10,11,12]. Therefore, the expression of HSPs is significantly increased in tumor cells as compared to normal cells [13]. HSPs are classified according to their molecular weights constituting various families—HSPH (HSP110), HSPC (HSP90), HSPA (HSP70), DNAJ (HSP40), small HSP (HSPB), human chaperonin families CCT (TRiC), and HSPD/E (Table 1) [14]. HSPs play a significant role in tumor cell proteostasis and evasion of apoptosis; they are involved in cancer cell division, DNA repair mechanisms, invasion, and metastasis [10,12,15,16].

Table 1.
Roles of major HSP family members in cell physiology.
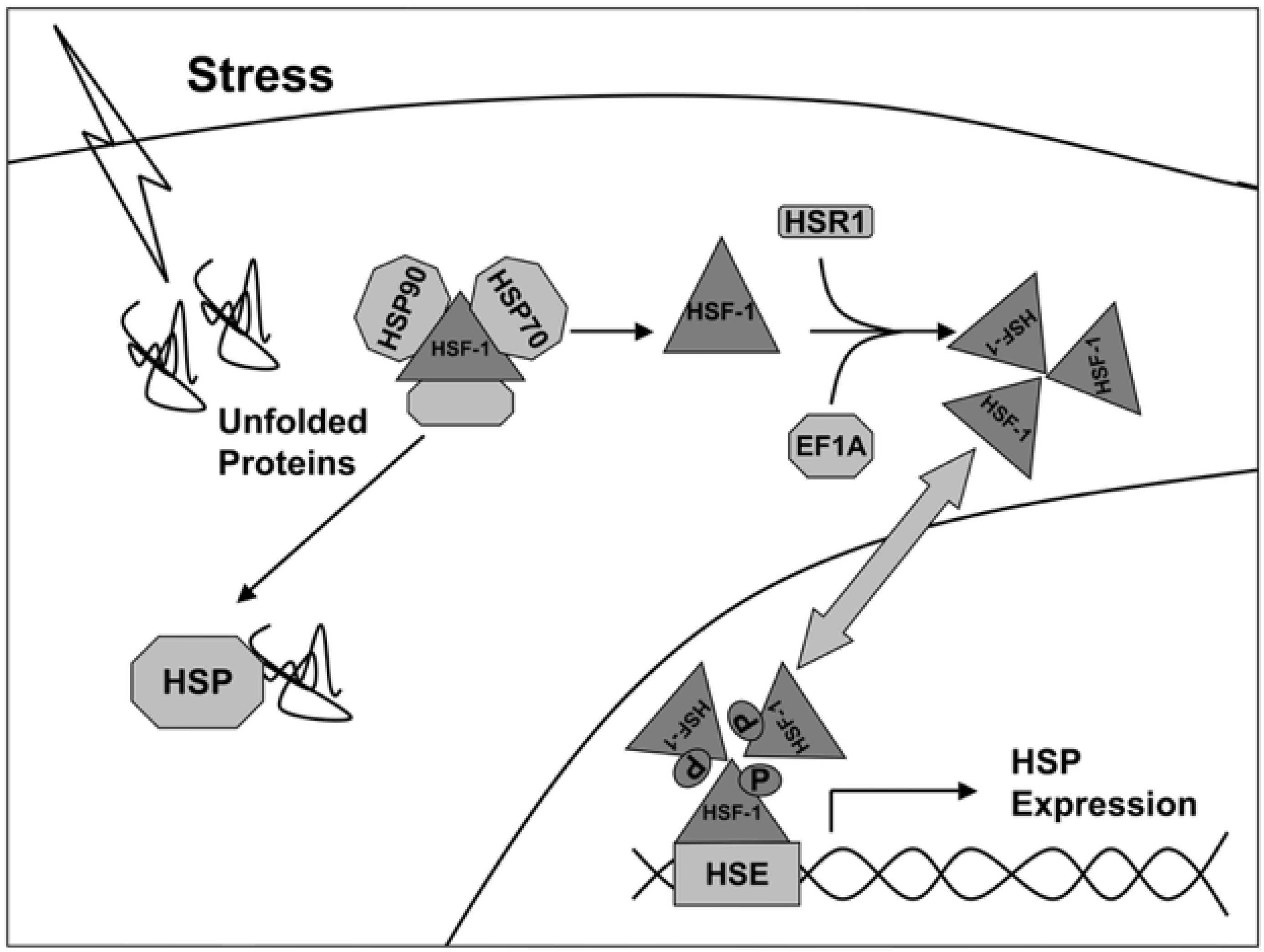
The transcription of genes of heat shock proteins is activated by heat shock factors (HSF), represented by dimeric structures recognizing the sequences -AGAAN- (Figure 1) [17]. Under normal conditions, HSF monomers are partially inactivated by binding to HSPs in the cytosol. Under stress, the affinity of HSP to denaturing proteins is higher, so HSF1 dissociate from the complex and are transported to the nucleus, where they induce transcription of HSP genes (including Hsp27, Hsp40, Hsp70, and Hsp90). Thus, the activity of synthesis of new chaperones depends on the state of the cell and external influences.
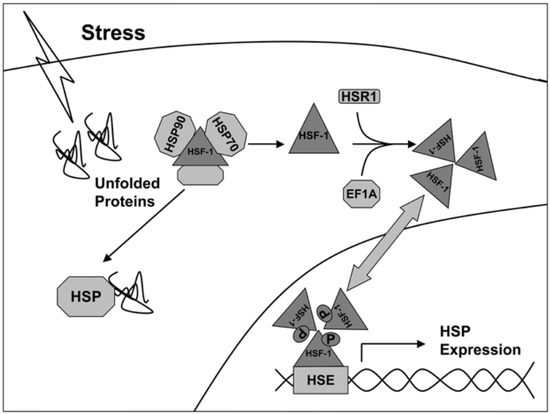
Figure 1.
Activation scheme of HSP gene expression under stress conditions [17].
Major chaperones, HSP70 and HSP90, function in the cytosol as refolding proteins, while small HSPs, including Hsp27, prevent aggregation of unfolded polypeptides and proteins [18,19]. A family of HSP40 containing a “J-domain” consists of three subclasses depending on the level of conservation of their J-domain [20]. The main role of HSP40 is the regulation of HSP70 activity [21]. Additionally, Hsp40 facilitates the binding of Hsp70 to the Hsp90-Hop (heat shock organizing protein) complex for further control of the protein folding [22,23,24]. Taking into consideration the protective role of HSPs, the overexpression of chaperones was reported in various types of solid and hematological malignancies [25,26,27,28,29]. High expression of HSPs also correlated to the chemo- and radioresistance of tumors indicated the possibility of employing these proteins as prognostic and/or diagnostic markers [26,30,31,32,33,34].
Many studies have reported that HSPs play an important role in regulating apoptosis and participating in autophagy [35,36,37,38]. Thus, Hsp27, a member of the small chaperones’ family, inhibits apoptosis by controlling the extrinsic apoptotic pathway [35]. It has also been reported that Hsp27 can directly associate with cytochrome c in the cytosol and inhibit the apoptosome formation [36]. Members of HSP70 and HSP90 families have also been reported to be involved in a pathway of apoptosis. Hsp70 can bind the death receptors and inhibit the TRAIL-induced assembly of the DISC at the pre-mitochondrial level [37]. Hsp90 has been reported to block apoptosis at a post-mitochondrial level. The authors demonstrated that HSP90 hampered the caspase activation [38].
Other effects of HSPs that could be employed for the development of anti-cancer therapies is their potential antiangiogenic properties which were reported by several studies [39,40,41,42]. HSP90 inhibitors exert antiangiogenic effects by affecting the PI-3K/AKT and eNOS signal transduction pathways in endothelial cells [43]. It was shown that the connection of HSPs to the eNOS pathway and the activation of PI-3K/AKT pathways encourages Ang-1-induced phosphorylation of eNOS and consequent angiogenesis [44].
Apart from the involvement of HSPs in proteomic homeostasis, it was demonstrated that these proteins exert immunomodulatory activities (reviewed in [45,46]). Thus Grp94(Gp96)-chaperoned peptides were shown to induce a protective T lymphocyte-mediated immune response that involved the uptake of HSP-peptide complexes by antigen-presenting cells with subsequent cross-presentation of these peptides on MHC I class molecules to cytotoxic CD8+ T-cells [45,47,48]. These immune properties of the HSPs were further employed for the generation of anti-cancer vaccines [49,50].
In the current review, we aimed to assess the prognostic/diagnostic values of HSPs expression in malignant brain tumors. Additionally, we analyzed the future perspectives of therapeutic approaches designed to target the HSPs in brain tumors, particularly when the combined treatment strategies were proposed.
2. Expression of HSPs in Brain Tumors
2.1. Small HSPs
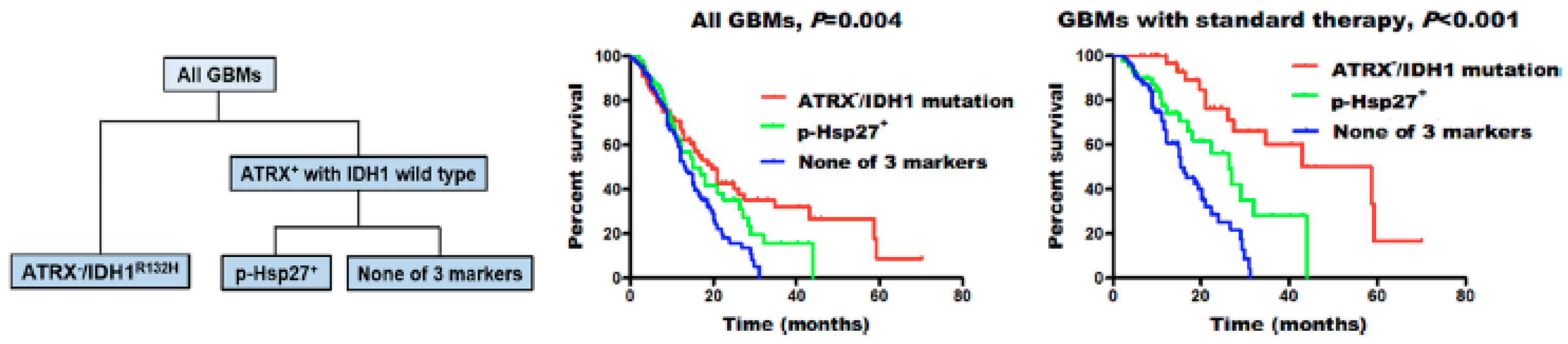
Among small heat shock proteins, Hsp27 is one of the most studied. Immunohistochemical analysis performed by several research groups has revealed a difference in the expression of HSP27 in different grades of glial tumors. The strongest expression was found in glioblastomas, followed by anaplastic astrocytomas, and only moderate expression in astrocytomas [51,52,53,54]. However, the extent of Hsp27 expression in astrocytomas is not definitive, as an immunohistochemical analysis found adjacent para-cancerous brain tissue to have higher expression of the chaperone than low-grade (grade II) glioma tumors [55]. No differentiation in the expression of the chaperone was found between recurrent and primary astrocytic tumors, nor did it vary by the sex of the patients [51]. The factor of age in the chaperone expression, however, was not as clear, as Assimakopoulou et al. did not find an association [51], while in the study by Cai et al., elder patients had higher Hsp27 and phosphorylated p-Hsp27 expression [53]. They also reported no correlation of Hsp27 expression with the survival of the patients [53]. Intriguingly, subsequent subgroup analysis of patients (n = 421) revealed that expression of phosphorylated p-Hsp27 (but not Hsp27) strongly correlated with ATRX (ATP-dependent helicase ATRX, X-linked helicase II) loss (ATRX-) and the isocitrate-dehydrogenase IDH1R132H mutation (Figure 2). This subgroup of patients showed an intermediate overall survival (15.0 vs. 13.1 months, p = 0.045). Better sensitivity for standard therapy was also reported in p-Hsp27+ GBM patients without the IDH1 mutation and ATRX loss (26.3 vs. 15.5 months, p = 0.008). As was shown previously, under different conditions, including stress, Hsp27 could be phosphorylated, which leads to its heterogenous oligomerization with subsequent loss of chaperone activity [56,57]. Presumably, this post-translational modification of p-Hsp27 could influence the tumor cell sensitivity towards applied therapies.
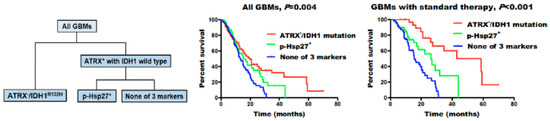
Figure 2.
Molecular classification of glioblastomas (GBMs). GBMs were separated into three groups: ATRX− /IDH1R132H, high p-Hsp27 expression (p-Hsp27+), and none of these three markers. Individuals with ATRX− /IDH1R132H showed the longest median survival, those with high p-Hsp27 expression had an intermediate prognosis, and those without any alteration in the three proteins had the poorest survival rates [53].
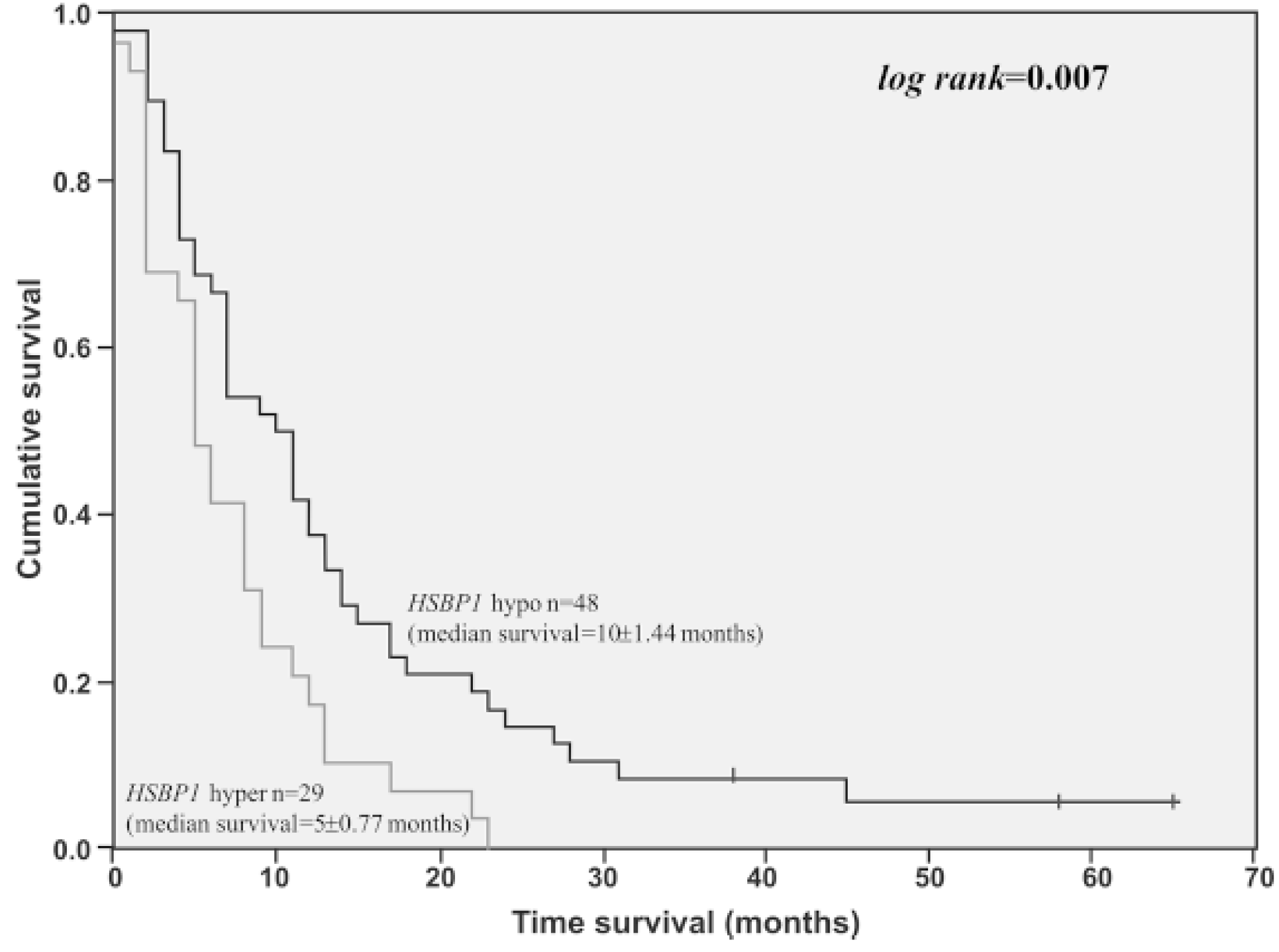
Another study employing quantitative proteomic analysis found Hsp27 (HSPB1) expression to be significantly higher in short-term survival patients when compared to long-term survival patients (Figure 3) [54].
