Focus on the Dynamics of Neutrophil-to-Lymphocyte Ratio in Cancer Patients Treated with Immune Checkpoint Inhibitors: A Meta-Analysis and Systematic Review
Abstract
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Search Strategy
2.2. Inclusion and Exclusion Criteria
2.3. Data Extraction
2.4. Statistical Analyses
3. Results
3.1. Characteristics of Included Studies
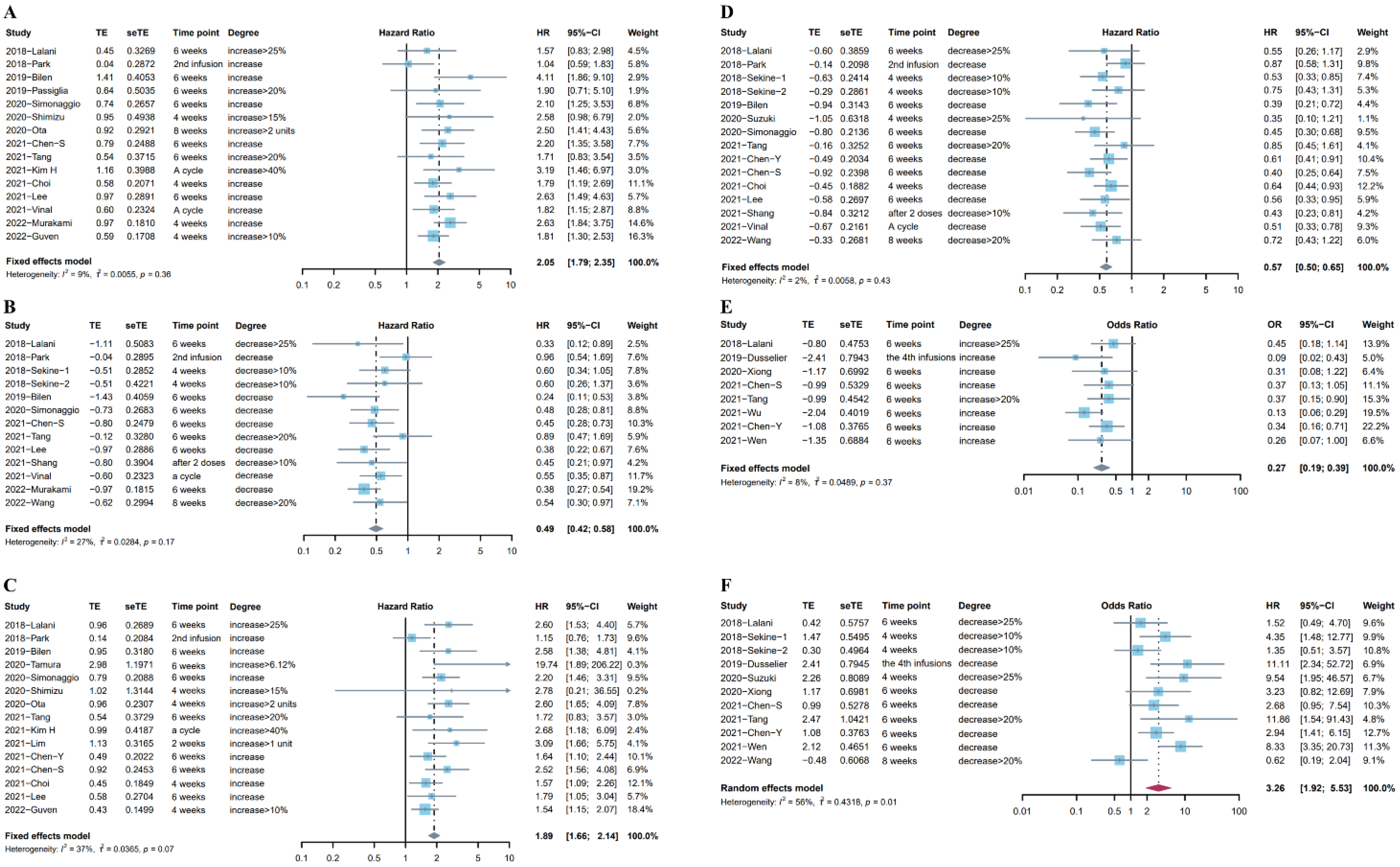
3.2. Change in NLR before and after ICI Treatment
3.3. Different Trends in NLR after ICI Treatment Were Associated with Different Prognoses
3.4. Post-Treatment High NLR Was Associated with More Impaired Survival Than Baseline High NLR
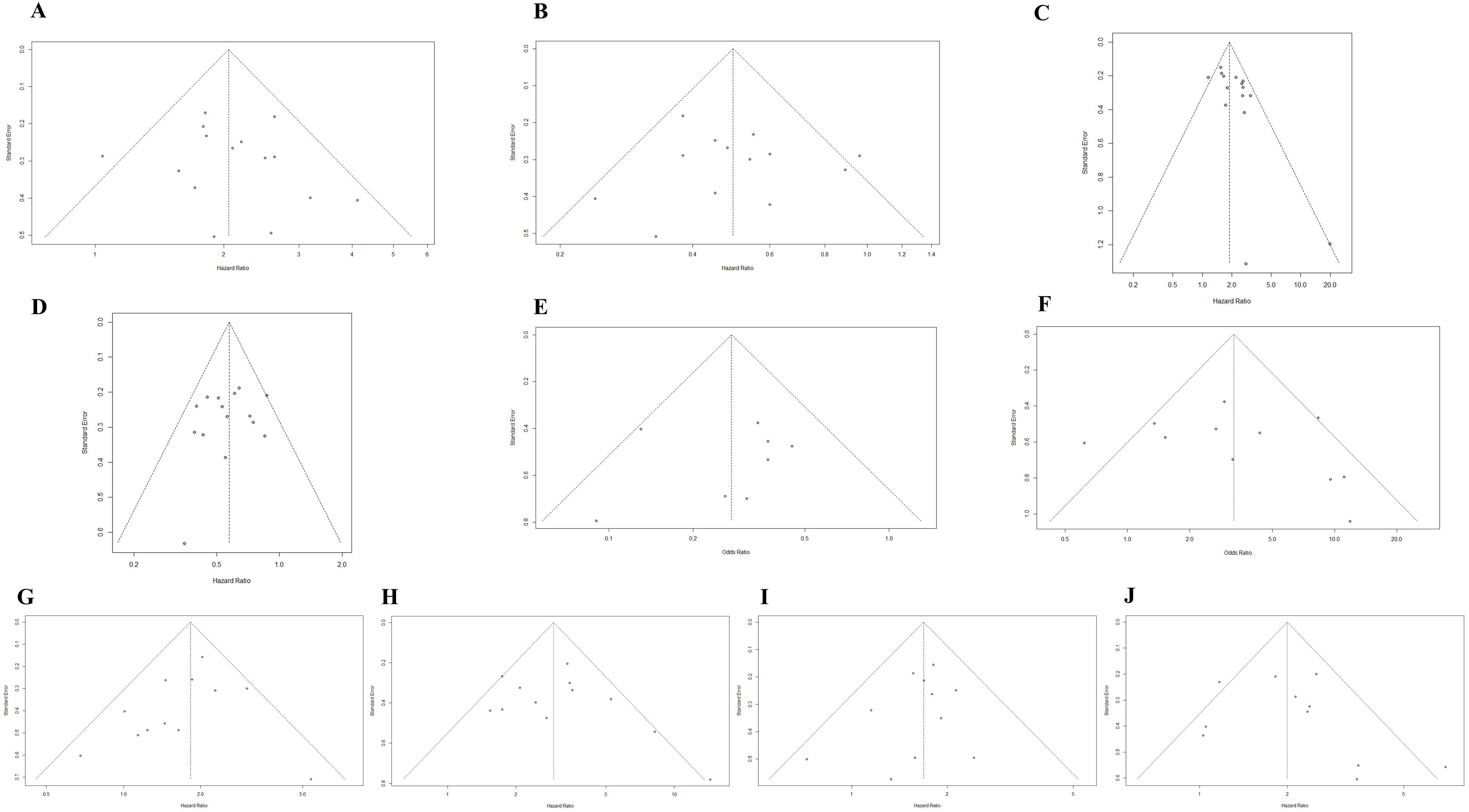
3.5. Publication Bias
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hartmann, J.; Schüßler-Lenz, M.; Bondanza, A.; Buchholz, C.J. Clinical development of CAR T cells-challenges and opportunities in translating innovative treatment concepts. EMBO Mol. Med. 2017, 9, 1183–1197. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Chen, M.; Liu, Z. Local biomaterials-assisted cancer immunotherapy to trigger systemic antitumor responses. Chem. Soc. Rev. 2019, 48, 5506–5526. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.A.; Minn, A.J. Combination Cancer Therapy with Immune Checkpoint Blockade: Mechanisms and Strategies. Immunity 2018, 48, 417–433. [Google Scholar] [CrossRef] [PubMed]
- Darnell, E.P.; Mooradian, M.J.; Baruch, E.N.; Yilmaz, M.; Reynolds, K.L. Immune-Related Adverse Events (irAEs): Diagnosis, Management, and Clinical Pearls. Curr. Oncol. Rep. 2020, 22, 39. [Google Scholar] [CrossRef] [PubMed]
- André, T.; Shiu, K.K.; Kim, T.W.; Jensen, B.V.; Jensen, L.H.; Punt, C.; Smith, D.; Garcia-Carbonero, R.; Benavides, M.; Gibbs, P.; et al. Pembrolizumab in Microsatellite-Instability-High Advanced Colorectal Cancer. N. Engl. J. Med. 2020, 383, 2207–2218. [Google Scholar] [CrossRef]
- Herbst, R.S.; Giaccone, G.; de Marinis, F.; Reinmuth, N.; Vergnenegre, A.; Barrios, C.H.; Morise, M.; Felip, E.; Andric, Z.; Geater, S.; et al. Atezolizumab for First-Line Treatment of PD-L1-Selected Patients with NSCLC. N. Engl. J. Med. 2020, 383, 1328–1339. [Google Scholar] [CrossRef]
- Büttner, R.; Longshore, J.W.; López-Ríos, F.; Merkelbach-Bruse, S.; Normanno, N.; Rouleau, E.; Penault-Llorca, F. Implementing TMB measurement in clinical practice: Considerations on assay requirements. ESMO Open 2019, 4, e000442. [Google Scholar] [CrossRef]
- Guven, D.C.; Sahin, T.K.; Erul, E.; Cakir, I.Y.; Ucgul, E.; Yildirim, H.C.; Aktepe, O.H.; Erman, M.; Kilickap, S.; Aksoy, S.; et al. The Association between Early Changes in Neutrophil-Lymphocyte Ratio and Survival in Patients Treated with Immunotherapy. J. Clin. Med. 2022, 11, 4523. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Liao, T.L.; Chen, Y.M.; Tang, K.T.; Chen, P.K.; Liu, H.J.; Chen, D.Y. MicroRNA-223 inhibits neutrophil extracellular traps formation through regulating calcium influx and small extracellular vesicles transmission. Sci. Rep. 2021, 11, 15676. [Google Scholar] [CrossRef]
- Oberg, H.H.; Wesch, D.; Kalyan, S.; Kabelitz, D. Regulatory Interactions Between Neutrophils, Tumor Cells and T Cells. Front. Immunol. 2019, 10, 1690. [Google Scholar] [CrossRef] [PubMed]
- Valero, C.; Lee, M.; Hoen, D.; Weiss, K.; Kelly, D.W.; Adusumilli, P.S.; Paik, P.K.; Plitas, G.; Ladanyi, M.; Postow, M.A.; et al. Pretreatment neutrophil-to-lymphocyte ratio and mutational burden as biomarkers of tumor response to immune checkpoint inhibitors. Nat. Commun. 2021, 12, 729. [Google Scholar] [CrossRef]
- Xie, X.; Liu, J.; Yang, H.; Chen, H.; Zhou, S.; Lin, H.; Liao, Z.; Ding, Y.; Ling, L.; Wang, X. Prognostic Value of Baseline Neutrophil-to-Lymphocyte Ratio in Outcome of Immune Checkpoint Inhibitors. Cancer Investig. 2019, 37, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Yang, L.; Liu, D.; Li, W. Association of the neutrophil to lymphocyte ratio and clinical outcomes in patients with lung cancer receiving immunotherapy: A meta-analysis. BMJ Open 2020, 10, e035031. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Jiang, J.; Tang, S.; Sun, G. Predictive value of neutrophil-lymphocyte ratio and platelet-lymphocyte ratio in non-small cell lung cancer patients treated with immune checkpoint inhibitors: A meta-analysis. Int. Immunopharmacol. 2020, 85, 106677. [Google Scholar] [CrossRef]
