Electroporation in Head-and-Neck Cancer: An Innovative Approach with Immunotherapy and Nanotechnology Combination
Abstract
Simple Summary
Abstract
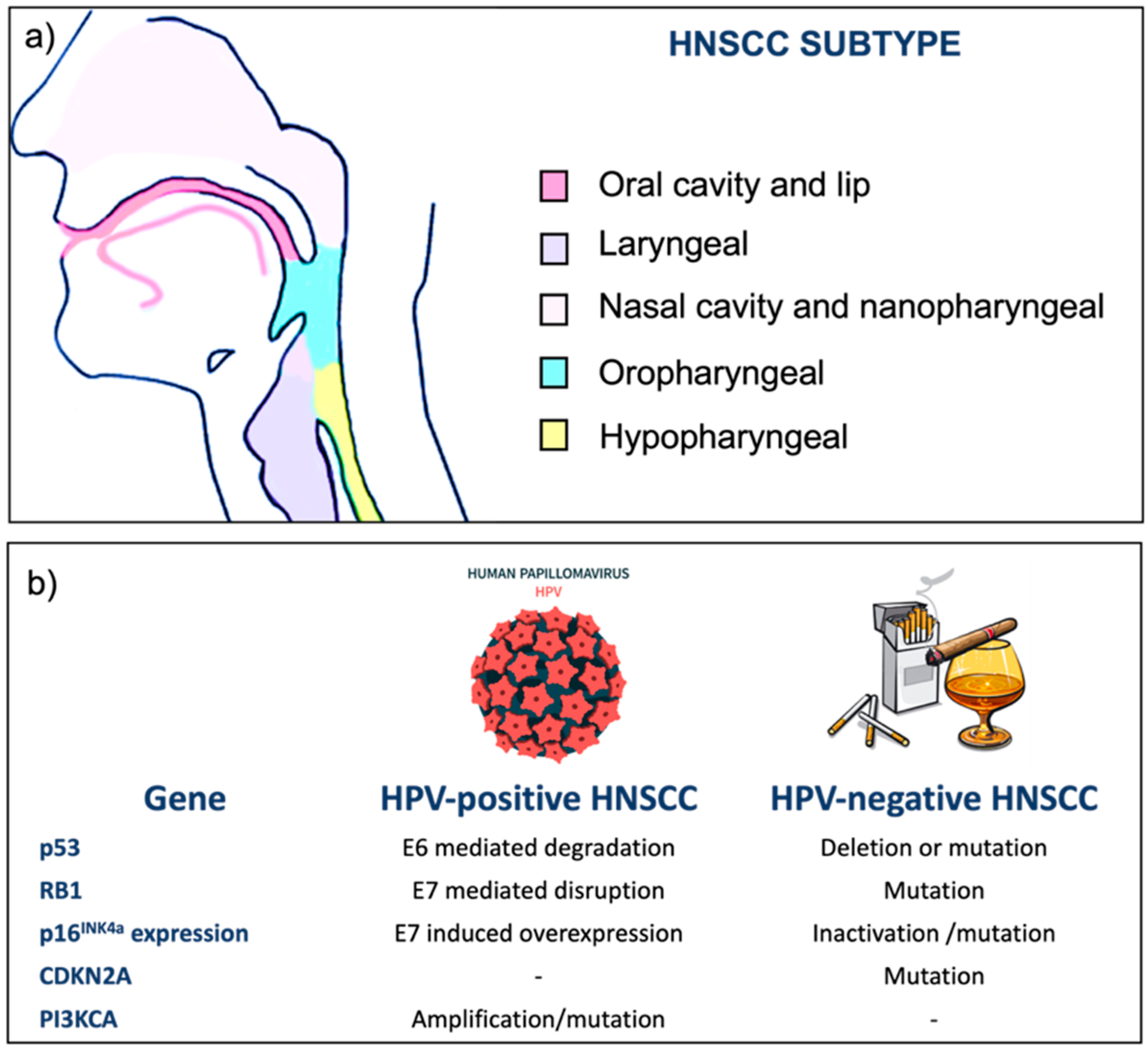
1. Head-and-Neck Cancers
- Epithelial cell hyperplasia characterized by loss of 9p21 and consequent downregulation of tumor suppressor genes (TSGs) such as CDKN2A.
- Dysplasia (mild, moderate, severe) marked by loss of 3p21 and 17p13 that regulates p53.
- In situ carcinoma characterized by loss of 11q13, 13q21, and 14q32.
- Invasive carcinoma in which is observed loss of 6p, 4q27, and 10q23.
- Second primary tumors (SPTs) and metastases localized at distinct anatomical sites (esophagus, lungs, skin) can express the same molecular abnormalities of the primary tumor or different markers.
2. Electroporation
- Reversible EP: The electric field is sufficient enough (≈1 kV/cm) to exceed the critical threshold, but the cell is still able to return to its initial state (resting potential). Due to its reversible pore formation in the microsecond time frame, membrane resealing happens over a range of minutes. In an in vivo experiment performed on mouse skeletal muscle tissue, it was found that a 63% resealing time (s) required approximately 9 min [65]. Due to it is not irreversible destructive action, this type of EP is used to insert molecules in the intracellular environment (i.e., Electrochemotherapy, Genetic transfer, and Calcium electroporation).
- Irreversible EP: The electric field is extremely high (>2–5 kV/cm), and the number of pores created induces cell osmotic imbalance or homeostasis loss, resulting in cell necrosis and death (i.e., non-thermal tissue ablation) [66]. Cell death due to irreversible electroporation is a function of electric field strength and pulse number [67].
- Thermal irreversible EP: Electric field intensity or exposing time is so high that Joule heating is observed (≈10 kV/cm and T° > 50 °C) [68].
2.1. Electrochemotherapy (ECT)
2.2. Gene Electroporation (GE)
2.3. Calcium Electroporation
2.4. Electroporation and Immunotherapy
2.5. Electroporation and Nanotechnology
3. Conclusions and Future Prospective
Author Contributions
Funding
Conflicts of Interest
Acronyms
| ADCC | Antibody-dependent cell cytotoxicity |
| APCs | Antigen-presenting cells |
| BLM | Bleomycin |
| Ca2+ | Calcium |
| CisPt | Cisplatin |
| CRT | Chemoradiotherapy |
| CSCs | Cancer stem cells |
| DAMPs | Damage-associated molecular patterns |
| ECT | Electrochemotherapy |
| EFPs | Electrical field pulses |
| EMA | European Medicines Agency |
| EP | Electroporation |
| EPR | Enhanced permeability and retention effect |
| ER | Endoplasmic reticulum |
| FDA | U.S. Food and Drug Administration |
| FU | 5- fluorouracil |
| GA | General anesthesia |
| GE | Gene electroporation |
| Gy | Gray |
| H&N | Head and neck |
| HNSCC | Head-and-neck squamous cell carcinoma |
| HPV | Human papillomavirus |
| K | Dielectric constant |
| IL | Interleukin |
| I.T. | Intra-tumor |
| I.V. | Intra-venous |
| LA | Local anesthesia |
| MCAM | Melanoma cell adhesion molecule |
| NMs | Nanomaterial |
| NV | Nanovector |
| NsEFPs | Nanosecond electrical field pulses |
| OS | Overall survival |
| PCs | Protein coronas |
| PD-L1 | Programmed death ligand 1 |
| PEG | poly-(ethylene glycol) |
| QoL | Quality of life |
| RB1 | Retinoblastoma-associated protein |
| RT | Radiotherapy |
| SOP | Standard operating procedure |
| SR | Sarcoplasmic reticulum |
| TMP | Transmembrane potential |
| Vm | Transmembrane voltage |
References
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Mathers, C.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int. J. Cancer 2019, 144, 1941–1953. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.E.; Burtness, B.; Leemans, C.R.; Lui, V.W.Y.; Bauman, J.E.; Grandis, J.R. Head and neck squamous cell carcinoma. Nat. Rev. Dis. Prim. 2020, 6, 92. [Google Scholar] [CrossRef] [PubMed]
- WHO. List of Classifications by Cancer Sites with Sufficient or Limited Evidence in Humans, IARC Monographs on the Identification of Carcinogenic Hazards to Humans; WHO: Geneva, Switzerland, 2020. [Google Scholar]
- Hennessey, P.T.; Westra, W.H.; Califano, J.A. Human papillomavirus and head and neck squamous cell carcinoma: Recent evidence and clinical implications. J. Dent. Res. 2009, 88, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Machiels, J.P.; Leemans, C.R.; Golusinski, W.; Grau, C.; Licitra, L.; Gregoire, V. Squamous cell carcinoma of the oral cavity, larynx, oropharynx and hypopharynx: EHNS-ESMO-ESTRO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2020, 31, 1462–1475. [Google Scholar] [CrossRef] [PubMed]
- Castilho, R.M.; Squarize, C.H.; Almeida, L.O. Epigenetic Modifications and Head and Neck Cancer: Implications for Tumor Progression and Resistance to Therapy. Int. J. Mol. Sci. 2017, 18, 1506. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.-M.; Wang, L.; Zhou, S.-B. Recent Progress in Shape Memory Polymers for Biomedical Applications. Chin. J. Polym. Sci. 2018, 36, 905–917. [Google Scholar] [CrossRef]
- Fakhry, C.; Lacchetti, C.; Rooper, L.M.; Jordan, R.C.; Rischin, D.; Sturgis, E.M.; Bell, D.; Lingen, M.W.; Harichand-Herdt, S.; Thibo, J.; et al. Human Papillomavirus Testing in Head and Neck Carcinomas: ASCO Clinical Practice Guideline Endorsement of the College of American Pathologists Guideline. J. Clin. Oncol. 2018, 36, 3152–3161. [Google Scholar] [CrossRef]
- Brierley, J.D.; Gospodarowicz, M.K.; Wittekind, C. TNM Classification of Malignant Tumours, 8th ed.; Wiley Blackwell: New York, NY, USA, 2016. [Google Scholar]
- National Cancer Institute. Drugs Approved for Head and Neck Cancer-NCI, 2021. Available online: https://www.cancer.gov/about-cancer/treatment/drugs/head-neck (accessed on 28 October 2022).
