The Renaissance of CDK Inhibitors in Breast Cancer Therapy: An Update on Clinical Trials and Therapy Resistance
Abstract
Simple Summary
Abstract
1. Introduction
2. Breast Cancer and Existing Targeted Therapy
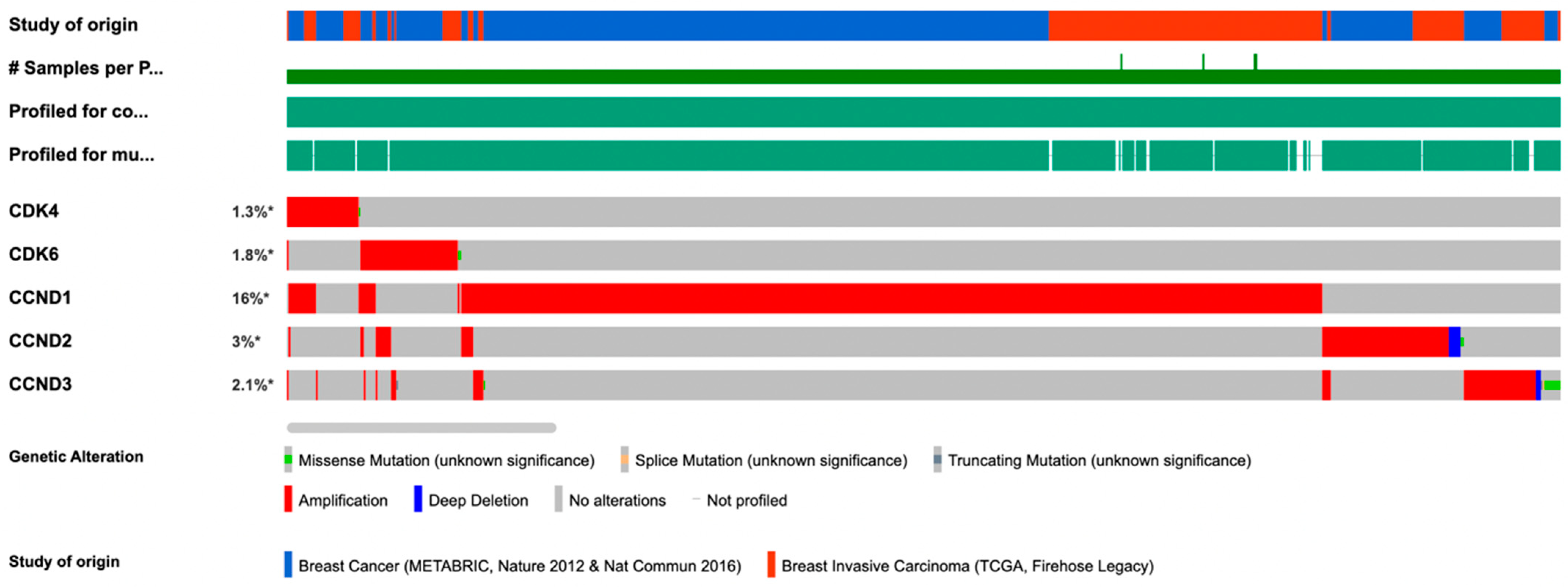
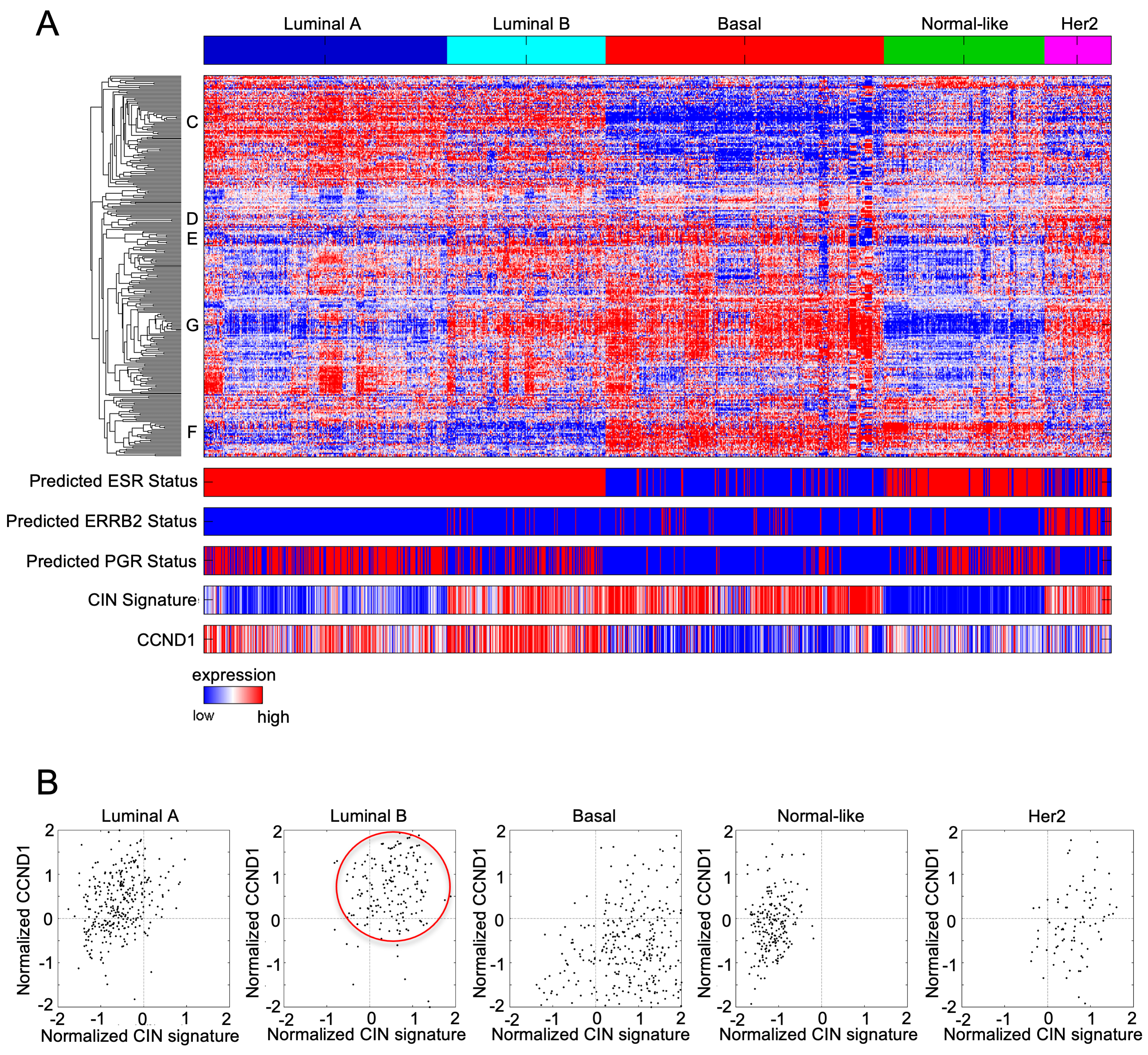
3. The Regulatory Cyclin D Subunit Are Frequently Amplified in Human Breast Cancer
4. CDK Inhibitors
5. Dose-Limiting Side Effects
6. Mechanisms of Resistance to CDK4/6 Inhibitors
7. Identifying Targeted Therapies for CDK4/6 Inhibitor-Resistant ER+ Breast Cancer
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Mayer, E.L. Targeting breast cancer with CDK inhibitors. Curr. Oncol. Rep. 2015, 17, 443. [Google Scholar] [CrossRef] [PubMed]
- Di Sante, G.; Page, J.; Jiao, X.; Nawab, O.; Cristofanilli, M.; Skordalakes, E.; Pestell, R.G. Recent advances with cyclin-dependent kinase inhibitors: Therapeutic agents for breast cancer and their role in immuno-oncology. Expert Rev. Anticancer Ther. 2019, 19, 569–587. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Yu, S.; Liu, Q.; Yuan, X.; Mani, S.; Pestell, R.G.; Wu, K. Recent advances of highly selective CDK4/6 inhibitors in breast cancer. J. Hematol. Oncol. 2017, 10, 97. [Google Scholar] [CrossRef] [PubMed]
- Sherr, C.J.; Beach, D.; Shapiro, G.I. Targeting CDK4 and CDK6: From Discovery to Therapy. Cancer Discov. 2016, 6, 353–367. [Google Scholar] [CrossRef] [PubMed]
- Shupp, A.; Casimiro, M.C.; Pestell, R.G. Biological functions of CDK5 and potential CDK5 targeted clinical treatments. Oncotarget 2017, 8, 17373–17382. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer Statistics, 2017. CA Cancer J. Clin. 2017, 67, 7–30. [Google Scholar] [CrossRef]
- Cancer Genome Atlas, N. Comprehensive molecular portraits of human breast tumours. Nature 2012, 490, 61–70. [Google Scholar] [CrossRef]
- Wang, G.; Gormley, M.; Qiao, J.; Zhao, Q.; Wang, M.; Di Sante, G.; Deng, S.; Dong, L.; Pestell, T.; Ju, X.; et al. Cyclin D1-mediated microRNA expression signature predicts breast cancer outcome. Theranostics 2018, 8, 2251–2263. [Google Scholar] [CrossRef]
- Schreiber, M.; Kolbus, A.; Piu, F.; Szabowski, A.; Möhle-Steinlein, U.; Tian, J.; Karin, M.; Angel, P.; Wagner, E.F. Control of cell cycle progression by c-Jun is p53 dependent. Genes Dev. 1999, 13, 607–619. [Google Scholar] [CrossRef]
- Fouad, Y.A.; Aanei, C. Revisiting the hallmarks of cancer. Am. J. Cancer Res. 2017, 7, 1016–1036. [Google Scholar]
- Csermely, P.; Korcsmaros, T.; Nussinov, R. Intracellular and intercellular signaling networks in cancer initiation, development and precision anti-cancer therapy: RAS acts as contextual signaling hub. Semin. Cell Dev. Biol. 2016, 58, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Gradishar, W.J.; Anderson, B.O.; Abraham, J.; Aft, R.; Agnese, D.; Allison, K.H.; Blair, S.L.; Burstein, H.J.; Dang, C.; Elias, A.D.; et al. Breast Cancer, Version 3.2020, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2020, 18, 452–478. [Google Scholar] [CrossRef] [PubMed]
- Stracquadanio, G.; Wang, X.; Wallace, M.D.; Grawenda, A.M.; Zhang, P.; Hewitt, J.; Zeron-Medina, J.; Castro-Giner, F.; Tomlinson, I.P.; Goding, C.R.; et al. The importance of p53 pathway genetics in inherited and somatic cancer genomes. Nat. Rev. Cancer 2016, 16, 251–265. [Google Scholar] [CrossRef] [PubMed]
- Upadhyaya, C.; Jiao, X.; Ashton, A.; Patel, K.; Kossenkov, A.V.; Pestell, R.G. The G protein coupled receptor CCR5 in cancer. Adv. Cancer Res. 2020, 145, 29–47. [Google Scholar] [CrossRef]
- Aine, M.; Boyaci, C.; Hartman, J.; Hakkinen, J.; Mitra, S.; Campos, A.B.; Nimeus, E.; Ehinger, A.; Vallon-Christersson, J.; Borg, A.; et al. Molecular analyses of triple-negative breast cancer in the young and elderly. Breast Cancer Res. 2021, 23, 20. [Google Scholar] [CrossRef]
- Li, Y.; Zhan, Z.; Yin, X.; Fu, S.; Deng, X. Targeted Therapeutic Strategies for Triple-Negative Breast Cancer. Front. Oncol. 2021, 11, 731535. [Google Scholar] [CrossRef]
- Gao, J.J.; Swain, S.M. Luminal A Breast Cancer and Molecular Assays: A Review. Oncologist 2018, 23, 556–565. [Google Scholar] [CrossRef]
- Li, Y.; Ma, L. Efficacy of chemotherapy for lymph node-positive luminal A subtype breast cancer patients: An updated meta-analysis. World J. Surg. Oncol. 2020, 18, 316. [Google Scholar] [CrossRef]
- Roulot, A.; Hequet, D.; Guinebretiere, J.M.; Vincent-Salomon, A.; Lerebours, F.; Dubot, C.; Rouzier, R. Tumoral heterogeneity of breast cancer. Ann. Biol. Clin. 2016, 74, 653–660. [Google Scholar] [CrossRef]
- Navale, P.; Bleiweiss, I.J.; Jaffer, S.; Nayak, A. Evaluation of Biomarkers in Multiple Ipsilateral Synchronous Invasive Breast Carcinomas. Arch. Pathol. Lab. Med. 2019, 143, 190–196. [Google Scholar] [CrossRef]
