Simple Summary
Diffuse large B-cell lymphoma (DLBCL) frequently occurs in HIV-infected patients. However, the effect of HIV infection on the outcome of the DLBCL population remains controversial. The aim of the present retrospective study was to compare clinical features, phenotypic markers and outcomes of BLBCL between HIV-infected and HIV-uninfected Chinese patients. Our study indicated HIV-infected DLBCL patients displayed high EBER expression but low CD20 and CD79a expression on histopathology. The overall response rate at end of chemotherapy and 1-year overall survival (OS) were low in HIV-infected patients, which may be associated with the higher incidence of leukopenia, neutropenia, thrombocytopenia and hypoalbuminemia, as well as high involvement of the central nervous system (CNS), gastrointestinal tract and bone marrow. Hypoalbuminemia and CNS involvement were independent risk factors for 1-year OS. HIV-infected DLBCL patients without CNS involvement had a favorable outcome if rituximab was included in the chemotherapy regimen.
Abstract
Background: The effect of HIV infection on the clinicopathological characteristics of diffuse large B-cell lymphoma (DLBCL) remains debatable. Methods: Fifty-three HIV-infected and ninety-three HIV-uninfected DLBCL patients were enrolled in the retrospective study by propensity score matching for sex, age, body mass index and international prognostic index (IPI) at a ratio of 1:2. The clinicopathological characteristics were compared between the two groups. Results: HIV-infected DLBCL patients had lower white blood cell counts [×109/L; 4.4 (3.4–5.6) vs. 6.1 (4.2–8.2), p < 0.001], platelet counts (×109/L; 184.7 ± 89.3 vs. 230.0 ± 113.9, p = 0.014) and serum albumin (g/L; 37.3 ± 6.9 vs. 41.3 ± 6.2, p < 0.001) but higher incidences of central nervous system (CNS) involvement (9.4% vs. 1.1%, p = 0.014), bone marrow involvement (24.5% vs. 11.5%, p = 0.044) and Epstein–Barr viremia (61.1% vs. 26.7%, p = 0.002) than HIV-uninfected patients. In terms of histopathology, HIV-infected patients had higher positivity of Epstein–Barr virus-encoded small RNA (EBER) (41.7% vs. 6.7%, p = 0.002), but lower CD20 (90.2% vs. 98.7%, p= 0.029) and CD79a (23.1% vs. 53.7%, p < 0.001) expression. The overall response rate (ORR) at the end of chemotherapy (70.2% vs. 87.8%, p= 0.012) and 1-year overall survival (OS) (61.7% vs. 84.2%, log-rank p = 0.006) in HIV-infected patients were significantly lower than those in HIV-uninfected patients. Multivariate analysis suggested IPI ≤2.0 [adjusted odds ratio (AOR) (95% confidence interval): 5.0 (1.2–21.2), p = 0.030] was associated with ORR, hypoalbuminemia [AOR: 3.3 (1.3–9.1), p = 0.018] and CNS involvement [AOR: 3.3 (1.0–10.5), p = 0.044] were associated with reduced 1-year OS in HIV-infected patients. Conclusion: HIV-infected DLBCL patients have unique blood profiles and phenotypic markers. Low ORR and 1-year OS were observed in HIV-infected DLBCL patients in our study, even in the HAART era.
1. Introduction
Human immunodeficiency virus (HIV) is associated with an increased incidence of malignances [1]. In particular, HIV-related non-Hodgkin lymphoma (NHL), including diffuse large B-cell lymphoma (DLBCL), is the most common hematological malignancy [2]. Although the incidence of NHL was significantly decreased in the highly active antiretroviral treatment (HAART) era, HIV-infected patients were at about 500-fold risk of NHL compared to HIV-uninfected patients [3,4]. Importantly, DLBCL is the most common subtype, accounting for 35–60% of all cases of reported NHL [5,6].
Currently, HIV infection is thought to contribute to lymphomagenesis through two indirect mechanisms as follows: (a) induction of B-cell activation and chronic inflammation. Some HIV-1 structural and regulatory proteins, such as gp120, Tat and p17 [7,8,9], induce chronic inflammation and persistent immune cell activation, which are key contributors to the development of NHL in the HIV-infected population [10,11]; and (b) loss of immunoregulatory control of oncogenic herpesviruses. HIV infection is associated with a high prevalence of Epstein–Barr virus (EBV) infection [11,12]. EBV expresses some oncogenic proteins, such as LMP1 and EBNA2, which have tumorigenic functions [13]. EBV infection is detected in a substantial proportion of HIV-related DLBCL patients [14]. Furthermore, the consequent attenuation of tumor surveillance caused by EBV and the HIV-related inflammatory environment increases the risk of lymphoma occurrence [12,15].
The effect of HIV infection on the outcome of the DLBCL population remains controversial. Some studies suggested that HIV infection was associated with lower overall survival rate. Meanwhile, some studies indicated HIV infection was not associated with poor prognosis of DLBCL patients [16,17,18]. In addition, HIV infection may alter phenotypic markers of DLBCL. For example, EBV-DNA and EBV-related virus proteins are more frequently detected in HIV-infected NHL patients than in HIV-uninfected NHL patients [11,13]. Exploring the different phenotypic characteristics between HIV-infected and HIV-uninfected DLBCL patients is helpful to understand the effect of HIV infection on DLBCL and even the outcome of patients.