- Shibata, H.; Saito, S.; Uppaluri, R. Immunotherapy for Head and Neck Cancer: A Paradigm Shift From Induction Chemotherapy to Neoadjuvant Immunotherapy. Front. Oncol. 2021, 11, 727433. [Google Scholar] [CrossRef]
- Bauman, J.E.; Ferris, R.L. Integrating novel therapeutic monoclonal antibodies into the management of head and neck cancer. Cancer 2014, 120, 624–632. [Google Scholar] [CrossRef]
- Lacas, B.; Bourhis, J.; Overgaard, J.; Zhang, Q.; Grégoire, V.; Nankivell, M.; Zackrisson, B.; Szutkowski, Z.; Suwiński, R.; Poulsen, M.; et al. Role of radiotherapy fractionation in head and neck cancers (MARCH): An updated meta-analysis. Lancet Oncol. 2017, 18, 1221–1237. [Google Scholar] [CrossRef]
- Goh, H.K.; Ng, Y.H.; Teo, D.T. Minimally invasive surgery for head and neck cancer. Lancet Oncol. 2010, 11, 281–286. [Google Scholar] [CrossRef]
- Kanetaka, K.; Eguchi, S. Regenerative medicine for the upper gastrointestinal tract. Regen. Ther. 2020, 15, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Pisani, S.; Croce, S.; Mauramati, S.; Marmonti, M.; Cobianchi, L.; Herman, I.; Dorati, R.; Avanzini, M.A.; Genta, I.; Benazzo, M.; et al. Engineered Full Thickness Electrospun Scaffold for Esophageal Tissue Regeneration: From In Vitro to In Vivo Approach. Pharmaceutics 2022, 14, 252. [Google Scholar] [CrossRef]
- Pisani, S.; Croce, S.; Chiesa, E.; Dorati, R.; Lenta, E.; Genta, I.; Bruni, G.; Mauramati, S.; Benazzo, A.; Cobianchi, L.; et al. Tissue Engineered Esophageal Patch by Mesenchymal Stromal Cells: Optimization of Electrospun Patch Engineering. Int. J. Mol. Sci. 2020, 21, 1764. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Grandis, J.R. Emerging drugs for head and neck cancer. Expert Opin. Emerg. Drugs 2015, 20, 313–329. [Google Scholar] [CrossRef] [PubMed]
- Kohno, N. The role of taxanes for head and neck cancer. Gan Kagaku Ryoho 2005, 32, 2035–2039. [Google Scholar]
- Osman, N.; Elamin, Y.Y.; Rafee, S.; O’Brien, C.; Stassen, L.F.; Timon, C.; Kinsella, J.; Brennan, S.; O’Byrne, K.J. Weekly cisplatin concurrently with radiotherapy in head and neck squamous cell cancer: A retrospective analysis of a tertiary institute experience. Eur. Arch. Otorhinolaryngol. 2014, 271, 2253–2259. [Google Scholar] [CrossRef]
- Taketa, C.; Shimosato, Y.; Nagano, A.; Washizu, K.; Matsuura, S.; Ono, I.; Ebihara, S. Effects of Bleomycin for Epidermoid Carcinoma of Head and Neck. Jpn. J. Clin. Oncol. 2010, 40, e41–e53. [Google Scholar] [CrossRef]
- Polovich, M.; Whitford, J.M.; Kelleher, L.O. (Eds.) Chemotherapy and Biotherapy Guidelines and Recommendations for Practice; Oncology Nursing Society: Pittsburgh, PA, USA, 2005. [Google Scholar]
- Drugs.com. Bleomycin-FDA Prescribing Information, Side Effects and Uses. 2022. Available online: https://www.drugs.com/pro/bleomycin.html (accessed on 28 October 2022).
- Employee Engagement Manager Agency. Bleomycin-Art. 29 Referral-Annex I, II, III, Article 29 of Directive 2001/83/EC. 2009. Available online: https://www.ema.europa.eu/en/documents/referral/bleomycin-article-29-referral-annex-i-ii-iii_en.pdf (accessed on 28 October 2022).
- Yu, S.S.; Cirillo, N. The molecular markers of cancer stem cells in head and neck tumors. J. Cell. Physiol. 2020, 235, 65–73. [Google Scholar] [CrossRef]
- Rehmani, H.S.; Issaeva, N. EGFR in head and neck squamous cell carcinoma: Exploring possibilities of novel drug combinations. Ann. Transl. Med. 2020, 8, 813. [Google Scholar] [CrossRef]
- Faber, A.; Barth, C.; Hörmann, K.; Kassner, S.; Schultz, J.D.; Sommer, U.; Stern-Straeter, J.; Thorn, C.; Goessler, U.R. CD44 as a stem cell marker in head and neck squamous cell carcinoma. Oncol. Rep. 2011, 26, 321–326. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Glumac, P.M.; LeBeau, A.M. The role of CD133 in cancer: A concise review. Clin. Transl. Med. 2018, 7, 18. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Shi, S.; Yen, Y.; Brown, J.; Ta, J.Q.; Le, A.D. A subpopulation of CD133(+) cancer stem-like cells characterized in human oral squamous cell carcinoma confer resistance to chemotherapy. Cancer Lett. 2010, 289, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.E.; O’Keefe, R.A.; Grandis, J.R. Targeting the IL-6/JAK/STAT3 signalling axis in cancer. Nat. Rev. Clin. Oncol. 2018, 15, 234–248. [Google Scholar] [CrossRef]
- Peyser, N.D.; Du, Y.; Li, H.; Lui, V.; Xiao, X.; Chan, T.A.; Grandis, J.R. Loss-of-Function PTPRD Mutations Lead to Increased STAT3 Activation and Sensitivity to STAT3 Inhibition in Head and Neck Cancer. PLoS ONE 2015, 10, e0135750. [Google Scholar] [CrossRef]
- Crosta, S.; Boldorini, R.; Bono, F.; Brambilla, V.; Dainese, E.; Fusco, N.; Gianatti, A.; L’Imperio, V.; Morbini, P.; Pagni, F. PD-L1 Testing and Squamous Cell Carcinoma of the Head and Neck: A Multicenter Study on the Diagnostic Reproducibility of Different Protocols. Cancers 2021, 13, 292. [Google Scholar] [CrossRef]
- Ritchie, K.E.; Nör, J.E. Perivascular stem cell niche in head and neck cancer. Cancer Lett. 2013, 338, 41–46. [Google Scholar] [CrossRef]
- Lenouvel, D.; González-Moles, M.; Talbaoui, A.; Ramos-García, P.; González-Ruiz, L.; Ruiz-Ávila, I.; Gil-Montoya, J.A. An update of knowledge on PD-L1 in head and neck cancers: Physiologic, prognostic and therapeutic perspectives. Oral Dis. 2020, 26, 511–526. [Google Scholar] [CrossRef]
- Chiou, S.H.; Yu, C.C.; Huang, C.Y.; Lin, S.C.; Liu, C.J.; Tsai, T.H.; Chou, S.H.; Chien, C.S.; Ku, H.H.; Lo, J.F. Positive correlations of Oct-4 and Nanog in oral cancer stem-like cells and high-grade oral squamous cell carcinoma. Clin. Cancer Res. 2008, 14, 4085–4095. [Google Scholar] [CrossRef]
- Cetuximab approved by FDA for treatment of head and neck squamous cell cancer. Cancer Biol. Ther. 2006, 5, 340–342.