- Sanmamed, M.F.; Perez-Gracia, J.L.; Schalper, K.A.; Fusco, J.P.; Gonzalez, A.; Rodriguez-Ruiz, M.E.; Oñate, C.; Perez, G.; Alfaro, C.; Martín-Algarra, S.; et al. Changes in serum interleukin-8 (IL-8) levels reflect and predict response to anti-PD-1 treatment in melanoma and non-small-cell lung cancer patients. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2017, 28, 1988–1995. [Google Scholar] [CrossRef]
- Boutsikou, E.; Domvri, K.; Hardavella, G.; Tsiouda, D.; Zarogoulidis, K.; Kontakiotis, T. Tumour necrosis factor, interferon-gamma and interleukins as predictive markers of antiprogrammed cell-death protein-1 treatment in advanced non-small cell lung cancer: A pragmatic approach in clinical practice. Ther. Adv. Med. Oncol. 2018, 10, 1758835918768238. [Google Scholar] [CrossRef]
- Lim, J.U.; Kang, H.S.; Yeo, C.D.; Kim, J.S.; Park, C.K.; Kim, J.W.; Kim, S.J.; Lee, S.H. Predictability of early changes in derived neutrophil-to-lymphocyte ratio and neutrophil-to-lymphocyte ratio in patients with advanced non-small cell lung cancer treated with immune checkpoint inhibitors. J. Thorac. Dis. 2021, 13, 2824–2832. [Google Scholar] [CrossRef]
- Wen, S.; Du, X.; Chen, Y.; Xia, J.; Wang, R.; Zhu, M.; Peng, W.; Spitaleri, G.; Hofman, P.; Bironzo, P.; et al. Association between changes in thioredoxin reductase and other peripheral blood biomarkers with response to PD-1 inhibitor-based combination immunotherapy in non-small cell lung cancer: A retrospective study. Transl. Lung Cancer Res. 2022, 11, 757–775. [Google Scholar] [CrossRef]
- Chen, Y.; Wen, S.; Xia, J.; Du, X.; Wu, Y.; Pan, B.; Zhu, W.; Shen, B. Association of Dynamic Changes in Peripheral Blood Indexes With Response to PD-1 Inhibitor-Based Combination Therapy and Survival Among Patients With Advanced Non-Small Cell Lung Cancer. Front. Immunol. 2021, 12, 672271. [Google Scholar] [CrossRef]
- Lalani, A.A.; Xie, W.; Martini, D.J.; Steinharter, J.A.; Norton, C.K.; Krajewski, K.M.; Duquette, A.; Bossé, D.; Bellmunt, J.; Van Allen, E.M.; et al. Change in Neutrophil-to-lymphocyte ratio (NLR) in response to immune checkpoint blockade for metastatic renal cell carcinoma. J. ImmunoTherapy Cancer 2018, 6, 5. [Google Scholar] [CrossRef] [PubMed]
- Sekine, K.; Kanda, S.; Goto, Y.; Horinouchi, H.; Fujiwara, Y.; Yamamoto, N.; Motoi, N.; Ohe, Y. Change in the lymphocyte-to-monocyte ratio is an early surrogate marker of the efficacy of nivolumab monotherapy in advanced non-small-cell lung cancer. Lung Cancer 2018, 124, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Ota, Y.; Takahari, D.; Suzuki, T.; Osumi, H.; Nakayama, I.; Oki, A.; Wakatsuki, T.; Ichimura, T.; Ogura, M.; Shinozaki, E.; et al. Changes in the neutrophil-to-lymphocyte ratio during nivolumab monotherapy are associated with gastric cancer survival. Cancer Chemother. Pharmacol. 2020, 85, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Li, Y.; Fan, Q.; Shu, Y.; Yang, L.; Cui, T.; Gu, K.; Tao, M.; Wang, X.; Cui, C.; et al. Clinical and biomarker analyses of sintilimab versus chemotherapy as second-line therapy for advanced or metastatic esophageal squamous cell carcinoma: A randomized, open-label phase 2 study (ORIENT-2). Nat. Commun. 2022, 13, 857. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Li, L.; Zhou, Y.; Yang, Z.; Wang, M.; Gao, Y.; Yang, Y.; Yang, F.; Liu, B.; Hong, X.; et al. Clinical Characteristics Correlate With Outcomes of Immunotherapy in Advanced Non-Small Cell Lung Cancer. J. Cancer 2020, 11, 7137–7145. [Google Scholar] [CrossRef]
- Shimizu, T.; Miyake, M.; Hori, S.; Ichikawa, K.; Omori, C.; Iemura, Y.; Owari, T.; Itami, Y.; Nakai, Y.; Anai, S.; et al. Clinical Impact of Sarcopenia and Inflammatory/Nutritional Markers in Patients with Unresectable Metastatic Urothelial Carcinoma Treated with Pembrolizumab. Diagnostics 2020, 10, 310. [Google Scholar] [CrossRef]
