Subclass Analysis of Malignant, Inflammatory and Degenerative Pathologies Based on Multiple Timepoint FAPI-PET Acquisitions Using FAPI-02, FAPI-46 and FAPI-74
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Characterization
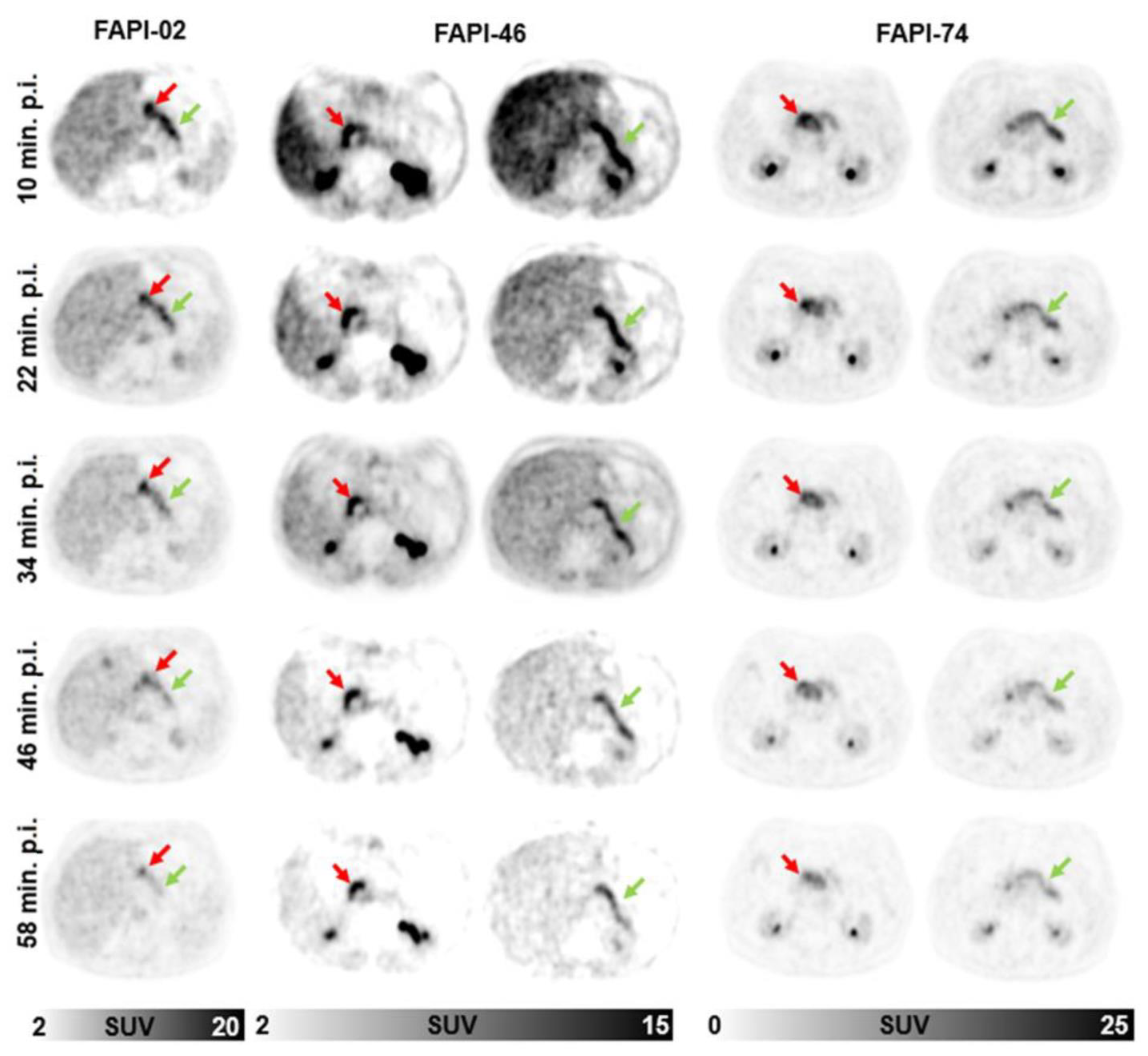
2.2. Repetitive FAPI-PET/CT Imaging
2.3. Image Analysis and Quantification
2.4. Statistical Analysis
3. Results
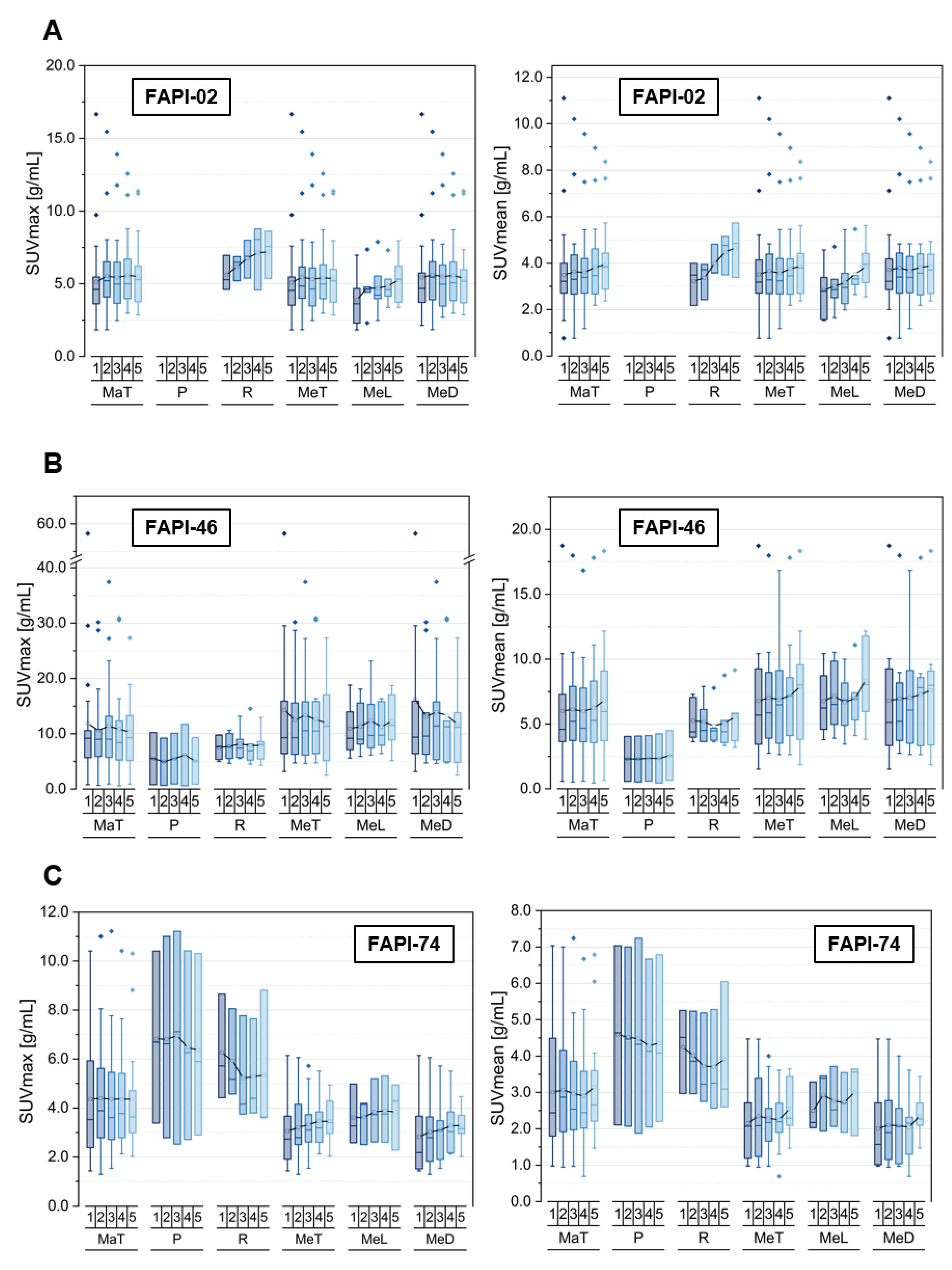
3.1. Tracer Uptake and TBRs of Malignant Manifestations over Time
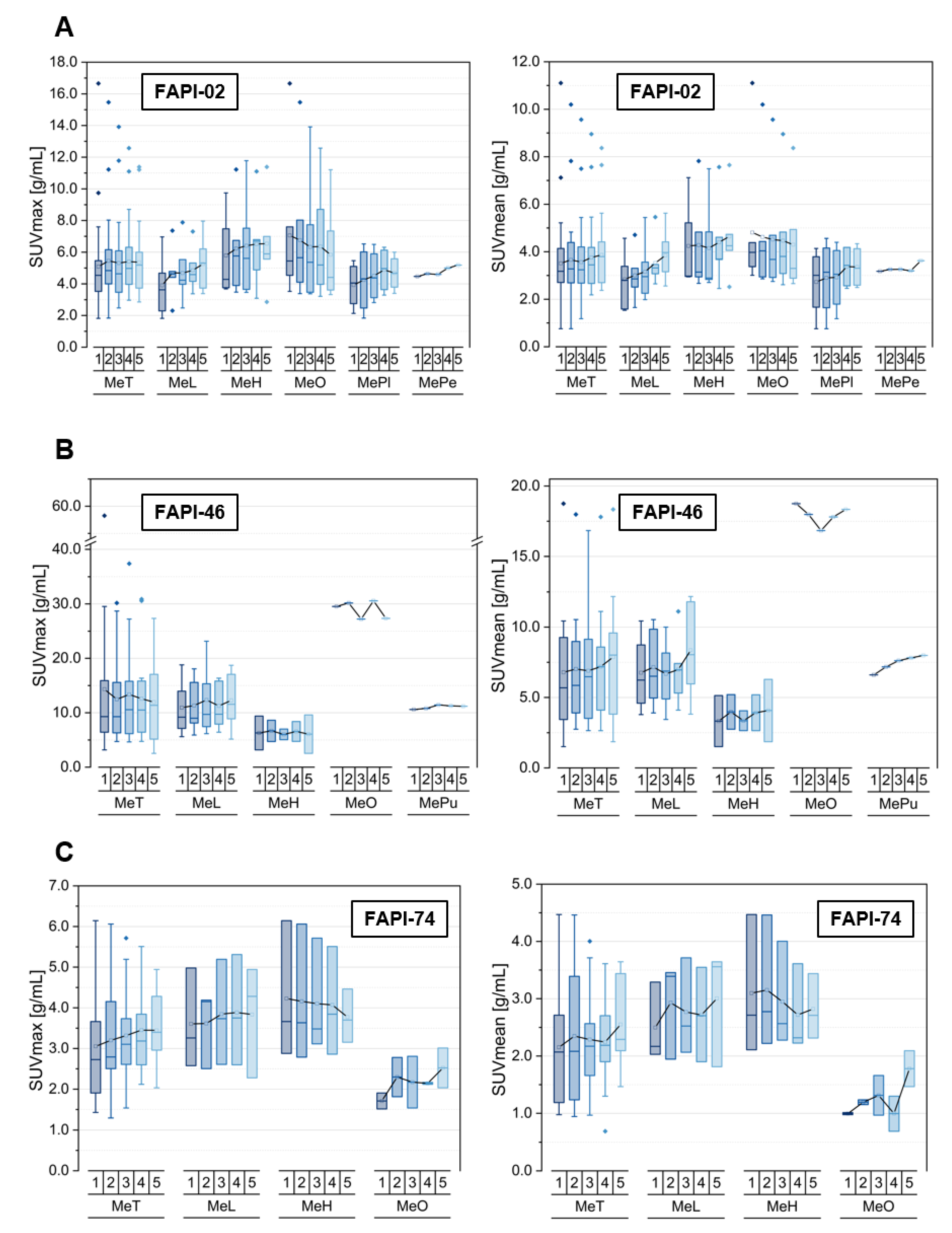
3.2. Tracer Uptake and TBRs of Metastases at Different Localizations over Time
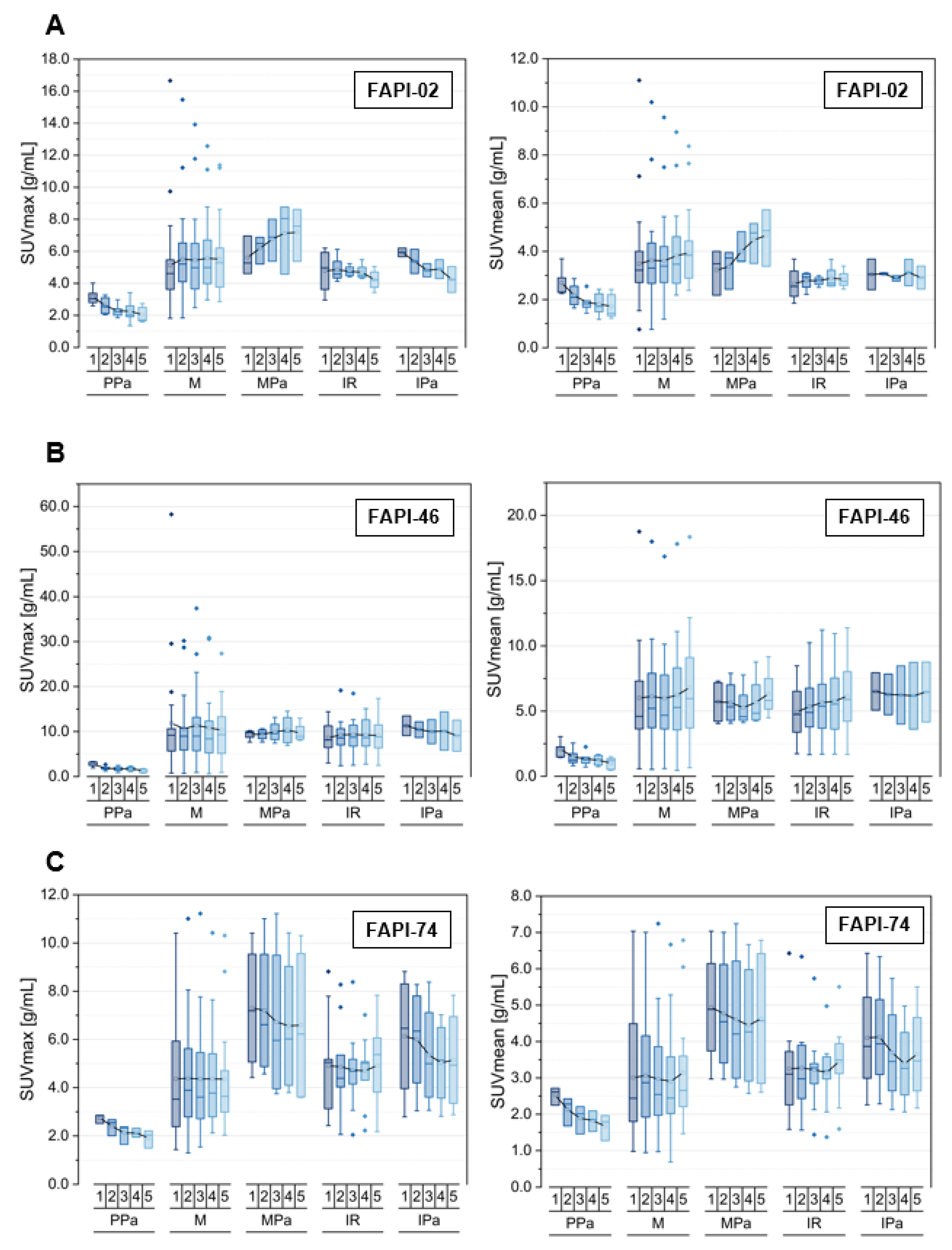
3.3. Tracer Uptake and TBRs of Inflammatory/Reactive Lesions over Time
3.4. Comparison of Physiological Pancreatic FAPI-Uptake, Pancreatitis- and Pancreatic Carcinoma-Associated FAPI-Uptakes and TBRs over Time
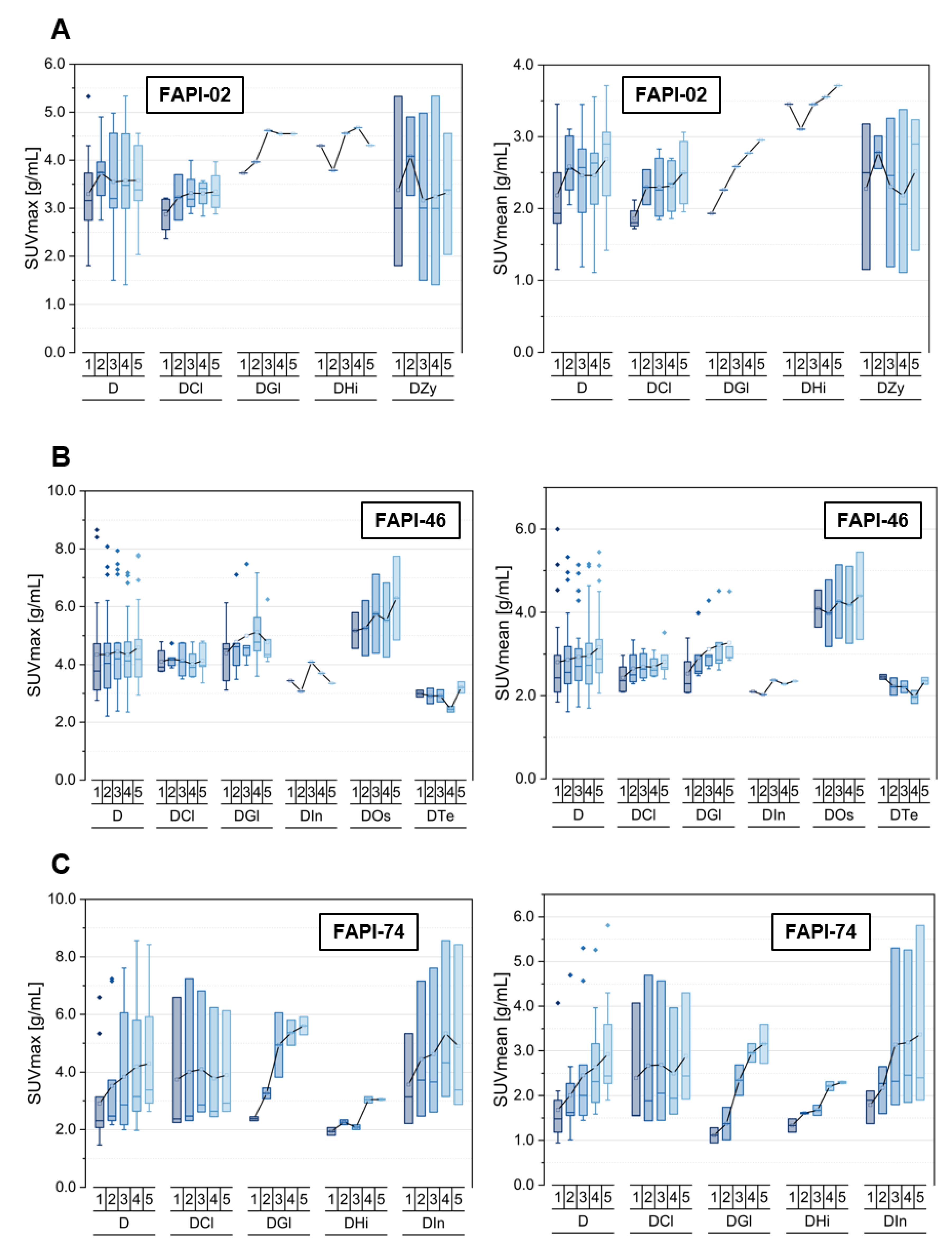
3.5. Tracer Uptake and TBRs of Degenerative Lesions over Time
3.6. Inter-Tracer Comparison of Uptake and TBRs Regarding Specific Malignant, Inflammatory and Degenerative Lesions over Time
4. Discussion
4.1. Summary of the Results
4.2. Differentiation of Malignant from Benign Lesions and the Diagnostic Benefit through Repetitive Early FAPI-PET/CT Imaging
4.3. Differentiation of Pancreatic Carcinoma from Inflammatory Lesions of the Pancreas
4.4. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- LeBleu, V.S.; Kalluri, R. A peek into cancer-associated fibroblasts: Origins, functions and translational impact. Dis. Model. Mech. 2018, 11, dmm029447. [Google Scholar] [CrossRef] [PubMed]