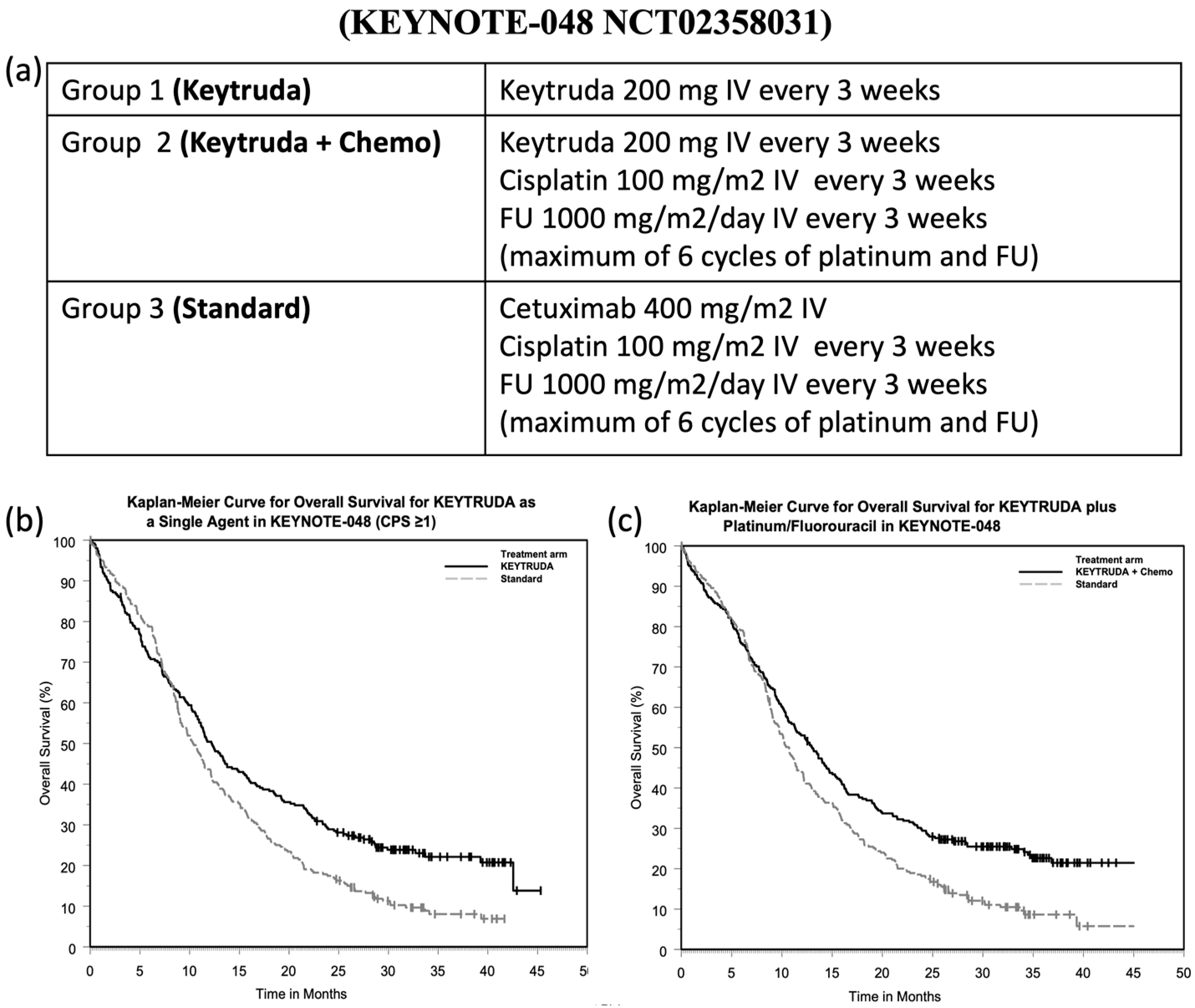
- Burtness, B.; Harrington, K.J.; Greil, R.; Soulières, D.; Tahara, M.; de Castro, G., Jr.; Psyrri, A.; Basté, N.; Neupane, P.; Bratland, Å.; et al. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): A randomised, open-label, phase 3 study. Lancet 2019, 394, 1915–1928. [Google Scholar] [CrossRef]
- Vos, J.L.; Elbers, J.B.W.; Krijgsman, O.; Traets, J.J.H.; Qiao, X.; van der Leun, A.M.; Lubeck, Y.; Seignette, I.M.; Smit, L.A.; Willems, S.M.; et al. Neoadjuvant immunotherapy with nivolumab and ipilimumab induces major pathological responses in patients with head and neck squamous cell carcinoma. Nat. Commun. 2021, 12, 7348. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.J.; Heron, D.E.; Stenson, K.; Ling, D.C.; Vargo, J.A. Locoregional Recurrent or Second Primary Head and Neck Cancer: Management Strategies and Challenges. Am. Soc. Clin. Oncol. Educ. Book 2016, 35, e284–e292. [Google Scholar] [CrossRef]
- Kabolizadeh, P.; Kubicek, G.J.; Heron, D.E.; Ferris, R.L.; Gibson, M.K. The role of cetuximab in the management of head and neck cancers. Expert Opin. Biol. Ther. 2012, 12, 517–528. [Google Scholar] [CrossRef]
- Trotta, A.M.; Ottaiano, A.; Romano, C.; Nasti, G.; Nappi, A.; de Divitiis, C.; Napolitano, M.; Zanotta, S.; Casaretti, R.; D’Alterio, C.; et al. Prospective Evaluation of Cetuximab-Mediated Antibody-Dependent Cell Cytotoxicity in Metastatic Colorectal Cancer Patients Predicts Treatment Efficacy. Cancer Immunol. Res. 2016, 4, 366–374. [Google Scholar] [CrossRef] [PubMed]
- Kimura, H.; Sakai, K.; Arao, T.; Shimoyama, T.; Tamura, T.; Nishio, K. Antibody-dependent cellular cytotoxicity of cetuximab against tumor cells with wild-type or mutant epidermal growth factor receptor. Cancer Sci. 2007, 98, 1275–1280. [Google Scholar] [CrossRef]
- Ochoa, M.C.; Minute, L.; Rodriguez, I.; Garasa, S.; Perez-Ruiz, E.; Inogés, S.; Melero, I.; Berraondo, P. Antibody-dependent cell cytotoxicity: Immunotherapy strategies enhancing effector NK cells. Immunol. Cell Biol. 2017, 95, 347–355. [Google Scholar] [CrossRef]
- Lattanzio, L.; Denaro, N.; Vivenza, D.; Varamo, C.; Strola, G.; Fortunato, M.; Chamorey, E.; Comino, A.; Monteverde, M.; Lo Nigro, C.; et al. Elevated basal antibody-dependent cell-mediated cytotoxicity (ADCC) and high epidermal growth factor receptor (EGFR) expression predict favourable outcome in patients with locally advanced head and neck cancer treated with cetuximab and radiotherapy. Cancer Immunol. Immunother. 2017, 66, 573–579. [Google Scholar] [CrossRef]
- FDA. KEYTRUDA® (pembrolizumab) Injection, for Intravenous Use; Merck & Co., Inc.: Whitehouse Station, NJ, USA, 2014. [Google Scholar]
- Qiao, X.-W.; Jiang, J.; Pang, X.; Huang, M.-C.; Tang, Y.-J.; Liang, X.-H.; Tang, Y.-L. The Evolving Landscape of PD-1/PD-L1 Pathway in Head and Neck Cancer. Front. Immunol. 2020, 11, 1721. [Google Scholar] [CrossRef]
- Pisani, P.; Airoldi, M.; Allais, A.; Valletti, P.A.; Battista, M.; Benazzo, M.; Briatore, R.; Cacciola, S.; Cocuzza, S.; Colombo, A.; et al. Metastatic disease in head & neck oncology. Acta Otorhinolaryngol. Ital. 2020, 40, S1–S86. [Google Scholar]
- Pucci, C.; Martinelli, C.; Ciofani, G. Innovative approaches for cancer treatment: Current perspectives and new challenges. Ecancermedicalscience 2019, 13, 961. [Google Scholar] [CrossRef] [PubMed]
- Stoeckli, S.J.; Broglie, M.A. Recent innovations in head and neck oncology: A report from the ICHNO. Expert Rev. Anticancer. Ther. 2013, 13, 535–539. [Google Scholar] [CrossRef]
- Qu, X.; Li, J.-W.; Chan, J.; Meehan, K. Extracellular Vesicles in Head and Neck Cancer: A Potential New Trend in Diagnosis, Prognosis, and Treatment. Int. J. Mol. Sci. 2020, 21, 8260. [Google Scholar] [CrossRef]
- El-Sayed, I.H. Nanotechnology in head and neck cancer: The race is on. Curr. Oncol. Rep. 2010, 12, 121–128. [Google Scholar] [CrossRef]
- Gao, S.; Zheng, M.; Ren, X.; Tang, Y.; Liang, X. Local hyperthermia in head and neck cancer: Mechanism, application and advance. Oncotarget 2016, 7, 57367–57378. [Google Scholar] [CrossRef] [PubMed]
- Krassowska, W.; Filev, P.D. Modeling electroporation in a single cell. Biophys. J. 2007, 92, 404–417. [Google Scholar] [CrossRef] [PubMed]
- Yadollahpour, A.; Rezaee, Z. Electroporation as a New Cancer Treatment Technique: A Review on the Mechanisms of Action. Biomed. Pharmacol. J. 2014, 7, 53–62. [Google Scholar] [CrossRef]
- Weaver, J.C. Electroporation: A general phenomenon for manipulating cells and tissues. J. Cell. Biochem. 1993, 51, 426–435. [Google Scholar] [CrossRef]
- Weaver, J.C. Electroporation in cells and tissues: A biophysical phenomenon due to electromagnetic fields. Radio Sci. 1995, 30, 205–221. [Google Scholar] [CrossRef]
- Gehl, J. Electroporation: Theory and methods, perspectives for drug delivery, gene therapy and research. Acta Physiol. Scand. 2003, 177, 437–447. [Google Scholar] [CrossRef]
- Carlson, B.M. Chapter 2-Tissues. In The Human Body; Carlson, B.M., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 27–63. [Google Scholar]
- Gabriel, B.; Teissié, J. Direct observation in the millisecond time range of fluorescent molecule asymmetrical interaction with the electropermeabilized cell membrane. Biophys. J. 1997, 73, 2630–2637. [Google Scholar] [CrossRef]
- Rols, M.-P.; Golzio, M.; Gabriel, B.; Teissié, J. Factors Controlling Electropermeabilisation of Cell Membranes. Technol. Cancer Res. Treat. 2002, 1, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Teissié, J.; Eynard, N.; Gabriel, B.; Rols, M.-P. Electropermeabilization of cell membranes. Adv. Drug Deliv. Rev. 1999, 35, 3–19. [Google Scholar] [CrossRef]
- Shi, J.; Ma, Y.; Zhu, J.; Chen, Y.; Sun, Y.; Yao, Y.; Yang, Z.; Xie, J. A Review on Electroporation-Based Intracellular Delivery. Molecules 2018, 23, 3044. [Google Scholar] [CrossRef]
- Teissie, J.; Golzio, M.; Rols, M.P. Mechanisms of cell membrane electropermeabilization: A minireview of our present (lack of ?) knowledge. Biochim. Biophys. Acta Gen. Subj. 2005, 1724, 270–280. [Google Scholar] [CrossRef]
- Kolosnjaj-Tabi, J.; Gibot, L.; Fourquaux, I.; Golzio, M.; Rols, M.-P. Electric field-responsive nanoparticles and electric fields: Physical, chemical, biological mechanisms and therapeutic prospects. Adv. Drug Deliv. Rev. 2019, 138, 56–67. [Google Scholar] [CrossRef]
- Bier, M.; Hammer, S.M.; Canaday, D.J.; Lee, R.C. Kinetics of sealing for transient electropores in isolated mammalian skeletal muscle cells. Bioelectromagnetics 1999, 20, 194–201. [Google Scholar] [CrossRef]
- Jourabchi, N.; Beroukhim, K.; Tafti, B.A.; Kee, S.T.; Lee, E.W. Irreversible electroporation (NanoKnife) in cancer treatment. Gastrointest. Interv. 2014, 3, 8–18. [Google Scholar] [CrossRef]
- Garcia, P.A.; Davalos, R.V.; Miklavcic, D. A numerical investigation of the electric and thermal cell kill distributions in electroporation-based therapies in tissue. PLoS ONE 2014, 9, e103083. [Google Scholar] [CrossRef]
- Van Gemert, M.J.; Wagstaff, P.G.; de Bruin, D.M.; van Leeuwen, T.G.; van der Wal, A.C.; Heger, M.; van der Geld, C.W. Irreversible electroporation: Just another form of thermal therapy? Prostate 2015, 75, 332–335. [Google Scholar] [CrossRef]
- Mir, L. Bases and rationale of the electrochemotherapy. EJC Suppl. 2006, 4, 38–44. [Google Scholar] [CrossRef]