- Zer, A.; Sung, M.R.; Walia, P.; Khoja, L.; Maganti, M.; Labbe, C.; Shepherd, F.A.; Bradbury, P.A.; Feld, R.; Liu, G.; et al. Correlation of Neutrophil to Lymphocyte Ratio and Absolute Neutrophil Count With Outcomes With PD-1 Axis Inhibitors in Patients With Advanced Non-Small-Cell Lung Cancer. Clin. Lung Cancer 2018, 19, 426–434.e421. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Terakawa, T.; Furukawa, J.; Harada, K.; Hinata, N.; Nakano, Y.; Fujisawa, M. C-reactive protein and the neutrophil-to-lymphocyte ratio are prognostic biomarkers in metastatic renal cell carcinoma patients treated with nivolumab. Int. J. Clin. Oncol. 2020, 25, 135–144. [Google Scholar] [CrossRef]
- Park, W.; Kwon, D.; Saravia, D.; Desai, A.; Vargas, F.; El Dinali, M.; Warsch, J.; Elias, R.; Chae, Y.K.; Kim, D.W.; et al. Developing a Predictive Model for Clinical Outcomes of Advanced Non-Small Cell Lung Cancer Patients Treated With Nivolumab. Clin. Lung Cancer 2018, 19, 280–288.e284. [Google Scholar] [CrossRef]
- Tang, Y.; Cui, Y.; Li, L.L.; Guan, Y.P.; Feng, D.F.; Yin, B.B.; Liang, X.F.; Yin, J.; Jiang, R.; Liang, J.; et al. Dynamics of Early Serum Tumour Markers and Neutrophil-to-Lymphocyte Ratio Predict Response to PD-1/PD-L1 Inhibitors in Advanced Non-Small-Cell Lung Cancer. Cancer Manag. Res. 2021, 13, 8241–8255. [Google Scholar] [CrossRef]
- Prelaj, A.; Rebuzzi, S.E.; Pizzutilo, P.; Bilancia, M.; Montrone, M.; Pesola, F.; Longo, V.; Del Bene, G.; Lapadula, V.; Cassano, F.; et al. EPSILoN: A Prognostic Score Using Clinical and Blood Biomarkers in Advanced Non-Small-cell Lung Cancer Treated With Immunotherapy. Clin. Lung Cancer 2020, 21, 365–377.e365. [Google Scholar] [CrossRef] [PubMed]
- Ikarashi, D.; Kato, Y.; Kato, R.; Kanehira, M.; Takata, R.; Obara, W. Inflammatory markers for predicting responses to nivolumab in patients with metastatic renal cell carcinoma. Int. J. Urol. Off. J. Jpn. Urol. Assoc. 2020, 27, 350–351. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.M.; Kim, J.Y.; Choi, J.; Lee, D.; Shim, J.H.; Lim, Y.S.; Lee, H.C.; Yoo, C.; Ryu, M.H.; Ryoo, B.Y.; et al. Kinetics of the neutrophil-lymphocyte ratio during PD-1 inhibition as a prognostic factor in advanced hepatocellular carcinoma. Liver Int. Off. J. Int. Assoc. Study Liver 2021, 41, 2189–2199. [Google Scholar] [CrossRef] [PubMed]
- Passiglia, F.; Galvano, A.; Castiglia, M.; Incorvaia, L.; Calò, V.; Listì, A.; Mazzarisi, S.; Perez, A.; Gallina, G.; Rizzo, S.; et al. Monitoring blood biomarkers to predict nivolumab effectiveness in NSCLC patients. Ther. Adv. Med. Oncol. 2019, 11, 1758835919839928. [Google Scholar] [CrossRef]
- Cassidy, M.R.; Wolchok, R.E.; Zheng, J.; Panageas, K.S.; Wolchok, J.D.; Coit, D.; Postow, M.A.; Ariyan, C. Neutrophil to Lymphocyte Ratio is Associated With Outcome During Ipilimumab Treatment. EBioMedicine 2017, 18, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Nishiyama, N.; Hirobe, M.; Kikushima, T.; Matsuki, M.; Takahashi, A.; Yanase, M.; Ichimatsu, K.; Egawa, M.; Hayashi, N.; Negishi, T.; et al. The neutrophil-lymphocyte ratio has a role in predicting the effectiveness of nivolumab in Japanese patients with metastatic renal cell carcinoma: A multi-institutional retrospective study. BMC Urol. 2020, 20, 110. [Google Scholar] [CrossRef] [PubMed]
- Ameratunga, M.; Chénard-Poirier, M.; Moreno Candilejo, I.; Pedregal, M.; Lui, A.; Dolling, D.; Aversa, C.; Ingles Garces, A.; Ang, J.E.; Banerji, U.; et al. Neutrophil-lymphocyte ratio kinetics in patients with advanced solid tumours on phase I trials of PD-1/PD-L1 inhibitors. Eur. J. Cancer 2018, 89, 56–63. [Google Scholar] [CrossRef]
- Ogata, T.; Satake, H.; Ogata, M.; Hatachi, Y.; Inoue, K.; Hamada, M.; Yasui, H. Neutrophil-to-lymphocyte ratio as a predictive or prognostic factor for gastric cancer treated with nivolumab: A multicenter retrospective study. Oncotarget 2018, 9, 34520–34527. [Google Scholar] [CrossRef]
- Dusselier, M.; Deluche, E.; Delacourt, N.; Ballouhey, J.; Egenod, T.; Melloni, B.; Vergnenègre, C.; Veillon, R.; Vergnenègre, A. Neutrophil-to-lymphocyte ratio evolution is an independent predictor of early progression of second-line nivolumab-treated patients with advanced non-small-cell lung cancers. PLoS ONE 2019, 14, e0219060. [Google Scholar] [CrossRef]
- Lee, P.Y.; Oen, K.Q.X.; Lim, G.R.S.; Hartono, J.L.; Muthiah, M.; Huang, D.Q.; Teo, F.S.W.; Li, A.Y.; Mak, A.; Chandran, N.S.; et al. Neutrophil-to-Lymphocyte Ratio Predicts Development of Immune-Related Adverse Events and Outcomes from Immune Checkpoint Blockade: A Case-Control Study. Cancers 2021, 13, 1308. [Google Scholar] [CrossRef]