- Kalluri, R. The biology and function of fibroblasts in cancer. Nat. Rev. Cancer 2016, 16, 582–598. [Google Scholar] [CrossRef] [PubMed]
- Sahai, E.; Astsaturov, I.; Cukierman, E.; DeNardo, D.G.; Egeblad, M.; Evans, R.M.; Fearon, D.; Greten, F.R.; Hingorani, S.R.; Hunter, T.; et al. A framework for advancing our understanding of cancer-associated fibroblasts. Nat. Rev. Cancer 2020, 20, 174–186. [Google Scholar] [CrossRef] [PubMed]
- Lindner, T.; Loktev, A.; Altmann, A.; Giesel, F.; Kratochwil, C.; Debus, J.; Jager, D.; Mier, W.; Haberkorn, U. Development of Quinoline-Based Theranostic Ligands for the Targeting of Fibroblast Activation Protein. J. Nucl. Med. 2018, 59, 1415–1422. [Google Scholar] [CrossRef]
- Henderson, N.C.; Rieder, F.; Wynn, T.A. Fibrosis: From mechanisms to medicines. Nature 2020, 587, 555–566. [Google Scholar] [CrossRef]
- Affo, S.; Yu, L.X.; Schwabe, R.F. The Role of Cancer-Associated Fibroblasts and Fibrosis in Liver Cancer. Annu. Rev. Pathol. 2017, 12, 153–186. [Google Scholar] [CrossRef]
- Pap, T.; Dankbar, B.; Wehmeyer, C.; Korb-Pap, A.; Sherwood, J. Synovial fibroblasts and articular tissue remodelling: Role and mechanisms. Semin. Cell Dev. Biol. 2020, 101, 140–145. [Google Scholar] [CrossRef]
- Luo, Y.; Pan, Q.; Xu, H.; Zhang, R.; Li, J.; Li, F. Active uptake of (68)Ga-FAPI in Crohn’s disease but not in ulcerative colitis. Eur J. Nucl. Med. Mol. Imaging 2021, 48, 1682–1683. [Google Scholar] [CrossRef]