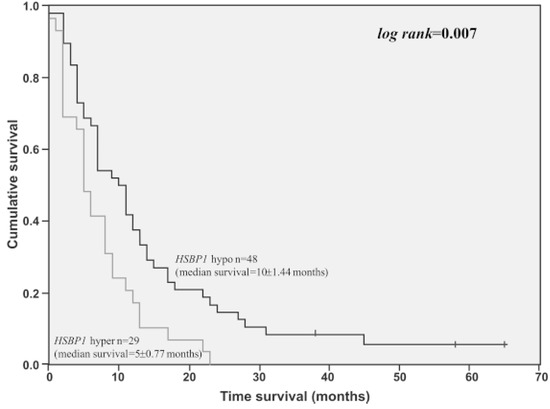
Figure 3.
Overall survival time of GBM cases presenting HSPB1 high expression level (3-fold of cut-off value determined by ROC curve) (n = 29) compared to GBM cases presenting lower HSPB1 expression level (n = 48). Log-rank = 0.007 [54].
In a study that examined Hsp27 expression along with Activator protein 1 (AP-1) transcription factor expression, it was found that HSP27 was only expressed in tumors that had c-Jun and c-Fos co-expression, which are major parts of AP-1. The authors conclude that there might be a relation between HSP27 and AP-1 activation, noting the correlation between AP-1/HSP27 co-expression and the rising grade of the tumor malignancy [58]. This suggests the involvement of Hsp27 in tumor cell survival, as AP-1 plays a major part in cell proliferation and growth [59]. Hsp27 also has an established client relationship with Androgen Receptor (AR) that is excessively expressed in the glioblastoma [60], which might explain the sex disparities in glioblastoma patients. By targeting HSP27, Li et al. demonstrated the potential for AR degradation by employing the HSP27 inhibition [61].
Given the involvement of Hsp27 in the processes ensuring tumor cells survival and with evidence of an increase of Hsp27 expression with the increase of glial tumor grade, the chaperone could serve as an indicator of tumor progression. However, more studies are needed to determine if Hsp27 could be used for the prognosis of the survival outcomes in the patients.
α-B-crystallin expression in glial tumors is more ambiguous, with some studies identifying high expression in the glioblastoma [62,63] and others finding heterogeneity in expression of the chaperone varying from high to undetectable [64,65,66]. Furthermore, a study found the expression of α-B-crystallin to be higher in grade I and II astrocytomas than in grades III and IV, although highly migratory glioma cells overexpressed α-Bc and were resistant to apoptosis [67]. However, no association between the patients’ survival was found with α-B-crystallin expression [66].
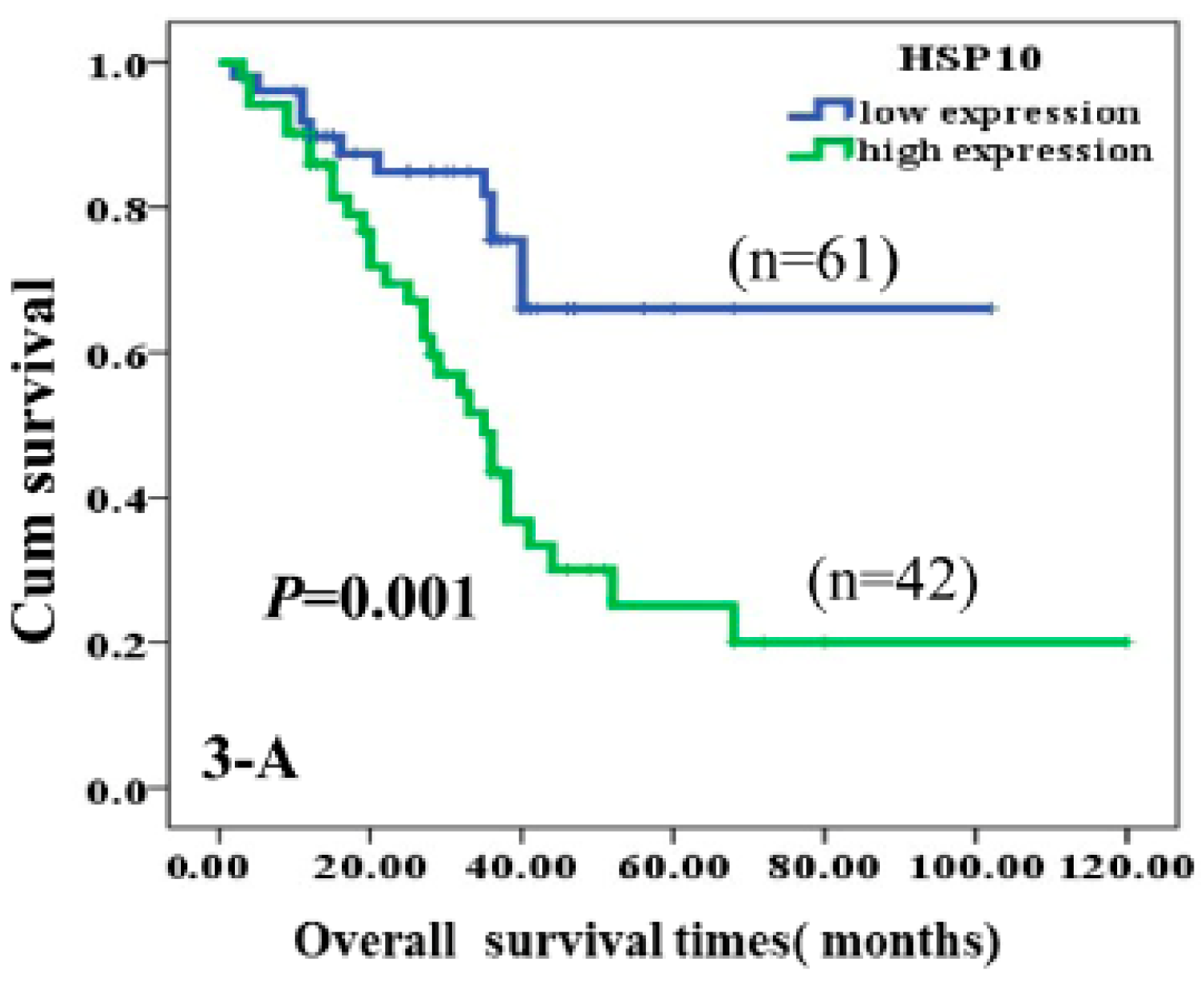
Immunohistochemical analyses showed higher expression of Hsp10 in astrocytoma tissue. Moreover, through multivariate analysis, Hsp10 was established as an independent factor negatively associated with poor prognosis when tumor grade, treatment received, tumor size, age, sex, and poly (ADP-ribose) polymerase (c-PARP) proteins were accounted for. The authors came to the conclusion that high expression of Hsp10 leads to inhibition of apoptosis in tumor cells and consequential poor survival of the patients (Figure 4) [68].
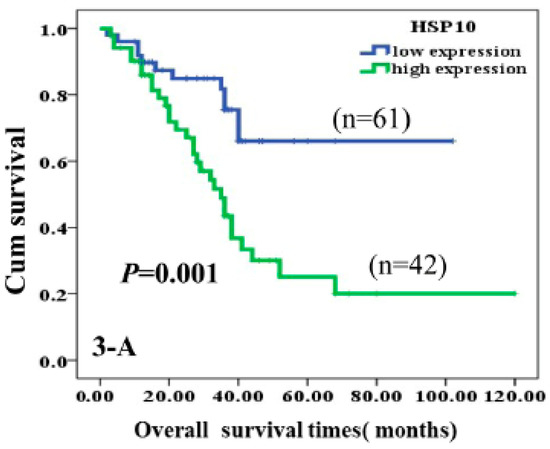
Figure 4.
Kaplan-Meier curves according to expression of HSP10 protein divided into high and low expression. High expression of HSP10 was significantly correlated to poor prognosis of astrocytoma patients (p = 0.001, two-sided) [68].
Small heat shock protein expression in brain tumors other than glial has been studied less frequently. Among the studied tumor types, medulloblastomas showed only a weak reaction to Hsp27 in immunohistochemical studies [63,69,70], and no association was established with the survival of the patients [70].
Anaplastic meningiomas were more reactive, with 24% of undifferentiated by-grade samples stained for Hsp27 antibodies [69]. Another study of meningiomas found significantly more intense staining of anaplastic meningiomas with HSP27 when compared to their lower-grade counterparts. However, no such difference was observed with α-B-crystallin, where both benign and malignant meningioma types showed weak reactivity [63].
Similarly, anaplastic ependymomas had intense staining for HSP27 and occasional reaction with α-B-crystallin [63], which is in line with the findings by Kato et al., who detected a minor expression of α-B-crystallin in ependymomas [69].
On the contrary, gangliogliomas did not express HSP27 and α-B-crystallin at the glial component of the tumor. Embryonal tumors (i.e., medulloblastoma and ependymoblastoma) showed weak reactions to both proteins; however, the sample size in the study was small (5 and 1, respectively) [63].
2.2. HSP40
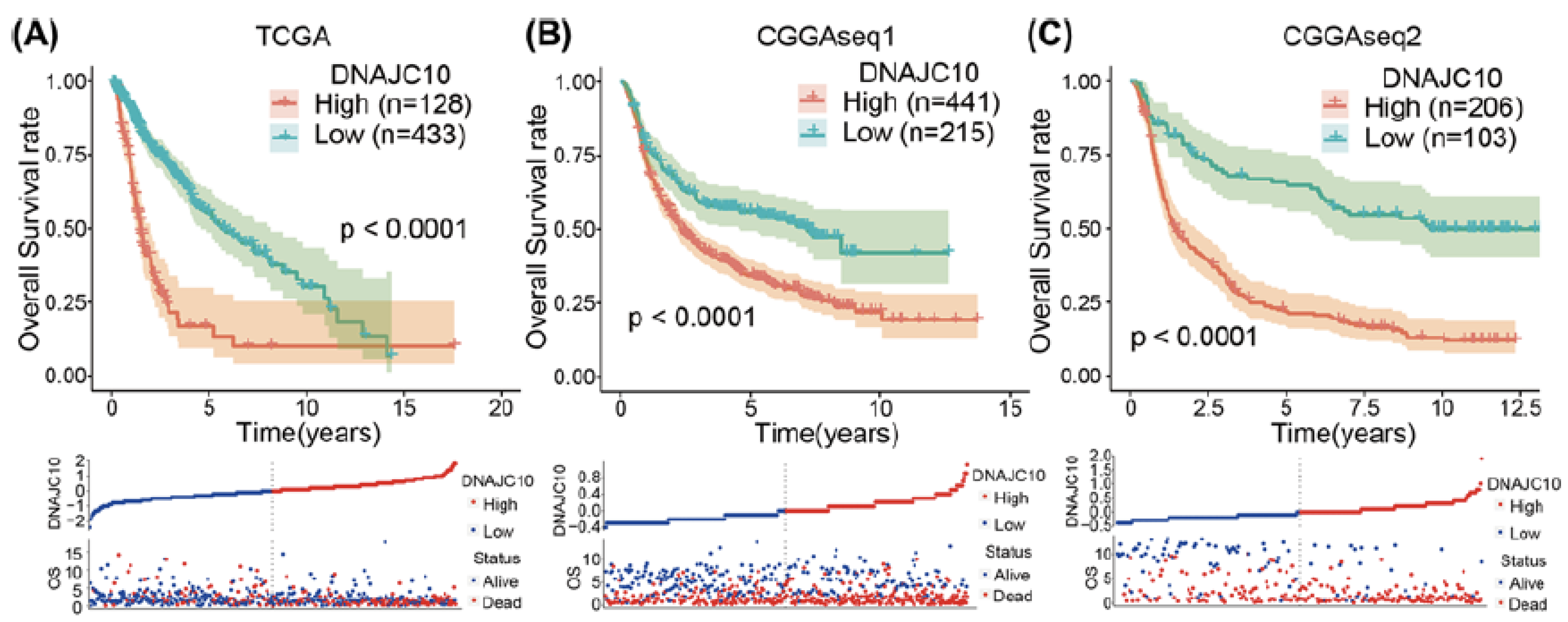
The family of 40 kDa heat shock proteins and their role in brain tumors is still comparatively under-investigated. A recent study by Liu et al. has found an upregulation of DNAJC10 mRNA, a member of the Hsp40 protein family, in both low-grade gliomas (WHO grade I and II) and high-grade gliomas (glioblastomas) in comparison to normal brain tissue [71]. The DNAJC10 protein expression increased with the increase of WHO glioma tumor grade. An association was found with DNAJC10 increased expression and an MGMT unmethylated status, IDH-wild type, and 1p/19q non-codeletion. Moreover, protein overexpression was associated with a poor survival prognosis for both LGG and glioblastomas (Figure 5) [71].
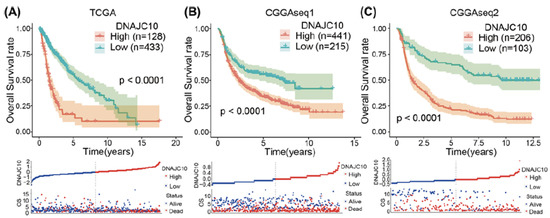
Figure 5.
High DNAJC10 expression indicates poor prognosis of glioma patients (A–C). Kaplan–Meier survival curves indicated that glioma patients with higher DNAJC10 expression levels showed shorter survival time and rate in three independent glioma cohorts (TCGA, CGGAseq1, and CGGAseq2) [71].
This finding is supported by the study of Sun et al., that found upregulation of DNAJC10 was associated with shorter survival time in patients [72]. The same study has also found overexpression of DNAJB6 and DNAJB1 to lead to worse survival outcomes and overexpression of DNAJA4, DNAJC6, and DNAJC12 to lead to better survival outcomes [72].