Currently, the data on HIV-infected Chinese DLBCL patients are uncomprehensive. Some studies illustrated well the basic clinical characteristics of HIV-related DLBCL based on a small Chinese population [19,20,21]. Thus, a further study is necessary to enrich the data of HIV-infected Chinese DLBCL patients. In an effort to clarify this, we conducted a study to better understand the role of HIV infection in DLBCL. In the present retrospective study, 53 HIV-infected and 93 HIV-uninfected patients were enrolled after propensity score matching for sex, age, body mass index (BMI) and international prognosis index (IPI) at a ratio of 1:2. We compared the differences in laboratory tests, molecular characteristics and 1-year outcomes between the two groups, aiming to outline the different clinical features of DLBCL between HIV-infected and HIV-uninfected Chinese patients.
2. Methods
2.1. Study Design and Patient Selection
Between January 2015 and December 2020, 342 (56 HIV-infected and 286 HIV-uninfected) DBBCL patients from the First Affiliated Hospital, College of Medicine, Zhejiang University, China, were eligible for enrollment in this retrospective study. Patients were excluded if they had liver cirrhosis, congestive heart failure, chronic kidney disorders, chronic obstructive pulmonary disease (COPD), solid organ malignancies or NHL relapse. DLBCL was diagnosed by pathological biopsy based on the 2016 revision of the World Health Organization classification of lymphoid neoplasms [22]. Propensity score matching for age, sex, BMI and IPI was used to select patients according to a ratio of 1:2 at a matching tolerance of 0.2. After 13 repeated cases were excluded, 53 HIV-infected and 93 HIV-uninfected patients were enrolled in the present study. The flowchart of patient selection is shown in Figure 1. Basic medical information of patients and chemotherapy regimens were recorded in the electronic medical records system (EMRS) of the hospital. Cases were followed up to December 2021 by clinical record, medical visiting and telephone interview.
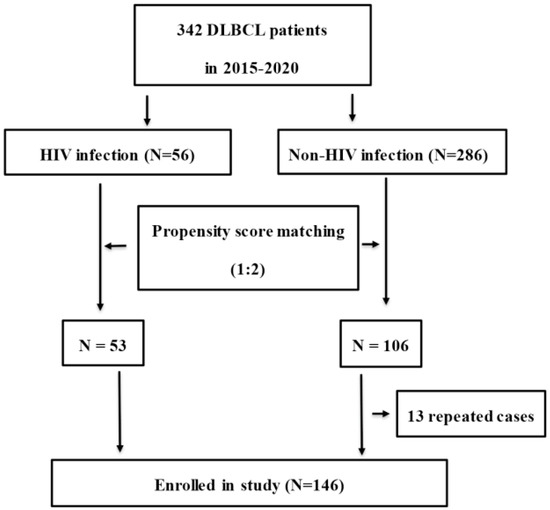
Figure 1.
Study flowchart for patient selection. (DLBCL: diffuse large B-cell lymphoma).
2.2. Chemotherapy
The following chemotherapy regimens were included in the present study: CHOP ± R, EPOCH ± R and hyper CVAD/MA. The dosage and protocol of each chemotherapy are listed in Supplementary Table S1. Chemotherapy response was classified as complete response (CR), partial response (PR), stable disease (SD) or progressive disease (PD) according to the 2014 revised-response criteria for malignant lymphoma [23]. Overall response rate (ORR) was calculated as (the numbers of patients with CR + numbers of patients with PR)/total numbers of patients who accepted chemotherapy.
2.3. Statistical Analysis
Continuous normal variables are presented as the mean ± standard deviation, and continuous abnormal variables are presented as the median (interquartile range). Categorical variables are presented as the percentage (number). Student’s t-test or the Mann–Whitney U test was used to compare continuous variables. Chi-square or Fisher’s exact test was used to compare categorical variables. A receiver operating characteristic (ROC) curve was used to select the best threshold value. Logistic regression was used for the multivariate analysis of ORR. Patient survival was analyzed by the Kaplan–Meier method and Cox proportional hazards model. Lymphoma-related death was defined as an ‘event’. Factors with significance (p < 0.200) in univariate analysis were further analyzed in the multivariate Cox proportional hazards model or logistic regression by the forward stepwise (likelihood ratio) method. The odds ratio (OR) and 95% confidence interval (CI) were used to assess differences in survival between HIV-infected and HIV-uninfected groups. p < 0.050 was considered significant. All statistical data were analyzed using SPSS software 26.0 (SPSS Institute, Chicago, IL, USA) and GraphPad Prism 7.0 (GraphPad Software, La Jolla, CA, USA).
2.4. Ethics Approval
The research protocols were in accordance with the 1975 Declaration of Helsinki and received ethical approval from the Ethics Committee of the First Affiliated Hospital, College of Medicine, Zhejiang University (No. 2021-139). All data were analyzed anonymously. The committee waived the requirement for written informed consent from the participants.