- Xu, T.; Zhao, Y.; Ding, H.; Cai, L.; Zhou, Z.; Song, Z.; Chen, Y. [(68)Ga]Ga-DOTA-FAPI-04 PET/CT imaging in a case of prostate cancer with shoulder arthritis. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 1254–1255. [Google Scholar] [CrossRef]
- Rohrich, M.; Naumann, P.; Giesel, F.L.; Choyke, P.; Staudinger, F.; Wefers, A.; Liew, D.P.; Kratochwil, C.; Rathke, H.; Liermann, J.; et al. Impact of (68)Ga-FAPI-PET/CT imaging on the therapeutic management of primary and recurrent pancreatic ductal adenocarcinomas. J. Nucl. Med. 2020, 62, 779–786. [Google Scholar] [CrossRef]
- Rohrich, M.; Leitz, D.; Glatting, F.M.; Wefers, A.K.; Weinheimer, O.; Flechsig, P.; Kahn, N.; Mall, M.A.; Giesel, F.L.; Kratochwil, C.; et al. Fibroblast Activation Protein-Specific PET/CT Imaging in Fibrotic Interstitial Lung Diseases and Lung Cancer: A Translational Exploratory Study. J. Nucl. Med. 2022, 63, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Schmidkonz, C.; Rauber, S.; Atzinger, A.; Agarwal, R.; Gotz, T.I.; Soare, A.; Cordes, M.; Prante, O.; Bergmann, C.; Kleyer, A.; et al. Disentangling inflammatory from fibrotic disease activity by fibroblast activation protein imaging. Ann. Rheum. Dis. 2020, 79, 1485–1491. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Pan, Q.; Yang, H.; Peng, L.; Zhang, W.; Li, F. Fibroblast Activation Protein-Targeted PET/CT with (68)Ga-FAPI for Imaging IgG4-Related Disease: Comparison to (18)F-FDG PET/CT. J. Nucl. Med. 2021, 62, 266–271. [Google Scholar] [CrossRef]