Expression of Hsp40 was found in medulloblastomas, but no difference in expression was observed between different medulloblastoma types [73]. Additionally, epigenetic silencing of methylation-controlled J protein MCJ (DNAJD1) through extensive methylation of CpG island was found, which indicates that the inactivation of MCJ might be involved in the tumor formation [74].
In conclusion, Hsp40 proteins as predictive and prognostic factors for glial tumors are a promising area. However, more research is needed to identify its prognostic and diagnostic values.
2.3. HSP60
Hsp60 is also abundant in brain tumors and is expressed higher than in normal tissues [75,76]. However, immunohistochemical analysis showed that the expression levels do not differ between glial tumor grades [77]. Neither did the Hsp60 expression in brain tumors differ from other disease tissues, such as cerebral infarct, amyotrophic lateral sclerosis, multiple sclerosis, and acute disseminated encephalomyelitis [78]. A more recent study by Hallal et al. that used cavitron ultrasonic surgical aspirator (CUSA) liquid tumor biopsies containing exosomes for analysis of the chaperonins showed differing results [79].
The Hsp60 family (also called chaperonins) form double-ring oligomeric protein complexes consisting of 60 kDa subunits, with a central cavity that facilitates adenosine triphosphate–dependent folding of polypeptides. The cytoplasmic chaperonin CCT group includes the eukaryotic cytoplasmic chaperonin CCT (chaperonin containing the T-complex polypeptide–1 [TCP1]) families [80]. Proteins belonging to T-ComplexProtein 1 Ring Complex (TRiC), a member of the Hsp60 protein family, were found to be more abundant in glioblastomas compared to low-grade gliomas. Specifically, TCP1, chaperonin containing tailless complexes—CCT2, CCT5, CCT6A, and CCT7-- had a statistically significant difference in expression. This was further checked with TCGA data in silico. All TRiC subunits were significantly increased compared to normal brain tissue, and CCT2, CCT3, CCT5, CCT6A, and CCT8 were increased relative to grade II–III astrocytomas as well. CCT6A specifically had the highest effect on the change of gene expression in glioblastoma samples, and it had an inverse relation to the patient survival [79]. Although elevated expression of the Hsp60 protein family is not unique to glial tumors, there is a potential for using it as a biomarker for tumor malignancy. However, more studies of the family proteins are required to find the optimal candidate.
Through the few studies done on other brain tumors, it was found that meningiomas have elevated levels of Hsp60 [75,77]. Ependymomas did not have a positive immunohistochemical reaction with Hsp60 [75]. Meanwhile, medulloblastomas had increased expression of Hsp60, although it did not associate with the survival outcomes for the patients [73].
2.4. HSP70
The expression of Hsp70 family proteins is well-studied in glioblastomas. However, the expression of this chaperone and the effect of protein on tumor survival are ambiguous. Although many studies found the expression of Hsp70 to be elevated in the tumor tissue when compared to normal brain cells [81,82,83], some did not find such differentiation [72,84]. Several studies were performed to understand the difference between recurrent and primary GMB with varying results. Muth et al. found the expression of the extracellular Hsp70 to be significantly lower in the primary GBM compared to the recurrent [81]. No such difference existed with intracellular Hsp70. They also found an elevation of Hsp70 expression to be associated with better prognostic outcomes. These findings contradict those of Thorsteinsdottir et al., who also found elevated expression of Hsp70 (cytosolic, plasma membrane-bound, and extracellular) in tumor tissue compared to healthy; however, expression was much higher in primary glioblastoma than in recurrent GBM, astrocytoma (GII and GIII), and control (epilepsy patients) (Figure 6) [82]. The study also did not find any association with progression-free survival or overall survival or O-6-methylguanine-DNA methyltransferase (MGMT) promoter status. Interestingly, immunoblot analysis in vitro and immunohistochemistry analysis in vivo showed worse survival in patients with high Hsp73 (i.e., constitutive cytosolic Hsc70) expression [66] and higher mRNA expression of HSPA6, which was downregulated in gliomas, also resulted in shorter survival [72].
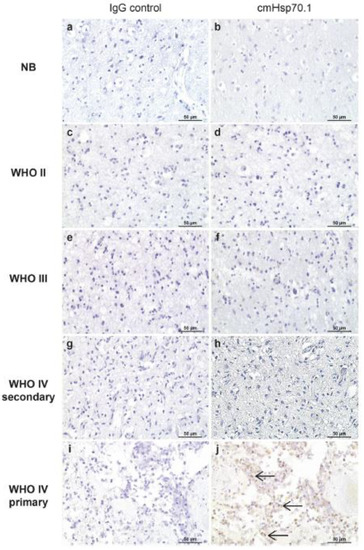
Figure 6.
Membrane Hsp70 expression in the non-neoplastic brain (b), diffuse astrocytoma (d), anaplastic astrocytoma (f), secondary GBM (h), and primary GBM (j) and corresponding negative controls (a,c,e,g,i) as determined by IHC. Magnification: ×20. Scale bar 50 µm [82].
Another study has found a significant increase in cytosolic Hsp70 expression in primary GBM compared to normal tissue and an association of high Hsp70 with better PFS and overall survival [85]. However, the effect on survival was negated in multivariate analysis when MGMT promoter methylation status was accounted for. The confounding effect suggests a relationship between MGMT methylation status and Hsp70 expression, potentially making Hsp70 a biomarker for MGMT methylation. However, the study has its limitations, such as an arbitrary cut-off point of staining 10% or more as an indication of high chaperone expression.
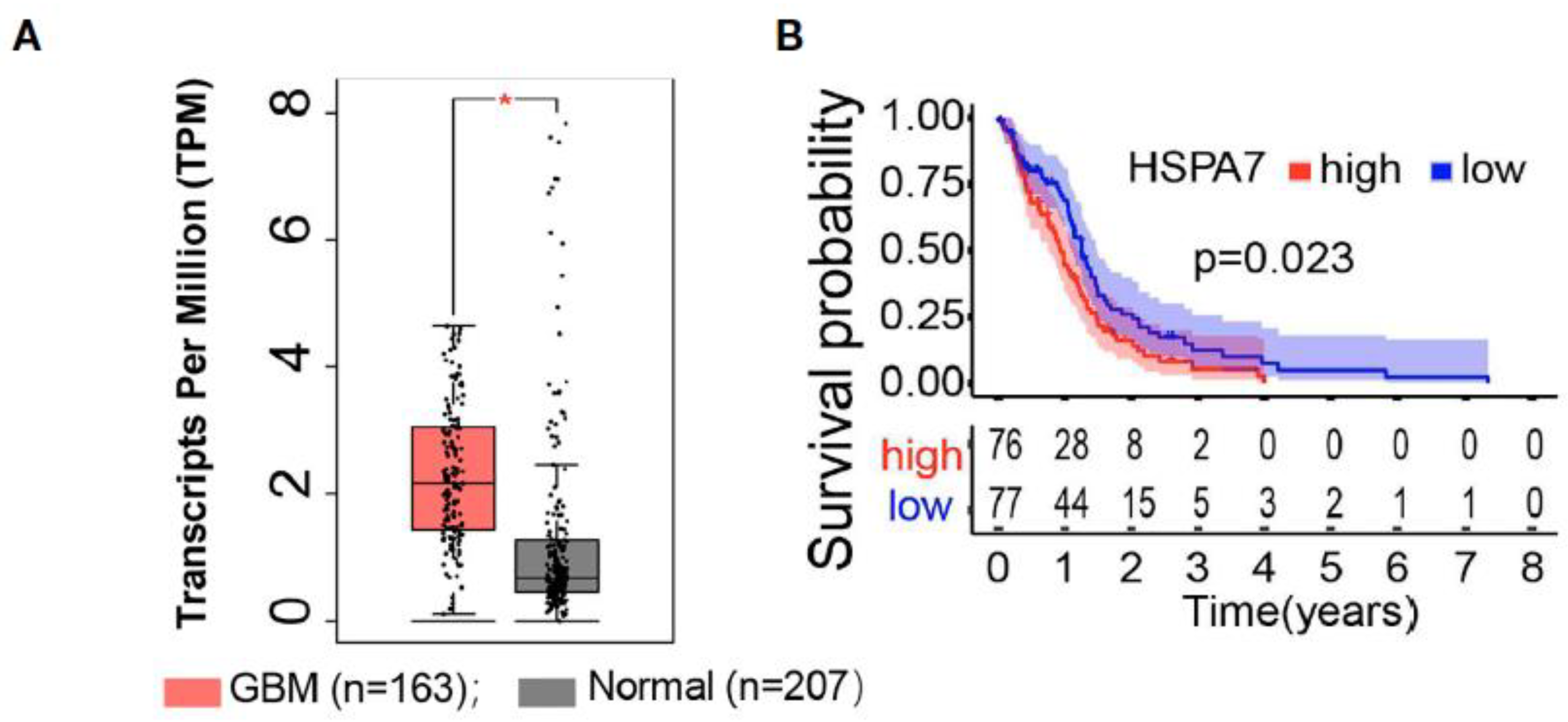
Analysis of HSPA7 (Hsp70) RNA expression showed significantly elevated levels in glioblastoma cell lines (U87MG, U251MG, A172, LN229) compared to normal astrocytes [86]. IDH-mutation types and CpG island methylator phenotype (G-CIMP) tumors had lower levels of HSPA7 expression, and HSPA7 expression was associated with worse survival prognosis after adjusting for other variables such as age, sex, IDH status, MGMT promoter methylation status, G-CIMP status (Figure 7) [86].
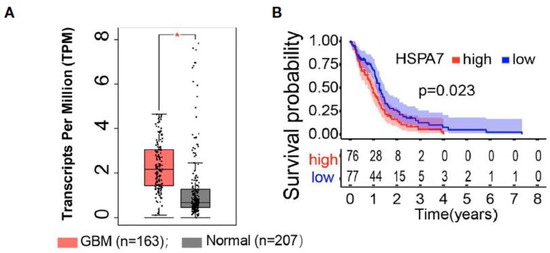
Figure 7.
HSPA7 as a novel prognostic factor in GBM. (A) The GEPIA database showed that HSAP7 was overexpressed significantly in GBM tissues compared with GETx normal brain tissues. (B) Kaplan–Meier survival curves show that HSPA7 is a prognostic risk factor in GBM [86].
Another important protein of the Hsp70 family, GRP78/BiP protein, is significantly overexpressed in glioblastoma, as was confirmed by the immunohistochemistry [87], proteomics [88], western blot analysis of cell lines, and immunohistochemistry of in vivo grown tumors [89], and in vivo in xenografted mice models [90]. It was also found that the expression of GRP78 mRNA is elevated even more in the recurrent GBMs [91]. This effect could be the result of a protective reaction to treatment with temozolomide and radiotherapy that recurrent GBM patients have undergone, as, for example, treatment of glioblastoma cells with cisplatin increased chaperone accumulation by three folds [92].
Expression of GRP78 was not only cytosolic but also on the surface of glioblastoma cells and was highest in GBM (T98G, A172, and U-87 MG) when compared to other tumors, including grade III anaplastic glioma cell line (Hs 683 and U-373 MG). The role of GRP78 in tumor survival was tested with polyclonal N-20 antibodies treatment. Neutralization of the chaperone resulted in the suppression of growth and survival of the tumors. However, the effect was much more noticeable in anaplastic glioma than in the glioblastoma [90].
GRP78 is also predictive of glioblastoma cells’ sensitivity to ubiquitin-like modifier activating enzyme 1 (UBA1) inhibitor TAK-243 [93]. Inhibition of the UBA1 leads to UPR and subsequent cell apoptosis, resulting in longer survival of glioblastoma-bearing mice. Tumors with high levels of GRP78 expression are resistant to treatment with TAK243. The inhibition of UBA1 by the latter causes an increase in GRP78 expression, creating a negative feedback [93]. Overall, GRP78 could be a target for the treatment of high-grade gliomas.
Heat shock cognate protein 70 (Hsc70) is overexpressed in glioma tissues, and its expression increases with the higher grade. Expression was 3-fold higher in grade IV glioma tissues when compared to normal brain tissue. Decreasing the expression of Hcs70 resulted in a slowing of tumor migration and invasion, and its knockdown stimulated apoptosis and reduced cell proliferation [94,95].
Mortalin, another member of the Hsp70 protein family, also has an elevated expression corresponding to increased malignancy in astrocyte tumors and is undetectable in normal brain tissue [96]. The overexpression, however, is not unique to glial tumors but was also detected in other brain tumor types (e.g., meningiomas, neurinomas, pituitary adenomas, and metastases). Thus, an increase in mortalin expression with grade might suggest the involvement of the protein in the malignant transformation [96].
Other brain tumor types were not as thoroughly studied. In medulloblastomas, one study found, among all the studied chaperons, the expression of Hsp70 to be the highest [70]. Another study found a significant increase in Hsp70 expression in large-cell medulloblastoma than in the classic subtype [73]. Meanwhile, immunohistochemical analysis by Rappa et al. did not find an increase in the expression of Hsp70 in the medulloblastomas [77]. None of the studies found an association between the chaperone and patients’ overall survival [70,73].
In meningiomas, expression levels of Hsp70 are elevated [75], regardless of grade, and compared to other tumors, such as neuroepithelial tumors and medulloblastoma, as well as normal brain tissue [77].
2.5. HSP90
Hsp90 is also overexpressed in glial tumors [66,84]. Significantly higher levels of Hsp90 expression were found in glioblastomas IDH-wildtype. Moreover, patients with low expression of EGRF and Hsp90, its chaperone, had worse prognostic outcomes [94]. Zhang et al. also found the association of Hsp90 expression with IDHwt status, particularly in low-grade gliomas (LGG) of grades II and III [97]. They also stratified LGG by survival time, taking 36 months as a cutoff point for the better prognosis group. They found elevated expression of HSP90AB1 to be associated with better survival outcomes. No such analysis was done for WHO grade IV gliomas, defined by them as high-grade gliomas (HGG), which had lower chaperone expression than LGG. They did however find higher expression of HSP90AA1 and HSP90AB1 in the recurrent glioblastomas [97].