3. Results
3.1. Baseline Characteristics of Patients before and after Propensity Score Matching
After propensity score matching, HIV-infected patients had lower WBCs [×109/L; 4.4 (3.4–5.6) vs. 6.1 (4.2–8.2), p < 0.001] and neutrophils [×109/L; 2.6 (1.5–3.8) vs. 3.6 (2.4–5.9), p < 0.001] compared to HIV-uninfected patients. There was no difference in hemoglobin between the two groups (p = 0.314). The platelet numbers in HIV-infected patients were also lower than those in HIV-uninfected patients [×109/L; 184.7 ± 89.3 vs. 230.0 ± 113.9, p = 0.014]. Moreover, the albumin levels in HIV-uninfected patients (41.3 ± 6.2 g/L) were significantly higher than the albumin levels in HIV-infected patients (37.3 ± 6.9 g/L) (p < 0.001). There were no significant differences in the levels of alanine aminotransferase (ALT) (p = 0.382) and creatinine (p = 0.643) between the two groups. The serum urea level in HIV-infected patients was lower than that in HIV-uninfected patients [mmol/L; 4.5 (3.5–5.4) vs. 5.6 (4.6–6.9), p = 0.005).
Of the available data for 81 patients, 61.1% (22/36) HIV-infected patients and 26.7% (12/45) HIV-uninfected patients were with Epstein–Barr viremia (p = 0.002). Furthermore, the level of Epstein–Barr DNA was 4.3 ± 1.1 copies/mL (log10) in HIV-infected patients and 2.9 ± 1.3 copies/mL (log10) in HIV-infected patients (p < 0.001).
HIV-infected patients had higher incidences of CNS involvement (9.4% vs. 1.1%, p = 0.014), digestive involvement (54.7% vs. 18.3%, p < 0.001) and bone marrow involvement [24.5% vs. 11.5%, p = 0.044] compared to HIV-uninfected patients. The detailed characteristics and demographic information before and after propensity score matching were listed in Table 1.

Table 1.
The demographic characteristics of NHL patients with and without HIV infection.
3.2. Phenotypic Markers and Histopathology of HIV-Infected and HIV-Uninfected Patients
In the present study, phenotypic markers (CD3, CD5, CD10, CD20, CD21, CD56, CD79a, CD30, Bcl-2, Bcl-6, ALK, MUM1, Ki-67, PAX-5, CyclinD1 and MYC) were assayed on histopathology (Table 2). EBER was assayed by in situ hybridization. Of these markers, HIV-infected DLBCL patients had a high expression rate of EBER (41.7% vs. 6.7%, p = 0.002) but low expression rates of CD20 (90.2% vs. 98.7%, p = 0.029) and CD79a (23.1% vs. 53.7%, p < 0.001). No obvious differences in the frequencies of other molecular marks were found. Additionally, CD10, Bcl-6 and MUM1 expression were used to divide NHL into germinal center B-cell-like (GCB) and non-germinal center B-cell-like (non-GCB) subgroups according to Hans algorithm. Among the DLBCL patients, 44.7% (21/47) of HIV-infected patients and 34.2% (26/76) of HIV-uninfected patients were considered GCB (p = 0.208).

Table 2.
Comparison of phenotypic markers in DLBCL patients with and without HIV infection.
3.3. Chemotherapy Associated ORR between HIV-Infected and HIV-Uninfected DLBCL Patients
Of 146 DLBCL patients, 137 (93.8%) patients accepted chemotherapy, and 9 (6.2%) patients did not accept chemotherapy. Notably, 6 (11.3%) of HIV-infected patients and 3 (3.2%) of HIV-uninfected patients abandoned treatment (p = 0.073).
Of 137 patients who accepted chemotherapy, 103 (75.2%) patients were treated with the CHOP ± R regimen, 9 (6.6%) patients were treated with the EOPCH ± R regimen, 2 (1.5%) patients were treated with the hyper CVAD/MA regimen, and 23 (16.8%) patients were treated with other chemotherapy regimens. Moreover, 112 (81.8%) patients achieved ORR (91 patients achieved CR and 21 patients achieved PR), but 25 (18.2%) patients did not benefit from treatments at the end of 6–8 cycles of chemotherapy. The ORR was close between the CHOP regimen and the non-CHOP regimen among patients who accepted chemotherapy (p = 0.152). The ORR was 70.2% (33/47) in HIV-infected patients and 87.8% (79/90) in HIV-uninfected patients (p = 0.012), and the ORR was 88.3% (83/94) in patients who were treated with rituximab and 59.6% (31/52) in patients who were treated without rituximab at the end of chemotherapy (p < 0.001).
Factors associated with ORR at the end of chemotherapy were analyzed in patients who accepted chemotherapy. In univariate analysis, serum albumin ≥35.0 g/L [OR: 3.0 (1.1–8.1), p = 0.026] and rituximab usage [OR: 3.6 (1.5–8.9), p = 0.003] were positively related to ORR, whereas HIV infection [OR: 0.3 (0.1–0.8), p = 0.012], CNS involvement [OR: 0.1 (0.0–0.8), p = 0.042] and gastrointestinal involvement [OR: 0.4 (0.2–1.0), p = 0.048] were negatively associated with ORR. In addition, BMI >21.0 kg/m2 [OR: 1.8 (0.8–4.5), p = 0.158] and IPI ≤ 2.0 [OR: 3.0 (1.0–1.4), p = 0.063] were marginally associated with ORR. Multivariate logistic regression analysis indicated that rituximab usage [AOR: 4.0 (1.5–10.2), p = 0.004], no gastrointestinal involvement [AOR: 2.7 (1.1–7.1), p = 0.039] and no CNS involvement [AOR: 9.2 (1.3–65.1), p = 0.027] were independent prognostic factors for ORR. HIV infection was not an independent risk for decreased ORR (p = 0.784) (Table 3).