- Zhou, Y.; Yang, X.; Liu, H.; Luo, W.; Liu, H.; Lv, T.; Wang, J.; Qin, J.; Ou, S.; Chen, Y. Value of [(68)Ga]Ga-FAPI-04 imaging in the diagnosis of renal fibrosis. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 3493–3501. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Pan, Q.; Yang, H.; Li, F.; Zhang, F. Inflammatory Arthritis Induced by Anti-Programmed Death-1 Shown in 68Ga-FAPI PET/CT in a Patient With Esophageal Carcinoma. Clin. Nucl. Med. 2021, 46, 431–432. [Google Scholar] [CrossRef] [PubMed]
- Kessler, L.; Ferdinandus, J.; Hirmas, N.; Zarrad, F.; Nader, M.; Kersting, D.; Weber, M.; Kazek, S.; Sraieb, M.; Hamacher, R.; et al. Pitfalls and Common Findings in (68)Ga-FAPI PET: A Pictorial Analysis. J. Nucl. Med. 2022, 63, 890–896. [Google Scholar] [CrossRef] [PubMed]
- Hotta, M.; Rieger, A.C.; Jafarvand, M.G.; Menon, N.; Farolfi, A.; Benz, M.R.; Calais, J. Non-oncologic incidental uptake on FAPI PET/CT imaging. Br. J. Radiol. 2022, 20220463. [Google Scholar] [CrossRef]
- Lang, M.; Spektor, A.M.; Hielscher, T.; Hoppner, J.; Glatting, F.M.; Bicu, F.; Hackert, T.; Heger, U.; Pausch, T.; Gutjahr, E.; et al. Static and dynamic (68)Ga-FAPI PET/CT for the detection of malignant transformation of intraductal papillary mucinous neoplasia of the pancreas. J. Nucl. Med. 2022. [Google Scholar] [CrossRef]
- Kratochwil, C.; Flechsig, P.; Lindner, T.; Abderrahim, L.; Altmann, A.; Mier, W.; Adeberg, S.; Rathke, H.; Rohrich, M.; Winter, H.; et al. (68)Ga-FAPI PET/CT: Tracer Uptake in 28 Different Kinds of Cancer. J. Nucl. Med. 2019, 60, 801–805. [Google Scholar] [CrossRef]