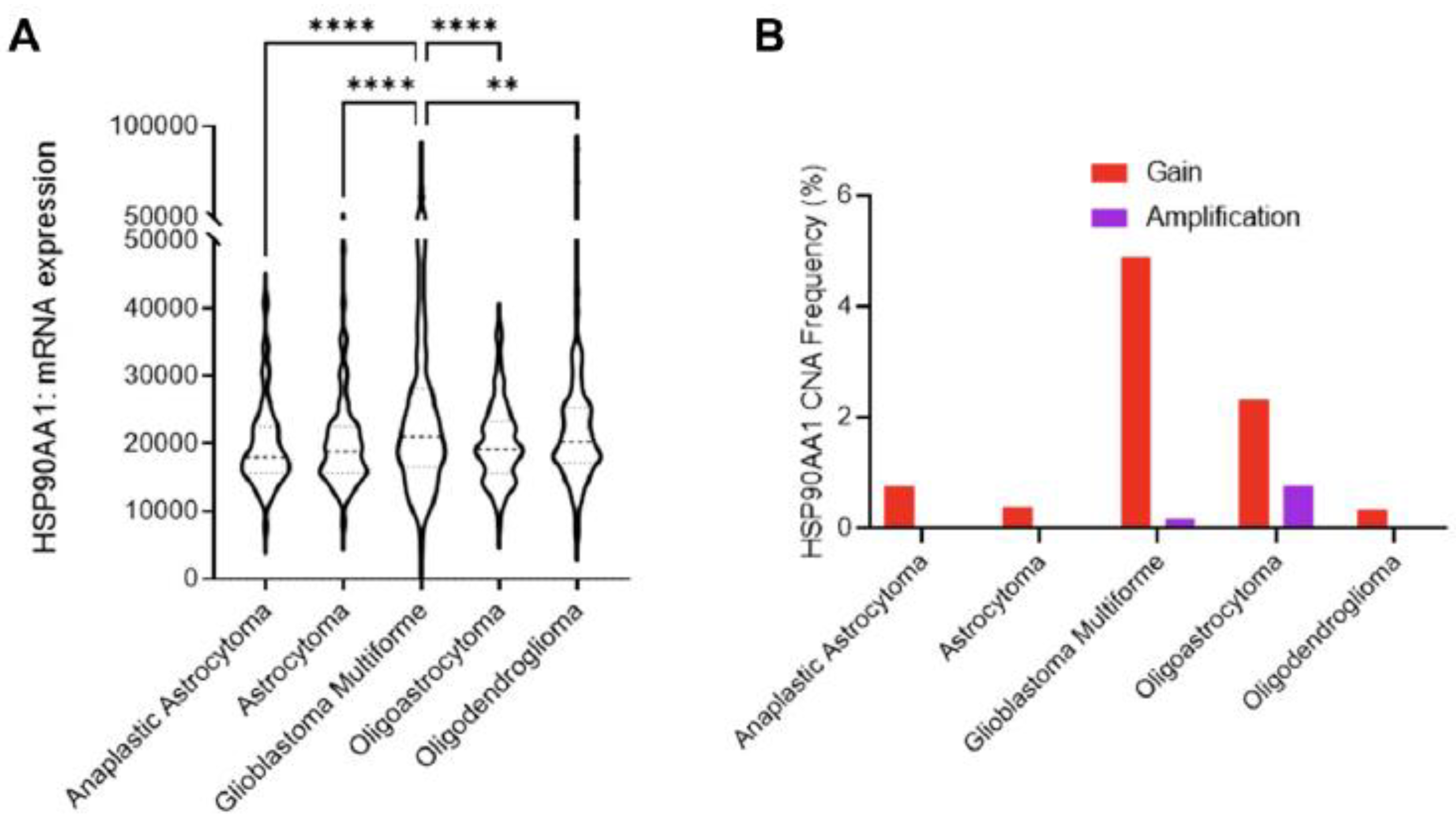
These results are the opposite of the study that employed a bioinformatics approach querying open repositories and pooling data from several clinical studies with over 1500 samples [98]. The study found mRNA expression of Hsp90 to be the highest in glioblastoma when compared to other tumor types, such as astrocytoma, and oligodendrogliomas, and the chaperon expression has the inverse correlation with disease-free survival (Figure 8) [98]. The same association with survival was found by Sun et al., where HSP90B1 increased expression led to a shorter survival time [72].
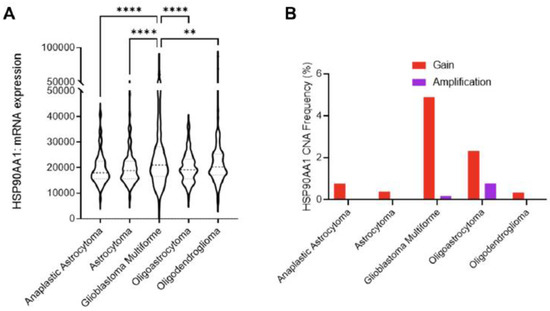
Figure 8.
Role of Hsp90 in glioblastoma multiforme (GBM) across CNS tumors. (A) HSP90AA1 mRNA expression (RNA Seq V2 RSEM) [log2(value + 1)] compared across five clinical studies (cBioPortal). ** p < 0.01, **** p < 0.0001 analyzed by ordinary one-way ANOVA with Tukey’s multiple-comparison test. (B) Copy number alteration (CNA) evaluated across seven clinical studies (cBioPortal) [98].
Despite the opposing findings on the effect of Hsp90 on the survival of patients, experimental studies show that inhibition of the Hsp90 ATPase domain with Shepherdin, a peptidomimetic antagonist of the complex between the chaperone and survivin, resulted in induction of autophagy in glioblastoma cells and suppression of tumor growth in mice model (immunocompromised CB17 SCID/beige female mice), as well as longer survival [99]. Moreover, inhibition of Hsp90 in glioblastoma cancer stem cell-like cells (T98G) increased their sensitization to the radiotherapy [100]. Clearly, inhibition of Hsp90 has shown a positive effect on survival in vivo and in vitro [101,102]. Acetylation of Hsp90 through HDAC enhanced its chaperone function, promoting the stability of HIF-1alpha, which is involved in cell angiogenesis, cell survival, and tumor invasion. Inhibition of HDAC (with LBH589) resulted in reduced cell proliferation, growth, and microvessel density [103].
Another important protein of the Hsp90 family is TRAP1, a mitochondrial protein found to be associated with chemotherapeutic drug resistance [104], and significantly elevated in glioblastoma cell lines when compared to normal brain tissue [99,105]. Higher-grade glioma tumor tissues have more intense TRAP1 staining than lower-grade tumors and have a higher expression (according to immunoblotting). TRAP1 expression is also associated with a low Karnofsky score and worse overall survival of the patients [106]. Knock-out of TRAP1 in glioblastoma cells sensitized them to temozolomide, decreased migration, induced cell apoptosis, and reduced cell proliferation [105].
Just as with other heat shock proteins, studies on Hsp90 and brain tumors other than glial are rare. It has been found that meningiomas had a positive reaction to Hsp90 with 26 to 45% of the samples reacting on immunohistology tests [75,107]. Medulloblastomas were also positive for Hsp90 to a varying degree [75,107]. However, no association with survival was found in the medulloblastomas [70,73].
3. Combination of HSPs with Other Treatment Modalities
Heat shock protein inhibition is emerging as a promising strategy for cancer therapy and demonstrates some promising results [108]. HSPs inhibitors can be effectively used as a monotherapy or in combination with conventional forms of treatment, such as radiotherapy and chemotherapy. A potentially effective treatment outcome has been observed in malignant tumors treated with a combination of HSPs inhibitors with traditional treatment options in malignant tumors [108,109]. Data collected from experimental and clinical studies of HSPs inhibitors and their combination with radio- and chemotherapy, and other treatment modalities in malignant brain tumors are presented in Table 2.

Table 2.
A combination of HSPs inhibitors with anti-tumor therapy in malignant brain tumors.
3.1. Combination of HSP90 Inhibitors with Other Treatment Modalities
HSP90 inhibitors are among the most studied and offer an effective therapeutic approach to the treatment of cancer. HSP90 family inhibitors have been discussed as an anti-cancer agent in the treatment of malignant brain tumors in combination with other treatment modalities. Several clinical trials have been initiated to test the safety, efficacy, and anti-cancer activity of the combinatorial treatment approaches [116].
Several studies have investigated the 17-allylamino-17-demethoxygeldanamycin (17-AAG or Tanespimycin) inhibitor of HSP90 as a therapeutic agent in combination with standard cancer treatment modalities against glioblastoma [110,112,113]. Thus Sauvageot et al. reported that HSP90 inhibitor 17-AAG might synergize with radiation and have a potential therapeutic effect on the treatment of newly diagnosed glioblastoma [112]. In a study, animals treated with 17-AAG survived longer in comparison with those that did not receive the inhibitor. Intriguingly, 50% of all 17-AAG treated animals survived while all the animals from the control group died [112]. The inhibitor was successful not only in combination with the standard treatment options but also showed the additive radiosensitivity effect in the complex treatment with olaparib, the inhibitor of poly (ADP-ribose) polymerase (PARP). 17-AAG enhances the PARP inhibition effect and thereby increases the radiosensitization of human glioma cells [110].
Another study has investigated the combined inhibitory effect of 17-AGG and ZD1839 (Iressa), an inhibitor of an epidermal growth factor receptor (EGFR) tyrosine, on glioma cells growth [113]. The overexpression of EGFR is a genetic alteration in primary glioblastoma and promotes aggressive tumor cell growth. HSP90 stabilizes Akt and oncogenic forms of mutant EGFR. Akt and mutant EGFR contribute to the growth of glioma cells. HSP90 inhibitors geldanamycin (GA) and Radicicol, also known as the first researched HSP90 inhibitors, decreased the expression of EGFRvIII in glioblastoma cells. The study reported that the effect of Iressa was potentiated in combination with the 17-AAG, which presumably reflects the inhibition of some signaling pathways important to cancer cell growth and survival [113].
Despite the positive outcomes of the 17-AAG combination with other treatment modalities, the therapeutic effect of 17-AAG is limited by its poor permeability through the blood-brain barrier (BBB) and high toxicity. Another inhibitor of HSP90, OSU-03012 (or AR-12), also demonstrated a certain therapeutic potency [117]. Additionally, another Hsp90 inhibitor, 17-DMAG (17-Dimethylaminoethylamino-17-demethoxygeldanamycin) (Alvespimycin), was shown to impact the DNA damage response to radiation and also enhanced tumor cell radiosensitivity [118].
Interestingly, the authors reported that the combined application of OSU-03012 and sildenafil further enhanced the therapeutic effect of inhibitor [119].
One of the inhibitors of the HSP90 family that can penetrate the BBB is HSP990. HSP990, in combination with a PI3-kinase inhibitor, BKM120, and radiotherapy, has demonstrated improved clinical outcomes in patients with glial tumors. HSP990 and BKM120, being effective agents against tumor cells on their own, showed individual induce rates of 75% (BKM120) and 59% (HSP990) for glioma cells death, creating an enhancement in radiosensitivity in combination, which induced 89% apoptosis of cancerous cells, resulting in improved clinical outcomes in patients with primary glioblastoma [114]. Future clinical trials should further prove the therapeutic potency of these agents.
Alongside HSP990, which can penetrate the BBB, another novel inhibitor of the 90-kDa heat-shock protein family—NXD30001-- could also easily cross the BBB and accumulate in the brain. The NXD30001 impairs the DNA Damage Response (DDR) after radiotherapy. In vivo study results show that a combination of radiotherapy and NXD30001 inhibitor extended survival from 31 days to 43 days in glioblastoma mice models (female athymic nude mice implanted with T4302 CD133+ cells) when compared to radiotherapy alone [101]. In line with these results, a previous study has described NXD30001 inhibitor as a molecule that can strike glioblastoma at the core of its drivers of tumorigenesis through apoptosis and degradation of HSP90 client proteins and therefore, can be used in the treatment of glioblastoma [120]. Therefore, the NXD30001 inhibitor could be a potential candidate for the treatment of glioblastoma in combination with radiation therapy.
An in vitro study of another Hsp90 inhibitor, NW457, has demonstrated that a combination of the inhibitor in a low dose with radiotherapy considerably interferes with DNA Damage Response (DDR) in cancerous cells [111]. DDR, in turn, is conducive to sensitization towards radiotherapy, creating a stronger effect of radiotherapy.
Other inhibitors of the HSP90 family that are rarely studied are PU-H71 and NVP-HSP990. The combined effect of PU-H71 and heavy ion radiation has been found to enhance the sensitivity in human cancer cells [121]. While NVP-HSP990 sensitized the U251 glioblastoma cell to an ionizing radiation [122].
3.2. Combination of HSP70 Inhibitors with Other Treatment Modalities
The 70 kDa heat shock protein Hsp70 is known to be localized in various cell compartments (including cytosol, nucleus, plasma membrane), and thus several targeting approaches were proposed [25]. Up-to-date several small molecule inhibitors were reported to suppress Hsp70 efficiently. However, the only inhibitor, MKT-077 (1-ethyl-2-[[3-ethyl-5-(3-methylbenzothiazolin-2-yliden)]-4-oxothiazolidin-2-ylidenemethyl] pyridinium chloride) (rhodacyanine dye analog), that entered clinical phase I trial in patients with chemo-resistant solid tumors due to high renal toxicity was halted [123,124]. Other inhibitors (such as flavonoids, 3′-sulfogalactolipids, and 15-deoxyspergulin) demonstrated only a modest anti-tumor therapeutic activity [125,126].
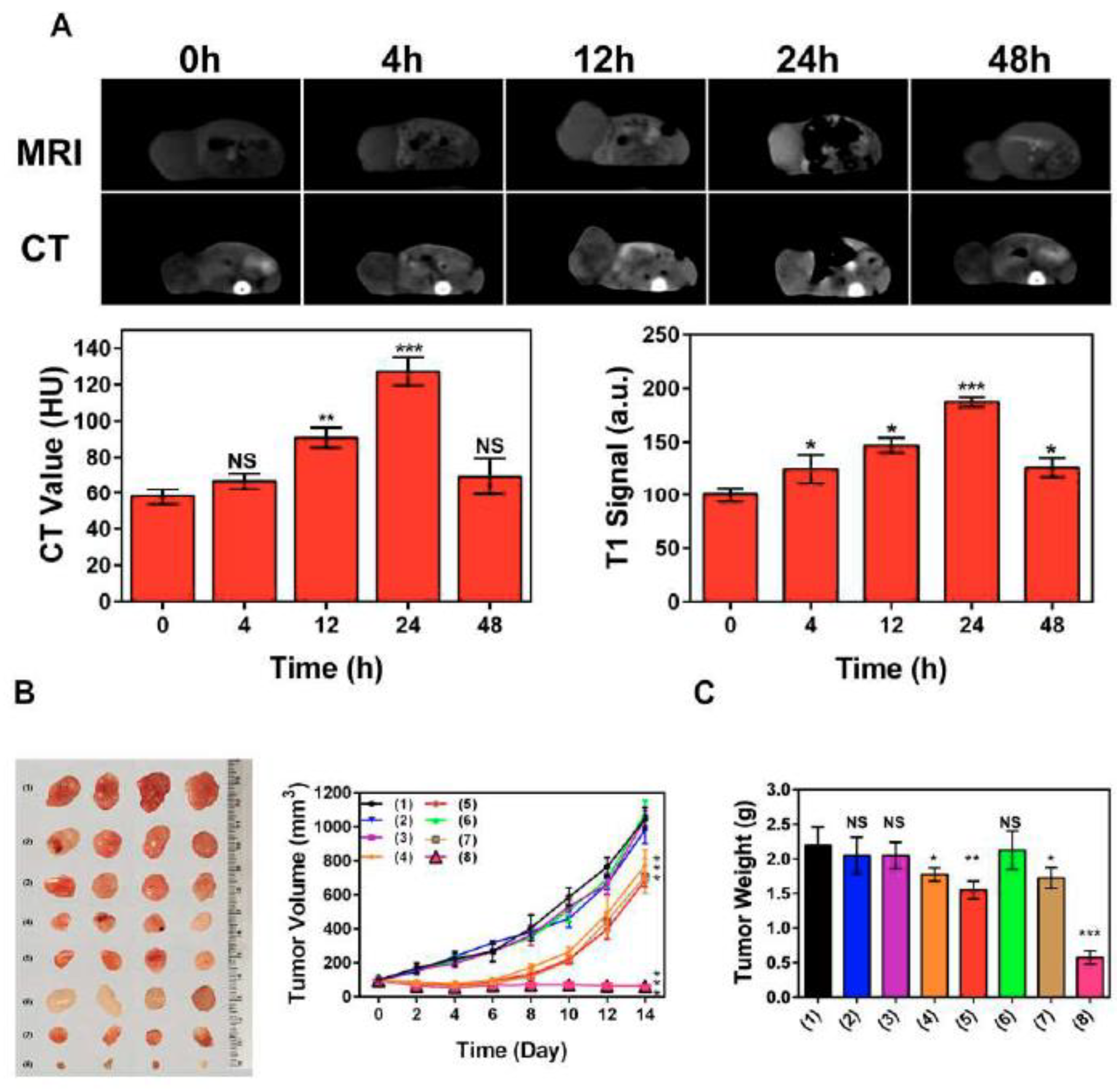
Other Hsp70 inhibitors that showed promising results, particularly when they we employed with other anti-tumor agents, included VER-155008, pifithrin-μ (PES), and MAL3-101 [127,128,129]. Recently, Zhu et al. proposed a nanoplatform (PES-Au@PDA) that could deliver PES, photothermal conversion agent polydopamine (PDA), and radiosensitizer (gold nanospheres, AuNS) for synergistic radiotherapy and photothermal therapy, as well as to visualize the tumor progression employing MRI and/or CT [130]. The preclinical studies demonstrated that PES-Au@PDA particles efficiently induced pro-apoptotic cascades, thus inhibiting tumor progression in human SW1783 glioblastoma-bearing mice (Figure 9) [130].