Table 3.
Risk factors for overall response rate in DLBCL patients who accepted chemotherapy.
Furthermore, the factors associated with ORR in HIV-infected patients were analyzed. We found that IPI ≤ 2.0 [OR: 1.9 (1.1–3.0), p = 0.023], no marrow involvement [OR: 2.2 (0.8–4.9), p = 0.163] and rituximab usage [OR: 2.4 (0.7–8.9), p = 0.170] were factors associated with ORR in univariate model, while multivariate logistic regression analysis indicated that only an IPI ≤ 2.0 [OR: 5.0 (1.2–21.2), p = 0.030] was related to the effectiveness of chemotherapy (Table 3).
3.4. One-Year Overall Survival (OS) of HIV-Infected and HIV-Uninfected DLBCL Patients
Among 137 DLBCL patients who accepted chemotherapy, 15.3% (21/137) died during the follow-up period, including 27.7% (13/47) of HIV-infected patients and 8.9% (8/90) of HIV-uninfected patients (p = 0.004). The 1-year OS was 66.2% in HIV-infected patients and 85.1% in HIV-uninfected patients (log-rank p = 0.006) (Figure 2).
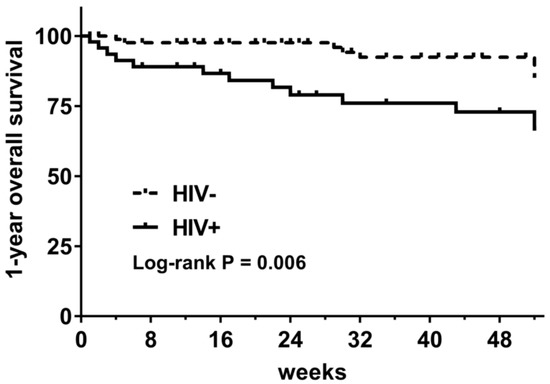
Figure 2.
One-year overall survival of DLBCL patients with and without HIV infection. (HIV−: HIV-uninfected patients; HIV+: HIV-infected patients).
The factors associated with 1-year mortality of patients who accepted chemotherapy were analyzed. In the univariate Cox proportional hazard model, HIV infection [OR: 3.2 (1.3–7.6), p = 0.010], CNS invasion [OR: 3.9 (2.1–13.2), p = 0.030], chemotherapy without rituximab [HR: 4.7 (1.9–11.5), p = 0.001], serum albumin < 35.0 g/L [OR: 4.7 (2.0–11.2), p < 0.001], hemoglobin < 110.0 g/L [OR: 2.0 (0.8–4.7), p = 0.120] and IPI > 2.0 [OR: 4.0 (1.0–5.7), p = 0.054] were closely associated with 1-year mortality. In the multivariate Cox proportional hazard model, we found that CNS invasion [AOR: 3.8 (1.1–13.8), p = 0.039], serum albumin < 35.0 g/L [AOR: 4.4 (1.8–10.9), p = 0.001] and chemotherapy without rituximab [AOR: 3.8 (1.5–9.4), p = 0.005] were independent risk factors for the 1-year mortality rate (Table 4).

Table 4.
Risk factors for 1-year mortality in DLBCL patients in univariate/multivariate Cox proportional hazards models.
Furthermore, survival analysis was performed in HIV-infected patients who received chemotherapy. In the univariate model, we found that only serum albumin ≥ 35.0 g/L [OR: 4.6 (1.5–13.7), p = 0.007] was associated with 1-year OS. In addition, hemoglobin < 110.0 g/L [OR: 2.5 (0.8–7.5), p = 0.101], CNS invasion [OR: 3.1 (0.9–11.3), p = 0.086] and IPI > 2.0 [OR: 2.6 (0.8–8.5), p = 0.111] were marginally related to 1-year mortality. In the multivariate model, CNS invasion [OR: 3.3 (1.0–10.5), p = 0.044] and serum <35.0 g/L [OR: 3.3 (1.3–9.1), p = 0.018] were independent risk factors for the 1-year mortality rate (Table 4).
In addition, among 43 HIV-infected DBLCL patients who accepted chemotherapy and were without CNS invasion, we found that the 1-year mortality was 9.1% (2/22) in those treated with rituximab and 38.1% (8/21) in those treated without rituximab [OR: 6.2 (1.1–33.7), p = 0.024], and the 1-year mortality was 50.0% (6/12) in patients with serum albumin < 35.0 g/L and 9.3% (4/31) in patients with serum albumin ≥35.0 g/L [OR: 6.8 (1.4–31.6), p = 0.010]. No difference in mortality was found between patients with different levels of hemoglobin (g/L; < 110.0 vs. ≥ 110.0, p = 0.131), IPI (≤ 2.0 vs. > 2.0, p = 0.174) and BMI (kg/m2; <21.0 vs. ≥ 21.0, p = 0.440).
4. Discussion
As early as the initial stage of the HIV/AIDS epidemic, the high incidence of AIDS-related malignances, including Kaposi sarcoma and non-Hodgkin’s lymphoma, were predominant hematological malignancies in HIV-infected patients. With the increasing accessibility of antiviral therapy and improving medical care for HIV/AIDS, AIDS-related cancers have dramatically decreased. Meanwhile, the incidence of non-AIDS-related cancers rise in HIV-infected patients in Western countries [24,25]. More recent data from studies of HIV-infected people in the US, Europe and Australia indicated that non-AIDS-related cancers increased, reflecting increasing rates in the general population [26,27]. In developing countries, including China, there are a large proportion of newly-HIV infected people, AIDS-related cancers are the most common malignances [24,28].