- Canatella, P.J.; Black, M.M.; Bonnichsen, D.M.; McKenna, C.; Prausnitz, M.R. Tissue electroporation: Quantification and analysis of heterogeneous transport in multicellular environments. Biophys. J. 2004, 86, 3260–3268. [Google Scholar] [CrossRef]
- Kotnik, T.; Frey, W.; Sack, M.; Meglič, S.H.; Peterka, M.; Miklavčič, D. Electroporation-based applications in biotechnology. Trends Biotechnol. 2015, 33, 480–488. [Google Scholar] [CrossRef] [PubMed]
- Gehl, J. Electroporation for drug and gene delivery in the clinic: Doctors go electric. Methods Mol. Biol. 2008, 423, 351–359. [Google Scholar]
- Gehl, J.; Skovsgaard, T.; Mir, L. Vascular reactions to in vivo electroporation: Characterization and consequences for drug and gene delivery. Biochim. Biophys. Acta 2002, 1569, 51–58. [Google Scholar] [CrossRef]
- Markelc, B.; Sersa, G.; Cemazar, M. Differential Mechanisms Associated with Vascular Disrupting Action of Electrochemotherapy: Intravital Microscopy on the Level of Single Normal and Tumor Blood Vessels. PLoS ONE 2013, 8, e59557. [Google Scholar] [CrossRef]
- Das, R.; Langou, S.; Le, T.T.; Prasad, P.; Lin, F.; Nguyen, T.D. Electrical Stimulation for Immune Modulation in Cancer Treatments. Front. Bioeng. Biotechnol. 2022, 9, 795300. [Google Scholar] [CrossRef]
- Geboers, B.; Scheffer, H.J.; Graybill, P.M.; Ruarus, A.H.; Nieuwenhuizen, S.; Puijk, R.S.; van den Tol, P.M.; Davalos, R.V.; Rubinsky, B.; de Gruijl, T.D.; et al. High-Voltage Electrical Pulses in Oncology: Irreversible Electroporation, Electrochemotherapy, Gene Electrotransfer, Electrofusion, and Electroimmunotherapy. Radiology 2020, 295, 254–272. [Google Scholar] [CrossRef]
- Tremble, L.F.; O’Brien, M.A.; Soden, D.M.; Forde, P.F. Electrochemotherapy with cisplatin increases survival and induces immunogenic responses in murine models of lung cancer and colorectal cancer. Cancer Lett. 2019, 442, 475–482. [Google Scholar] [CrossRef]
- Arnold, C.E.; Rajnicek, A.M.; Hoare, J.I.; Pokharel, S.M.; McCaig, C.D.; Barker, R.N.; Wilson, H.M. Physiological strength electric fields modulate human T cell activation and polarisation. Sci. Rep. 2019, 9, 17604. [Google Scholar] [CrossRef]
- Hernández-Bule, M.L.; Trillo, M.; Úbeda, A. Molecular mechanisms underlying antiproliferative and differentiating responses of hepatocarcinoma cells to subthermal electric stimulation. PLoS ONE 2014, 9, e84636. [Google Scholar] [CrossRef] [PubMed]
- Enokida, T.; Tahara, M. Electrochemotherapy in the Treatment of Head and Neck Cancer: Current Conditions and Future Directions. Cancers 2021, 13, 1418. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, C.; Huebener, P.; Schwabe, R.F. Damage-associated molecular patterns in cancer: A double-edged sword. Oncogene 2016, 35, 5931–5941. [Google Scholar] [CrossRef] [PubMed]
- Di Barba, P.; Mognaschi, M.E.; Forzan, M.; Sgarbossa, P.; Sieni, E. Numerical models for designing ECT applications. In Proceedings of the 2019 19th International Symposium on Electromagnetic Fields in Mechatronics, Electrical and Electronic Engineering (ISEF), Nancy, France, 29–31 August 2019. [Google Scholar]
- Giardino, R.; Fini, M.; Bonazzi, V.; Cadossi, R.; Nicolini, A.; Carpi, A. Electrochemotherapy a novel approach to the treatment of metastatic nodules on the skin and subcutaneous tissues. Biomed. Pharmacother. 2006, 60, 458–462. [Google Scholar] [CrossRef]
- Condello, M.; D’Avack, G.; Spugnini, E.P.; Meschini, S. Electrochemotherapy: An Alternative Strategy for Improving Therapy in Drug-Resistant SOLID Tumors. Cancers 2022, 14, 4341. [Google Scholar] [CrossRef]
- Heller, R.; Gilbert, R.; Jaroszeski, M.J. Clinical applications of electrochemotherapy. Adv. Drug Deliv. Rev. 1999, 35, 119–129. [Google Scholar] [CrossRef]
- Möller, M.G.; Salwa, S.; Soden, D.M.; O’Sullivan, G.C. Electrochemotherapy as an adjunct or alternative to other treatments for unresectable or in-transit melanoma. Expert Rev. Anticancer. Ther. 2009, 9, 1611–1630. [Google Scholar] [CrossRef]
- Hampton, T. Electric Pulses Help With Chemotherapy, May Open New Paths for Other Agents. JAMA 2011, 305, 549–551. [Google Scholar] [CrossRef]
- Gehl, J.; Sersa, G.; Matthiessen, L.W.; Muir, T.; Soden, D.; Occhini, A.; Quaglino, P.; Curatolo, P.; Campana, L.G.; Kunte, C.; et al. Updated standard operating procedures for electrochemotherapy of cutaneous tumours and skin metastases. Acta Oncol. 2018, 57, 874–882. [Google Scholar] [CrossRef]
- Mir, L.; Gehl, J.; Sersa, G.; Collins, C.; Garbay, J.-R.; Billard, V.; Geertsen, P.; Rudolf, Z.; O’Sullivan, G.C.; Marty, M. Standard operating procedures of the electrochemotherapy: Instructions for the use of bleomycin or cisplatin adminstered either systemically or locally and electric pulses delivered by the Cliniporator (TM) by means of invasive or non-invasive electrodes. EJC Suppl. 2006, 4, 14–25. [Google Scholar] [CrossRef]
- Gehl, J.; Sersa, G.; Garbay, J.; Soden, D.; Rudolf, Z.; Marty, M.; O’Sullivan, G.; Geertsen, P.F.; Mir, L.M. Results of the ESOPE (European Standard Operating Procedures on Electrochemotherapy) study: Efficient, highly tolerable and simple palliative treatment of cutaneous and subcutaneous metastases from cancers of any histology. J. Clin. Oncol. 2006, 24, 8047. [Google Scholar] [CrossRef]
- Allegretti, J.P.; Panje, W.R. Electroporation therapy for head and neck cancer including carotid artery involvement. Laryngoscope 2001, 111, 52–56. [Google Scholar] [CrossRef] [PubMed]
- Corovic, S.; Sakere, B.A.; Haddad, V.; Miklavcic, D.; Mir, L.M. Importance of contact surface between electrodes and treated tissue in electrochemotherapy. Technol. Cancer Res. Treat. 2008, 7, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Campana, L.; di Barba, P.; Dughiero, F.; Forzan, M.; Mognaschi, M.E.; Rizzo, R.; Sieni, E. Non-parallellism of needles in electroporation: 3D computational model and experimental analysis. COMPEL Int. J. Comput. Math. Electr. Electron. Eng. 2018, 38. in press. [Google Scholar] [CrossRef]
- Campana, L.; di Barba, P.; Dughiero, F.; Rossi, C.; Sieni, E. Optimal Needle Positioning for Electrochemotherapy: A Constrained Multiobjective Strategy. IEEE Trans. Magn. 2013, 49, 2141–2144. [Google Scholar] [CrossRef]
- Ho, E.; Leonard, E.; Tih-Shih, L.; Meredith, G. Thermal burns in electroconvulsive therapy. Proc. Singap. Healthc. 2021. [Google Scholar] [CrossRef]
- Plaschke, C.C.; Bertino, G.; McCaul, J.A.; Grau, J.J.; de Bree, R.; Sersa, G.; Occhini, A.; Groselj, A.; Langdon, C.; Heuveling, D.A.; et al. European Research on Electrochemotherapy in Head and Neck Cancer (EURECA) project: Results from the treatment of mucosal cancers. Eur. J. Cancer 2017, 87, 172–181. [Google Scholar] [CrossRef]
- Plaschke, C.C.; Gothelf, A.; Gehl, J.; Wessel, I. Electrochemotherapy of mucosal head and neck tumors: A systematic review. Acta Oncol. 2016, 55, 1266–1272. [Google Scholar] [CrossRef] [PubMed]
- Marty, M.; Sersa, G.; Garbay, J.R.; Gehl, J.; Collins, C.G.; Snoj, M.; Billard, V.; Geertsen, P.F.; Larkin, J.O.; Miklavcic, D.; et al. Electrochemotherapy–An easy, highly effective and safe treatment of cutaneous and subcutaneous metastases: Results of ESOPE (European Standard Operating Procedures of Electrochemotherapy) study. Eur. J. Cancer Suppl. 2006, 4, 3–13. [Google Scholar] [CrossRef]
- National Institute for Health and Care Excellence (NICE). Electrochemotherapy for Primary Basal Cell Carcinoma and Primary Squamous Cell Carcinoma. Interventional Procedures Guidance. 2014. Available online: www.nice.org.uk/guidance/ipg478 (accessed on 28 October 2022).