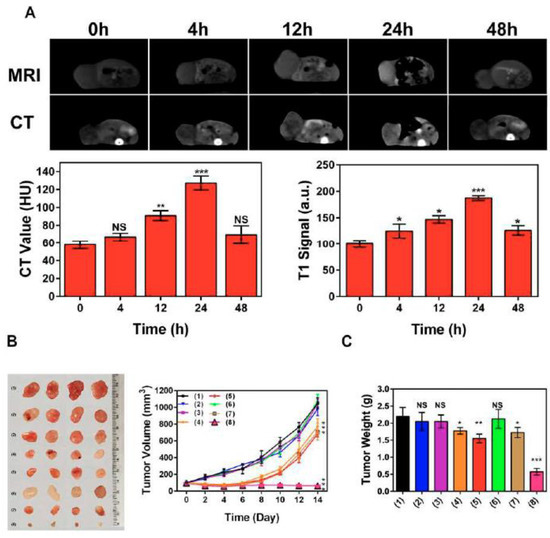
Figure 9.
PES-Au@PDA NPs synergistic GBM treatment and multimodal imaging in vivo. (A) CT and 3 T1-weighted MR images were acquired at the indicated times (0, 4, 12, 24, 48 h) following intravenous injection of 12 nM•kg−1 PES-Au@PDA NPs. (B) Ex vivo tumor images and tumor growth curves following different treatments (n = 4 per group): (1) PBS (control), (2) RT (4 Gy), (3) PES-Au@PDA (12 nM•kg−1), (4) PES-Au@PDA(12 nM•kg−1) + RT (4 Gy), (5) Au@PDA(12 nM•kg−1) + RT (4 Gy), (6) laser (808 nm, 1.0 W•cm−2, 5 min) + RT (4 Gy), (7) Au@PDA(12 nM•kg−1) + laser (808 nm, 1.0 W•cm−2, 5 min) + RT (4 Gy), (8) PES-Au@PDA(12 nM•kg−1) + laser (808 nm, 1.0 W•cm−2, 5 min) + RT (4 Gy). (C) Tumor weights on the 14th day 9 after the treatments as indicated in (B). HU represents Hounsfield units, RT represents radiotherapy. Experiments 10 were repeated three times, and the data are expressed as the mean ± SEM. * p < 0.05, ** p < 0.01, *** p < 0.001. NS—not significant [130].
Another agent, cannabinoid agonist WIN55-212-2, also demonstrated efficacy in vitro (human GAMG and U251 glioblastoma cell lines) and in vivo by altering the expression of p53, cathepsin D and Hsp70 [131]. Another inhibitor, AEAC (N-amino-ethylamino derivate of colchicine), that suppressed substrate-binding and refolding activities of Hsp70 was shown to accumulate in C6 glioma in vivo, further potentiating the effect of doxorubicin that resulted in the reduction of tumor growth rate and prolongation of animal survival by 12 ± 2.2 days [132].
Tumor-specific expression of the plasma membrane-bound Hsp70 provides a possibility to apply, apart from small molecular inhibitors, other targeting agents, including aptamers, peptides, anticalins, and antibodies [25,133,134]. Thus, by the group of Prof. Carmen Garrido (University of Burgundy, Dijon, France), two peptide aptamers—A8 and A17—were designed to bind substrate-binding (SBD) and nucleotide-binding (NBD) domains of Hsp70, respectively. These aptamers specifically inhibited the Hsp70 chaperone activity, thereby promoting the tumor cells’ sensitivity to therapeutic agents that resulted in the regression of subcutaneous tumors in animals [133]. Furthermore, inhibition of Hsp70 by A17 aptamer potentiated the radiosensitizing effects of Hsp90 inhibitor NVP-AUY922 [135]. Application of anti-Hsp70 antibodies conjugated with superparamagnetic iron oxide nanoparticles (SPIONs) also demonstrated high diagnostic potency in the orthotopic C6 model in rats (when MRI was applied) [136]. Furthermore, when nanoparticles were decorated with serine protease granzyme B, which is known to specifically bind membrane-bound Hsp70 [137], magnetic particles could accumulate in the human U87 glioma tissues and induce the apoptotic cell death [138]. The combination of GrB-SPIONs with a single dose (10 Gy) radiotherapy further increased the therapeutic anti-glioma potency of nanoparticles [138].
3.3. Combination of HSP40 Inhibitors with Other Treatment Modalities
As was shown previously, HSP47 (encoded by SERPINH1 gene) is overexpressed in primary GBM cells where it functions as a chaperone protein for collagen-promoting tumor cells invasion, angiogenesis, and stem-cell-like properties via the TGF-β pathway [139,140]. Subsequent blockade of this pathway abrogated the tumor-supportive effects of HSP47 [140]. Due to the limited knowledge of the involvement of HSP40 in GBM tumorigenesis, there are few studies demonstrating the therapeutic potency of its inhibition. One of the molecules reported to bind Hsp40 was phenoxy-N-arylcetamides (IC50 = 130 nM) that also disrupted the Hsp70-mediated luciferase refolding activity [141]. Other inhibitors were also reported, including benzylidene lactam compound (KNK437, N-formyl-3,4-methylenedioxy-benzylidene-γbutyrolactam) [142], natural compound-derived plumbagin derivate (PLIHZ, plumbagin-isoniazid analog) [143], and bioflavonoid quercetin (3,3′,4′,5,7-pentahydroxyflavon) [144]. Presumably, future preclinical trials will elucidate the therapeutic efficacy of HSP40 inhibitors in neuro-oncology.
3.4. Combination of HSP27 Inhibitors with Other Treatment Modalities
The member of the small HSPs family is HSP27 (HspB1), and its expression is increased in glioma cells. It has been reported that using resveratrol, a natural compound with antitumor effects, against glioblastoma, in combination with temozolomide might increase the sensitivity of the cancerous cells through the inhibition of HSP27 [115]. A study has demonstrated the potential of Hsp27 siRNA and rosmarinic acid combination applied to human glioma cells as an effective Hsp27 inhibitor and inducer of apoptosis [145].
It was demonstrated that androgen receptor (AR) mutations promote cancer development in glioblastoma, and silencing of AR in glial cells had a potential antitumor effect [60,146]. Thus Li et al. inhibited AR expression in GBM cells by suppressing HSP27 using Compound I that resulted in the suppression of tumor cell growth with IC50 values of 5 nM. Subsequent in vivo experiments in the GBM xenograft model demonstrated an acceptable toxicity profile of the compound and therapeutic potency [61]. Furthermore, it should be noted that treatment of GBM cells with temozolomide (TMZ) increases the expression of HSPB1 (HSP27), contributing to the chemoresistance [61,147]. To overcome this challenge, Rajesh et al. applied Friend leukemia integration 1 (Fli-1) as a mediator of HSPB1 and suggested that via the regulation of Fli-1, one could affect the resistance of glioblastoma to TMZ [148]. The authors reported that lumefantrine (FDA-approved anti-malarial medicine which can pass BBB and inhibit Fli-1) has a therapeutic potential against radio- and TMZ-resistant GBM cells [148].
4. Conclusions
Molecular chaperones, due to their important role in the physiological processes in cells, are highly expressed in brain tumors, and the level of HSPs expression strongly correlates with a malignancy grade (higher in GBM), invasive potential, as well as resistance to radiochemotherapy. For certain HSPs representatives (i.e., HSP10, HSPB1, DNAJC10, HSPA7, HSP90), a direct correlation between the protein level expression (based on IHC analysis) and poor overall survival prognosis for patients with glial tumors was identified. This indicates the prognostic values of these markers that could be included in the future included into the diagnostic panel in the neuropathological examination of a tumor sample. One of the limitations is an absence of standardized protocols for HSPs detection when mostly IHC analysis (for evaluation of HSPs cytosolic and nuclear expression) and flow cytometry (for detection of HSPs plasma-membrane bound forms) are employed. Moreover, there is still no agreement between the researchers on whether to separately assess the cytosolic and nuclear expression of HSPs on paraffin IHC sections and their prognostic and diagnostic values. Most of the studies report HSPs expression without such a distinction. However, as was previously shown, upon various stress conditions, HPSs do migrate into the cell nucleus (although the list is known about their function in the nucleus) [149,150]. Presumably, in tumor sections, these two patterns of HSPs expression should be evaluated independently.
Taking into consideration the importance of HSPs in the maintenance of proteostasis, signal transduction, regulation of apoptosis, and autophagy inhibition of chaperones might have therapeutic potential. However, most of the reported studies were performed with other malignancies where the inhibitors demonstrated a therapeutic potential either as a monotherapeutic agent or in combination with other treatment modalities. This gives hope for future studies that these inhibitors could also be beneficial when used for targeting brain tumors.
Author Contributions
Conceptualization, S.A. and M.S.; methodology, A.B., K.M., T.B., A.D., S.A. and M.S.; validation, S.A. and M.S.; investigation, A.B., K.M., T.B., A.D., S.A. and M.S.; resources, A.B., K.M., T.B., A.D., S.A. and M.S.; data curation, A.D., S.A. and M.S.; writing—original draft preparation, A.B., K.M., T.B., A.D., S.A. and M.S.; writing—review and editing, A.B., K.M., T.B., A.D., S.A. and M.S.; visualization, A.B., K.M., T.B., A.D., S.A. and M.S.; supervision, S.A. and M.S.; project administration, A.D., S.A. and M.S.; funding acquisition, A.D., S.A. and M.S. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the program “Development of a program of molecular-cytogenetic studies and the creation of a biobank of tumors of the central nervous system” (BR10965225) (Ministry of Education and Science of the Republic of Kazakhstan. MS was financially supported by the Ministry of Science and Higher Education of the Russian Federation (Agreement No. 075-15-2022-301).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Boele, F.W.; Klein, M.; Reijneveld, J.C.; Verdonck-de Leeuw, I.M.; Heimans, J.J. Symptom management and quality of life in glioma patients. CNS Oncol. 2014, 3, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A summary. Neuro Oncol. 2021, 23, 1231–1251. [Google Scholar] [CrossRef] [PubMed]
- Stupp, R.; Hegi, M.E.; Mason, W.P.; van den Bent, M.J.; Taphoorn, M.J.; Janzer, R.C.; Ludwin, S.K.; Allgeier, A.; Fisher, B.; Belanger, K.; et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009, 10, 459–466. [Google Scholar] [CrossRef]
- Stupp, R.; Taillibert, S.; Kanner, A.; Read, W.; Steinberg, D.; Lhermitte, B.; Toms, S.; Idbaih, A.; Ahluwalia, M.S.; Fink, K.; et al. Effect of Tumor-Treating Fields Plus Maintenance Temozolomide vs Maintenance Temozolomide Alone on Survival in Patients with Glioblastoma: A Randomized Clinical Trial. JAMA 2017, 318, 2306–2316. [Google Scholar] [CrossRef] [PubMed]
- Śledzińska, P.; Bebyn, M.; Furtak, J.; Koper, A.; Koper, K. Current and promising treatment strategies in glioma. Rev. Neurosci. 2022. [Google Scholar] [CrossRef]
- Liu, H.; Qiu, W.; Sun, T.; Wang, L.; Du, C.; Hu, Y.; Liu, W.; Feng, F.; Chen, Y.; Sun, H. Therapeutic strategies of glioblastoma (GBM): The current advances in the molecular targets and bioactive small molecule compounds. Acta Pharm. Sin. B 2022, 12, 1781–1804. [Google Scholar] [CrossRef]
- Van Ommeren, R.; Staudt, M.D.; Xu, H.; Hebb, M.O. Advances in HSP27 and HSP90-targeting strategies for glioblastoma. J. Neurooncol. 2016, 127, 209–219. [Google Scholar] [CrossRef]
- Iglesia, R.P.; Fernandes, C.F.L.; Coelho, B.P.; Prado, M.B.; Melo Escobar, M.I.; Almeida, G.; Lopes, M.H. Heat Shock Proteins in Glioblastoma Biology: Where Do We Stand? Int. J. Mol. Sci. 2019, 20, 5794. [Google Scholar] [CrossRef]
- Hu, C.; Yang, J.; Qi, Z.; Wu, H.; Wang, B.; Zou, F.; Mei, H.; Liu, J.; Wang, W.; Liu, Q. Heat shock proteins: Biological functions, pathological roles, and therapeutic opportunities. MedComm 2022, 3, e161. [Google Scholar] [CrossRef]
- Yang, J.; Carra, S.; Zhu, W.G.; Kampinga, H.H. The regulation of the autophagic network and its implications for human disease. Int. J. Biol. Sci. 2013, 9, 1121–1133. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Liu, T.; Rios, Z.; Mei, Q.; Lin, X.; Cao, S. Heat Shock Proteins and Cancer. Trends Pharmacol. Sci. 2017, 38, 226–256. [Google Scholar] [CrossRef] [PubMed]
- Lang, B.J.; Prince, T.L.; Okusha, Y.; Bunch, H.; Calderwood, S.K. Heat shock proteins in cell signaling and cancer. Biochim. Biophys. Acta Mol. Cell Res. 2022, 1869, 119187. [Google Scholar] [CrossRef] [PubMed]