- Afghahi, A.; Timms, K.M.; Vinayak, S.; Jensen, K.C.; Kurian, A.W.; Carlson, R.W.; Chang, P.J.; Schackmann, E.; Hartman, A.R.; Ford, J.M.; et al. Tumor BRCA1 Reversion Mutation Arising during Neoadjuvant Platinum-Based Chemotherapy in Triple-Negative Breast Cancer Is Associated with Therapy Resistance. Clin. Cancer Res. 2017, 23, 3365–3370. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.S.; Zhao, Z.; Yang, Z.N.; Xu, F.; Lu, H.J.; Zhu, Z.Y.; Shi, W.; Jiang, J.; Yao, P.P.; Zhu, H.P. Risk Factors and Preventions of Breast Cancer. Int. J. Biol. Sci. 2017, 13, 1387–1397. [Google Scholar] [CrossRef] [PubMed]
- Sobhani, N.; D’Angelo, A.; Pittacolo, M.; Roviello, G.; Miccoli, A.; Corona, S.P.; Bernocchi, O.; Generali, D.; Otto, T. Updates on the CDK4/6 Inhibitory Strategy and Combinations in Breast Cancer. Cells 2019, 8, 321. [Google Scholar] [CrossRef] [PubMed]
- Haque, M.M.; Desai, K.V. Pathways to Endocrine Therapy Resistance in Breast Cancer. Front. Endocrinol. 2019, 10, 573. [Google Scholar] [CrossRef]
- Rani, A.; Stebbing, J.; Giamas, G.; Murphy, J. Endocrine Resistance in Hormone Receptor Positive Breast Cancer-From Mechanism to Therapy. Front. Endocrinol. 2019, 10, 245. [Google Scholar] [CrossRef]
- Goel, S.; DeCristo, M.J.; McAllister, S.S.; Zhao, J.J. CDK4/6 Inhibition in Cancer: Beyond Cell Cycle Arrest. Trends Cell Biol. 2018, 28, 911–925. [Google Scholar] [CrossRef] [PubMed]
- Di Sante, G.; Di Rocco, A.; Pupo, C.; Casimiro, M.C.; Pestell, R.G. Hormone-induced DNA damage response and repair mediated by cyclin D1 in breast and prostate cancer. Oncotarget 2017, 8, 81803–81812. [Google Scholar] [CrossRef]
- Sherr, C.J.; Roberts, J.M. CDK inhibitors: Positive and negative regulators of G1-phase progression. Genes Dev. 1999, 13, 1501–1512. [Google Scholar] [CrossRef]
- Casimiro, M.C.; Di Sante, G.; Crosariol, M.; Loro, E.; Dampier, W.; Ertel, A.; Yu, Z.; Saria, E.A.; Papanikolaou, A.; Li, Z.; et al. Kinase-independent role of cyclin D1 in chromosomal instability and mammary tumorigenesis. Oncotarget 2015, 6, 8525–8538. [Google Scholar] [CrossRef] [PubMed]
- Piezzo, M.; Cocco, S.; Caputo, R.; Cianniello, D.; Gioia, G.D.; Lauro, V.D.; Fusco, G.; Martinelli, C.; Nuzzo, F.; Pensabene, M.; et al. Targeting Cell Cycle in Breast Cancer: CDK4/6 Inhibitors. Int. J. Mol. Sci. 2020, 21, 6479. [Google Scholar] [CrossRef]
- Johnson, J.; Thijssen, B.; McDermott, U.; Garnett, M.; Wessels, L.F.; Bernards, R. Targeting the RB-E2F pathway in breast cancer. Oncogene 2016, 35, 4829–4835. [Google Scholar] [CrossRef] [PubMed]
- Schoninger, S.F.; Blain, S.W. The Ongoing Search for Biomarkers of CDK4/6 Inhibitor Responsiveness in Breast Cancer. Mol. Cancer Ther. 2020, 19, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Spring, L.M.; Wander, S.A.; Zangardi, M.; Bardia, A. CDK 4/6 Inhibitors in Breast Cancer: Current Controversies and Future Directions. Curr. Oncol. Rep. 2019, 21, 25. [Google Scholar] [CrossRef] [PubMed]
- DeMichele, A.; Cristofanilli, M.; Brufsky, A.; Liu, X.; Mardekian, J.; McRoy, L.; Layman, R.M.; Emir, B.; Torres, M.A.; Rugo, H.S.; et al. Comparative effectiveness of first-line palbociclib plus letrozole versus letrozole alone for HR+/HER2- metastatic breast cancer in US real-world clinical practice. Breast Cancer Res. 2021, 23, 37. [Google Scholar] [CrossRef]
- Finn, R.S.; Boer, K.; Bondarenko, I.; Patel, R.; Pinter, T.; Schmidt, M.; Shparyk, Y.V.; Thummala, A.; Voitko, N.; Bananis, E.; et al. Overall survival results from the randomized phase 2 study of palbociclib in combination with letrozole versus letrozole alone for first-line treatment of ER+/HER2- advanced breast cancer (PALOMA-1, TRIO-18). Breast Cancer Res. Treat. 2020, 183, 419–428. [Google Scholar] [CrossRef]
- Wilson, F.R.; Varu, A.; Mitra, D.; Cameron, C.; Iyer, S. Systematic review and network meta-analysis comparing palbociclib with chemotherapy agents for the treatment of postmenopausal women with HR-positive and HER2-negative advanced/metastatic breast cancer. Breast Cancer Res. Treat. 2017, 166, 167–177. [Google Scholar] [CrossRef]
- Finn, R.S.; Aleshin, A.; Slamon, D.J. Targeting the cyclin-dependent kinases (CDK) 4/6 in estrogen receptor-positive breast cancers. Breast Cancer Res. 2016, 18, 17. [Google Scholar] [CrossRef]
- Fry, D.W.; Harvey, P.J.; Keller, P.R.; Elliott, W.L.; Meade, M.; Trachet, E.; Albassam, M.; Zheng, X.; Leopold, W.R.; Pryer, N.K.; et al. Specific inhibition of cyclin-dependent kinase 4/6 by PD 0332991 and associated antitumor activity in human tumor xenografts. Mol. Cancer Ther. 2004, 3, 1427–1438. [Google Scholar] [CrossRef]