- Wu, X.; Han, R.; Zhong, Y.; Weng, N.; Zhang, A. Post treatment NLR is a predictor of response to immune checkpoint inhibitor therapy in patients with esophageal squamous cell carcinoma. Cancer Cell Int. 2021, 21, 356. [Google Scholar] [CrossRef] [PubMed]
- Khunger, M.; Patil, P.D.; Khunger, A.; Li, M.; Hu, B.; Rakshit, S.; Basu, A.; Pennell, N.; Stevenson, J.P.; Elson, P.; et al. Post-treatment changes in hematological parameters predict response to nivolumab monotherapy in non-small cell lung cancer patients. PLoS ONE 2018, 13, e0197743. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Q.; Huang, Z.; Xin, L.; Qin, B.; Zhao, X.; Zhang, J.; Shi, W.; Yang, B.; Zhang, G.; Hu, Y. Post-treatment neutrophil-to-lymphocyte ratio (NLR) predicts response to anti-PD-1/PD-L1 antibody in SCLC patients at early phase. Cancer Immunol. Immunother. CII 2021, 70, 713–720. [Google Scholar] [CrossRef] [PubMed]
- Bilen, M.A.; Martini, D.J.; Liu, Y.; Lewis, C.; Collins, H.H.; Shabto, J.M.; Akce, M.; Kissick, H.T.; Carthon, B.C.; Shaib, W.L.; et al. The prognostic and predictive impact of inflammatory biomarkers in patients who have advanced-stage cancer treated with immunotherapy. Cancer 2019, 125, 127–134. [Google Scholar] [CrossRef]
- Wang, L.; Zhu, Y.; Zhang, B.; Wang, X.; Mo, H.; Jiao, Y.; Xu, J.; Huang, J. Prognostic and predictive impact of neutrophil-to-lymphocyte ratio and HLA-I genotyping in advanced esophageal squamous cell carcinoma patients receiving immune checkpoint inhibitor monotherapy. Thorac. Cancer 2022, 13, 1631–1641. [Google Scholar] [CrossRef]
- Tamura, D.; Jinnouchi, N.; Abe, M.; Ikarashi, D.; Matsuura, T.; Kato, R.; Maekawa, S.; Kato, Y.; Kanehira, M.; Takata, R.; et al. Prognostic outcomes and safety in patients treated with pembrolizumab for advanced urothelial carcinoma: Experience in real-world clinical practice. Int. J. Clin. Oncol. 2020, 25, 899–905. [Google Scholar] [CrossRef]
- Chen, S.; Li, R.; Zhang, Z.; Huang, Z.; Cui, P.; Jia, W.; Zhang, S.; Tao, H.; Wang, L.; Li, X.; et al. Prognostic value of baseline and change in neutrophil-to-lymphocyte ratio for survival in advanced non-small cell lung cancer patients with poor performance status receiving PD-1 inhibitors. Transl. Lung Cancer Res. 2021, 10, 1397–1407. [Google Scholar] [CrossRef]
- Viñal, D.; Gutierrez-Sainz, L.; Martinez, D.; Garcia-Cuesta, J.A.; Pedregosa, J.; Villamayor, J.; Ostios, L.; Sanchez-Cabrero, D.; Higuera, O.; Pinto, A.; et al. Prognostic value of neutrophil-to-lymphocyte ratio in advanced cancer patients receiving immunotherapy. Clin. Transl. Oncol. Off. Publ. Fed. Span. Oncol. Soc. Natl. Cancer Inst. Mex. 2021, 23, 1185–1192. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Yatsuda, J.; Shimokawa, M.; Fuji, N.; Aoki, A.; Sakano, S.; Yamamoto, M.; Suga, A.; Tei, Y.; Yoshihiro, S.; et al. Prognostic value of pre-treatment risk stratification and post-treatment neutrophil/lymphocyte ratio change for pembrolizumab in patients with advanced urothelial carcinoma. Int. J. Clin. Oncol. 2021, 26, 169–177. [Google Scholar] [CrossRef]
- Kim, J.H.; Ahn, B.; Hong, S.M.; Jung, H.Y.; Kim, D.H.; Choi, K.D.; Ahn, J.Y.; Lee, J.H.; Na, H.K.; Kim, J.H.; et al. Real-World Efficacy Data and Predictive Clinical Parameters for Treatment Outcomes in Advanced Esophageal Squamous Cell Carcinoma Treated with Immune Checkpoint Inhibitors. Cancer Res. Treat. 2022, 54, 505–516. [Google Scholar] [CrossRef]
- Murakami, Y.; Tamiya, A.; Taniguchi, Y.; Adachi, Y.; Enomoto, T.; Azuma, K.; Inagaki, Y.; Kouno, S.; Matsuda, Y.; Okishio, K.; et al. Retrospective analysis of long-term survival factors in patients with advanced non-small cell lung cancer treated with nivolumab. Thorac. Cancer 2022, 13, 593–601. [Google Scholar] [CrossRef] [PubMed]
- Shang, J.; Han, X.; Zha, H.; Tao, H.; Li, X.; Yuan, F.; Chen, G.; Wang, L.; Ma, J.; Hu, Y. Systemic Immune-Inflammation Index and Changes of Neutrophil-Lymphocyte Ratio as Prognostic Biomarkers for Patients With Pancreatic Cancer Treated With Immune Checkpoint Blockade. Front. Oncol. 2021, 11, 585271. [Google Scholar] [CrossRef] [PubMed]