- National Institute for Health and Care Excellence (NICE). Electrochemotherapy for Metastases in the Skin from Tumours of Non-Skin Origin and Melanoma, Interventional Procedures Guidance [IPG446]. 2013. Available online: http://publi-cationsniceorguk/electrochemotherapy-for-metastases-in-the-skin-from-tumours-of-non-skin-origin-and-melanoma-ipg446 (accessed on 28 October 2022).
- Sersa, G.; Cemazar, M.; Snoj, M. Electrochemotherapy of solid tumors-preclinical and clinical experience. In Proceedings of the 2011 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Boston, MA, USA, 30 August–3 September 2011; pp. 728–731. [Google Scholar]
- Matthiessen, L.W.; Keshtgar, M.; Curatolo, P.; Kunte, C.; Grischke, E.M.; Odili, J.; Muir, T.; Mowatt, D.; Clover, J.P.; Liew, S.H.; et al. Electrochemotherapy for Breast Cancer-Results From the INSPECT Database. Clin. Breast Cancer 2018, 18, e909–e917. [Google Scholar] [CrossRef]
- De Virgilio, A.; Ralli, M.; Longo, L.; Mancini, P.; Attanasio, G.; Atturo, F.; de Vincentiis, M.; Greco, A. Electrochemotherapy in head and neck cancer: A review of an emerging cancer treatment. Oncol Lett. 2018, 16, 3415–3423. [Google Scholar] [CrossRef]
- Campana, L.G.; Miklavčič, D.; Bertino, G.; Marconato, R.; Valpione, S.; Imarisio, I.; Dieci, M.V.; Granziera, E.; Cemazar, M.; Alaibac, M.; et al. Electrochemotherapy of superficial tumors–Current status:: Basic principles, operating procedures, shared indications, and emerging applications. Semin. Oncol. 2019, 46, 173–191. [Google Scholar] [CrossRef]
- Landström, F.J.; Reizenstein, J.A.; Nilsson, C.O.; Beckerath, M.V.; Löfgren, A.L.; Adamsson, G.B.; Möller, C. Electrochemotherapy-possible benefits and limitations to its use in the head and neck region. Acta Otolaryngol. 2015, 135, 90–95. [Google Scholar] [CrossRef]
- Chisholm, E.; Bapat, U.; Chisholm, C.; Alusi, G.; Vassaux, G. Gene therapy in head and neck cancer: A review. Postgrad. Med. J. 2007, 83, 731–737. [Google Scholar] [CrossRef]
- Xi, S.; Grandis, J.R. Gene Therapy for the Treatment of Oral Squamous Cell Carcinoma. J. Dent. Res. 2003, 82, 11–16. [Google Scholar] [CrossRef]
- Kranjc Brezar, S.; Mrak, V.; Bosnjak, M.; Savarin, M.; Sersa, G.; Cemazar, M. Intratumoral Gene Electrotransfer of Plasmid DNA Encoding shRNA against Melanoma Cell Adhesion Molecule Radiosensitizes Tumors by Antivascular Effects and Activation of an Immune Response. Vaccines 2020, 8, 135. [Google Scholar] [CrossRef]
- Blankenstein, T.; Coulie, P.G.; Gilboa, E.; Jaffee, E.M. The determinants of tumour immunogenicity. Nat. Rev. Cancer 2012, 12, 307–313. [Google Scholar] [CrossRef]
- Sedlar, A.; Dolinsek, T.; Markelc, B.; Prosen, L.; Kranjc, S.; Bosnjak, M.; Blagus, T.; Cemazar, M.; Sersa, G. Potentiation of electrochemotherapy by intramuscular IL-12 gene electrotransfer in murine sarcoma and carcinoma with different immunogenicity. Radiol. Oncol. 2012, 46, 302–311. [Google Scholar] [CrossRef]
- Daud, A.I.; DeConti, R.C.; Andrews, S.; Urbas, P.; Riker, A.I.; Sondak, V.K.; Munster, P.N.; Sullivan, D.M.; Ugen, K.E.; Messina, J.L.; et al. Phase I trial of interleukin-12 plasmid electroporation in patients with metastatic melanoma. J. Clin. Oncol. 2008, 26, 5896–5903. [Google Scholar] [CrossRef]
- Chopra, S.; Satkauskas, S. Electrotransfer of Cytokine Genes for Cancer Treatment. CBU Int. Conf. Proc. 2018, 6, 1036–1041. [Google Scholar] [CrossRef][Green Version]
- Best, S.R.; Peng, S.; Juang, C.M.; Hung, C.F.; Hannaman, D.; Saunders, J.R.; Wu, T.C.; Pai, S.I. Administration of HPV DNA vaccine via electroporation elicits the strongest CD8+ T cell immune responses compared to intramuscular injection and intradermal gene gun delivery. Vaccine 2009, 27, 5450–5459. [Google Scholar] [CrossRef]
- Diehl, M.C.; Lee, J.C.; Daniels, S.E.; Tebas, P.; Khan, A.S.; Giffear, M.; Sardesai, N.Y.; Bagarazzi, M.L. Tolerability of intramuscular and intradermal delivery by CELLECTRA(®) adaptive constant current electroporation device in healthy volunteers. Hum. Vaccin. Immunother. 2013, 9, 2246–2252. [Google Scholar] [CrossRef]
- Berridge, M.J.; Bootman, M.D.; Roderick, H.L. Calcium signalling: Dynamics, homeostasis and remodelling. Nat. Rev. Mol. Cell Biol. 2003, 4, 517–529. [Google Scholar] [CrossRef]
- Lytton, J.; Westlin, M.; Burk, S.E.; Shull, G.E.; MacLennan, D.H. Functional comparisons between isoforms of the sarcoplasmic or endoplasmic reticulum family of calcium pumps. J. Biol. Chem. 1992, 267, 14483–14489. [Google Scholar] [CrossRef]
- Plaschke, C.C.; Gehl, J.; Johannesen, H.H.; Fischer, B.M.; Kjaer, A.; Lomholt, A.F.; Wessel, I. Calcium electroporation for recurrent head and neck cancer: A clinical phase I study. Laryngoscope Investig. Otolaryngol. 2019, 4, 49–56. [Google Scholar] [CrossRef]
- Staresinic, B.; Jesenko, T.; Kamensek, U.; Frandsen, S.K.; Sersa, G.; Gehl, J.; Cemazar, M. Effect of calcium electroporation on tumour vasculature. Sci. Rep. 2018, 8, 9412. [Google Scholar] [CrossRef]
- Falk, H.; Matthiessen, L.W.; Wooler, G.; Gehl, J. Calcium electroporation for treatment of cutaneous metastases; a randomized double-blinded phase II study, comparing the effect of calcium electroporation with electrochemotherapy. Acta Oncol. 2018, 57, 311–319. [Google Scholar] [CrossRef]
- Frandsen, S.K.; Vissing, M.; Gehl, J. A Comprehensive Review of Calcium Electroporation-A Novel Cancer Treatment Modality. Cancers 2020, 12, 290. [Google Scholar] [CrossRef]
- Vissing, M.; Ploen, J.; Pervan, M.; Vestergaard, K.; Schnefeldt, M.; Frandsen, S.K.; Rafaelsen, S.R.; Lindhardt, C.L.; Jensen, L.H.; Rody, A.; et al. Study protocol designed to investigate tumour response to calcium electroporation in cancers affecting the skin: A non-randomised phase II clinical trial. BMJ Open 2021, 11, e046779. [Google Scholar] [CrossRef]
- Mir-Bonafé, J.M.; Vilalta, A.; Alarcón, I.; Carrera, C.; Puig, S.; Malvehy, J.; Rull, R.; Bennàssar, A. Electrochemotherapy in the Treatment of Melanoma Skin Metastases: A Report on 31 Cases. Actas Dermo-Sifiliográficas 2015, 106, 285–291. [Google Scholar] [CrossRef]
- Miklavcic, D.; Dj, A.; Belehradek, J.; Mir, L. Host’s immune response in electrotherapy of murine tumors by direct current. Eur. Cytokine Netw. 1997, 8, 275–279. [Google Scholar]
- Zhang, X.; Zhang, Y.; Chen, J.; Wu, Y.; Zhang, J.; Wang, J. Nanosecond pulsed electric field inhibits malignant melanoma growth by inducing the change of systemic immunity. Med. Oral Patol. Oral Cir. Bucal. 2019, 24, e555–e561. [Google Scholar] [CrossRef]
- Longo, F.; Perri, F.; Caponigro, F.; Scarpati, G.D.V.; Guida, A.; Pavone, E.; Aversa, C.; Muto, P.; Giuliano, M.; Ionna, F.; et al. Boosting the Immune Response with the Combination of Electrochemotherapy and Immunotherapy: A New Weapon for Squamous Cell Carcinoma of the Head and Neck? Cancers 2020, 12, 2781. [Google Scholar] [CrossRef]
- Brizio, M.; Fava, P.; Astrua, C.; Cavaliere, G.; Savoia, P. Complete regression of melanoma skin metastases after electrochemotherapy plus ipilimumab treatment: An unusual clinical presentation. Eur. J. Dermatol. 2015, 25, 271–272. [Google Scholar] [CrossRef]
- Mozzillo, N.; Simeone, E.; Benedetto, L.; Curvietto, M.; Giannarelli, D.; Gentilcore, G.; Camerlingo, R.; Capone, M.; Madonna, G.; Festino, L.; et al. Assessing a novel immuno-oncology-based combination therapy: Ipilimumab plus electrochemotherapy. Oncoimmunology 2015, 4, e1008842. [Google Scholar] [CrossRef]
- Heppt, M.V.; Eigentler, T.K.; Kähler, K.C.; Herbst, R.A.; Göppner, D.; Gambichler, T.; Ulrich, J.; Dippel, E.; Loquai, C.; Schell, B.; et al. Immune checkpoint blockade with concurrent electrochemotherapy in advanced melanoma: A retrospective multicenter analysis. Cancer Immunol. Immunother. 2016, 65, 951–959. [Google Scholar] [CrossRef]
- Karaca, B.; Yayla, G.; Erdem, M.; Gürler, T. Electrochemotherapy with anti-PD-1 treatment induced durable complete response in heavily pretreated metastatic melanoma patient. Anticancer. Drugs 2018, 29, 190–196. [Google Scholar] [CrossRef]
- Campana, L.G.; Peric, B.; Mascherini, M.; Spina, R.; Kunte, C.; Kis, E.; Rozsa, P.; Quaglino, P.; Jones, R.P.; Clover, A.J.P.; et al. Combination of Pembrolizumab with Electrochemotherapy in Cutaneous Metastases from Melanoma: A Comparative Retrospective Study from the InspECT and Slovenian Cancer Registry. Cancers 2021, 13, 4289. [Google Scholar] [CrossRef]
- National Nanotechnology Initiative (NNI). Available online: https://www.nano.gov/about-nanotechnology (accessed on 28 October 2022).