- Kampinga, H.H.; Hageman, J.; Vos, M.J.; Kubota, H.; Tanguay, R.M.; Bruford, E.A.; Cheetham, M.E.; Chen, B.; Hightower, L.E. Guidelines for the nomenclature of the human heat shock proteins. Cell Stress Chaperones 2009, 14, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Sarto, C.; Binz, P.A.; Mocarelli, P. Heat shock proteins in human cancer. Electrophoresis 2000, 21, 1218–1226. [Google Scholar] [CrossRef]
- Hasan, A.; Rizvi, S.F.; Parveen, S.; Mir, S.S. Molecular chaperones in DNA repair mechanisms: Role in genomic instability and proteostasis in cancer. Life Sci. 2022, 306, 120852. [Google Scholar] [CrossRef]
- Stice, J.P.; Knowlton, A.A. Estrogen, NFkappaB, and the heat shock response. Mol. Med. 2008, 14, 517–527. [Google Scholar] [CrossRef]
- Frydman, J.; Nimmesgern, E.; Ohtsuka, K.; Hartl, F.U. Folding of nascent polypeptide chains in a high molecular mass assembly with molecular chaperones. Nature 1994, 370, 111–117. [Google Scholar] [CrossRef]
- Hernández, M.P.; Chadli, A.; Toft, D.O. HSP40 binding is the first step in the HSP90 chaperoning pathway for the progesterone receptor. J. Biol. Chem. 2002, 277, 11873–11881. [Google Scholar] [CrossRef]
- Mitra, A.; Shevde, L.A.; Samant, R.S. Multi-faceted role of HSP40 in cancer. Clin. Exp. Metastasis 2009, 26, 559–567. [Google Scholar] [CrossRef]
- Qiu, X.B.; Shao, Y.M.; Miao, S.; Wang, L. The diversity of the DnaJ/Hsp40 family, the crucial partners for Hsp70 chaperones. Cell. Mol. Life Sci. 2006, 63, 2560–2570. [Google Scholar] [CrossRef]
- Hernández, M.P.; Sullivan, W.P.; Toft, D.O. The assembly and intermolecular properties of the hsp70-Hop-hsp90 molecular chaperone complex. J. Biol. Chem. 2002, 277, 38294–38304. [Google Scholar] [CrossRef] [PubMed]
- Pratt, W.B.; Toft, D.O. Regulation of signaling protein function and trafficking by the hsp90/hsp70-based chaperone machinery. Exp. Biol. Med. 2003, 228, 111–133. [Google Scholar] [CrossRef] [PubMed]
- Edkins, A.L.; Boshoff, A. General Structural and Functional Features of Molecular Chaperones. Adv. Exp. Med. Biol. 2021, 1340, 11–73. [Google Scholar] [CrossRef] [PubMed]
- Shevtsov, M.; Balogi, Z.; Khachatryan, W.; Gao, H.; Vígh, L.; Multhoff, G. Membrane-Associated Heat Shock Proteins in Oncology: From Basic Research to New Theranostic Targets. Cells 2020, 9, 1263. [Google Scholar] [CrossRef] [PubMed]
- Stangl, S.; Tontcheva, N.; Sievert, W.; Shevtsov, M.; Niu, M.; Schmid, T.E.; Pigorsch, S.; Combs, S.E.; Haller, B.; Balermpas, P.; et al. Heat shock protein 70 and tumor-infiltrating NK cells as prognostic indicators for patients with squamous cell carcinoma of the head and neck after radiochemotherapy: A multicentre retrospective study of the German Cancer Consortium Radiation Oncology Group (DKTK-ROG). Int. J. Cancer 2018, 142, 1911–1925. [Google Scholar] [CrossRef] [PubMed]
- Tustumi, F.; Agareno, G.A.; Galletti, R.P.; da Silva, R.B.R.; Quintas, J.G.; Sesconetto, L.A.; Szor, D.J.; Wolosker, N. The Role of the Heat-Shock Proteins in Esophagogastric Cancer. Cells 2022, 11, 2664. [Google Scholar] [CrossRef]
- Javid, H.; Hashemian, P.; Yazdani, S.; Sharbaf Mashhad, A.; Karimi-Shahri, M. The role of heat shock proteins in metastatic colorectal cancer: A review. J. Cell. Biochem. 2022. [Google Scholar] [CrossRef]
- Kliková, K.; Pilchova, I.; Stefanikova, A.; Hatok, J.; Dobrota, D.; Racay, P. The Role of Heat Shock Proteins in Leukemia. Klin Onkol 2016, 29, 29–38. [Google Scholar] [CrossRef]
- Xue, N.; Du, T.; Lai, F.; Jin, J.; Ji, M.; Chen, X. Secreted HSP90α-LRP1 Signaling Promotes Tumor Metastasis and Chemoresistance in Pancreatic Cancer. Int. J. Mol. Sci. 2022, 23, 5532. [Google Scholar] [CrossRef]
- Abi Zamer, B.; El-Huneidi, W.; Eladl, M.A.; Muhammad, J.S. Ins and Outs of Heat Shock Proteins in Colorectal Carcinoma: Its Role in Carcinogenesis and Therapeutic Perspectives. Cells 2021, 10, 2862. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Xu, C.; Li, G.; Lv, P.; Gu, J. Incomplete radiofrequency ablation induced chemoresistance by up-regulating heat shock protein 70 in hepatocellular carcinoma. Exp. Cell Res. 2021, 409, 112910. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.; Li, Y.; Tan, X.; Fu, L. Small Heat Shock Proteins in Cancers: Functions and Therapeutic Potential for Cancer Therapy. Int. J. Mol. Sci. 2020, 21, 6611. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Min, H.Y.; Yong, Y.S.; Ann, J.; Nguyen, C.T.; La, M.T.; Hyun, S.Y.; Le, H.T.; Kim, H.; Kwon, H.; et al. A novel C-terminal heat shock protein 90 inhibitor that overcomes STAT3-Wnt-β-catenin signaling-mediated drug resistance and adverse effects. Theranostics 2022, 12, 105–125. [Google Scholar] [CrossRef] [PubMed]
- Shan, R.; Liu, N.; Yan, Y.; Liu, B. Apoptosis, autophagy and atherosclerosis: Relationships and the role of Hsp27. Pharmacol. Res. 2021, 166, 105169. [Google Scholar] [CrossRef]
- Bruey, J.M.; Ducasse, C.; Bonniaud, P.; Ravagnan, L.; Susin, S.A.; Diaz-Latoud, C.; Gurbuxani, S.; Arrigo, A.P.; Kroemer, G.; Solary, E.; et al. Hsp27 negatively regulates cell death by interacting with cytochrome c. Nat. Cell Biol. 2000, 2, 645–652. [Google Scholar] [CrossRef]
- Joly, A.L.; Wettstein, G.; Mignot, G.; Ghiringhelli, F.; Garrido, C. Dual role of heat shock proteins as regulators of apoptosis and innate immunity. J. Innate Immun. 2010, 2, 238–247. [Google Scholar] [CrossRef]
- Pandey, P.; Saleh, A.; Nakazawa, A.; Kumar, S.; Srinivasula, S.M.; Kumar, V.; Weichselbaum, R.; Nalin, C.; Alnemri, E.S.; Kufe, D.; et al. Negative regulation of cytochrome c-mediated oligomerization of Apaf-1 and activation of procaspase-9 by heat shock protein 90. EMBO J. 2000, 19, 4310–4322. [Google Scholar] [CrossRef]
- Sanderson, S.; Valenti, M.; Gowan, S.; Patterson, L.; Ahmad, Z.; Workman, P.; Eccles, S.A. Benzoquinone ansamycin heat shock protein 90 inhibitors modulate multiple functions required for tumor angiogenesis. Mol. Cancer Ther. 2006, 5, 522–532. [Google Scholar] [CrossRef]
- Kaur, G.; Belotti, D.; Burger, A.M.; Fisher-Nielson, K.; Borsotti, P.; Riccardi, E.; Thillainathan, J.; Hollingshead, M.; Sausville, E.A.; Giavazzi, R. Antiangiogenic properties of 17-(dimethylaminoethylamino)-17-demethoxygeldanamycin: An orally bioavailable heat shock protein 90 modulator. Clin. Cancer Res. 2004, 10, 4813–4821. [Google Scholar] [CrossRef] [PubMed]
- Lang, S.A.; Klein, D.; Moser, C.; Gaumann, A.; Glockzin, G.; Dahlke, M.H.; Dietmaier, W.; Bolder, U.; Schlitt, H.J.; Geissler, E.K.; et al. Inhibition of heat shock protein 90 impairs epidermal growth factor-mediated signaling in gastric cancer cells and reduces tumor growth and vascularization in vivo. Mol. Cancer Ther. 2007, 6, 1123–1132. [Google Scholar] [CrossRef] [PubMed]
- Qi, S.; Deng, S.; Lian, Z.; Yu, K. Novel Drugs with High Efficacy against Tumor Angiogenesis. Int. J. Mol. Sci. 2022, 23, 6934. [Google Scholar] [CrossRef] [PubMed]
- Staufer, K.; Stoeltzing, O. Implication of heat shock protein 90 (HSP90) in tumor angiogenesis: A molecular target for anti-angiogenic therapy? Curr. Cancer Drug Targets 2010, 10, 890–897. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.X.; Lawrence, M.L.; Cunningham, G.; Christman, B.W.; Meyrick, B. HSP90 and Akt modulate Ang-1-induced angiogenesis via NO in coronary artery endothelium. J. Appl. Physiol. 2004, 96, 612–620. [Google Scholar] [CrossRef] [PubMed]
- Shevtsov, M.; Multhoff, G. Heat Shock Protein-Peptide and HSP-Based Immunotherapies for the Treatment of Cancer. Front. Immunol. 2016, 7, 171. [Google Scholar] [CrossRef] [PubMed]
- Taha, E.A.; Ono, K.; Eguchi, T. Roles of Extracellular HSPs as Biomarkers in Immune Surveillance and Immune Evasion. Int. J. Mol. Sci. 2019, 20, 4588. [Google Scholar] [CrossRef]
- Srivastava, P.K.; Menoret, A.; Basu, S.; Binder, R.J.; McQuade, K.L. Heat shock proteins come of age: Primitive functions acquire new roles in an adaptive world. Immunity 1998, 8, 657–665. [Google Scholar] [CrossRef]
- Strbo, N.; Garcia-Soto, A.; Schreiber, T.H.; Podack, E.R. Secreted heat shock protein gp96-Ig: Next-generation vaccines for cancer and infectious diseases. Immunol. Res. 2013, 57, 311–325. [Google Scholar] [CrossRef]
- Baldin, A.V.; Zamyatnin, A.A., Jr.; Bazhin, A.V.; Xu, W.H.; Savvateeva, L.V. Advances in the Development of Anticancer HSP-based Vaccines. Curr. Med. Chem. 2019, 26, 427–445. [Google Scholar] [CrossRef]
- Kelly, M.; McNeel, D.; Fisch, P.; Malkovsky, M. Immunological considerations underlying heat shock protein-mediated cancer vaccine strategies. Immunol. Lett. 2018, 193, 1–10. [Google Scholar] [CrossRef]
- Assimakopoulou, M.; Sotiropoulou-Bonikou, G.; Maraziotis, T.; Varakis, I. Prognostic significance of Hsp-27 in astrocytic brain tumors: An immunohistochemical study. Anticancer Res. 1997, 17, 2677–2682. [Google Scholar] [PubMed]
- Khalid, H.; Tsutsumi, K.; Yamashita, H.; Kishikawa, M.; Yasunaga, A.; Shibata, S. Expression of the small heat shock protein (hsp) 27 in human astrocytomas correlates with histologic grades and tumor growth fractions. Cell. Mol. Neurobiol. 1995, 15, 257–268. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.Q.; Wang, P.F.; Zhang, H.P.; Cheng, Z.J.; Li, S.W.; He, J.; Zhang, Y.; Hao, J.J.; Wang, M.R.; Yan, C.X.; et al. Phosphorylated Hsp27 is mutually exclusive with ATRX loss and the IDH1(R132H) mutation and may predict better prognosis among glioblastomas without the IDH1 mutation and ATRX loss. J. Clin. Pathol. 2018, 71, 702–707. [Google Scholar] [CrossRef] [PubMed]
- Gimenez, M.; Marie, S.K.; Oba-Shinjo, S.; Uno, M.; Izumi, C.; Oliveira, J.B.; Rosa, J.C. Quantitative proteomic analysis shows differentially expressed HSPB1 in glioblastoma as a discriminating short from long survival factor and NOVA1 as a differentiation factor between low-grade astrocytoma and oligodendroglioma. BMC Cancer 2015, 15, 481. [Google Scholar] [CrossRef] [PubMed]
- Shen, G.; Liang, S.; Xu, Z.; Zhou, L.; Xiao, S.; Xia, X.; Li, R.; Liao, Y.; You, C.; Wei, Y. Downregulated expression of HSP27 in human low-grade glioma tissues discovered by a quantitative proteomic analysis. Proteome Sci. 2010, 8, 17. [Google Scholar] [CrossRef] [PubMed]
- Hayes, D.; Napoli, V.; Mazurkie, A.; Stafford, W.F.; Graceffa, P. Phosphorylation dependence of hsp27 multimeric size and molecular chaperone function. J. Biol. Chem. 2009, 284, 18801–18807. [Google Scholar] [CrossRef] [PubMed]
- Koteiche, H.A.; McHaourab, H.S. Mechanism of chaperone function in small heat-shock proteins. Phosphorylation-induced activation of two-mode binding in alphaB-crystallin. J. Biol. Chem. 2003, 278, 10361–10367. [Google Scholar] [CrossRef]
- Assimakopoulou, M.; Varakis, J. AP-1 and heat shock protein 27 expression in human astrocytomas. J. Cancer Res. Clin. Oncol. 2001, 127, 727–732. [Google Scholar] [CrossRef]
- Cai, M.; Chen, N. The Roles of IRF-8 in Regulating IL-9-Mediated Immunologic Mechanisms in the Development of DLBCL: A State-of-the-Art Literature Review. Front. Oncol. 2022, 12, 817069. [Google Scholar] [CrossRef]
- Zalcman, N.; Canello, T.; Ovadia, H.; Charbit, H.; Zelikovitch, B.; Mordechai, A.; Fellig, Y.; Rabani, S.; Shahar, T.; Lossos, A.; et al. Androgen receptor: A potential therapeutic target for glioblastoma. Oncotarget 2018, 9, 19980–19993. [Google Scholar] [CrossRef]
- Li, Y.; Orahoske, C.M.; Geldenhuys, W.J.; Bhattarai, A.; Sabbagh, A.; Bobba, V.; Salem, F.M.; Zhang, W.; Shukla, G.C.; Lathia, J.D.; et al. Small-Molecule HSP27 Inhibitor Abolishes Androgen Receptors in Glioblastoma. J. Med. Chem. 2021, 64, 1570–1583. [Google Scholar] [CrossRef] [PubMed]
- Odreman, F.; Vindigni, M.; Gonzales, M.L.; Niccolini, B.; Candiano, G.; Zanotti, B.; Skrap, M.; Pizzolitto, S.; Stanta, G.; Vindigni, A. Proteomic studies on low- and high-grade human brain astrocytomas. J. Proteome Res. 2005, 4, 698–708. [Google Scholar] [CrossRef] [PubMed]