- Giesel, F.L.; Kratochwil, C.; Schlittenhardt, J.; Dendl, K.; Eiber, M.; Staudinger, F.; Kessler, L.; Fendler, W.P.; Lindner, T.; Koerber, S.A.; et al. Head-to-head intra-individual comparison of biodistribution and tumor uptake of (68)Ga-FAPI and (18)F-FDG PET/CT in cancer patients. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 4377–4385. [Google Scholar] [CrossRef]
- Glatting, F.M.; Hoppner, J.; Liew, D.P.; van Genabith, A.; Spektor, A.M.; Steinbach, L.; Hubert, A.; Kratochwil, C.; Giesel, F.L.; Dendl, K.; et al. Repetitive early FAPI-PET acquisition comparing FAPI-02, FAPI-46 and FAPI-74: Methodological and diagnostic implications for malignant, inflammatory and degenerative lesions. J. Nucl. Med. 2022. [Google Scholar] [CrossRef]
- Loktev, A.; Lindner, T.; Burger, E.M.; Altmann, A.; Giesel, F.; Kratochwil, C.; Debus, J.; Marme, F.; Jager, D.; Mier, W.; et al. Development of Fibroblast Activation Protein-Targeted Radiotracers with Improved Tumor Retention. J. Nucl. Med. 2019, 60, 1421–1429. [Google Scholar] [CrossRef]
- Giesel, F.L.; Adeberg, S.; Syed, M.; Lindner, T.; Jiménez-Franco, L.D.; Mavriopoulou, E.; Staudinger, F.; Tonndorf-Martini, E.; Regnery, S.; Rieken, S.; et al. FAPI-74 PET/CT Using Either 18F-AlF or Cold-Kit 68Ga Labeling: Biodistribution, Radiation Dosimetry, and Tumor Delineation in Lung Cancer Patients. J. Nucl. Med. 2020, 62, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Pang, Y.; Zhao, L.; Shang, Q.; Meng, T.; Zhao, L.; Feng, L.; Wang, S.; Guo, P.; Wu, X.; Lin, Q.; et al. Positron emission tomography and computed tomography with [(68)Ga]Ga-fibroblast activation protein inhibitors improves tumor detection and staging in patients with pancreatic cancer. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 1322–1337. [Google Scholar] [CrossRef] [PubMed]

| Tracer Variant | Pathology | Number (%) | ||||||
|---|---|---|---|---|---|---|---|---|
| FAPI-02 | Total | 34 (69.4) | ||||||
| P | 0 (0.0) | |||||||
| R | 3 (8.8) | |||||||
| MeT | 31 (91.2) | |||||||
| MeL | 7 (22.6) | |||||||
| MeD | 24 (77.4) | |||||||
| MeH | 5 (20.8) | |||||||
| MeO | 7 (29.2) | |||||||
| MePl | 4 (16.7) | |||||||
| MePe | 1 (4.2) | |||||||
| other | 7 (29.2) | |||||||
| FAPI-46 | Total | 21 (40.4) | ||||||
| P | 2 (9.5) | |||||||
| R | 5 (23.8) | |||||||
| MeT | 14 (66.7) | |||||||
| MeL | 5 (35.7) | |||||||
| MeD | 9 (64.3) | |||||||
| MeH | 2 (22.2) | |||||||
| MeO | 1 (11.1) | |||||||
| MePu | 1 (11.1) | |||||||
| other | 5 (55.6) | |||||||
| FAPI-74 | Total | 16 (44.4) | ||||||
| P | 3 (18.8) | |||||||
| R | 3 (18.8) | |||||||
| MeT | 10 (62.5) | |||||||
| MeL | 3 (30.0) | |||||||
| MeD | 7 (70.0) | |||||||
| MeH | 3 (42.9) | |||||||
| MeO | 2 (28.6) | |||||||
| other | 2 (28.6) | |||||||
| Tracer Variant | Pathology | Number (%) | ||||
|---|---|---|---|---|---|---|
| FAPI-02 | Total | 4 (100.0) | ||||
| I | 2 (50.0) | |||||
| IPa | 2 (100.0) | |||||
| IOe * | 0 (0.0) | |||||
| Other | 0 (0.0) | |||||
| R | 2 (50.0) | |||||
| RPo | 2 (100.0) | |||||
| Other | 0 (0.0) | |||||
| FAPI-46 | Total | 8 (100.0) | ||||
| I | 4 (50.0) | |||||