- Hortobagyi, G.N.; Stemmer, S.M.; Burris, H.A.; Yap, Y.S.; Sonke, G.S.; Paluch-Shimon, S.; Campone, M.; Blackwell, K.L.; Andre, F.; Winer, E.P.; et al. Ribociclib as First-Line Therapy for HR-Positive, Advanced Breast Cancer. N. Engl. J. Med. 2016, 375, 1738–1748. [Google Scholar] [CrossRef]
- Tripathy, D.; Bardia, A.; Sellers, W.R. Ribociclib (LEE011): Mechanism of Action and Clinical Impact of This Selective Cyclin-Dependent Kinase 4/6 Inhibitor in Various Solid Tumors. Clin. Cancer Res. 2017, 23, 3251–3262. [Google Scholar] [CrossRef]
- Patnaik, A.; Rosen, L.S.; Tolaney, S.M.; Tolcher, A.W.; Goldman, J.W.; Gandhi, L.; Papadopoulos, K.P.; Beeram, M.; Rasco, D.W.; Hilton, J.F.; et al. Efficacy and Safety of Abemaciclib, an Inhibitor of CDK4 and CDK6, for Patients with Breast Cancer, Non-Small Cell Lung Cancer, and Other Solid Tumors. Cancer Discov. 2016, 6, 740–753. [Google Scholar] [CrossRef] [PubMed]
- Dickler, M.N.; Tolaney, S.M.; Rugo, H.S.; Cortes, J.; Dieras, V.; Patt, D.; Wildiers, H.; Hudis, C.A.; O’Shaughnessy, J.; Zamora, E.; et al. MONARCH 1, A Phase II Study of Abemaciclib, a CDK4 and CDK6 Inhibitor, as a Single Agent, in Patients with Refractory HR(+)/HER2(−) Metastatic Breast Cancer. Clin. Cancer Res. 2017, 23, 5218–5224. [Google Scholar] [CrossRef] [PubMed]
- Gelbert, L.M.; Cai, S.; Lin, X.; Sanchez-Martinez, C.; Del Prado, M.; Lallena, M.J.; Torres, R.; Ajamie, R.T.; Wishart, G.N.; Flack, R.S.; et al. Preclinical characterization of the CDK4/6 inhibitor LY2835219: In-vivo cell cycle-dependent/independent anti-tumor activities alone/in combination with gemcitabine. Investig. New Drugs 2014, 32, 825–837. [Google Scholar] [CrossRef]
- Ma, C.X.; Gao, F.; Luo, J.; Northfelt, D.W.; Goetz, M.; Forero, A.; Hoog, J.; Naughton, M.; Ademuyiwa, F.; Suresh, R.; et al. NeoPalAna: Neoadjuvant Palbociclib, a Cyclin-Dependent Kinase 4/6 Inhibitor, and Anastrozole for Clinical Stage 2 or 3 Estrogen Receptor-Positive Breast Cancer. Clin. Cancer Res. 2017, 23, 4055–4065. [Google Scholar] [CrossRef]
- Bisi, J.E.; Sorrentino, J.A.; Jordan, J.L.; Darr, D.D.; Roberts, P.J.; Tavares, F.X.; Strum, J.C. Preclinical development of G1T38: A novel, potent and selective inhibitor of cyclin dependent kinases 4/6 for use as an oral antineoplastic in patients with CDK4/6 sensitive tumors. Oncotarget 2017, 8, 42343–42358. [Google Scholar] [CrossRef] [PubMed]
- Lukasik, P.; Baranowska-Bosiacka, I.; Kulczycka, K.; Gutowska, I. Inhibitors of Cyclin-Dependent Kinases: Types and Their Mechanism of Action. Int. J. Mol. Sci. 2021, 22, 2806. [Google Scholar] [CrossRef]
- Liao, X.; Hong, Y.; Mao, Y.; Chen, N.; Wang, Q.; Wang, Z.; Zhang, L.; Wang, L.; Shi, C.; Shi, W.; et al. SPH3643: A novel cyclin-dependent kinase 4/6 inhibitor with good anticancer efficacy and strong blood-brain barrier permeability. Cancer Sci. 2020, 111, 1761–1773. [Google Scholar] [CrossRef]
- Chen, F.; Liu, C.; Zhang, J.; Xu, W.; Zhang, Y. Progress of CDK4/6 Inhibitor Palbociclib in the Treatment of Cancer. Anticancer Agents Med. Chem. 2018, 18, 1241–1251. [Google Scholar] [CrossRef]
- Roskoski, R., Jr. Cyclin-dependent protein kinase inhibitors including palbociclib as anticancer drugs. Pharmacol. Res. 2016, 107, 249–275. [Google Scholar] [CrossRef]
- Xu, B.; Li, H.; Zhang, Q.; Sun, W.; Yu, Y.; Li, W.; Wang, S.; Liao, N.; Shen, P.; Liu, Y.; et al. Pharmacokinetics, safety, activity, and biomarker analysis of palbociclib plus letrozole as first-line treatment for ER+/HER2− advanced breast cancer in Chinese women. Cancer Chemother. Pharmacol. 2021, 88, 131–141. [Google Scholar] [CrossRef]
- Saha, S.; Dey, S.; Nath, S. Steroid Hormone Receptors: Links with Cell Cycle Machinery and Breast Cancer Progression. Front. Oncol. 2021, 11, 620214. [Google Scholar] [CrossRef] [PubMed]
- Palumbo, R.; Torrisi, R.; Sottotetti, F.; Presti, D.; Rita Gambaro, A.; Collova, E.; Ferzi, A.; Agostinetto, E.; Maria Teragni, C.; Saltalamacchia, G.; et al. Patterns of treatment and outcome of palbociclib plus endocrine therapy in hormone receptor-positive/HER2 receptor-negative metastatic breast cancer: A real-world multicentre Italian study. Ther. Adv. Med. Oncol. 2021, 13, 1758835920987651. [Google Scholar] [CrossRef] [PubMed]
- Rossi, V.; Berchialla, P.; Giannarelli, D.; Nistico, C.; Ferretti, G.; Gasparro, S.; Russillo, M.; Catania, G.; Vigna, L.; Mancusi, R.L.; et al. Should All Patients with HR-Positive HER2-Negative Metastatic Breast Cancer Receive CDK 4/6 Inhibitor As First-Line Based Therapy? A Network Meta-Analysis of Data from the PALOMA 2, MONALEESA 2, MONALEESA 7, MONARCH 3, FALCON, SWOG and FACT Trials. Cancers 2019, 11, 1661. [Google Scholar] [CrossRef] [PubMed]
- Finn, R.S.; Crown, J.P.; Lang, I.; Boer, K.; Bondarenko, I.M.; Kulyk, S.O.; Ettl, J.; Patel, R.; Pinter, T.; Schmidt, M.; et al. The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): A randomised phase 2 study. Lancet Oncol. 2015, 16, 25–35. [Google Scholar] [CrossRef]