- Hitotsumatsu, T.; Iwaki, T.; Fukui, M.; Tateishi, J. Distinctive immunohistochemical profiles of small heat shock proteins (heat shock protein 27 and alpha B-crystallin) in human brain tumors. Cancer 1996, 77, 352–361. [Google Scholar] [CrossRef]
- Aoyama, A.; Steiger, R.H.; Fröhli, E.; Schäfer, R.; von Deimling, A.; Wiestler, O.D.; Klemenz, R. Expression of alpha B-crystallin in human brain tumors. Int. J. Cancer 1993, 55, 760–764. [Google Scholar] [CrossRef] [PubMed]
- Iwaki, T.; Iwaki, A.; Miyazono, M.; Goldman, J.E. Preferential expression of alpha B-crystallin in astrocytic elements of neuroectodermal tumors. Cancer 1991, 68, 2230–2240. [Google Scholar] [CrossRef]
- Hermisson, M.; Strik, H.; Rieger, J.; Dichgans, J.; Meyermann, R.; Weller, M. Expression and functional activity of heat shock proteins in human glioblastoma multiforme. Neurology 2000, 54, 1357–1365. [Google Scholar] [CrossRef] [PubMed]
- Goplen, D.; Bougnaud, S.; Rajcevic, U.; Bøe, S.O.; Skaftnesmo, K.O.; Voges, J.; Enger, P.; Wang, J.; Tysnes, B.B.; Laerum, O.D.; et al. αB-crystallin is elevated in highly infiltrative apoptosis-resistant glioblastoma cells. Am. J. Pathol. 2010, 177, 1618–1628. [Google Scholar] [CrossRef]
- Fan, W.; Fan, S.S.; Feng, J.; Xiao, D.; Fan, S.; Luo, J. Elevated expression of HSP10 protein inhibits apoptosis and associates with poor prognosis of astrocytoma. PLoS ONE 2017, 12, e0185563. [Google Scholar] [CrossRef]
- Kato, M.; Herz, F.; Kato, S.; Hirano, A. Expression of stress-response (heat-shock) protein 27 in human brain tumors: An immunohistochemical study. Acta Neuropathol. 1992, 83, 420–422. [Google Scholar] [CrossRef]
- Hauser, P.; Hanzély, Z.; Jakab, Z.; Oláh, L.; Szabó, E.; Jeney, A.; Schuler, D.; Fekete, G.; Bognár, L.; Garami, M. Expression and prognostic examination of heat shock proteins (HSP 27, HSP 70, and HSP 90) in medulloblastoma. J. Pediatr. Hematol. Oncol. 2006, 28, 461–466. [Google Scholar] [CrossRef]
- Liu, F.; Tu, Z.; Liu, J.; Long, X.; Xiao, B.; Fang, H.; Huang, K.; Zhu, X. DNAJC10 correlates with tumor immune characteristics and predicts the prognosis of glioma patients. Biosci. Rep. 2022, 42, BSR20212378. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Zou, H.Y.; Cai, X.Y.; Zhou, H.F.; Li, X.Q.; Xie, W.J.; Xie, W.M.; Du, Z.P.; Xu, L.Y.; Li, E.M.; et al. Network Analyses of the Differential Expression of Heat Shock Proteins in Glioma. DNA Cell Biol. 2020, 39, 1228–1242. [Google Scholar] [CrossRef] [PubMed]
- Alexiou, G.A.; Vartholomatos, G.; Stefanaki, K.; Patereli, A.; Dova, L.; Karamoutsios, A.; Lallas, G.; Sfakianos, G.; Moschovi, M.; Prodromou, N. Expression of heat shock proteins in medulloblastoma. J. Neurosurg. Pediatr. 2013, 12, 452–457. [Google Scholar] [CrossRef] [PubMed]
- Lindsey, J.C.; Lusher, M.E.; Strathdee, G.; Brown, R.; Gilbertson, R.J.; Bailey, S.; Ellison, D.W.; Clifford, S.C. Epigenetic inactivation of MCJ (DNAJD1) in malignant paediatric brain tumours. Int. J. Cancer 2006, 118, 346–352. [Google Scholar] [CrossRef] [PubMed]
- Kato, S.; Kato, M.; Hirano, A.; Takikawa, M.; Ohama, E. The immunohistochemical expression of stress-response protein (srp) 60 in human brain tumours: Relationship of srp 60 to the other five srps, proliferating cell nuclear antigen and p53 protein. Histol. Histopathol. 2001, 16, 809–820. [Google Scholar] [CrossRef] [PubMed]
- Khalil, A.A. Biomarker discovery: A proteomic approach for brain cancer profiling. Cancer Sci. 2007, 98, 201–213. [Google Scholar] [CrossRef]
- Rappa, F.; Unti, E.; Baiamonte, P.; Cappello, F.; Scibetta, N. Different immunohistochemical levels of Hsp60 and Hsp70 in a subset of brain tumors and putative role of Hsp60 in neuroepithelial tumorigenesis. Eur. J. Histochem. 2013, 57, e20. [Google Scholar] [CrossRef]
- Gáti, I.; Leel-Ossy, L. Heat shock protein 60 in corpora amylacea. Pathol. Oncol. Res. 2001, 7, 140–144. [Google Scholar] [CrossRef]
- Hallal, S.; Russell, B.P.; Wei, H.; Lee, M.Y.T.; Toon, C.W.; Sy, J.; Shivalingam, B.; Buckland, M.E.; Kaufman, K.L. Extracellular Vesicles from Neurosurgical Aspirates Identifies Chaperonin Containing TCP1 Subunit 6A as a Potential Glioblastoma Biomarker with Prognostic Significance. Proteomics 2019, 19, e1800157. [Google Scholar] [CrossRef]
- Lewis, V.A.; Hynes, G.M.; Zheng, D.; Saibil, H.; Willison, K. T-complex polypeptide-1 is a subunit of a heteromeric particle in the eukaryotic cytosol. Nature 1992, 358, 249–252. [Google Scholar] [CrossRef]
- Muth, C.; Rubner, Y.; Semrau, S.; Rühle, P.F.; Frey, B.; Strnad, A.; Buslei, R.; Fietkau, R.; Gaipl, U.S. Primary glioblastoma multiforme tumors and recurrence: Comparative analysis of the danger signals HMGB1, HSP70, and calreticulin. Strahlenther. Und Onkol. 2016, 192, 146–155. [Google Scholar] [CrossRef] [PubMed]
- Thorsteinsdottir, J.; Stangl, S.; Fu, P.; Guo, K.; Albrecht, V.; Eigenbrod, S.; Erl, J.; Gehrmann, M.; Tonn, J.C.; Multhoff, G.; et al. Overexpression of cytosolic, plasma membrane bound and extracellular heat shock protein 70 (Hsp70) in primary glioblastomas. J. Neurooncol. 2017, 135, 443–452. [Google Scholar] [CrossRef] [PubMed]
- Beaman, G.M.; Dennison, S.R.; Chatfield, L.K.; Phoenix, D.A. Reliability of HSP70 (HSPA) expression as a prognostic marker in glioma. Mol. Cell. Biochem. 2014, 393, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Alberti, G.; Campanella, C.; Paladino, L.; Porcasi, R.; Bavisotto, C.C.; Pitruzzella, A.; Graziano, F.; Florena, A.M.; Argo, A.; de Macario, E.C.; et al. The chaperone system in glioblastoma multiforme and derived cell lines: Diagnostic and mechanistic implications. Front. Biosci. 2022, 27, 97. [Google Scholar] [CrossRef] [PubMed]
- Lämmer, F.; Delbridge, C.; Würstle, S.; Neff, F.; Meyer, B.; Schlegel, J.; Kessel, K.A.; Schmid, T.E.; Schilling, D.; Combs, S.E. Cytosolic Hsp70 as a biomarker to predict clinical outcome in patients with glioblastoma. PLoS ONE 2019, 14, e0221502. [Google Scholar] [CrossRef]
- Zhao, R.; Li, B.; Zhang, S.; He, Z.; Pan, Z.; Guo, Q.; Qiu, W.; Qi, Y.; Zhao, S.; Wang, S.; et al. The N(6)-Methyladenosine-Modified Pseudogene HSPA7 Correlates With the Tumor Microenvironment and Predicts the Response to Immune Checkpoint Therapy in Glioblastoma. Front. Immunol. 2021, 12, 653711. [Google Scholar] [CrossRef]
- Pyrko, P.; Schönthal, A.H.; Hofman, F.M.; Chen, T.C.; Lee, A.S. The unfolded protein response regulator GRP78/BiP as a novel target for increasing chemosensitivity in malignant gliomas. Cancer Res. 2007, 67, 9809–9816. [Google Scholar] [CrossRef]
- Banerjee, H.N.; Hyman, G.; Evans, S.; Manglik, V.; Gwebu, E.; Banerjee, A.; Vaughan, D.; Medley, J.; Krauss, C.; Wilkins, J.; et al. Identification of the Transmembrane Glucose Regulated Protein 78 as a Biomarker for the Brain Cancer Glioblastoma Multiforme by Gene Expression and Proteomic Studies. J. Membr. Sci. Technol. 2014, 4, 1000126. [Google Scholar] [CrossRef]
- Graner, M.W.; Cumming, R.I.; Bigner, D.D. The heat shock response and chaperones/heat shock proteins in brain tumors: Surface expression, release, and possible immune consequences. J. Neurosci. 2007, 27, 11214–11227. [Google Scholar] [CrossRef]
- Kang, B.R.; Yang, S.H.; Chung, B.R.; Kim, W.; Kim, Y. Cell surface GRP78 as a biomarker and target for suppressing glioma cells. Sci. Rep. 2016, 6, 34922. [Google Scholar] [CrossRef]
- Wen, X.; Chen, X.; Chen, X. Increased Expression of GRP78 Correlates with Adverse Outcome in Recurrent Glioblastoma Multiforme Patients. Turk. Neurosurg. 2020, 30, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, H.; Hayashi, S.; Shioura, H.; Ohtsubo, T.; Ohnishi, T.; Kano, E. Suppression of heat-induced hsp72 accumulation by cisplatin in human glioblastoma cells. Cancer Lett. 1996, 110, 253–257. [Google Scholar] [CrossRef]
- Liu, G.; Yu, J.; Wu, R.; Shi, L.; Zhang, X.; Zhang, W.; Zhong, X.; Wang, Y.; Li, H.; Shen, Y.; et al. GRP78 determines glioblastoma sensitivity to UBA1 inhibition-induced UPR signaling and cell death. Cell Death Dis. 2021, 12, 733. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.; Cao, Y.; Xu, Y.; Huai, D.; Chen, P.; Guo, J.; Li, M.; Dai, Y. Overexpression of Hsc70 promotes proliferation, migration, and invasion of human glioma cells. J. Cell. Biochem. 2019, 120, 10707–10714. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.; Cao, Y.; Dai, X.; Li, M.; Guo, J. Hsc70 Interacts with β4GalT5 to Regulate the Growth of Gliomas. Neuromol. Med. 2019, 21, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Takano, S.; Wadhwa, R.; Yoshii, Y.; Nose, T.; Kaul, S.C.; Mitsui, Y. Elevated levels of mortalin expression in human brain tumors. Exp. Cell Res. 1997, 237, 38–45. [Google Scholar] [CrossRef]
- Zhang, M.; Pan, Y.; Qi, X.; Liu, Y.; Dong, R.; Zheng, D.; Chang, Q.; Zhang, J.; Fang, W.; Zhong, Y. Identification of New Biomarkers Associated with IDH Mutation and Prognosis in Astrocytic Tumors Using NanoString nCounter Analysis System. Appl. Immunohistochem. Mol. Morphol. 2018, 26, 101–107. [Google Scholar] [CrossRef]
- Saha, T.; van Vliet, A.A.; Cui, C.; Macias, J.J.; Kulkarni, A.; Pham, L.N.; Lawler, S.; Spanholtz, J.; Georgoudaki, A.M.; Duru, A.D.; et al. Boosting Natural Killer Cell Therapies in Glioblastoma Multiforme Using Supramolecular Cationic Inhibitors of Heat Shock Protein 90. Front. Mol. Biosci. 2021, 8, 754443. [Google Scholar] [CrossRef]
- Siegelin, M.D.; Plescia, J.; Raskett, C.M.; Gilbert, C.A.; Ross, A.H.; Altieri, D.C. Global targeting of subcellular heat shock protein-90 networks for therapy of glioblastoma. Mol. Cancer Ther. 2010, 9, 1638–1646. [Google Scholar] [CrossRef]
- Tani, T.; Tojo, N.; Ohnishi, K. Preferential radiosensitization to glioblastoma cancer stem cell-like cells by a Hsp90 inhibitor, N-vinylpyrrolidone-AUY922. Oncol. Lett. 2022, 23, 102. [Google Scholar] [CrossRef]
- Chen, H.; Gong, Y.; Ma, Y.; Thompson, R.C.; Wang, J.; Cheng, Z.; Xue, L. A Brain-Penetrating Hsp90 Inhibitor NXD30001 Inhibits Glioblastoma as a Monotherapy or in Combination with Radiation. Front. Pharmacol. 2020, 11, 974. [Google Scholar] [CrossRef] [PubMed]
- Canella, A.; Welker, A.M.; Yoo, J.Y.; Xu, J.; Abas, F.S.; Kesanakurti, D.; Nagarajan, P.; Beattie, C.E.; Sulman, E.P.; Liu, J.; et al. Efficacy of Onalespib, a Long-Acting Second-Generation HSP90 Inhibitor, as a Single Agent and in Combination with Temozolomide against Malignant Gliomas. Clin. Cancer Res. 2017, 23, 6215–6226. [Google Scholar] [CrossRef] [PubMed]
- Yao, Z.G.; Li, W.H.; Hua, F.; Cheng, H.X.; Zhao, M.Q.; Sun, X.C.; Qin, Y.J.; Li, J.M. LBH589 Inhibits Glioblastoma Growth and Angiogenesis Through Suppression of HIF-1α Expression. J. Neuropathol. Exp. Neurol. 2017, 76, 1000–1007. [Google Scholar] [CrossRef] [PubMed]
- Costantino, E.; Maddalena, F.; Calise, S.; Piscazzi, A.; Tirino, V.; Fersini, A.; Ambrosi, A.; Neri, V.; Esposito, F.; Landriscina, M. TRAP1, a novel mitochondrial chaperone responsible for multi-drug resistance and protection from apoptotis in human colorectal carcinoma cells. Cancer Lett. 2009, 279, 39–46. [Google Scholar] [CrossRef]
- Wu, J.; Liu, Y.; Cho, K.; Dong, X.; Teng, L.; Han, D.; Liu, H.; Chen, X.; Chen, X.; Hou, X.; et al. Downregulation of TRAP1 sensitizes glioblastoma cells to temozolomide chemotherapy through regulating metabolic reprogramming. Neuroreport 2016, 27, 136–144. [Google Scholar] [CrossRef]
- Li, S.; Lv, Q.; Sun, H.; Xue, Y.; Wang, P.; Liu, L.; Li, Z.; Li, Z.; Tian, X.; Liu, Y.H. Expression of TRAP1 predicts poor survival of malignant glioma patients. J. Mol. Neurosci. 2015, 55, 62–68. [Google Scholar] [CrossRef]
- Kato, S.; Morita, T.; Takenaka, T.; Kato, M.; Hirano, A.; Herz, F.; Ohama, E. Stress-response (heat-shock) protein 90 expression in tumors of the central nervous system: An immunohistochemical study. Acta Neuropathol. 1995, 89, 184–188. [Google Scholar] [CrossRef]