| IPa | 2 (50.0) | |||||
| IOe * | 2 (50.0) | |||||
| Other | ||||||
| R | 4 (50.0) | |||||
| RPo | 3 (75.0) | |||||
| Other | 1 (25.0) | |||||
| FAPI-74 | Total | 9 (100.0) | ||||
| I | 5 (55.6) | |||||
| IPa | 4 (80.0) | |||||
| IOe * | 0 (0.0) | |||||
| Other | 1 (20.0) | |||||
| R | 4 (44.4) | |||||
| RPo | 3 (75.0) | |||||
| Other | 1 (25.0) | |||||
| Tracer Variant | Pathology | Number (%) | ||
|---|---|---|---|---|
| FAPI-02 | PPA | 5 | ||
| M | 34 (100.0) | |||
| MPa | 3 (8.8) | |||
| IR | 4 (100.0) | |||
| IPa | 2 (50.0) | |||
| FAPI-46 | PPA | 5 | ||
| M | 21 (100.0) | |||
| MPa | 4 (19.0) | |||
| IR | 8 (100.0) | |||
| IPa | 2 (25.0) | |||
| FAPI-74 | PPA | 3 | ||
| M | 16 (100.0) | |||
| MPa | 4 (25.0) | |||
| IR | 9 (100.0) | |||
| IPa | 4 (44.4) | |||
| Tracer Variant | Pathology | Number (%) | ||
|---|---|---|---|---|
| FAPI-02 | Total | 9 (100.0) | ||
| DCl | 4 (44.4) | |||
| DZy | 3 (33.3) | |||
| DGl | 1 (11.1) | |||
| DHi | 1 (11.1) | |||
| Dos * | 0 (0.0) | |||
| DTe * | 0 (0.0) | |||
| Din * | 0 (0.0) | |||
| DSp * | 0 (0.0) | |||
| Other * | 0 (0.0) | |||
| FAPI-46 | Total | 22 (100.0) | ||
| DCl | 6 (27.3) | |||
| DZy | 1 (4.5) | |||
| DGl | 5 (22.7) | |||
| DHi * | 0 (0.0) | |||
| DOs | 2 (9.1) | |||
| DTe | 2 (9.1) | |||
| DIn | 1 (4.5) | |||
| DSp * | 2 (9.1) | |||
| Other * | 3 (13.6) | |||
| FAPI-74 | Total | 11 (100.0) | ||
| DCl | 3 (27.3) | |||
| DZy * | 0 (0.0) | |||
| DGl | 2 (18.2) | |||
| DHi | 2 (18.2) | |||
| Dos * | 0 (0.0) | |||
| DTe * | 0 (0.0) | |||
| DIn | 3 (27.3) | |||