- Finn, R.S.; Martin, M.; Rugo, H.S.; Jones, S.; Im, S.A.; Gelmon, K.; Harbeck, N.; Lipatov, O.N.; Walshe, J.M.; Moulder, S.; et al. Palbociclib and Letrozole in Advanced Breast Cancer. N. Engl. J. Med. 2016, 375, 1925–1936. [Google Scholar] [CrossRef]
- Turner, N.C.; Ro, J.; Andre, F.; Loi, S.; Verma, S.; Iwata, H.; Harbeck, N.; Loibl, S.; Huang Bartlett, C.; Zhang, K.; et al. Palbociclib in Hormone-Receptor-Positive Advanced Breast Cancer. N. Engl. J. Med. 2015, 373, 209–219. [Google Scholar] [CrossRef]
- Loibl, S.; Turner, N.C.; Ro, J.; Cristofanilli, M.; Iwata, H.; Im, S.A.; Masuda, N.; Loi, S.; Andre, F.; Harbeck, N.; et al. Palbociclib Combined with Fulvestrant in Premenopausal Women with Advanced Breast Cancer and Prior Progression on Endocrine Therapy: PALOMA-3 Results. Oncologist 2017, 22, 1028–1038. [Google Scholar] [CrossRef]
- Turner, N.C.; Slamon, D.J.; Ro, J.; Bondarenko, I.; Im, S.A.; Masuda, N.; Colleoni, M.; DeMichele, A.; Loi, S.; Verma, S.; et al. Overall Survival with Palbociclib and Fulvestrant in Advanced Breast Cancer. N. Engl. J. Med. 2018, 379, 1926–1936. [Google Scholar] [CrossRef]
- Gnant, M.; Dueck, A.C.; Frantal, S.; Martin, M.; Burstein, H.J.; Greil, R.; Fox, P.; Wolff, A.C.; Chan, A.; Winer, E.P.; et al. Adjuvant Palbociclib for Early Breast Cancer: The PALLAS Trial Results (ABCSG-42/AFT-05/BIG-14-03). J. Clin. Oncol. 2022, 40, 282–293. [Google Scholar] [CrossRef]
- Loibl, S.; Marme, F.; Martin, M.; Untch, M.; Bonnefoi, H.; Kim, S.B.; Bear, H.; McCarthy, N.; Mele Olive, M.; Gelmon, K.; et al. Palbociclib for Residual High-Risk Invasive HR-Positive and HER2-Negative Early Breast Cancer-The Penelope-B Trial. J. Clin. Oncol. 2021, 39, 1518–1530. [Google Scholar] [CrossRef]
- Schettini, F.; Giudici, F.; Giuliano, M.; Cristofanilli, M.; Arpino, G.; Del Mastro, L.; Puglisi, F.; De Placido, S.; Paris, I.; De Placido, P.; et al. Overall Survival of CDK4/6-Inhibitor-Based Treatments in Clinically Relevant Subgroups of Metastatic Breast Cancer: Systematic Review and Meta-Analysis. J. Natl. Cancer Inst. 2020, 112, 1089–1097. [Google Scholar] [CrossRef]
- Cristofanilli, M.; Rugo, H.S.; Im, S.A.; Slamon, D.J.; Harbeck, N.; Bondarenko, I.; Masuda, N.; Colleoni, M.; DeMichele, A.; Loi, S.; et al. Overall Survival with Palbociclib and Fulvestrant in Women with HR+/HER2− ABC: Updated Exploratory Analyses of PALOMA-3, a Double-blind, Phase III Randomized Study. Clin. Cancer Res. 2022, 28, 3433–3442. [Google Scholar] [CrossRef] [PubMed]
- Im, S.A.; Lu, Y.S.; Bardia, A.; Harbeck, N.; Colleoni, M.; Franke, F.; Chow, L.; Sohn, J.; Lee, K.S.; Campos-Gomez, S.; et al. Overall Survival with Ribociclib plus Endocrine Therapy in Breast Cancer. N. Engl. J. Med. 2019, 381, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Slamon, D.J.; Neven, P.; Chia, S.; Fasching, P.A.; De Laurentiis, M.; Im, S.A.; Petrakova, K.; Bianchi, G.V.; Esteva, F.J.; Martin, M.; et al. Phase III Randomized Study of Ribociclib and Fulvestrant in Hormone Receptor-Positive, Human Epidermal Growth Factor Receptor 2-Negative Advanced Breast Cancer: MONALEESA-3. J. Clin. Oncol. 2018, 36, 2465–2472. [Google Scholar] [CrossRef] [PubMed]
- Slamon, D.J.; Neven, P.; Chia, S.; Fasching, P.A.; De Laurentiis, M.; Im, S.A.; Petrakova, K.; Bianchi, G.V.; Esteva, F.J.; Martin, M.; et al. Overall Survival with Ribociclib plus Fulvestrant in Advanced Breast Cancer. N. Engl. J. Med. 2020, 382, 514–524. [Google Scholar] [CrossRef] [PubMed]
- Sledge, G.W., Jr.; Toi, M.; Neven, P.; Sohn, J.; Inoue, K.; Pivot, X.; Burdaeva, O.; Okera, M.; Masuda, N.; Kaufman, P.A.; et al. MONARCH 2: Abemaciclib in Combination with Fulvestrant in Women with HR+/HER2- Advanced Breast Cancer Who Had Progressed While Receiving Endocrine Therapy. J. Clin. Oncol. 2017, 35, 2875–2884. [Google Scholar] [CrossRef]
- Sledge, G.W., Jr.; Toi, M.; Neven, P.; Sohn, J.; Inoue, K.; Pivot, X.; Burdaeva, O.; Okera, M.; Masuda, N.; Kaufman, P.A.; et al. The Effect of Abemaciclib Plus Fulvestrant on Overall Survival in Hormone Receptor-Positive, ERBB2-Negative Breast Cancer That Progressed on Endocrine Therapy-MONARCH 2: A Randomized Clinical Trial. JAMA Oncol. 2020, 6, 116–124. [Google Scholar] [CrossRef]
- Goetz, M.P.; Toi, M.; Campone, M.; Sohn, J.; Paluch-Shimon, S.; Huober, J.; Park, I.H.; Tredan, O.; Chen, S.C.; Manso, L.; et al. MONARCH 3: Abemaciclib As Initial Therapy for Advanced Breast Cancer. J. Clin. Oncol. 2017, 35, 3638–3646. [Google Scholar] [CrossRef]
- Lam, S.Y.; Liu, W.S.A.; Lee, C.-S. A Review of CDK4/6 Inhibitors. Available online: https://www.uspharmacist.com/article/a-review-of-cdk4-6-inhibitors (accessed on 12 April 2021).