- Zhao, C.-Y.; Cheng, R.; Yang, Z.; Tian, Z.-M. Nanotechnology for Cancer Therapy Based on Chemotherapy. Molecules 2018, 23, 826. [Google Scholar] [CrossRef]
- Gmeiner, W.H.; Ghosh, S. Nanotechnology for cancer treatment. Nanotechnol. Rev. 2015, 3, 111–122. [Google Scholar] [CrossRef]
- Bregoli, L.; Movia, D.; Gavigan-Imedio, J.D.; Lysaght, J.; Reynolds, J.; Prina-Mello, A. Nanomedicine applied to translational oncology: A future perspective on cancer treatment. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 81–103. [Google Scholar] [CrossRef] [PubMed]
- Volovat, S.R.; Ursulescu, C.L.; Moisii, L.G.; Volovat, C.; Boboc, D.; Scripcariu, D.; Amurariti, F.; Stefanescu, C.; Stolniceanu, C.R.; Agop, M.; et al. The Landscape of Nanovectors for Modulation in Cancer Immunotherapy. Pharmaceutics 2022, 14, 397. [Google Scholar] [CrossRef] [PubMed]
- Kiran, A.V.V.V.R.; Kumari, G.K.; Krishnamurthy, P.T.; Khaydarov, R.R. Tumor microenvironment and nanotherapeutics: Intruding the tumor fort. Biomater. Sci. 2021, 9, 7667–7704. [Google Scholar] [CrossRef]
- Jackson, M.A.; Werfel, T.A.; Curvino, E.J.; Yu, F.; Kavanaugh, T.E.; Sarett, S.M.; Dockery, M.D.; Kilchrist, K.V.; Jackson, A.N.; Giorgio, T.D.; et al. Zwitterionic Nanocarrier Surface Chemistry Improves siRNA Tumor Delivery and Silencing Activity Relative to Polyethylene Glycol. ACS Nano 2017, 6, 5680–5696. [Google Scholar] [CrossRef]
- Fan, Z.; Li, P.Y.; Deng, J.; Bady, S.C.; Cheng, H. Cell membrane coating for reducing nanoparticle-induced inflammatory responses to scaffold constructs. Nano Res. 2018, 11, 5573–5583. [Google Scholar] [CrossRef] [PubMed]
- Gobbo, O.L.; Sjaastad, K.; Radomski, M.W.; Volkov, Y.; Prina-Mello, A. Magnetic Nanoparticles in Cancer Theranostics. Theranostics 2015, 5, 1249–1263. [Google Scholar] [CrossRef] [PubMed]
- Qian, X.L.; Li, J.; Wei, R.; Lin, H.; Xiong, L.X. Internal and External Triggering Mechanism of “Smart” Nanoparticle-Based DDSs in Targeted Tumor Therapy. Curr. Pharm. Des. 2018, 24, 1639–1651. [Google Scholar] [CrossRef]
- ETPN-Nanomedicine European Technology Platform. Nanomedicine Strategic Research & Innovation Agenda 2016–2030: Creating Junctions for Healthcare; ETPN-Nanomedicine European Technology Platform: Paris, France, 2016; pp. 1–31. [Google Scholar]
- Viegas, C.; Pereira, D.S.M.; Fonte, P. Insights into Nanomedicine for Head and Neck Cancer Diagnosis and Treatment. Materials 2022, 15, 2086. [Google Scholar] [CrossRef] [PubMed]
- Aghebati-Maleki, A.; Dolati, S.; Ahmadi, M.; Baghbanzhadeh, A.; Asadi, M.; Fotouhi, A.; Yousefi, M.; Aghebati-Maleki, L. Nanoparticles and cancer therapy: Perspectives for application of nanoparticles in the treatment of cancers. J. Cell. Physiol. 2020, 235, 1962–1972. [Google Scholar] [CrossRef]
- Zhao, Y.; Chen, H.; Chen, X.; Hollett, G.; Gu, Z.; Wu, J.; Liu, X. Targeted nanoparticles for head and neck cancers: Overview and perspectives. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2017, 9, e1469. [Google Scholar] [CrossRef]
- Melancon, M.P.; Appleton Figueira, T.; Fuentes, D.T.; Tian, L.; Qiao, Y.; Gu, J.; Gagea, M.; Ensor, J.E.; Muñoz, N.M.; Maldonado, K.L.; et al. Development of an Electroporation and Nanoparticle-based Therapeutic Platform for Bone Metastases. Radiology 2018, 286, 149–157. [Google Scholar] [CrossRef]
- Kim, K.; Lee, W.G. Electroporation for nanomedicine: A review. J. Mater. Chem. B 2017, 5, 2726–2738. [Google Scholar] [CrossRef]
- Alshehri, M.A.; Wierzbicki, P.M.; Kaboo, H.F.; Nasr, M.S.M.; Amer, M.E.; Abuamara, T.M.M.; Badr, D.A.; Saleh, K.A.; Fazary, A.E.; Mohamed, A.F. In vitro evaluation of electroporated gold nanoparticles and extremely-low frequency electromagnetic field anticancer activity against Hep-2 laryngeal cancer cells. Folia Histochem. Cytobiol. 2019, 57, 159–167. [Google Scholar] [CrossRef]
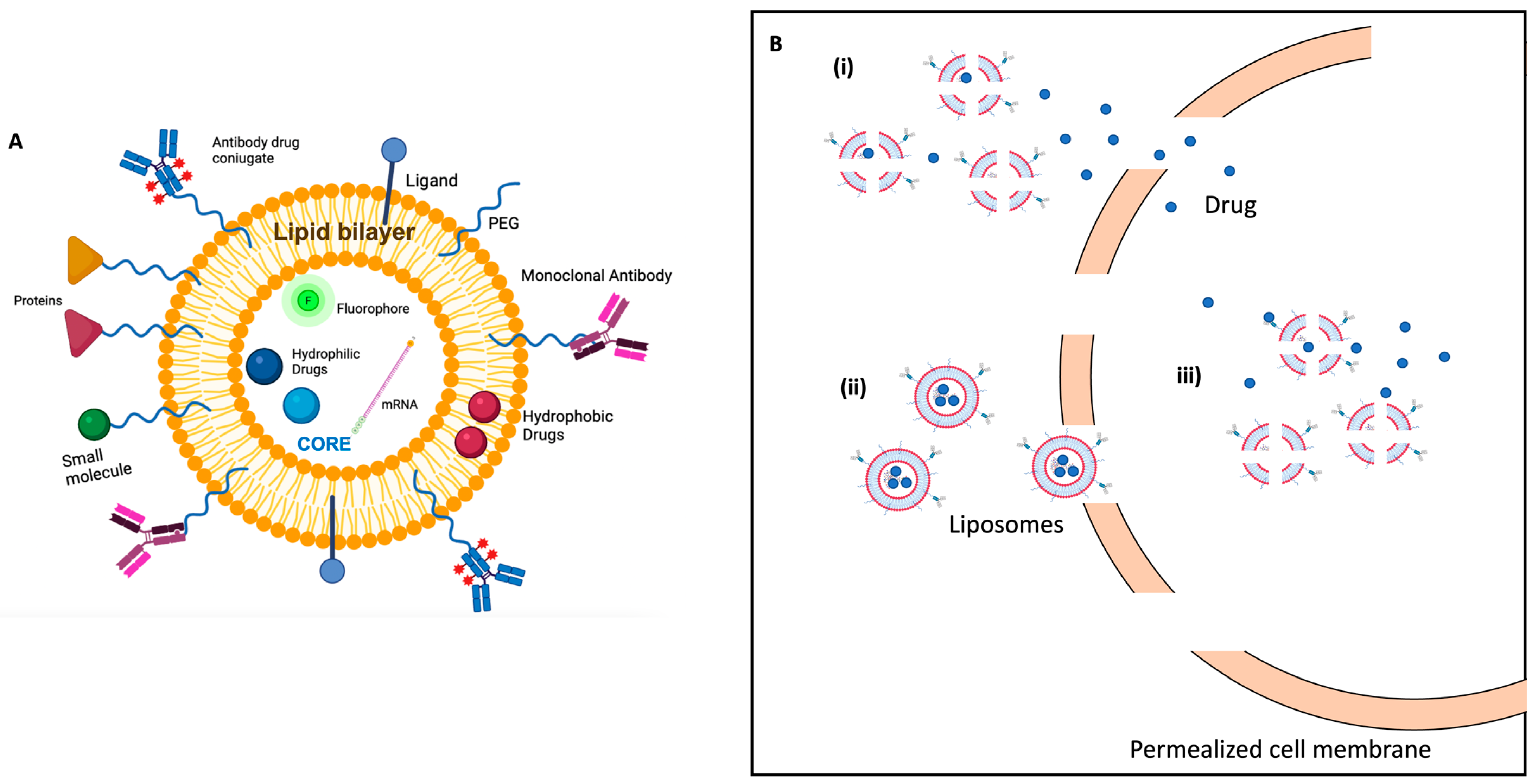
- Barenholz, Y. Doxil®--the first FDA-approved nano-drug: Lessons learned. J. Control. Release 2012, 160, 117–134. [Google Scholar] [CrossRef]
- Bulbake, U.; Doppalapudi, S.; Kommineni, N.; Khan, W. Liposomal Formulations in Clinical Use: An Updated Review. Pharmaceutics 2017, 9, 12. [Google Scholar] [CrossRef]
- Beltrán-Gracia, E.; López-Camacho, A.; Higuera-Ciapara, I.; Velázquez-Fernández, J.B.; Vallejo-Cardona, A.A. Nanomedicine review: Clinical developments in liposomal applications. Cancer Nanotechnol. 2019, 10, 11. [Google Scholar] [CrossRef]
- Caracciolo, G. Clinically approved liposomal nanomedicines: Lessons learned from the biomolecular corona. Nanoscale 2018, 10, 4167–4172. [Google Scholar] [CrossRef]
- Su, S.; Kang, P.M. Recent Advances in Nanocarrier-Assisted Therapeutics Delivery Systems. Pharmaceutics 2020, 12, 837. [Google Scholar] [CrossRef]
- Bozzuto, G.; Molinari, A. Liposomes as nanomedical devices. Int. J. Nanomed. 2015, 10, 975–995. [Google Scholar] [CrossRef]
- Lamichhane, N.; Udayakumar, T.S.; D’Souza, W.D.; Simone, C.B.; Raghavan, S.R., II; Polf, J.; Mahmood, J. Liposomes: Clinical Applications and Potential for Image-Guided Drug Delivery. Molecules 2018, 23, 288. [Google Scholar] [CrossRef]
- Balaure, P.C.; Gudovan, D.; Gudovan, I.A. Smart Triggered Release in Controlled Drug Delivery. Curr. Drug Targets 2018, 19, 318–327. [Google Scholar] [CrossRef] [PubMed]
- Chaurasiya, A.; Gorajiya, A.; Panchal, K.; Katke, S.; Singh, A.K. A review on multivesicular liposomes for pharmaceutical applications: Preparation, characterization, and translational challenges. Drug Deliv. Transl. Res. 2022, 12, 1569–1587. [Google Scholar] [CrossRef] [PubMed]
- Mishra, H.; Chauhan, V.; Kumar, K.; Teotia, D. A comprehensive review on Liposomes: A novel drug delivery system. Univers. J. Pharm. Res. 2018, 8, 400–404. [Google Scholar] [CrossRef]
- Srimathveeravalli, G.; Abdel-Atti, D.; Pérez-Medina, C.; Takaki, H.; Solomon, S.B.; Mulder, W.J.M.; Reiner, T. Reversible Electroporation-Mediated Liposomal Doxorubicin Delivery to Tumors Can Be Monitored with (89)Zr-Labeled Reporter Nanoparticles. Mol. Imaging 2018, 17, 1536012117749726. [Google Scholar] [CrossRef]
- Caramazza, L.; Nardoni, M.; de Angelis, A.; Paolicelli, P.; Liberti, M.; Apollonio, F.; Petralito, S. Proof-of-Concept of Electrical Activation of Liposome Nanocarriers: From Dry to Wet Experiments. Front. Bioeng. Biotechnol. 2020, 8, 819. [Google Scholar] [CrossRef]