| DSp * | 1 (9.1) | |||
| Other * | 0 (0.0) | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Glatting, F.M.; Hoppner, J.; Kauczor, H.-U.; Huber, P.E.; Kratochwil, C.; Giesel, F.L.; Haberkorn, U.; Röhrich, M. Subclass Analysis of Malignant, Inflammatory and Degenerative Pathologies Based on Multiple Timepoint FAPI-PET Acquisitions Using FAPI-02, FAPI-46 and FAPI-74. Cancers 2022, 14, 5301. https://doi.org/10.3390/cancers14215301
Glatting FM, Hoppner J, Kauczor H-U, Huber PE, Kratochwil C, Giesel FL, Haberkorn U, Röhrich M. Subclass Analysis of Malignant, Inflammatory and Degenerative Pathologies Based on Multiple Timepoint FAPI-PET Acquisitions Using FAPI-02, FAPI-46 and FAPI-74. Cancers. 2022; 14(21):5301. https://doi.org/10.3390/cancers14215301
Chicago/Turabian StyleGlatting, Frederik M., Jorge Hoppner, Hans-Ulrich Kauczor, Peter E. Huber, Clemens Kratochwil, Frederik L. Giesel, Uwe Haberkorn, and Manuel Röhrich. 2022. "Subclass Analysis of Malignant, Inflammatory and Degenerative Pathologies Based on Multiple Timepoint FAPI-PET Acquisitions Using FAPI-02, FAPI-46 and FAPI-74" Cancers 14, no. 21: 5301. https://doi.org/10.3390/cancers14215301
APA StyleGlatting, F. M., Hoppner, J., Kauczor, H.-U., Huber, P. E., Kratochwil, C., Giesel, F. L., Haberkorn, U., & Röhrich, M. (2022). Subclass Analysis of Malignant, Inflammatory and Degenerative Pathologies Based on Multiple Timepoint FAPI-PET Acquisitions Using FAPI-02, FAPI-46 and FAPI-74. Cancers, 14(21), 5301. https://doi.org/10.3390/cancers14215301