- Yip, H.Y.K.; Papa, A. Signaling Pathways in Cancer: Therapeutic Targets, Combinatorial Treatments, and New Developments. Cells 2021, 10, 659. [Google Scholar] [CrossRef]
- Lee, H.J.; Lee, W.K.; Kang, C.W.; Ku, C.R.; Cho, Y.H.; Lee, E.J. A selective cyclin-dependent kinase 4, 6 dual inhibitor, Ribociclib (LEE011) inhibits cell proliferation and induces apoptosis in aggressive thyroid cancer. Cancer Lett. 2018, 417, 131–140. [Google Scholar] [CrossRef]
- Li, T.; Xiong, Y.; Wang, Q.; Chen, F.; Zeng, Y.; Yu, X.; Wang, Y.; Zhou, F.; Zhou, Y. Ribociclib (LEE011) suppresses cell proliferation and induces apoptosis of MDA-MB-231 by inhibiting CDK4/6-cyclin D-Rb-E2F pathway. Artif. Cells Nanomed. Biotechnol. 2019, 47, 4001–4011. [Google Scholar] [CrossRef] [PubMed]
- Konecny, G.E. Cyclin-dependent kinase pathways as targets for women’s cancer treatment. Curr. Opin. Obstet. Gynecol. 2016, 28, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Kumarasamy, V.; Vail, P.; Nambiar, R.; Witkiewicz, A.K.; Knudsen, E.S. Functional Determinants of Cell Cycle Plasticity and Sensitivity to CDK4/6 Inhibition. Cancer Res. 2021, 81, 1347–1360. [Google Scholar] [CrossRef] [PubMed]
- Jansen, V.M.; Bhola, N.E.; Bauer, J.A.; Formisano, L.; Lee, K.M.; Hutchinson, K.E.; Witkiewicz, A.K.; Moore, P.D.; Estrada, M.V.; Sanchez, V.; et al. Kinome-Wide RNA Interference Screen Reveals a Role for PDK1 in Acquired Resistance to CDK4/6 Inhibition in ER-Positive Breast Cancer. Cancer Res. 2017, 77, 2488–2499. [Google Scholar] [CrossRef]
- Chen, P.; Lee, N.V.; Hu, W.; Xu, M.; Ferre, R.A.; Lam, H.; Bergqvist, S.; Solowiej, J.; Diehl, W.; He, Y.A.; et al. Spectrum and Degree of CDK Drug Interactions Predicts Clinical Performance. Mol. Cancer Ther. 2016, 15, 2273–2281. [Google Scholar] [CrossRef]
- Raub, T.J.; Wishart, G.N.; Kulanthaivel, P.; Staton, B.A.; Ajamie, R.T.; Sawada, G.A.; Gelbert, L.M.; Shannon, H.E.; Sanchez-Martinez, C.; De Dios, A. Brain Exposure of Two Selective Dual CDK4 and CDK6 Inhibitors and the Antitumor Activity of CDK4 and CDK6 Inhibition in Combination with Temozolomide in an Intracranial Glioblastoma Xenograft. Drug Metab. Dispos. 2015, 43, 1360–1371. [Google Scholar] [CrossRef]
- Cersosimo, R.J. Cyclin-dependent kinase 4/6 inhibitors for the management of advanced or metastatic breast cancer in women. Am. J. Health Syst. Pharm. 2019, 76, 1183–1202. [Google Scholar] [CrossRef]
- Knudsen, E.S.; Hutcheson, J.; Vail, P.; Witkiewicz, A.K. Biological specificity of CDK4/6 inhibitors: Dose response relationship, in vivo signaling, and composite response signature. Oncotarget 2017, 8, 43678–43691. [Google Scholar] [CrossRef]
- Spring, L.M.; Zangardi, M.L.; Moy, B.; Bardia, A. Clinical Management of Potential Toxicities and Drug Interactions Related to Cyclin-Dependent Kinase 4/6 Inhibitors in Breast Cancer: Practical Considerations and Recommendations. Oncologist 2017, 22, 1039–1048. [Google Scholar] [CrossRef]
- Braal, C.L.; Jongbloed, E.M.; Wilting, S.M.; Mathijssen, R.H.J.; Koolen, S.L.W.; Jager, A. Inhibiting CDK4/6 in Breast Cancer with Palbociclib, Ribociclib, and Abemaciclib: Similarities and Differences. Drugs 2021, 81, 317–331. [Google Scholar] [CrossRef]
- Tate, S.C.; Burke, T.F.; Hartman, D.; Kulanthaivel, P.; Beckmann, R.P.; Cronier, D.M. Optimising the combination dosing strategy of abemaciclib and vemurafenib in BRAF-mutated melanoma xenograft tumours. Br. J. Cancer 2016, 114, 669–679. [Google Scholar] [CrossRef] [PubMed]
- Finn, R.S.; Crown, J.P.; Ettl, J.; Schmidt, M.; Bondarenko, I.M.; Lang, I.; Pinter, T.; Boer, K.; Patel, R.; Randolph, S.; et al. Efficacy and safety of palbociclib in combination with letrozole as first-line treatment of ER-positive, HER2-negative, advanced breast cancer: Expanded analyses of subgroups from the randomized pivotal trial PALOMA-1/TRIO-18. Breast Cancer Res. 2016, 18, 67. [Google Scholar] [CrossRef] [PubMed]
- Johnston, S.R.D.; Harbeck, N.; Hegg, R.; Toi, M.; Martin, M.; Shao, Z.M.; Zhang, Q.Y.; Martinez Rodriguez, J.L.; Campone, M.; Hamilton, E.; et al. Abemaciclib Combined with Endocrine Therapy for the Adjuvant Treatment of HR+, HER2-, Node-Positive, High-Risk, Early Breast Cancer (monarchE). J. Clin. Oncol. 2020, 38, 3987–3998. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K. Differences of cyclin-dependent kinase 4/6 inhibitor, palbociclib and abemaciclib, in breast cancer. Jpn. J. Clin. Oncol. 2019, 49, 993–998. [Google Scholar] [CrossRef]
- Corona, S.P.; Generali, D. Abemaciclib: A CDK4/6 inhibitor for the treatment of HR+/HER2- advanced breast cancer. Drug Des. Devel. Ther. 2018, 12, 321–330. [Google Scholar] [CrossRef]
- Fasching, P.A.; Beck, J.T.; Chan, A.; De Laurentiis, M.; Esteva, F.J.; Jerusalem, G.; Neven, P.; Pivot, X.; Bianchi, G.V.; Martin, M.; et al. Ribociclib plus fulvestrant for advanced breast cancer: Health-related quality-of-life analyses from the MONALEESA-3 study. Breast 2020, 54, 148–154. [Google Scholar] [CrossRef]
- Casimiro, M.C.; Crosariol, M.; Loro, E.; Ertel, A.; Yu, Z.; Dampier, W.; Saria, E.A.; Papanikolaou, A.; Stanek, T.J.; Li, Z.; et al. ChIP sequencing of cyclin D1 reveals a transcriptional role in chromosomal instability in mice. J. Clin. Investig. 2012, 122, 833–843. [Google Scholar] [CrossRef]
- Duffy, S.; Fam, H.K.; Wang, Y.K.; Styles, E.B.; Kim, J.H.; Ang, J.S.; Singh, T.; Larionov, V.; Shah, S.P.; Andrews, B.; et al. Overexpression screens identify conserved dosage chromosome instability genes in yeast and human cancer. Proc. Natl. Acad. Sci. USA 2016, 113, 9967–9976. [Google Scholar] [CrossRef]
- Caruso, J.A.; Duong, M.T.; Carey, J.P.W.; Hunt, K.K.; Keyomarsi, K. Low-Molecular-Weight Cyclin E in Human Cancer: Cellular Consequences and Opportunities for Targeted Therapies. Cancer Res. 2018, 78, 5481–5491. [Google Scholar] [CrossRef]
- Guiley, K.Z.; Stevenson, J.W.; Lou, K.; Barkovich, K.J.; Kumarasamy, V.; Wijeratne, T.U.; Bunch, K.L.; Tripathi, S.; Knudsen, E.S.; Witkiewicz, A.K.; et al. p27 allosterically activates cyclin-dependent kinase 4 and antagonizes palbociclib inhibition. Science 2019, 366, eaaw2106. [Google Scholar] [CrossRef]