- Retelj, L.; Pucihar, G.; Miklavcic, D. Electroporation of intracellular liposomes using nanosecond electric pulses--a theoretical study. IEEE Trans. Biomed. Eng. 2013, 60, 2624–2635. [Google Scholar] [CrossRef]
- Denzi, A.; Della Valle, E.; Apollonio, F.; Breton, M.; Mir, L.M.; Liberti, M. Exploring the Applicability of Nano-Poration for Remote Control in Smart Drug Delivery Systems. J. Membr. Biol. 2017, 250, 31–40. [Google Scholar] [CrossRef]
- Denzi, A.; della Valle, E.; Esposito, G.; Mir, L.M.; Apollonio, F.; Liberti, M. Technological and Theoretical Aspects for Testing Electroporation on Liposomes. BioMed Res. Int. 2017, 2017, 5092704. [Google Scholar] [CrossRef]
- Raffy, S.; Teissié, J. Control of Lipid Membrane Stability by Cholesterol Content. Biophys. J. 1999, 76, 2072–2080. [Google Scholar] [CrossRef]
- Ogiso, T.; Yamaguchi, T.; Iwaki, M.; Tanino, T.; Miyake, Y. Effect of positively and negatively charged liposomes on skin permeation of drugs. J. Drug Target 2001, 9, 49–59. [Google Scholar] [CrossRef]
- Riaz, M.K.; Riaz, M.A.; Zhang, X.; Lin, C.; Wong, K.H.; Chen, X.; Zhang, G.; Lu, A.; Yang, Z. Surface Functionalization and Targeting Strategies of Liposomes in Solid Tumor Therapy: A Review. Int. J. Mol. Sci. 2018, 19, 195. [Google Scholar] [CrossRef] [PubMed]
- Kotnik, T.; Rems, L.; Tarek, M.; Miklavčič, D. Membrane Electroporation and Electropermeabilization: Mechanisms and Models. Annu. Rev. Biophys. 2019, 48, 63–91. [Google Scholar] [CrossRef] [PubMed]
- Jara-Quijada, E.; Pérez-Won, M.; Tabilo-Munizaga, G.; González-Cavieres, L.; Lemus-Mondaca, R. An Overview Focusing on Food Liposomes and Their Stability to Electric Fields. Food Eng. Rev. 2022, 14, 292–306. [Google Scholar] [CrossRef]
- Kotnik, T.; Miklavcic, D. Second-order model of membrane electric field induced by alternating external electric fields. IEEE Trans. Biomed. Eng. 2000, 47, 1074–1081. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Wang, L.; Qiao, Y.; Lu, L.; Lee, P.; Chang, A.; Ravi, S.; Rogers, T.A.; Melancon, M.P. Antitumor Efficacy of Liposome-Encapsulated NVP-BEZ235 Combined with Irreversible Electroporation for Head and Neck Cancer. Molecules 2019, 24, 3560. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Idris, R.A.M.; Hanaffi, W.N.W.; Perumal, K.; Boer, J.C.; Plebanski, M.; Jaafar, J.; Lim, J.K.; Mohamud, R. Cancer Nanomedicine and Immune System—Interactions and Challenges. Front. Nanotechnol. 2021, 3, 681305. [Google Scholar] [CrossRef]

| Biomarker | Activity | Ref. | |
|---|---|---|---|
| EGFR | Epidermal growth factor receptor | Controlling gene expression, proliferation, angiogenesis, apoptosis inhibition, cell motility, metastasis, adhesion, and angiogenesis | [26] |
| CD44 | Surface receptor for hyaluronic acid and matrix metalloproteinases (MMPs) | Intercellular interactions and cell migration | [27] |
| CD133 | Transmembrane glycoprotein | Invasiveness and metastasis | [28,29] |
| ALDH1 | Intracellular enzyme able to convert retinol into retinoic acid Cellular detoxification | Marker for both normal stem cells and CSCs | [25] |
| STAT3 | Protein transcription factor | It drives expression of genes promoting cellular proliferation and survival and genes encoding growth factors and cytokines promoting immunosuppression (IL-6, IL-10, and TGF-beta) | [30] |
| PTPRs | Protein tyrosine phosphatase receptors | It causes STAT3 hyperactivation in H&N | [31] |
| PD-L1 | Programmed death-ligand transmembrane protein | Biding receptor PD-1 suppresses the adaptive immune system | [32] |
| Monoclonal Antibody | Commercial Name | Mechanism of Action | Clinical Indication | Note |
|---|---|---|---|---|
| Cetuximab | Erbitux®; Merck | Binds with high affinity to the extracellular domain of human EGFR inhibiting receptor activity targets cytotoxic immune effector cells towards EGFR-expressing tumor cells (antibody-dependent cell-mediated cytotoxicity) | Patients with recurrent or metastatic disease Cisplatin-ineligible patients | FDA and EMA approved for HNSCC [36] |
| Pembrolizumab | KEYTRUDA®, Merck | Targeted programmed cell death protein PD-L1 (immune checkpoint inhibitors) | Cisplatin-sensitive HNSCC Patients with metastatic or unresectable recurrent HNSCC | FDA; EMA approved for HNSCC [37] |
| Nivolumab | Opdivo ® Bristol-Myers Squibb | Binds to the PD-1 receptor and blocks its interaction with PD-L1 and PD-L2 (immune checkpoint inhibitors) | Cisplatin-refractory recurrent or metastatic HNSCC | FDA and EMA approved for cancer treatments and in combined therapy for HNSCC [38] |
| Ipilimumab | Yervoy ® Bristol-Myers Squibb | Binds CTLA-4 inhibitory signal, activating immune system | Loco-regionally advanced HNSCC | Evaluated in combination with HNSCC treatment [38]. Not yet approved by EMA |
| NCT Number | Title | Interventions | Phase | Last Update | Location |
|---|---|---|---|---|---|
| NCT03051269 | Calcium electroporation for head-and-neck cancer | Drug: calcium chloride device: electroporation | 1 | 2017 | Department of Otorhinolaryngology, Rigshospitalet, Copenhagen University Hospital, Copenhagen, Denmark |
| NCT02549742 | Electrochemotherapy on head-and-neck cancer | Procedure: Electrochemotherapy Device: Cliniporator •Drug: bleomycin | 2 | 2017 | Department of Otorhinolaryngology, Rigshospitalet, Copenhagen University Hospital, Copenhagen, Denmark |
| NCT00198315 | Medpulser Electroporation with Bleomycin Study to treat anterior head-and-neck squamous cell carcinoma | Combination product: Medpulser electroporation with bleomycin Procedure: tumor surgical excision | 3 | 2017 | Inovio Biomedical Corporation, San Diego, California, United States |
| NCT00198263 | Study using the Medpulser electroporation system with Bleomycin to treat head-and-neck cancer | Combination product: Medpulser electroporation with bleomycin | 4 | 2017 | Inovio Biomedical Corporation, San Diego, California, United States |
| NCT01493154 | Safety study of HPV DNA vaccine to treat head-and-neck cancer patients | Biological: DNA vaccine drug: cyclophosphamide | 1 | 2018 | Sidney Kimmel Comprehensive Cancer Center, Johns Hopkins Hospital, Baltimore, Maryland, United States |