- Jego, G.; Hazoumé, A.; Seigneuric, R.; Garrido, C. Targeting heat shock proteins in cancer. Cancer Lett. 2013, 332, 275–285. [Google Scholar] [CrossRef]
- Kondo, T.; Shibamoto, Y.; Kawai, T.; Sugie, C.; Wang, Z.; Nakamura, K.; Murai, T.; Manabe, Y.; Nakashima, M.; Matsuo, M. Effects of a combined treatment regimen consisting of Hsp90 inhibitor DS-2248 and radiation in vitro and in a tumor mouse model. Transl. Cancer Res. 2021, 10, 2767–2776. [Google Scholar] [CrossRef]
- Dungey, F.A.; Caldecott, K.W.; Chalmers, A.J. Enhanced radiosensitization of human glioma cells by combining inhibition of poly(ADP-ribose) polymerase with inhibition of heat shock protein 90. Mol. Cancer Ther. 2009, 8, 2243–2254. [Google Scholar] [CrossRef]
- Orth, M.; Albrecht, V.; Seidl, K.; Kinzel, L.; Unger, K.; Hess, J.; Kreutzer, L.; Sun, N.; Stegen, B.; Nieto, A.; et al. Inhibition of HSP90 as a Strategy to Radiosensitize Glioblastoma: Targeting the DNA Damage Response and Beyond. Front. Oncol. 2021, 11, 612354. [Google Scholar] [CrossRef] [PubMed]
- Sauvageot, C.M.; Weatherbee, J.L.; Kesari, S.; Winters, S.E.; Barnes, J.; Dellagatta, J.; Ramakrishna, N.R.; Stiles, C.D.; Kung, A.L.; Kieran, M.W.; et al. Efficacy of the HSP90 inhibitor 17-AAG in human glioma cell lines and tumorigenic glioma stem cells. Neuro Oncol. 2009, 11, 109–121. [Google Scholar] [CrossRef] [PubMed]
- Premkumar, D.R.; Arnold, B.; Pollack, I.F. Cooperative inhibitory effect of ZD1839 (Iressa) in combination with 17-AAG on glioma cell growth. Mol. Carcinog. 2006, 45, 288–301. [Google Scholar] [CrossRef] [PubMed]
- Wachsberger, P.R.; Lawrence, Y.R.; Liu, Y.; Rice, B.; Feo, N.; Leiby, B.; Dicker, A.P. Hsp90 inhibition enhances PI-3 kinase inhibition and radiosensitivity in glioblastoma. J. Cancer Res. Clin. Oncol. 2014, 140, 573–582. [Google Scholar] [CrossRef]
- Önay Uçar, E.; Şengelen, A. Resveratrol and siRNA in combination reduces Hsp27 expression and induces caspase-3 activity in human glioblastoma cells. Cell Stress Chaperones 2019, 24, 763–775. [Google Scholar] [CrossRef]
- Banerji, U. Heat shock protein 90 as a drug target: Some like it hot. Clin. Cancer Res. 2009, 15, 9–14. [Google Scholar] [CrossRef]
- Booth, L.; Cruickshanks, N.; Ridder, T.; Chen, C.S.; Grant, S.; Dent, P. OSU-03012 interacts with lapatinib to kill brain cancer cells. Cancer Biol. Ther. 2012, 13, 1501–1511. [Google Scholar] [CrossRef]
- Dote, H.; Burgan, W.E.; Camphausen, K.; Tofilon, P.J. Inhibition of hsp90 compromises the DNA damage response to radiation. Cancer Res. 2006, 66, 9211–9220. [Google Scholar] [CrossRef] [PubMed]
- Booth, L.; Roberts, J.L.; Tavallai, M.; Nourbakhsh, A.; Chuckalovcak, J.; Carter, J.; Poklepovic, A.; Dent, P. OSU-03012 and Viagra Treatment Inhibits the Activity of Multiple Chaperone Proteins and Disrupts the Blood-Brain Barrier: Implications for Anti-Cancer Therapies. J. Cell. Physiol. 2015, 230, 1982–1998. [Google Scholar] [CrossRef]
- Zhu, H.; Woolfenden, S.; Bronson, R.T.; Jaffer, Z.M.; Barluenga, S.; Winssinger, N.; Rubenstein, A.E.; Chen, R.; Charest, A. The novel Hsp90 inhibitor NXD30001 induces tumor regression in a genetically engineered mouse model of glioblastoma multiforme. Mol. Cancer Ther. 2010, 9, 2618–2626. [Google Scholar] [CrossRef]
- Lee, Y.; Li, H.K.; Masaoka, A.; Sunada, S.; Hirakawa, H.; Fujimori, A.; Nickoloff, J.A.; Okayasu, R. The purine scaffold Hsp90 inhibitor PU-H71 sensitizes cancer cells to heavy ion radiation by inhibiting DNA repair by homologous recombination and non-homologous end joining. Radiother. Oncol. 2016, 121, 162–168. [Google Scholar] [CrossRef]
- Milanović, D.; Firat, E.; Grosu, A.L.; Niedermann, G. Increased radiosensitivity and radiothermosensitivity of human pancreatic MIA PaCa-2 and U251 glioblastoma cell lines treated with the novel Hsp90 inhibitor NVP-HSP990. Radiat. Oncol. 2013, 8, 42. [Google Scholar] [CrossRef] [PubMed]
- Propper, D.J.; Braybrooke, J.P.; Taylor, D.J.; Lodi, R.; Styles, P.; Cramer, J.A.; Collins, W.C.; Levitt, N.C.; Talbot, D.C.; Ganesan, T.S.; et al. Phase I trial of the selective mitochondrial toxin MKT077 in chemo-resistant solid tumours. Ann. Oncol. 1999, 10, 923–927. [Google Scholar] [CrossRef]
- Rousaki, A.; Miyata, Y.; Jinwal, U.K.; Dickey, C.A.; Gestwicki, J.E.; Zuiderweg, E.R. Allosteric drugs: The interaction of antitumor compound MKT-077 with human Hsp70 chaperones. J. Mol. Biol. 2011, 411, 614–632. [Google Scholar] [CrossRef] [PubMed]
- Whetstone, H.; Lingwood, C. 3′Sulfogalactolipid binding specifically inhibits Hsp70 ATPase activity in vitro. Biochemistry 2003, 42, 1611–1617. [Google Scholar] [CrossRef] [PubMed]
- Fewell, S.W.; Smith, C.M.; Lyon, M.A.; Dumitrescu, T.P.; Wipf, P.; Day, B.W.; Brodsky, J.L. Small molecule modulators of endogenous and co-chaperone-stimulated Hsp70 ATPase activity. J. Biol. Chem. 2004, 279, 51131–51140. [Google Scholar] [CrossRef]
- Braunstein, M.J.; Scott, S.S.; Scott, C.M.; Behrman, S.; Walter, P.; Wipf, P.; Coplan, J.D.; Chrico, W.; Joseph, D.; Brodsky, J.L.; et al. Antimyeloma Effects of the Heat Shock Protein 70 Molecular Chaperone Inhibitor MAL3-101. J. Oncol. 2011, 2011, 232037. [Google Scholar] [CrossRef]
- Kaiser, M.; Kühnl, A.; Reins, J.; Fischer, S.; Ortiz-Tanchez, J.; Schlee, C.; Mochmann, L.H.; Heesch, S.; Benlasfer, O.; Hofmann, W.K.; et al. Antileukemic activity of the HSP70 inhibitor pifithrin-μ in acute leukemia. Blood Cancer J. 2011, 1, e28. [Google Scholar] [CrossRef]
- Chatterjee, M.; Andrulis, M.; Stühmer, T.; Müller, E.; Hofmann, C.; Steinbrunn, T.; Heimberger, T.; Schraud, H.; Kressmann, S.; Einsele, H.; et al. The PI3K/Akt signaling pathway regulates the expression of Hsp70, which critically contributes to Hsp90-chaperone function and tumor cell survival in multiple myeloma. Haematologica 2013, 98, 1132–1141. [Google Scholar] [CrossRef]
- Zhu, H.; Cao, X.; Cai, X.; Tian, Y.; Wang, D.; Qi, J.; Teng, Z.; Lu, G.; Ni, Q.; Wang, S.; et al. Pifithrin-μ incorporated in gold nanoparticle amplifies pro-apoptotic unfolded protein response cascades to potentiate synergistic glioblastoma therapy. Biomaterials 2020, 232, 119677. [Google Scholar] [CrossRef]
- Silva, A.G.; Lopes, C.F.B.; Carvalho Júnior, C.G.; Thomé, R.G.; Dos Santos, H.B.; Reis, R.; Ribeiro, R. WIN55,212-2 induces caspase-independent apoptosis on human glioblastoma cells by regulating HSP70, p53 and Cathepsin D. Toxicol In Vitro 2019, 57, 233–243. [Google Scholar] [CrossRef] [PubMed]
- Lazarev, V.F.; Sverchinsky, D.V.; Mikhaylova, E.R.; Semenyuk, P.I.; Komarova, E.Y.; Niskanen, S.A.; Nikotina, A.D.; Burakov, A.V.; Kartsev, V.G.; Guzhova, I.V.; et al. Sensitizing tumor cells to conventional drugs: HSP70 chaperone inhibitors, their selection and application in cancer models. Cell Death Dis. 2018, 9, 41. [Google Scholar] [CrossRef] [PubMed]
- Rérole, A.L.; Gobbo, J.; De Thonel, A.; Schmitt, E.; Pais de Barros, J.P.; Hammann, A.; Lanneau, D.; Fourmaux, E.; Demidov, O.N.; Micheau, O.; et al. Peptides and aptamers targeting HSP70: A novel approach for anticancer chemotherapy. Cancer Res. 2011, 71, 484–495. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, L.; Kornberger, P.; Mendler, C.T.; Multhoff, G.; Schwaiger, M.; Skerra, A. Selection of an Anticalin® against the membrane form of Hsp70 via bacterial surface display and its theranostic application in tumour models. Biol. Chem. 2018, 399, 235–252. [Google Scholar] [CrossRef]
- Schilling, D.; Garrido, C.; Combs, S.E.; Multhoff, G. The Hsp70 inhibiting peptide aptamer A17 potentiates radiosensitization of tumor cells by Hsp90 inhibition. Cancer Lett. 2017, 390, 146–152. [Google Scholar] [CrossRef]
- Shevtsov, M.A.; Nikolaev, B.P.; Ryzhov, V.A.; Yakovleva, L.Y.; Marchenko, Y.Y.; Parr, M.A.; Rolich, V.I.; Mikhrina, A.L.; Dobrodumov, A.V.; Pitkin, E.; et al. Ionizing radiation improves glioma-specific targeting of superparamagnetic iron oxide nanoparticles conjugated with cmHsp70.1 monoclonal antibodies (SPION-cmHsp70.1). Nanoscale 2015, 7, 20652–20664. [Google Scholar] [CrossRef] [PubMed]
- Gehrmann, M.; Stangl, S.; Kirschner, A.; Foulds, G.A.; Sievert, W.; Doss, B.T.; Walch, A.; Pockley, A.G.; Multhoff, G. Immunotherapeutic targeting of membrane Hsp70-expressing tumors using recombinant human granzyme B. PLoS ONE 2012, 7, e41341. [Google Scholar] [CrossRef]
- Shevtsov, M.; Stangl, S.; Nikolaev, B.; Yakovleva, L.; Marchenko, Y.; Tagaeva, R.; Sievert, W.; Pitkin, E.; Mazur, A.; Tolstoy, P.; et al. Granzyme B Functionalized Nanoparticles Targeting Membrane Hsp70-Positive Tumors for Multimodal Cancer Theranostics. Small 2019, 15, e1900205. [Google Scholar] [CrossRef]
- Jiang, X.; Zhou, T.; Wang, Z.; Qi, B.; Xia, H. HSP47 Promotes Glioblastoma Stemlike Cell Survival by Modulating Tumor Microenvironment Extracellular Matrix through TGF-β Pathway. ACS Chem. Neurosci. 2017, 8, 128–134. [Google Scholar] [CrossRef]
- Wu, Z.B.; Cai, L.; Qiu, C.; Zhang, A.L.; Lin, S.J.; Yao, Y.; Xu, J.; Zhou, L.F. CTL responses to HSP47 associated with the prolonged survival of patients with glioblastomas. Neurology 2014, 82, 1261–1265. [Google Scholar] [CrossRef]
- Cassel, J.A.; Ilyin, S.; McDonnell, M.E.; Reitz, A.B. Novel inhibitors of heat shock protein Hsp70-mediated luciferase refolding that bind to DnaJ. Bioorg. Med. Chem. 2012, 20, 3609–3614. [Google Scholar] [CrossRef]
- Yokota, S.; Kitahara, M.; Nagata, K. Benzylidene lactam compound, KNK437, a novel inhibitor of acquisition of thermotolerance and heat shock protein induction in human colon carcinoma cells. Cancer Res. 2000, 60, 2942–2948. [Google Scholar]
- Alalem, M.; Bhosale, M.; Ranjan, A.; Yamamoto, S.; Kaida, A.; Nishikawa, S.; Parrales, A.; Farooki, S.; Anant, S.; Padhye, S.; et al. Mutant p53 Depletion by Novel Inhibitors for HSP40/J-Domain Proteins Derived from the Natural Compound Plumbagin. Cancers 2022, 14, 4187. [Google Scholar] [CrossRef]
- Xu, F.; Proft, J.; Gibbs, S.; Winkfein, B.; Johnson, J.N.; Syed, N.; Braun, J.E. Quercetin targets cysteine string protein (CSPalpha) and impairs synaptic transmission. PLoS ONE 2010, 5, e11045. [Google Scholar] [CrossRef]
- Şengelen, A.; Önay-Uçar, E. Rosmarinic acid and siRNA combined therapy represses Hsp27 (HSPB1) expression and induces apoptosis in human glioma cells. Cell Stress Chaperones 2018, 23, 885–896. [Google Scholar] [CrossRef]
- Bao, D.; Cheng, C.; Lan, X.; Xing, R.; Chen, Z.; Zhao, H.; Sun, J.; Wang, Y.; Niu, C.; Zhang, B.; et al. Regulation of p53wt glioma cell proliferation by androgen receptor-mediated inhibition of small VCP/p97-interacting protein expression. Oncotarget 2017, 8, 23142–23154. [Google Scholar] [CrossRef]
- Castro, G.N.; Cayado-Gutiérrez, N.; Zoppino, F.C.; Fanelli, M.A.; Cuello-Carrión, F.D.; Sottile, M.; Nadin, S.B.; Ciocca, D.R. Effects of temozolomide (TMZ) on the expression and interaction of heat shock proteins (HSPs) and DNA repair proteins in human malignant glioma cells. Cell Stress Chaperones 2015, 20, 253–265. [Google Scholar] [CrossRef]
- Rajesh, Y.; Biswas, A.; Kumar, U.; Banerjee, I.; Das, S.; Maji, S.; Das, S.K.; Emdad, L.; Cavenee, W.K.; Mandal, M.; et al. Lumefantrine, an antimalarial drug, reverses radiation and temozolomide resistance in glioblastoma. Proc. Natl. Acad. Sci. USA 2020, 117, 12324–12331. [Google Scholar] [CrossRef]
- Borrelli, M.J.; Lepock, J.R.; Frey, H.E.; Lee, Y.J.; Corry, P.M. Excess protein in nuclei isolated from heat-shocked cells results from a reduced extractability of nuclear proteins. J. Cell. Physiol. 1996, 167, 369–379. [Google Scholar] [CrossRef]
- Knowlton, A.A.; Salfity, M. Nuclear localization and the heat shock proteins. J. Biosci. 1996, 21, 123–132. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).