To date, the differences in the clinical and molecular characteristics of HIV-infected and HIV-uninfected DLBCL patients are controversial. The present retrospective study had the following findings: (1) in general, HIV-infected patients were predominantly male, younger and had a lower BMI; (2) after propensity score matching for age, sex, BMI and IPI at a ratio of 1:2 with HIV-uninfected patients, HIV-infected patients had a higher incidence of leukopenia, thrombocytopenia, hypoalbuminemia and Epstein–Barr viremia; (3) the EBER expression rate was high in HIV-infected patients, but the CD20 and CD79a expression rates were low on histopathology in HIV-infected patients; (4) ORR at end of chemotherapy and 1-year OS in HIV-infected DLBCL patients who accepted chemotherapy were lower in the univariate model, though those results were not confirmed in the multivariate model; (5) high IPI was related to decreased ORR. Meanwhile, hypoalbuminemia and CNS involvement were independent risk factors for decreased 1-year OS in HIV-infected DLBCL patients.
In general, HIV-infected NHL patients had lower BMI and were younger than HIV-uninfected patients. Even after propensity score matching, HIV-infected patients had high incidence of leukopenia, thrombocytopenia, hypoalbuminemia, CNS invasion, bone marrow involvement and Epstein–Barr viremia.
Our data showed that HIV-infected patients displayed high EBER expression but low CD20 and CD79a expression according to histopathological analysis. It seemed that HIV-associated lymphoma frequently occurs at a much higher frequency in EBV-DNA positive patients than in EBV-DNA negative patients [29,30]. CD20 and CD79a are the most widely used B-cell markers [31]. According to histopathological analysis, our data showed that the positivity of CD20 (90.2%) and CD79a (23.1%) was lower in HIV-infected patients. It was reported that 2–26% of HIV-infected DLBCL patients were negative for CD20 [32,33]. The exact mechanisms for loss of CD20 and CD79a expression were not clearly defined. Some factors associated with the loss of CD20 were inflection of receptor, bias of laboratory test and differences on biopsy site [34]. However, it was also suggested that CD20-negative patients presented with more extranodal disease, bone involvement, conversion of low-grade lymphoma to high-grade lymphoma, and even inferior survival rate [34,35]. Clinicopathological data showed that CD79a-negative expression was observed in patients with decreased platelet numbers or with high-grade lymphoma [36]. Our data indicated that HIV-infected DLBCL patients had high incidence of bone involvement and thrombocytopenia, which might relate to low expression of CD20 and CD79a on histopathology.
Although HIV infection was not a factor for decreased ORR and 1-year OS in multivariate analysis, our data indicated that both ORR and 1-year OS of HIV-infected patients were generally lower than in HIV-uninfected patients. The ORR in the present study was close to the ORR previously reported in other studies in China [19]. IPI > 2.0 was the only factor associated with decreased ORR in the multivariate analysis. In addition, the 1-year OS (66.2%) in HIV-infected patients was also close to the data reported in other studies in the Chinese population [19,21]. Importantly, our data demonstrated that hypoalbuminemia (<35.0 g/L) and CNS involvement were independent risk factors for reduced 1-year OS in HIV-infected BLDCL patients. Some studies indicated that hypoalbuminemia was associated with increased mortality in patients with lymphoma [37,38]. Consistent with other studies [39,40], our data also suggested that CNS involvement was an independent risk factor for poor OS in DLBCL patients. Therefore, it is rational that hypoalbuminemia and CNS invasion were linked to low 1-year OS in HIV-infected DLBCL patients.
There were some other potential factors related to the high mortality of HIV-infected DLBCL patients. We found that HIV-infected DLBCL patients had a higher incidence of leukopenia, neutropenia, thrombocytopenia, hypoalbuminemia, bone marrow involvement and Epstein–Barr viremia. These hematological abnormalities are mainly related to decreased production of precursor cells and increased destruction of peripheral differentiated cells resulting from a higher incidence of lymphoma invasion into the bone marrow in HIV-infected patients. Previous studies have shown that hypoalbuminemia, leukopenia and Epstein–Barr viremia were associated with a worse outcome of HIV-infected DLBCL patients [41,42].
Considering the substantial overweight of CNS involvement on mortality in multivariate analysis, we revaluated the factors associated with 1-year OS in HIV-infected patients without CNS involvement. Our study demonstrated that the odds ratio of mortality was 6.2-fold for patients who accepted chemotherapy without rituximab and 6.8-fold for patients with hypoalbuminemia. These data implied that administration of rituximab intravenously may effectively improve the outcome of BLBCL patients without CNS involvement.
The present study had several limitations. First, the present study indicated that HIV-infected patients had different phenotypic markers from HIV-uninfected patients. For example, histopathological analysis indicated a high positive rate of EBER. However, we did not discuss the effect of some phenotypic markers (such as Bcl-2 and c-Myc) on patient outcomes due to inadequate frequencies of those markers. Second, the present study was based on a small number of cases and a short observation period. Some potential risk factors (such as HIV-RNA load) were not analyzed, which may have produced potential effects on our results and conclusions. Third, we only provided 1-year OS rather than progression-free survival of patients. Thus, a further study based on large population is necessary for clarifying the detailed causes of mortality in HIV-infected DLBCL patients.