- Gomatou, G.; Trontzas, I.; Ioannou, S.; Drizou, M.; Syrigos, N.; Kotteas, E. Mechanisms of resistance to cyclin-dependent kinase 4/6 inhibitors. Mol. Biol. Rep. 2021, 48, 915–925. [Google Scholar] [CrossRef] [PubMed]
- Herrera-Abreu, M.T.; Palafox, M.; Asghar, U.; Rivas, M.A.; Cutts, R.J.; Garcia-Murillas, I.; Pearson, A.; Guzman, M.; Rodriguez, O.; Grueso, J.; et al. Early Adaptation and Acquired Resistance to CDK4/6 Inhibition in Estrogen Receptor-Positive Breast Cancer. Cancer Res. 2016, 76, 2301–2313. [Google Scholar] [CrossRef] [PubMed]
- O’Leary, B.; Finn, R.S.; Turner, N.C. Treating cancer with selective CDK4/6 inhibitors. Nat. Rev. Clin. Oncol. 2016, 13, 417–430. [Google Scholar] [CrossRef] [PubMed]
- Guarducci, C.; Bonechi, M.; Boccalini, G.; Benelli, M.; Risi, E.; Di Leo, A.; Malorni, L.; Migliaccio, I. Mechanisms of Resistance to CDK4/6 Inhibitors in Breast Cancer and Potential Biomarkers of Response. Breast Care 2017, 12, 304–308. [Google Scholar] [CrossRef]
- Malorni, L.; Piazza, S.; Ciani, Y.; Guarducci, C.; Bonechi, M.; Biagioni, C.; Hart, C.D.; Verardo, R.; Di Leo, A.; Migliaccio, I. A gene expression signature of retinoblastoma loss-of-function is a predictive biomarker of resistance to palbociclib in breast cancer cell lines and is prognostic in patients with ER positive early breast cancer. Oncotarget 2016, 7, 68012–68022. [Google Scholar] [CrossRef]
- Condorelli, R.; Spring, L.; O’Shaughnessy, J.; Lacroix, L.; Bailleux, C.; Scott, V.; Dubois, J.; Nagy, R.J.; Lanman, R.B.; Iafrate, A.J.; et al. Polyclonal RB1 mutations and acquired resistance to CDK 4/6 inhibitors in patients with metastatic breast cancer. Ann. Oncol. 2018, 29, 640–645. [Google Scholar] [CrossRef]
- Gao, X.; Leone, G.W.; Wang, H. Cyclin D-CDK4/6 functions in cancer. Adv. Cancer Res. 2020, 148, 147–169. [Google Scholar] [CrossRef]
- Milioli, H.H.; Alexandrou, S.; Lim, E.; Caldon, C.E. Cyclin E1 and cyclin E2 in ER + breast cancer: Prospects as biomarkers and therapeutic targets. Endocr. Relat. Cancer 2020, 27, R93–R112. [Google Scholar] [CrossRef]
- Turner, N.C.; Liu, Y.; Zhu, Z.; Loi, S.; Colleoni, M.; Loibl, S.; DeMichele, A.; Harbeck, N.; Andre, F.; Bayar, M.A.; et al. Cyclin E1 Expression and Palbociclib Efficacy in Previously Treated Hormone Receptor-Positive Metastatic Breast Cancer. J. Clin. Oncol. 2019, 37, 1169–1178. [Google Scholar] [CrossRef]
- Finn, R.S.; Liu, Y.; Zhu, Z.; Martin, M.; Rugo, H.S.; Dieras, V.; Im, S.A.; Gelmon, K.A.; Harbeck, N.; Lu, D.R.; et al. Biomarker Analyses of Response to Cyclin-Dependent Kinase 4/6 Inhibition and Endocrine Therapy in Women with Treatment-Naive Metastatic Breast Cancer. Clin. Cancer Res. 2020, 26, 110–121. [Google Scholar] [CrossRef]
- O’Leary, B.; Cutts, R.J.; Liu, Y.; Hrebien, S.; Huang, X.; Fenwick, K.; Andre, F.; Loibl, S.; Loi, S.; Garcia-Murillas, I.; et al. The Genetic Landscape and Clonal Evolution of Breast Cancer Resistance to Palbociclib plus Fulvestrant in the PALOMA-3 Trial. Cancer Discov. 2018, 8, 1390–1403. [Google Scholar] [CrossRef]
- Wei, G.; Wang, Y.; Zhang, P.; Lu, J.; Mao, J.H. Evaluating the prognostic significance of FBXW7 expression level in human breast cancer by a meta-analysis of transcriptional profiles. J. Cancer Sci. Ther. 2012, 4, 299–305. [Google Scholar] [CrossRef]
- Arnedos, M.; Bayar, M.A.; Cheaib, B.; Scott, V.; Bouakka, I.; Valent, A.; Adam, J.; Leroux-Kozal, V.; Marty, V.; Rapinat, A.; et al. Modulation of Rb phosphorylation and antiproliferative response to palbociclib: The preoperative-palbociclib (POP) randomized clinical trial. Ann. Oncol. 2018, 29, 1755–1762. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zou, W.; Zhang, J.; Zhang, Y.; Xu, Q.; Li, S.; Chen, C. Mechanisms of CDK4/6 Inhibitor Resistance in Luminal Breast Cancer. Front. Pharmacol. 2020, 11, 580251. [Google Scholar] [CrossRef] [PubMed]
- Pandey, K.; Park, N.; Park, K.S.; Hur, J.; Cho, Y.B.; Kang, M.; An, H.J.; Kim, S.; Hwang, S.; Moon, Y.W. Combined CDK2 and CDK4/6 Inhibition Overcomes Palbociclib Resistance in Breast Cancer by Enhancing Senescence. Cancers 2020, 12, 3566. [Google Scholar] [CrossRef] [PubMed]
- Sharifi, M.N.; Anandan, A.; Grogan, P.; O’Regan, R.M. Therapy after cyclin-dependent kinase inhibition in metastatic hormone receptor-positive breast cancer: Resistance mechanisms and novel treatment strategies. Cancer 2020, 126, 3400–3416. [Google Scholar] [CrossRef]
- Hui, R.; Macmillan, R.D.; Kenny, F.S.; Musgrove, E.A.; Blamey, R.W.; Nicholson, R.I.; Robertson, J.F.; Sutherland, R.L. INK4a gene expression and methylation in primary breast cancer: Overexpression of p16INK4a messenger RNA is a marker of poor prognosis. Clin. Cancer Res. 2000, 6, 2777–2787. [Google Scholar]
- Lebok, P.; Roming, M.; Kluth, M.; Koop, C.; Özden, C.; Taskin, B.; Hussein, K.; Leveau, A.; Witzel, I.; Wölber, L.; et al. p16 overexpression and 9p21 deletion are linked to unfavorable tumor phenotype in breast cancer. Oncotarget 2016, 7, 81322–81331. [Google Scholar] [CrossRef]
- Herman, J.G.; Merlo, A.; Mao, L.; Lapidus, R.G.; Issa, J.P.; Davidson, N.E.; Sidransky, D.; Baylin, S.B. Inactivation of the CDKN2/p16/MTS1 gene is frequently associated with aberrant DNA methylation in all common human cancers. Cancer Res. 1995, 55, 4525–4530. [Google Scholar]
- D’Amico, M.; Wu, K.; Di Vizio, D.; Reutens, A.T.; Stahl, M.; Fu, M.; Albanese, C.; Russell, R.G.; Muller, W.J.; White, M.; et al. The role of Ink4a/Arf in ErbB2 mammary gland tumorigenesis. Cancer Res. 2003, 63, 3395–3402. [Google Scholar]
- Hart, C.D.; Migliaccio, I.; Malorni, L.; Guarducci, C.; Biganzoli, L.; Di Leo, A. Challenges in the management of advanced, ER-positive, HER2-negative breast cancer. Nat. Rev. Clin. Oncol. 2015, 12, 541–552. [Google Scholar] [CrossRef] [PubMed]
- Kollmann, K.; Heller, G.; Schneckenleithner, C.; Warsch, W.; Scheicher, R.; Ott, R.G.; Schafer, M.; Fajmann, S.; Schlederer, M.; Schiefer, A.I.; et al. A kinase-independent function of CDK6 links the cell cycle to tumor angiogenesis. Cancer Cell 2013, 24, 167–181. [Google Scholar] [CrossRef] [PubMed]