- Namikawa, T.; Yokota, K.; Tanioka, N.; Fukudome, I.; Iwabu, J.; Munekage, M.; Uemura, S.; Maeda, H.; Kitagawa, H.; Kobayashi, M.; et al. Systemic inflammatory response and nutritional biomarkers as predictors of nivolumab efficacy for gastric cancer. Surg. Today 2020, 50, 1486–1495. [Google Scholar] [CrossRef] [PubMed]
- Simonaggio, A.; Elaidi, R.; Fournier, L.; Fabre, E.; Ferrari, V.; Borchiellini, D.; Thouvenin, J.; Barthelemy, P.; Thibault, C.; Tartour, E.; et al. Variation in neutrophil to lymphocyte ratio (NLR) as predictor of outcomes in metastatic renal cell carcinoma (mRCC) and non-small cell lung cancer (mNSCLC) patients treated with nivolumab. Cancer Immunol. Immunother. CII 2020, 69, 2513–2522. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Suh, K.J.; Kim, S.H.; Kim, Y.J.; Kim, M.; Keam, B.; Kim, T.M.; Kim, D.W.; Heo, D.S.; Lee, J.S. Post-treatment neutrophil-to-lymphocyte ratio at week 6 is prognostic in patients with advanced non-small cell lung cancers treated with anti-PD-1 antibody. Cancer Immunol. Immunother. CII 2018, 67, 459–470. [Google Scholar] [CrossRef]
- Wang, R.; Lin, N.; Mao, B.; Wu, Q. The efficacy of immune checkpoint inhibitors in advanced hepatocellular carcinoma: A meta-analysis based on 40 cohorts incorporating 3697 individuals. J. Cancer Res. Clin. Oncol. 2022, 148, 1195–1210. [Google Scholar] [CrossRef]
- Takenaka, Y.; Oya, R.; Takemoto, N.; Inohara, H. Neutrophil-to-lymphocyte ratio as a prognostic marker for head and neck squamous cell carcinoma treated with immune checkpoint inhibitors: Meta-analysis. Head Neck 2022, 44, 1237–1245. [Google Scholar] [CrossRef]
- Tan, Q.; Liu, S.; Liang, C.; Han, X.; Shi, Y. Pretreatment hematological markers predict clinical outcome in cancer patients receiving immune checkpoint inhibitors: A meta-analysis. Thorac. Cancer 2018, 9, 1220–1230. [Google Scholar] [CrossRef]
- Liu, N.; Mao, J.; Tao, P.; Chi, H.; Jia, W.; Dong, C. The relationship between NLR/PLR/LMR levels and survival prognosis in patients with non-small cell lung carcinoma treated with immune checkpoint inhibitors. Medicine 2022, 101, e28617. [Google Scholar] [CrossRef]
- Yanagisawa, T.; Mori, K.; Katayama, S.; Mostafaei, H.; Quhal, F.; Laukhtina, E.; Rajwa, P.; Motlagh, R.S.; Aydh, A.; König, F.; et al. Hematological prognosticators in metastatic renal cell cancer treated with immune checkpoint inhibitors: A meta-analysis. Immunotherapy 2022, 14, 709–725. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Meng, F.; Jiang, R. Neutrophil-to-Lymphocyte Ratio as a Prognostic Biomarker for Patients With Metastatic Renal Cell Carcinoma Treated With Immune Checkpoint Inhibitors: A Systematic Review and Meta-Analysis. Front. Oncol. 2021, 11, 746976. [Google Scholar] [CrossRef] [PubMed]
- Nunno, V.D.; Mollica, V.; Gatto, L.; Santoni, M.; Cosmai, L.; Porta, C.; Massari, F. Prognostic impact of neutrophil-to-lymphocyte ratio in renal cell carcinoma: A systematic review and meta-analysis. Immunotherapy 2019, 11, 631–643. [Google Scholar] [CrossRef] [PubMed]
- Platini, H.; Ferdinand, E.; Kohar, K.; Prayogo, S.A.; Amirah, S.; Komariah, M.; Maulana, S. Neutrophil-to-Lymphocyte Ratio and Platelet-to-Lymphocyte Ratio as Prognostic Markers for Advanced Non-Small-Cell Lung Cancer Treated with Immunotherapy: A Systematic Review and Meta-Analysis. Medicina 2022, 58, 1069. [Google Scholar] [CrossRef] [PubMed]
- Yanagisawa, T.; Mori, K.; Katayama, S.; Mostafaei, H.; Quhal, F.; Laukhtina, E.; Rajwa, P.; Motlagh, R.S.; Aydh, A.; König, F.; et al. Pretreatment clinical and hematologic prognostic factors of metastatic urothelial carcinoma treated with pembrolizumab: A systematic review and meta-analysis. Int. J. Clin. Oncol. 2022, 27, 59–71. [Google Scholar] [CrossRef]
- Galle, P.R.; Finn, R.S.; Qin, S.; Ikeda, M.; Zhu, A.X.; Kim, T.Y.; Kudo, M.; Breder, V.; Merle, P.; Kaseb, A.; et al. Patient-reported outcomes with atezolizumab plus bevacizumab versus sorafenib in patients with unresectable hepatocellular carcinoma (IMbrave150): An open-label, randomised, phase 3 trial. Lancet Oncol. 2021, 22, 991–1001. [Google Scholar] [CrossRef]
- Escudier, B.; Sharma, P.; McDermott, D.F.; George, S.; Hammers, H.J.; Srinivas, S.; Tykodi, S.S.; Sosman, J.A.; Procopio, G.; Plimack, E.R.; et al. CheckMate 025 Randomized Phase 3 Study: Outcomes by Key Baseline Factors and Prior Therapy for Nivolumab Versus Everolimus in Advanced Renal Cell Carcinoma. Eur. Urol. 2017, 72, 962–971. [Google Scholar] [CrossRef]