5. Conclusions
In conclusion, we found that HIV-infected DLBCL patients had different blood and histopathological profiles from HIV-uninfected patients. HIV-infected DLBCL patients had low ORR and 1-year OS, which may be associated with the higher incidence of leukopenia, neutropenia, thrombocytopenia and hypoalbuminemia as well as high involvement of the CNS, gastrointestinal tract and bone marrow. A high IPI was associated with poor ORR, and hypoalbuminemia and CNS involvement were independent risk factors for 1-year OS. HIV-infected DLBCL patients without CNS involvement had a favorable outcome if rituximab was included in the chemotherapy regimen.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cancers14215380/s1, Table S1: The dosage and protocol for each chemotherapy regimen.
Author Contributions
L.X. designed the study and revised the manuscript. M.Z., H.Z. and J.Q. collected the data and wrote the draft manuscript. J.C. interpreted the histopathological results. W.Y., M.Y., G.L., R.T. and Q.C. performed the study. M.Z. and L.X. analyzed and interpreted the data. Y.H. performed the follow-ups. B.Z. supervised the study. All authors reviewed the final manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by National Key R&D Program of China (Grant number 2021YFC2301900-2021YFC2301901).
Institutional Review Board Statement
The research protocols were in accordance with the 1975 Declaration of Helsinki and received ethical approval from the Ethics Committee of the First Affiliated Hospital, College of Medicine, Zhejiang University (No. 2021-139, 13 March 2021).
Informed Consent Statement
The Ethics Committee of the First Affiliated Hospital, College of Medicine, Zhejiang University, waived the requirement for written informed consent from the participants.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Acknowledgments
We thank the staff of the HIV/AIDS ward in the First Affiliated Hospital, College of Medicine, Zhejiang University. This article is dedicated to all medical staff at the forefront of the battle against the novel coronavirus, 2019–2022.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Puronen, C.E.; Ford, E.S.; Uldrick, T.S. Immunotherapy in People With HIV and Cancer. Front. Immunol. 2019, 10, 2060. [Google Scholar] [CrossRef] [PubMed]
- Khwaja, J.; Burns, J.E.; Ahmed, N.; Cwynarski, K. HIV-associated lymphoma—Advances in clinical management. Ann. Lymphoma 2021, 5, 26. [Google Scholar] [CrossRef]
- Howlader, N.; Shiels, M.S.; Mariotto, A.B.; Engels, E.A. Contributions of HIV to Non-Hodgkin Lymphoma Mortality Trends in the United States. Cancer Epidemiol Biomark. Prev. 2016, 25, 1289–1296. [Google Scholar] [CrossRef] [PubMed]
- Atallah-Yunes, S.A.; Murphy, D.J.; Noy, A. HIV-associated Burkitt lymphoma. Lancet Haematol. 2020, 7, e594–e600. [Google Scholar] [CrossRef]
- Ekberg, S.; Smedby, K.E.; Glimelius, I.; Nilsson-Ehle, H.; Goldkuhl, C.; Lewerin, C.; Jerkeman, M.; Eloranta, S. Trends in the prevalence, incidence and survival of non-Hodgkin lymphoma subtypes during the 21st century—A Swedish lymphoma register study. Br. J. Haematol. 2020, 189, 1083–1092. [Google Scholar] [CrossRef]
- Bellitti, E.; Masciopinto, P.; Musto, P.; Arcuti, E.; Mastracci, L.; Opinto, G.; Ciavarella, S.; Guarini, A.; Cazzato, G.; Specchia, G.; et al. Diffuse Large B Cell Lymphoma Arising in Patients with Preexisting Hodgkin Lymphoma. Curr. Oncol. 2022, 29, 6115–6124. [Google Scholar] [CrossRef]
- He, B.; Qiao, X.; Klasse, P.J.; Chiu, A.; Chadburn, A.; Knowles, D.M.; Moore, J.P.; Cerutti, A. HIV-1 envelope triggers polyclonal Ig class switch recombination through a CD40-independent mechanism involving BAFF and C-type lectin receptors. J. Immunol. 2006, 176, 3931–3941. [Google Scholar] [CrossRef]
- Kundu, R.K.; Sangiorgi, F.; Wu, L.Y.; Pattengale, P.K.; Hinton, D.R.; Gill, P.S.; Maxson, R. Expression of the human immunodeficiency virus-Tat gene in lymphoid tissues of transgenic mice is associated with B-cell lymphoma. Blood 1999, 94, 275–282. [Google Scholar] [CrossRef]
- Dandachi, D.; Morón, F. Effects of HIV on the Tumor Microenvironment. Adv. Exp. Med. Biol. 2020, 1263, 45–54. [Google Scholar] [CrossRef]