| NCT02960594 | hTERT immunotherapy alone or in combination with IL-12 DNA followed by electroporation in adults with solid tumors (H&N cancer and esophageal cancer) at high risk of relapse (TRT-001) | Biological: INO-1400 Biological: INO-9012 Biological: INO-1401 | 1 | 2018 | Barbara Ann Karmanos Cancer Institute Detroit, Michigan, United States Mayo Clinic Rochester, Minnesota, United States And more. |
| NCT02345330 | Trial of pIL-12 electroporation in squamous cll carcinoma of the head and neck (IL12HNSCC) | Biological: Tavokinogene Telseplasmid (tavo) Device: OncoSec Medical System (OMS) | 2 | 2018 | UCSF Helen Diller Family Comprehensive Cancer Center, San Francisco, California, United States University of Chicago Medical Center, Chicago, Illinois, United States |
| NCT01941901 | Calcium electroporation for treatment of cutaneous metastases | Drug: calcium electroporation Drug: electrochemotherapy with bleomycin | 2 | 2019 | Department of Oncology, Copenhagen University Hospital, Herlev, Herlev, Denmark |
| NCT03823131 | Optimizing antitumor immunity using plasmid electroporation, Pembrolizumab, and Epacadostat | Device: ImmunoPulse Drug: Epacadostat Drug: Pembrolizumab Biological: CORVax Drug: Tavokinogene telseplasmid | 2 | 2021 | University of California, San Francisco, San Francisco, California, United States |
| NCT02163057 | Study of HPV-specific immunotherapy in participants with HPV-associated head-and-neck squamous cell carcinoma | Biological: INO-3112 •Device: CELLECTRATM-5P | 2 | 2021 | University of Pennsylvania, Philadelphia, Pennsylvania, United States |
| NCT03448666 | ECT-Pembrolizumab in patients with unresectable melanoma with superficial or superficial and visceral metastases | Combination product: Pembrolizumab | 2 | 2021 | IEO Istituto Europeo di Oncologia, Milan, Italy |
| NCT03162224 | Safety and efficacy of MEDI0457 and Durvalumab in patients with HPV associated recurrent/metastatic head-and-neck cancer | Drug: MEDI0457 Device: CELLECTRA®5P device (CELLECTRA 2000) Drug: Durvalumab | 2 | 2021 | San Francisco, California, United States Orlando, Florida, United States Atlanta, Georgia, United States Indianapolis, Indiana, United States Baltimore, Maryland, United States Baltimore, Maryland, United States And more. |
| NCT Number | Title | Interventions | Phase | Last Update | Location |
|---|---|---|---|---|---|
| NCT00252889 | Doxil Topotecan doublet cancer Study (H&N cancer, Esophageal cancer) | Drug: Topotecan and pegylated doxorubicin | 1 | 2009 | Christiana Care Health Services, Newark, Delaware, United States |
| NCT00022594 | Liposomal Lurtotecan in treating patients with metastatic or locally recurrent head-and-neck cancer | Drug: liposomal lurtotecan | 2 | 2012 | Kaiser Franz Josef Hospital, Vienna (Wien), Austria Universitair Ziekenhuis Antwerpen, Edegem, Belgium Centre Jean Perrin, Clermont-Ferrand, France And more |
| NCT00009841 | Gene therapy in treating patients with advanced head-and-neck cancer | Biological: EGFR antisense DNA Biological: growth factor antagonist therapy Drug: DC-cholesterol liposome | 1 | 2016 | University of Pittsburgh Cancer Institute, Pittsburgh, Pennsylvania, United States |
| NCT02262455 | Effects of STM 434 alone or in combination with liposomal doxorubicin in patients with ovarian cancer or other advanced solid tumors (H&N cancer) | Drug: STM 434 (inhibitor of activin A) Drug: liposomal doxorubicin | 1 | 2017 | Dana Farber Cancer Institute Boston, Massachusetts, United States Memorial Sloan Kettering Cancer Center New York, New York, United States And more |
| NCT03076372 | A study evaluating MM-310 in patients with solid tumors (HNSCC) | MM-310 is a liposomal formulation of a docetaxel | 1 | 2018 | Honor Health, Scottsdale, Arizona, United States University California San Francisco, San Francisco, California, United States Mayo Clinic, Rochester, Minnesota, United States |
| NCT00006036 | Liposomal Lurtotecan plus Cisplatin in treating patients with advanced or metastatic solid tumors | Drug: cisplatin Drug: lurtotecan liposome | 1 | 2020 | British Columbia Cancer Agency, Vancouver, British Columbia, Canada Cancer Care Ontario-Hamilton Regional Cancer Centre, Hamilton, Ontario, Canada Toronto General Hospital, Toronto, Ontario, Canada |
| NCT04902027 | A study of Mitoxantrone Hydrochloride liposome injection in the treatment of recurrent/metastatic head-and-neck cancers | Drug: mitoxantrone hydrochloride liposome, intravenous injection (IV) | 1 | 2021 | - |
| NCT04244552 | A Phase 1b trial of ATRC-101 in adults with advanced solid malignancies (HNSCC) | Biological: ATRC-101(engineered fully human immunoglobulin G, subclass 1 (IgG1) antibody) Biological: Pembrolizumab Drug: pegylated liposomal doxorubicin (PLD) | 1 | 2021 | Mayo Clinic Phoenix, Arizona, United States The University of Arizona Cancer Center Tucson, Arizona, United States And more |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pisani, S.; Bertino, G.; Prina-Mello, A.; Locati, L.D.; Mauramati, S.; Genta, I.; Dorati, R.; Conti, B.; Benazzo, M. Electroporation in Head-and-Neck Cancer: An Innovative Approach with Immunotherapy and Nanotechnology Combination. Cancers 2022, 14, 5363. https://doi.org/10.3390/cancers14215363
Pisani S, Bertino G, Prina-Mello A, Locati LD, Mauramati S, Genta I, Dorati R, Conti B, Benazzo M. Electroporation in Head-and-Neck Cancer: An Innovative Approach with Immunotherapy and Nanotechnology Combination. Cancers. 2022; 14(21):5363. https://doi.org/10.3390/cancers14215363
Chicago/Turabian StylePisani, Silvia, Giulia Bertino, Adriele Prina-Mello, Laura Deborah Locati, Simone Mauramati, Ida Genta, Rossella Dorati, Bice Conti, and Marco Benazzo. 2022. "Electroporation in Head-and-Neck Cancer: An Innovative Approach with Immunotherapy and Nanotechnology Combination" Cancers 14, no. 21: 5363. https://doi.org/10.3390/cancers14215363
APA StylePisani, S., Bertino, G., Prina-Mello, A., Locati, L. D., Mauramati, S., Genta, I., Dorati, R., Conti, B., & Benazzo, M. (2022). Electroporation in Head-and-Neck Cancer: An Innovative Approach with Immunotherapy and Nanotechnology Combination. Cancers, 14(21), 5363. https://doi.org/10.3390/cancers14215363