- Rini, B.I.; Powles, T.; Atkins, M.B.; Escudier, B.; McDermott, D.F.; Suarez, C.; Bracarda, S.; Stadler, W.M.; Donskov, F.; Lee, J.L.; et al. Atezolizumab plus bevacizumab versus sunitinib in patients with previously untreated metastatic renal cell carcinoma (IMmotion151): A multicentre, open-label, phase 3, randomised controlled trial. Lancet 2019, 393, 2404–2415. [Google Scholar] [CrossRef]
- Reck, M.; Mok, T.S.K.; Nishio, M.; Jotte, R.M.; Cappuzzo, F.; Orlandi, F.; Stroyakovskiy, D.; Nogami, N.; Rodríguez-Abreu, D.; Moro-Sibilot, D.; et al. Atezolizumab plus bevacizumab and chemotherapy in non-small-cell lung cancer (IMpower150): Key subgroup analyses of patients with EGFR mutations or baseline liver metastases in a randomised, open-label phase 3 trial. Lancet Respir. Med. 2019, 7, 387–401. [Google Scholar] [CrossRef]
- Kiriu, T.; Yamamoto, M.; Nagano, T.; Hazama, D.; Sekiya, R.; Katsurada, M.; Katsurada, N.; Tachihara, M.; Kobayashi, K.; Nishimura, Y. Pseudo-Progression and the Neutrophil-to-Lymphocyte Ratio in Non-Small Cell Lung Cancer Treated with Immune Checkpoint Inhibitors: A Case-Control Study. Onco Targets Ther. 2019, 12, 10559–10568. [Google Scholar] [CrossRef]
- Kim, C.G.; Kim, C.; Yoon, S.E.; Kim, K.H.; Choi, S.J.; Kang, B.; Kim, H.R.; Park, S.H.; Shin, E.C.; Kim, Y.Y.; et al. Hyperprogressive disease during PD-1 blockade in patients with advanced hepatocellular carcinoma. J. Hepatol. 2021, 74, 350–359. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.P.; Ju, L.; Chen, C.; Liu, T.; Li, S.; Wang, X. DNA Methylation-Based Panel Predicts Survival of Patients With Clear Cell Renal Cell Carcinoma and Its Correlations With Genomic Metrics and Tumor Immune Cell Infiltration. Front. Cell Dev. Biol. 2020, 8, 572628. [Google Scholar] [CrossRef]
- Yang, J.; Xu, H.; Guo, X.; Zhang, J.; Ye, X.; Yang, Y.; Ma, X. Pretreatment Inflammatory Indexes as Prognostic Predictors for Survival in Colorectal Cancer Patients Receiving Neoadjuvant Chemoradiotherapy. Sci. Rep. 2018, 8, 3044. [Google Scholar] [CrossRef] [PubMed]
- Anker, J.F.; Naseem, A.F.; Mok, H.; Schaeffer, A.J.; Abdulkadir, S.A.; Thumbikat, P. Multi-faceted immunomodulatory and tissue-tropic clinical bacterial isolate potentiates prostate cancer immunotherapy. Nat. Commun. 2018, 9, 1591. [Google Scholar] [CrossRef] [PubMed]
- Kalafati, L.; Mitroulis, I.; Verginis, P.; Chavakis, T.; Kourtzelis, I. Neutrophils as Orchestrators in Tumor Development and Metastasis Formation. Front. Oncol. 2020, 10, 581457. [Google Scholar] [CrossRef] [PubMed]
- Takakura, K.; Ito, Z.; Suka, M.; Kanai, T.; Matsumoto, Y.; Odahara, S.; Matsudaira, H.; Haruki, K.; Fujiwara, Y.; Saito, R.; et al. Comprehensive assessment of the prognosis of pancreatic cancer: Peripheral blood neutrophil-lymphocyte ratio and immunohistochemical analyses of the tumour site. Scand. J. Gastroenterol. 2016, 51, 610–617. [Google Scholar] [CrossRef] [PubMed]
- Ohki, S.; Shibata, M.; Gonda, K.; Machida, T.; Shimura, T.; Nakamura, I.; Ohtake, T.; Koyama, Y.; Suzuki, S.; Ohto, H.; et al. Circulating myeloid-derived suppressor cells are increased and correlate to immune suppression, inflammation and hypoproteinemia in patients with cancer. Oncol. Rep. 2012, 28, 453–458. [Google Scholar] [CrossRef]
- Restifo, N.P.; Dudley, M.E.; Rosenberg, S.A. Adoptive immunotherapy for cancer: Harnessing the T cell response. Nat. Rev. Immunol. 2012, 12, 269–281. [Google Scholar] [CrossRef]
- Havel, J.J.; Chowell, D.; Chan, T.A. The evolving landscape of biomarkers for checkpoint inhibitor immunotherapy. Nat. Rev. Cancer 2019, 19, 133–150. [Google Scholar] [CrossRef]


| Year | Author | Region | Treatment | Tumor | Number of Patients | Time Point After Immunotherapy | Outcome | Gender (Male/Female) | Newcastle–Ottawa Scale | Study Design | Reference Number |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 2017 | Michael R Cassidy | USA | Ipilimumab | Melanoma | 197 | 3 weeks and 6 weeks | OS; PFS | 125/72 | 9 | Retrospective | [35] |
| 2018 | Aly-Khan A Lalani | USA | Anti-PD-1 and anti-PD-L1 | mRCC | 192 | 6 weeks | OS; PFS; ORR | 101/41 | 9 | Retrospective | [21] |