- Vendrame, E.; Hussain, S.K.; Breen, E.C.; Magpantay, L.I.; Widney, D.P.; Jacobson, L.P.; Variakojis, D.; Knowlton, E.R.; Bream, J.H.; Ambinder, R.F.; et al. Serum levels of cytokines and biomarkers for inflammation and immune activation, and HIV-associated non-Hodgkin B-cell lymphoma risk. Cancer Epidemiol. Biomarkers Prev. 2014, 23, 343–349. [Google Scholar] [CrossRef]
- Re, A.; Cattaneo, C.; Rossi, G. HIV and Lymphoma: From Epidemiology to Clinical Management. Mediterr. J. Hematol. Infect. Dis. 2019, 11, e2019004. [Google Scholar] [CrossRef] [PubMed]
- Cesarman, E. Pathology of lymphoma in HIV. Curr. Opin. Oncol. 2013, 25, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Cesarman, E. Gammaherpesvirus and lymphoproliferative disorders in immunocompromised patients. Cancer Lett. 2011, 305, 163–174. [Google Scholar] [CrossRef] [PubMed]
- Noy, A. HIV Lymphoma and Burkitts Lymphoma. Cancer J. 2020, 26, 260–268. [Google Scholar] [CrossRef]
- Grogg, K.L.; Miller, R.F.; Dogan, A. HIV infection and lymphoma. J. Clin. Pathol. 2007, 60, 1365–1372. [Google Scholar] [CrossRef]
- Barta, S.K.; Samuel, M.S.; Xue, X.; Wang, D.; Lee, J.Y.; Mounier, N.; Ribera, J.-M.; Spina, M.; Tirelli, U.; Weiss, R.; et al. Changes in the influence of lymphoma- and HIV-specific factors on outcomes in AIDS-related non-Hodgkin lymphoma. Ann. Oncol. 2015, 26, 958–966. [Google Scholar] [CrossRef]
- Cingolani, A.; Cozzi Lepri, A.; Teofili, L.; Galli, L.; Mazzotta, V.; Baldin, G.M.; Hohaus, S.; Bandera, A.; Alba, L.; Galizzi, N.; et al. Survival and predictors of death in people with HIV-associated lymphoma compared to those with a diagnosis of lymphoma in general population. PLoS ONE 2017, 12, e0186549. [Google Scholar]
- Shen, Y.; Zhang, R.; Liu, L.; Shen, Y.; Song, W.; Qi, T.; Tang, Y.; Wang, Z.; Guan, L.; Lu, H. Clinical and prognostic analysis of 78 patients with human immuno-deficiency virus associated non-Hodgkin’s lymphoma in Chinese population. Infect. Agent. Cancer 2017, 12, 7. [Google Scholar] [CrossRef]
- Wu, J.; Miao, Y.; Qian, C.; Tao, P.; Wang, X.; Dong, X.; Li, X.; Lou, J.; Liang, J.; Xu, W.; et al. Clinical characteristics and outcomes in HIV-associated diffuse large B-cell lymphoma in China: A retrospective single-center study. J. Cancer 2021, 12, 2903–2911. [Google Scholar] [CrossRef]
- Zeng, J.; Zhang, X.; Jia, L.; Wu, Y.; Tian, Y.; Zhang, Y. Pretreatment lymphocyte-to-monocyte ratios predict AIDS-related diffuse large B-cell lymphoma overall survival. J. Med. Virol. 2021, 93, 3907–3914. [Google Scholar] [CrossRef]
- Sun, J.J.; Liu, L.; Wang, J.R.; Shen, Y.Z.; Qi, T.K.; Wang, Z.Y.; Tang, Y.; Song, W.; Chen, J.; Zhang, R.F. Clinical outcomes of people living with human immunodeficiency virus (HIV) with diffuse large B-cell lymphoma (DLBCL) in Shanghai, China. Chin. Med. J. 2020, 133, 2796–2802. [Google Scholar] [CrossRef] [PubMed]
- Swerdlow, S.H.; Campo, E.; Pileri, S.A.; Harris, N.L.; Stein, H.; Siebert, R.; Advani, R.; Ghielmini, M.; Salles, G.A.; Zelenetz, A.D.; et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood 2016, 127, 2375–2390. [Google Scholar] [CrossRef] [PubMed]
- Cheson, B.D.; Fisher, R.I.; Barrington, S.F.; Cavalli, F.; Schwartz, L.H.; Zucca, E.; Lister, T.A. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: The Lugano classification. J. Clin. Oncol. 2014, 32, 3059–3068. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Lu, H. Malignancies in HIV-Infected and AIDS Patients. Adv. Exp. Med. Biol. 2017, 1018, 167–179. [Google Scholar] [CrossRef]
- Shiels, M.S.; Engels, E.A. Evolving epidemiology of HIV-associated malignancies. Curr. Opin. HIV AIDS 2017, 12, 6–11. [Google Scholar] [CrossRef]
- Park, L.S.; Tate, J.P.; Sigel, K.; Rimland, D.; Crothers, K.; Gibert, C.; Rodriguez-Barradas, M.C.; Goetz, M.B.; Bedimo, R.J.; Brown, S.T.; et al. Time trends in cancer incidence in persons living with HIV/AIDS in the antiretroviral therapy era: 1997–2012. AIDS 2016, 30, 1795–1806. [Google Scholar] [CrossRef]