- Gacche, R.N.; Assaraf, Y.G. Redundant angiogenic signaling and tumor drug resistance. Drug Resist. Updates 2018, 36, 47–76. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Li, Z.; Bhatt, T.; Dickler, M.; Giri, D.; Scaltriti, M.; Baselga, J.; Rosen, N.; Chandarlapaty, S. Acquired CDK6 amplification promotes breast cancer resistance to CDK4/6 inhibitors and loss of ER signaling and dependence. Oncogene 2017, 36, 2255–2264. [Google Scholar] [CrossRef]
- Kwon, N.H.; Lee, J.Y.; Ryu, Y.L.; Kim, C.; Kong, J.; Oh, S.; Kang, B.S.; Ahn, H.W.; Ahn, S.G.; Jeong, J.; et al. Stabilization of Cyclin-Dependent Kinase 4 by Methionyl-tRNA Synthetase in p16(INK4a)-Negative Cancer. ACS Pharmacol. Transl. Sci. 2018, 1, 21–31. [Google Scholar] [CrossRef]
- Du, Q.; Guo, X.; Wang, M.; Li, Y.; Sun, X.; Li, Q. The application and prospect of CDK4/6 inhibitors in malignant solid tumors. J. Hematol. Oncol. 2020, 13, 41. [Google Scholar] [CrossRef]
- Jafarzadeh, M.; Mousavizadeh, K.; Joghataei, M.T.; Bahremani, M.H.; Safa, M.; Asghari, S.M. A Fibroblast Growth Factor Antagonist Peptide Inhibits Breast Cancer in BALB/c Mice. Open Life Sci. 2018, 13, 348–354. [Google Scholar] [CrossRef]
- Sahores, A.; May, M.; Sequeira, G.R.; Fuentes, C.; Jacobsen, B.; Lanari, C.; Lamb, C.A. Targeting FGFR with BGJ398 in Breast Cancer: Effect on Tumor Growth and Metastasis. Curr. Cancer Drug Targets 2018, 18, 979–987. [Google Scholar] [CrossRef]
- Drago, J.Z.; Formisano, L.; Juric, D.; Niemierko, A.; Servetto, A.; Wander, S.A.; Spring, L.M.; Vidula, N.; Younger, J.; Peppercorn, J.; et al. FGFR1 Amplification Mediates Endocrine Resistance but Retains TORC Sensitivity in Metastatic Hormone Receptor-Positive (HR(+)) Breast Cancer. Clin. Cancer Res. 2019, 25, 6443–6451. [Google Scholar] [CrossRef]
- Lv, Q.; Guan, S.; Zhu, M.; Huang, H.; Wu, J.; Dai, X. FGFR1 Is Associated with Tamoxifen Resistance and Poor Prognosis of ER-Positive Breast Cancers by Suppressing ER Protein Expression. Technol. Cancer Res. Treat. 2021, 20, 15330338211004935. [Google Scholar] [CrossRef]
- Takeshita, T.; Yamamoto, Y.; Yamamoto-Ibusuki, M.; Tomiguchi, M.; Sueta, A.; Murakami, K.; Iwase, H. Clinical significance of plasma cell-free DNA mutations in PIK3CA, AKT1, and ESR1 gene according to treatment lines in ER-positive breast cancer. Mol. Cancer 2018, 17, 67. [Google Scholar] [CrossRef]
- Michaloglou, C.; Crafter, C.; Siersbaek, R.; Delpuech, O.; Curwen, J.O.; Carnevalli, L.S.; Staniszewska, A.D.; Polanska, U.M.; Cheraghchi-Bashi, A.; Lawson, M.; et al. Combined Inhibition of mTOR and CDK4/6 Is Required for Optimal Blockade of E2F Function and Long-term Growth Inhibition in Estrogen Receptor-positive Breast Cancer. Mol. Cancer Ther. 2018, 17, 908–920. [Google Scholar] [CrossRef] [PubMed]
- Ye, N.; Ding, Y.; Wild, C.; Shen, Q.; Zhou, J. Small molecule inhibitors targeting activator protein 1 (AP-1). J. Med. Chem. 2014, 57, 6930–6948. [Google Scholar] [CrossRef] [PubMed]
- Pandey, K.; An, H.J.; Kim, S.K.; Lee, S.A.; Kim, S.; Lim, S.M.; Kim, G.M.; Sohn, J.; Moon, Y.W. Molecular mechanisms of resistance to CDK4/6 inhibitors in breast cancer: A review. Int. J. Cancer 2019, 145, 1179–1188. [Google Scholar] [CrossRef] [PubMed]
- Katiyar, S.; Casimiro, M.C.; Dettin, L.; Ju, X.; Wagner, E.F.; Tanaka, H.; Pestell, R.G. C-jun inhibits mammary apoptosis in vivo. Mol. Biol. Cell 2010, 21, 4264–4274. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Vleugel, M.M.; Greijer, A.E.; Bos, R.; van der Wall, E.; van Diest, P.J. c-Jun activation is associated with proliferation and angiogenesis in invasive breast cancer. Hum. Pathol. 2006, 37, 668–674. [Google Scholar] [CrossRef]
- Jiao, X.; Katiyar, S.; Willmarth, N.E.; Liu, M.; Ma, X.; Flomenberg, N.; Lisanti, M.P.; Pestell, R.G. c-Jun induces mammary epithelial cellular invasion and breast cancer stem cell expansion. J. Biol. Chem. 2010, 285, 8218–8226. [Google Scholar] [CrossRef]
- Katiyar, S.; Jiao, X.; Wagner, E.; Lisanti, M.P.; Pestell, R.G. Somatic excision demonstrates that c-Jun induces cellular migration and invasion through induction of stem cell factor. Mol. Cell Biol. 2007, 27, 1356–1369. [Google Scholar] [CrossRef][Green Version]
- Jiao, X.; Katiyar, S.; Liu, M.; Mueller, S.C.; Lisanti, M.P.; Li, A.; Pestell, T.G.; Wu, K.; Ju, X.; Li, Z.; et al. Disruption of c-Jun reduces cellular migration and invasion through inhibition of c-Src and hyperactivation of ROCK II kinase. Mol. Biol. Cell 2008, 19, 1378–1390. [Google Scholar] [CrossRef][Green Version]
- Pestell, T.G.; Jiao, X.; Kumar, M.; Peck, A.R.; Prisco, M.; Deng, S.; Li, Z.; Ertel, A.; Casimiro, M.C.; Ju, X.; et al. Stromal cyclin D1 promotes heterotypic immune signaling and breast cancer growth. Oncotarget 2017, 8, 81754–81775. [Google Scholar] [CrossRef]
- Gaynor, N.; Crown, J.; Collins, D.M. Immune checkpoint inhibitors: Key trials and an emerging role in breast cancer. Semin. Cancer Biol. 2022, 79, 44–57. [Google Scholar] [CrossRef] [PubMed]
- Demuth, C.; Andersen, M.N.; Jakobsen, K.R.; Madsen, A.T.; Sorensen, B.S. Increased PD-L1 expression in erlotinib-resistant NSCLC cells with MET gene amplification is reversed upon MET-TKI treatment. Oncotarget 2017, 8, 68221–68229. [Google Scholar] [CrossRef] [PubMed]
- Goel, S.; DeCristo, M.J.; Watt, A.C.; BrinJones, H.; Sceneay, J.; Li, B.B.; Khan, N.; Ubellacker, J.M.; Xie, S.; Metzger-Filho, O.; et al. CDK4/6 inhibition triggers anti-tumour immunity. Nature 2017, 548, 471–475. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Wang, E.S.; Jenkins, R.W.; Li, S.; Dries, R.; Yates, K.; Chhabra, S.; Huang, W.; Liu, H.; Aref, A.R.; et al. CDK4/6 Inhibition Augments Antitumor Immunity by Enhancing T-cell Activation. Cancer Discov. 2018, 8, 216–233. [Google Scholar] [CrossRef]
- Fallah, Y.; Demas, D.M.; Jin, L.; He, W.; Shajahan-Haq, A.N. Targeting WEE1 Inhibits Growth of Breast Cancer Cells That Are Resistant to Endocrine Therapy and CDK4/6 Inhibitors. Front. Oncol. 2021, 11, 681530. [Google Scholar] [CrossRef]
- Shih, I.M.; Zhou, W.; Goodman, S.N.; Lengauer, C.; Kinzler, K.W.; Vogelstein, B. Evidence that genetic instability occurs at an early stage of colorectal tumorigenesis. Cancer Res. 2001, 61, 818–822. [Google Scholar]
- Kops, G.J.; Weaver, B.A.; Cleveland, D.W. On the road to cancer: Aneuploidy and the mitotic checkpoint. Nat. Rev. Cancer 2005, 5, 773–785. [Google Scholar] [CrossRef]