| 2018 | Katsutoshi Sekine | Japan | Nivolumab (Cohort 1) | NSCLC | 87 | 4 weeks | OS; PFS; ORR | 54/33 | 8 | Retrospective | [22] |
| 2018 | Katsutoshi Sekine | Japan | Nivolumab (Cohort 2) | NSCLC | 75 | 4 weeks | OS; PFS; ORR | 50/25 | 8 | Retrospective | [22] |
| 2018 | Takatsugu Ogata | Japan | Nivolumab | Gastric cancer | 26 | Two weeks after the first administration | OS; PFS; ORR | 19/7 | 6 | Retrospective | [38] |
| 2018 | Alona Zer | Canada | Anti-PD-L1 | NSCLC | 88 | 8 weeks | Change in NLR before and after treatment | 43/45 | 7 | Retrospective | [27] |
| 2018 | Malaka Ameratunga | The United Kingdom, Spain, and Australia | Anti-PD-1 and anti-PD-L1 | Advanced solid tumors | 165 | Each cycle of therapy | Change in NLR before and after treatment | 91/74 | 9 | Retrospective | [37] |
| 2018 | Wungki Park | USA | Nivolumab | NSCLC | 159 | Before the second nivolumab infusion | OS; PFS | 82/77 | 7 | Retrospective | [29] |
| 2018 | Monica Khunger | USA | Nivolumab | NSCLC | 109 | After 2 cycles of treatment | Change in NLR before and after treatment | 56/53 | 7 | Retrospective | [42] |
| 2019 | Mehmet A Bilen | USA | / | Melanoma, gastrointestinal cancer, lung/head and neck cancer, breast cancer, and others | 90 | 6 weeks | Change in NLR before and after treatment; OS; PFS | 53/37 | 8 | Retrospective | [44] |
| 2019 | Francesco Passiglia | Italy | Nivolumab | NSCLC | 45 | 6 weeks | OS | 32/13 | 7 | Retrospective | [34] |
| 2019 | Matthieu Dusselier | France | Nivolumab | NSCLC | 59 | The 4th nivolumab infusions | Change in NLR before and after treatment | 44/15 | 7 | Retrospective | [39] |
| 2020 | Arsela Prelaj | Italy | Nivolumab and pembrolizumab | NSCLC | 154 | The second cycle and third cycle | OS; PFS | 126/28 | 7 | Retrospective | [31] |
| 2020 | Kotaro Suzuki | Japan | Nivolumab | mRCC | 65 | 4 weeks | OS; PFS; ORR | 47/18 | 7 | Retrospective | [28] |
| 2020 | A Simonaggio | France | Nivolumab | mNSCLC and mRCC | 161 | 6 weeks | Change in NLR before and after treatment; OS; PFS | 114/47 | 9 | Retrospective | [54] |
| 2020 | Takuto Shimizu | Japan | Pembrolizumab | Metastatic urothelial carcinoma | 27 | 4 weeks | OS; PFS | 23/4 | 6 | Retrospective | [26] |
| 2020 | Yumiko Ota | Japan | Nivolumab | Gastric cancer | 98 | 4 weeks (PFS) and 8 weeks (OS) | OS; PFS | 68/30 | 7 | Retrospective | [23] |
| 2020 | Naotaka Nishiyama | Japan | Nivolumab | mRCC | 52 | 4 weeks | OS; PFS | 36/16 | 7 | Retrospective | [36] |
| 2020 | Lan Huang | China | Nivolumab, pembrolizumab, atezolizumab and ipilimumab | NSCLC | 61 | The fourth cycle of treatment | OS; PFS | 38/23 | 7 | Retrospective | [25] |
| 2020 | Tsutomu Namikawa | Japan | Nivolumab | Gastric cancer | 29 | 2 weeks, 4 weeks, 6 weeks, and 8 weeks | OS; PFS | 19/10 | 6 | Retrospective | [53] |
| 2020 | Daichi Tamura | Japan | Pembrolizumab | Urothelial carcinoma | 41 | 6 weeks | PFS | 29/12 | 6 | Retrospective | [46] |
| 2020 | Daiki Ikarashi | Japan | Nivolumab | mRCC | 45 | 6 weeks | Change in NLR before and after treatment | 30/15 | 6 | Retrospective | [32] |
| 2021 | Yin Tang | China | Anti-PD-1 and anti-PD-L1 | NSCLC | 124 | 6 weeks | OS; PFS; ORR | 89/35 | 8 | Retrospective | [30] |
| 2021 | Xianbin Wu | China | Anti-PD-1 | Esophageal squamous cell carcinoma | 119 | 6 weeks | Change in NLR before and after treatment; ORR | 102/17 | 7 | Retrospective | [41] |
| 2021 | Jeong Uk Lim | Korea | Nivolumab, pembrolizumab, and atezolizumab | NSCLC | 89 | 2 weeks | PFS | 62/27 | 7 | Retrospective | [18] |
| 2021 | Yuzhong Chen | China | Pembrolizumab, Sintilimab and Toripalimab | NSCLC | 151 | 6 weeks and 12 weeks | Change in NLR before and after treatment; OS; PFS; ORR | 115/36 | 8 | Retrospective | [20] |
| 2021 | Won-Mook Choi | Korea | Nivolumab | HCC | 194 | 2 weeks, 4 weeks, and 6 weeks | Change in NLR before and after treatment; OS; PFS | 159/35 | 8 | Retrospective | [33] |
| 2021 | Shixue Chen | China | Nivolumab, pembrolizumab, and others | NSCLC | 101 | 6 weeks | Change in NLR before and after treatment; OS; PFS; ORR | 72/29 | 8 | Retrospective | [47] |