- Wong, I.K.J.; Grulich, A.E.; Poynten, I.M.; Polizzotto, M.N.; van Leeuwen, M.T.; Amin, J.; McGregor, S.; Law, M.; Templeton, D.J.; Vajdic, C.M.; et al. Time trends in cancer incidence in Australian people living with HIV between 1982 and 2012. HIV Med. 2022, 23, 134–145. [Google Scholar] [CrossRef]
- Jani, C.; Al Omari, O.; Singh, H.; Walker, A.; Patel, K.; Mouchati, C.; Radwan, A.; Pandit, Z.; Hanbury, G.; Crowley, C.; et al. Trends of HIV-Related Cancer Mortality between 2001 and 2018: An Observational Analysis. Trop. Med. Infect. Dis. 2021, 6, 213. [Google Scholar] [CrossRef]
- Lang, F.; Pei, Y.; Lamplugh, Z.L.; Robertson, E.S. Molecular Biology of EBV in Relationship to HIV/AIDS-Associated Oncogenesis. Cancer Treat. Res. 2019, 177, 81–103. [Google Scholar] [CrossRef]
- Chao, C.; Silverberg, M.J.; Martínez-Maza, O.; Chi, M.; Abrams, D.I.; Haque, R.; Zha, H.D.; McGuire, M.; Xu, L.; Said, J. Epstein-Barr virus infection and expression of B-cell oncogenic markers in HIV-related diffuse large B-cell Lymphoma. Clin. Cancer Res. 2012, 18, 4702–4712. [Google Scholar] [CrossRef]
- Liu, Y.; Barta, S.K. Diffuse large B-cell lymphoma: 2019 update on diagnosis, risk stratification, and treatment. Am. J. Hematol. 2019, 94, 604–616. [Google Scholar] [CrossRef] [PubMed]
- Castillo, J.J.; Winer, E.S.; Stachurski, D.; Perez, K.; Jabbour, M.; Milani, C.; Colvin, G.; Butera, J.N. Clinical and pathological differences between human immunodeficiency virus-positive and human immunodeficiency virus-negative patients with plasmablastic lymphoma. Leuk. Lymphoma 2010, 51, 2047–2053. [Google Scholar] [CrossRef] [PubMed]
- Xicoy, B.; Ribera, J.M.; Mate, J.L.; Tapia, G.; Morgades, M.; Navarro, J.T.; Sanz, C.; Ariza, A.; Feliu, E. Immunohistochemical expression profile and prognosis in patients with diffuse large B-cell lymphoma with or without human immunodeficiency virus infection. Leuk. Lymphoma 2010, 51, 2063–2069. [Google Scholar] [CrossRef] [PubMed]
- Rasheed, A.A.; Samad, A.; Raheem, A.; Hirani, S.I.; Shabbir-Moosajee, M. CD20 Expression and Effects on Outcome of Relapsed/Refractory Diffuse Large B Cell Lymphoma after Treatment with Rituximab. Asian Pac. J. Cancer Prev. APJCP 2018, 19, 331–335. [Google Scholar] [CrossRef]
- Johnson, N.A.; Boyle, M.; Bashashati, A.; Leach, S.; Brooks-Wilson, A.; Sehn, L.H.; Chhanabhai, M.; Brinkman, R.R.; Connors, J.M.; Weng, A.P.; et al. Diffuse large B-cell lymphoma: Reduced CD20 expression is associated with an inferior survival. Blood 2009, 113, 3773–3780. [Google Scholar] [CrossRef]
- Tanaka, T.; Ichimura, K.; Sato, Y.; Takata, K.; Morito, T.; Tamura, M.; Chhanabhai, M.; Brinkman, R.; Connors, J.M.; Weng, A.P.; et al. Frequent downregulation or loss of CD79a expression in plasma cell myelomas: Potential clue for diagnosis. Pathol. Int. 2009, 59, 804–808. [Google Scholar] [CrossRef]
- Bairey, O.; Shacham-Abulafia, A.; Shpilberg, O.; Gurion, R. Serum albumin level at diagnosis of diffuse large B-cell lymphoma: An important simple prognostic factor. Hematol. Oncol. 2016, 34, 184–192. [Google Scholar] [CrossRef]
- Wei, X.; Zheng, J.; Zhang, Z.; Liu, Q.; Zhan, M.; Huang, W.; Chen, J.; Wei, Q.; Wei, Y.; Feng, R. Consecutive Hypoalbuminemia Predicts Inferior Outcome in Patients With Diffuse Large B-Cell Lymphoma. Front. Oncol. 2020, 10, 610681. [Google Scholar] [CrossRef]
- Grommes, C.; Rubenstein, J.L.; DeAngelis, L.M.; Ferreri, A.J.M.; Batchelor, T.T. Comprehensive approach to diagnosis and treatment of newly diagnosed primary CNS lymphoma. Neuro-Oncol. 2019, 21, 296–305. [Google Scholar] [CrossRef]
- Schaff, L.R.; Grommes, C. Primary Central Nervous System Lymphoma. Blood 2021, 140, 971–979. [Google Scholar] [CrossRef]
- Tate, J.P.; Sterne, J.A.C.; Justice, A.C. Albumin, white blood cell count, and body mass index improve discrimination of mortality in HIV-positive individuals. AIDS 2019, 33, 903–912. [Google Scholar] [CrossRef] [PubMed]
- Tisi, M.C.; Cupelli, E.; Santangelo, R.; Maiolo, E.; Alma, E.; Giachelia, M.; Martini, M.; Bellesi, S.; D’Alò, F.; Voso, M.T.; et al. Whole blood EBV-DNA predicts outcome in diffuse large B-cell lymphoma. Leuk. Lymphoma 2016, 57, 628–634. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).