- Spruck, C.H.; Won, K.A.; Reed, S.I. Deregulated cyclin E induces chromosome instability. Nature 1999, 401, 297–300. [Google Scholar] [CrossRef]
- Nelsen, C.J.; Kuriyama, R.; Hirsch, B.; Negron, V.C.; Lingle, W.L.; Goggin, M.M.; Stanley, M.W.; Albrecht, J.H. Short term cyclin D1 overexpression induces centrosome amplification, mitotic spindle abnormalities, and aneuploidy. J. Biol. Chem. 2005, 280, 768–776. [Google Scholar] [CrossRef]
- Aggarwal, P.; Lessie, M.D.; Lin, D.I.; Pontano, L.; Gladden, A.B.; Nuskey, B.; Goradia, A.; Wasik, M.A.; Klein-Szanto, A.J.; Rustgi, A.K.; et al. Nuclear accumulation of cyclin D1 during S phase inhibits Cul4-dependent Cdt1 proteolysis and triggers p53-dependent DNA rereplication. Genes Dev. 2007, 21, 2908–2922. [Google Scholar] [CrossRef]
- Del Rey, J.; Prat, E.; Ponsa, I.; Lloreta, J.; Gelabert, A.; Algaba, F.; Camps, J.; Miro, R. Centrosome clustering and cyclin D1 gene amplification in double minutes are common events in chromosomal unstable bladder tumors. BMC Cancer 2010, 10, 280. [Google Scholar] [CrossRef] [PubMed]
- Manchado, E.; Malumbres, M. Targeting aneuploidy for cancer therapy. Cell 2011, 144, 465–466. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tang, Y.C.; Williams, B.R.; Siegel, J.J.; Amon, A. Identification of aneuploidy-selective antiproliferation compounds. Cell 2011, 144, 499–512. [Google Scholar] [CrossRef] [PubMed]
- Knudsen, E.S.; Kumarasamy, V.; Nambiar, R.; Pearson, J.D.; Vail, P.; Rosenheck, H.; Wang, J.; Eng, K.; Bremner, R.; Schramek, D.; et al. CDK/cyclin dependencies define extreme cancer cell-cycle heterogeneity and collateral vulnerabilities. Cell Rep. 2022, 38, 110448. [Google Scholar] [CrossRef]
- Kumarasamy, V.; Nambiar, R.; Wang, J.; Rosenheck, H.; Witkiewicz, A.K.; Knudsen, E.S. RB loss determines selective resistance and novel vulnerabilities in ER-positive breast cancer models. Oncogene 2022, 41, 3524–3538. [Google Scholar] [CrossRef]


| Drug | Mechanism of Action IC50 (nM) | Recommended Dose Half-Life Tmax | Drug Interactions IC50 against Bone Marrow Mononuclear Cells (nM) | References |
|---|---|---|---|---|
| Palbociclib | Similar potency against cyclin D1/CDK4 and cyclin D2/CDK6 | 125 mg po once a day for 21 days in a 28-day cycle with food | CYP3A4 substrate 240 ± 43 | [34,35,36,37,38] |
| CDK4–cyclin D1 | 11 | |||
| CDK6–cyclin D1/2/3 | 16 | Half-life | ||
| CDK1–cyclin B | >10,000 | 26–27 h | ||
| CDK2–cyclin A/E | >10,000 | Tmax | ||
| CDK5–p25 | >10,000 | 6–12 h | ||
| Ribociclib | Greater potency against CDK4 than CDK 6 | 600 mg po once daily for 21 days in a 28- day cycle with or without food | CYP3A4 substrate 1700 ± 231 | [39,40] |
| CDK4–cyclin D1 | 10 | |||
| CDK6–cyclin D1/2/3 | 39 | Half-life | ||
| CDK1–cyclin B | 113,000 | 33–42 h | ||
| CDK2–cyclin A/E | 76,000 | Tmax | ||
| CDK5–p25 | 43,900 | 1–5 h | ||
| Abemaciclib | Greater potency against CDK4 than CDK 6 (CDK4 and CDK6 with IC50 of 2 nM and 10 nM | 150 mg or 200 mg po BID with or without food. | CYP3A4 substrate, BCRP, Pgp 230 ± 27 | [4,41,42,43] |
| CDK4–cyclin D1 | 2 | |||
| CDK6–cyclin D1/2/3 | 10 | Half-life | ||
| CDK1–cyclin B | 1627 | 17–38 h | ||
| CDK2–cyclin A/E | 504 | Tmax | ||
| CDK5–p25 | 355 | 4–6 h |
| DRUG | TRIAL | TARGET POPULATION | EXPERIMENTALGROUP | CONTROL GROUP | CHANGE IN PFS | CHANGE IN OS |
|---|---|---|---|---|---|---|
| PALBOCICLIB | PALOMA-1 | 165 postmenopausal | +Letrozole | Letrozole monotherapy | 20.2 vs. 12.9 months [34,35,53,54,61] | [34,35,53,54,61] |
| PALOMA-2 | 666 postmenopausal | Placebo + Letrozole | 24.8 vs. 14.5 months [34,35,53,61] | |||
| PALOMA-3 | 521 pre/peri/post-menopausal | +Fulvestrant | Placebo + Fulvestrant | 9.2 vs. 3.8 months [34,35,53,57,61] | 6 years OS, 19.1% vs. 12.9% [62] | |
| PALLAS | 5796 (5761 included in analysis) | +Endocrine adjuvant | Endocrine adjuvant | N/A [34,35,53,59] | ||
| PENELOPE-B | 1250 included in analysis | +standard endocrine adjuvant | Placebo + standard endocrine adjuvant | N/A [60] | ||
| RIBOCICLIB | MONALEESA-2 | 668 postmenopausal | +Letrozole | Placebo + Letrozole | 20.5 vs. 12.8 months [53,61,63,64] [53,63,64] | |
| MONALEESA-3 | 726 men and postmenopausal women with prior exposure to ET | +Fulvestrant | Placebo + Fulvestrant | 42 months OS 57.8% vs. 45.9% [65] | ||
| MONALEESA-7 | 672 pre/peri-menopausal | +Fulvestrant + Goserelin | ET + Goserelin | 23.8 vs. 13.0 months [53,61,63,64] | ||
| ABEMACICLIB | MONARCH-1 | 132 with prior ET or chemo exposure | Monotherapy | N/A | 16.4 vs. 9.3 months [42,53,61] [53,66] | |
| MONARCH-2 | 669 with prior ET exposure | +Fulvestrant | Placebo + Fulvestrant | 46.7 months OS vs. 37.3 months [67] | ||
| MONARCH-3 | 493 postmenopausal | +Letrozole | Placebo + Letrozole | 28.2 vs. 14.8 months [53,61,68] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdelmalak, M.; Singh, R.; Anwer, M.; Ivanchenko, P.; Randhawa, A.; Ahmed, M.; Ashton, A.W.; Du, Y.; Jiao, X.; Pestell, R. The Renaissance of CDK Inhibitors in Breast Cancer Therapy: An Update on Clinical Trials and Therapy Resistance. Cancers 2022, 14, 5388. https://doi.org/10.3390/cancers14215388
Abdelmalak M, Singh R, Anwer M, Ivanchenko P, Randhawa A, Ahmed M, Ashton AW, Du Y, Jiao X, Pestell R. The Renaissance of CDK Inhibitors in Breast Cancer Therapy: An Update on Clinical Trials and Therapy Resistance. Cancers. 2022; 14(21):5388. https://doi.org/10.3390/cancers14215388
Chicago/Turabian StyleAbdelmalak, Mary, Rajanbir Singh, Mohammed Anwer, Pavel Ivanchenko, Amritdeep Randhawa, Myra Ahmed, Anthony W. Ashton, Yanming Du, Xuanmao Jiao, and Richard Pestell. 2022. "The Renaissance of CDK Inhibitors in Breast Cancer Therapy: An Update on Clinical Trials and Therapy Resistance" Cancers 14, no. 21: 5388. https://doi.org/10.3390/cancers14215388
APA StyleAbdelmalak, M., Singh, R., Anwer, M., Ivanchenko, P., Randhawa, A., Ahmed, M., Ashton, A. W., Du, Y., Jiao, X., & Pestell, R. (2022). The Renaissance of CDK Inhibitors in Breast Cancer Therapy: An Update on Clinical Trials and Therapy Resistance. Cancers, 14(21), 5388. https://doi.org/10.3390/cancers14215388