| 2021 | Pei Yi Lee | Singapore | Nivolumab, pebrolizumab, atezolimumab, avelumab, durvalumab, and tremelimumab | Lung cancer, colorectal cancer, nasopharyngeal carcinoma, gastric cancer, hepatocellular carcinoma | 147 | 6 weeks | OS; PFS | 99/48 | 7 | Retrospective | [40] |
| 2021 | Jin Shang | China | Nivolumab, pembrolizumab, atezolimab, ipilimumab, and sintilimab | Pancreatic Cancer | 122 | After 2 doses | OS; PFS | 87/35 | 7 | Retrospective | [52] |
| 2021 | Qi Xiong | China | Nivolumab, pembrolizumab, atezolizumab, and toripalimab | SCLC | 41 | 6 weeks | Change in NLR before and after treatment; OS; PFS; ORR | 36/5 | 7 | Retrospective | [43] |
| 2021 | D Viñal | Spain | / | Lung cancer, melanoma, kidney cancer, bladder cancer, others | 211 | A treatment cycle | Change in NLR before and after treatment; OS; PFS | 136/75 | 7 | Retrospective | [48] |
| 2021 | Yoshiaki Yamamoto | Japan | Pembrolizumab | Urothelial carcinoma | 121 | 6 weeks | Change in NLR before and after treatment | 87/34 | 7 | Retrospective | [49] |
| 2021 | Jwa Hoon Kim | Korea | Nivolumab and pembrolizumab | Esophageal squamous cell carcinoma | 60 | A treatment cycle | OS; PFS | 56/4 | 7 | Retrospective | [50] |
| 2022 | Shaodi Wen | China | Pembrolizumab, sintilimab, toripalimab | NSCLC | 90 | 6 weeks and 12 weeks | Change in NLR before and after treatment; ORR | 69/21 | 7 | Retrospective | [19] |
| 2022 | Yusuke Murakami | Japan | Nivolumab | NSCLC | 162 | 4 weeks | Change in NLR before and after treatment; OS | 111/51 | 8 | Retrospective | [51] |
| 2022 | Lin Wang | China | Camrelizumab | Esophageal squamous cell carcinoma | 69 | 8 weeks | OS; PFS; ORR | 64/5 | 8 | Retrospective | [45] |
| 2022 | Jianming Xu | China | Sintilimab | Esophageal squamous cell carcinoma | 97 | 6 weeks | OS; PFS | 88/7 | 8 | Prospective | [24] |
| 2022 | Deniz Can Guven | Turkey | Nivolumab, atezolizumab, pembrolizumab, ipilimumab, and avelumab | RCC, melanoma, NSCLC and others | 231 | 4 weeks | OS; PFS | 155/76 | 7 | Retrospective | [8] |
| Description | p-Value of Egger Test | p-Value of Begg Test | Corresponding Funnel Plot | Corresponding Forest Plot |
|---|---|---|---|---|
| The association between the upward trend in NLR and OS | 0.5542 | 0.2550 | Figure 4A | Figure 2A |
| The association between the downward trend in NLR and OS | 0.8197 | 1.0000 | Figure 4B | Figure 2B |
| The association between the upward trend in NLR and PFS | 0.0184 | 0.0536 | Figure 4C | Figure 2C |
| The association between the downward trend in NLR and PFS | 0.4286 | 0.7290 | Figure 4D | Figure 2D |
| The association between the upward trend in NLR and ORR | 0.6975 | 0.3223 | Figure 4E | Figure 2E |
| The association between the downward trend in NLR and ORR | 0.4106 | 0.4835 | Figure 4F | Figure 2F |
| The association between the baseline NLR and OS | 0.2993 | 0.1702 | Figure 4G | Figure 3A |
| The association between the post-treatment NLR and OS | 0.3730 | 0.3370 | Figure 4H | Figure 3A |
| The association between the baseline NLR and PFS | 0.3288 | 0.3115 | Figure 4I | Figure 3B |
| The association between the post-treatment NLR and PFS | 0.4003 | 0.3115 | Figure 4J | Figure 3B |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, Y.; Xiang, D.; Wan, J.; Yang, L.; Zheng, C. Focus on the Dynamics of Neutrophil-to-Lymphocyte Ratio in Cancer Patients Treated with Immune Checkpoint Inhibitors: A Meta-Analysis and Systematic Review. Cancers 2022, 14, 5297. https://doi.org/10.3390/cancers14215297
Guo Y, Xiang D, Wan J, Yang L, Zheng C. Focus on the Dynamics of Neutrophil-to-Lymphocyte Ratio in Cancer Patients Treated with Immune Checkpoint Inhibitors: A Meta-Analysis and Systematic Review. Cancers. 2022; 14(21):5297. https://doi.org/10.3390/cancers14215297
Chicago/Turabian StyleGuo, Yusheng, Dongqiao Xiang, Jiayu Wan, Lian Yang, and Chuansheng Zheng. 2022. "Focus on the Dynamics of Neutrophil-to-Lymphocyte Ratio in Cancer Patients Treated with Immune Checkpoint Inhibitors: A Meta-Analysis and Systematic Review" Cancers 14, no. 21: 5297. https://doi.org/10.3390/cancers14215297
APA StyleGuo, Y., Xiang, D., Wan, J., Yang, L., & Zheng, C. (2022). Focus on the Dynamics of Neutrophil-to-Lymphocyte Ratio in Cancer Patients Treated with Immune Checkpoint Inhibitors: A Meta-Analysis and Systematic Review. Cancers, 14(21), 5297. https://doi.org/10.3390/cancers14215297







