A Novel Plasma-Based Methylation Panel for Upper Gastrointestinal Cancer Early Detection
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Cell Free DNA Extraction, Bisulfite Treatment
2.3. Quantitative Methylation-Specific PCR
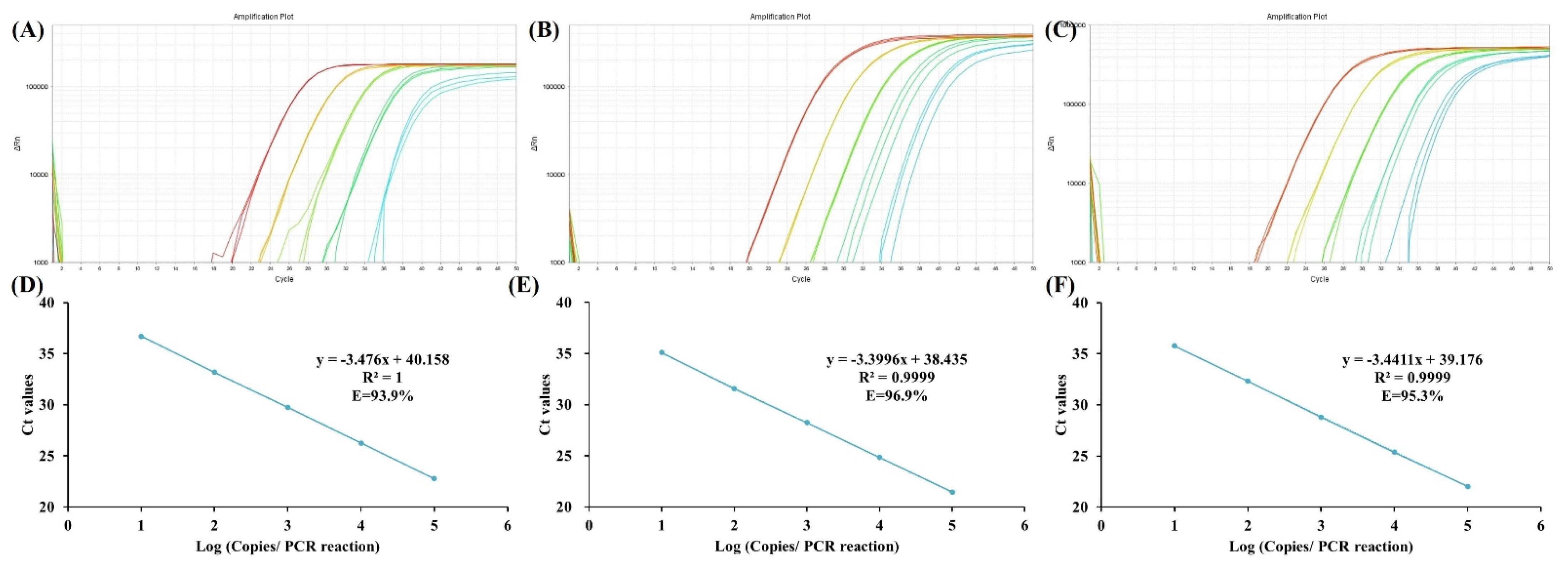
2.4. Analytic Performance Analysis
2.5. Data Analysis
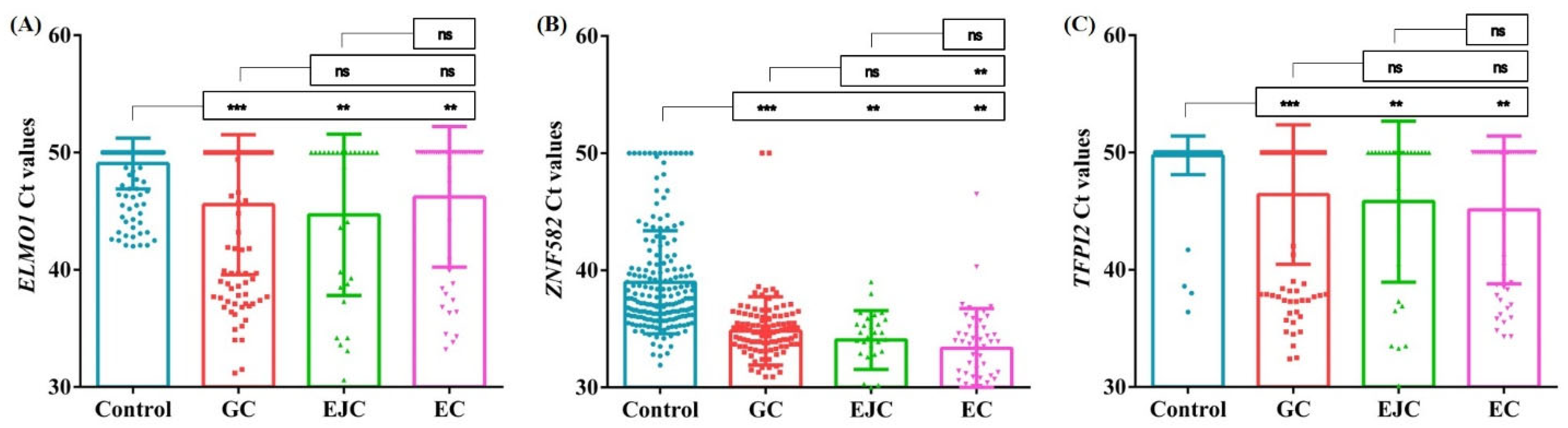
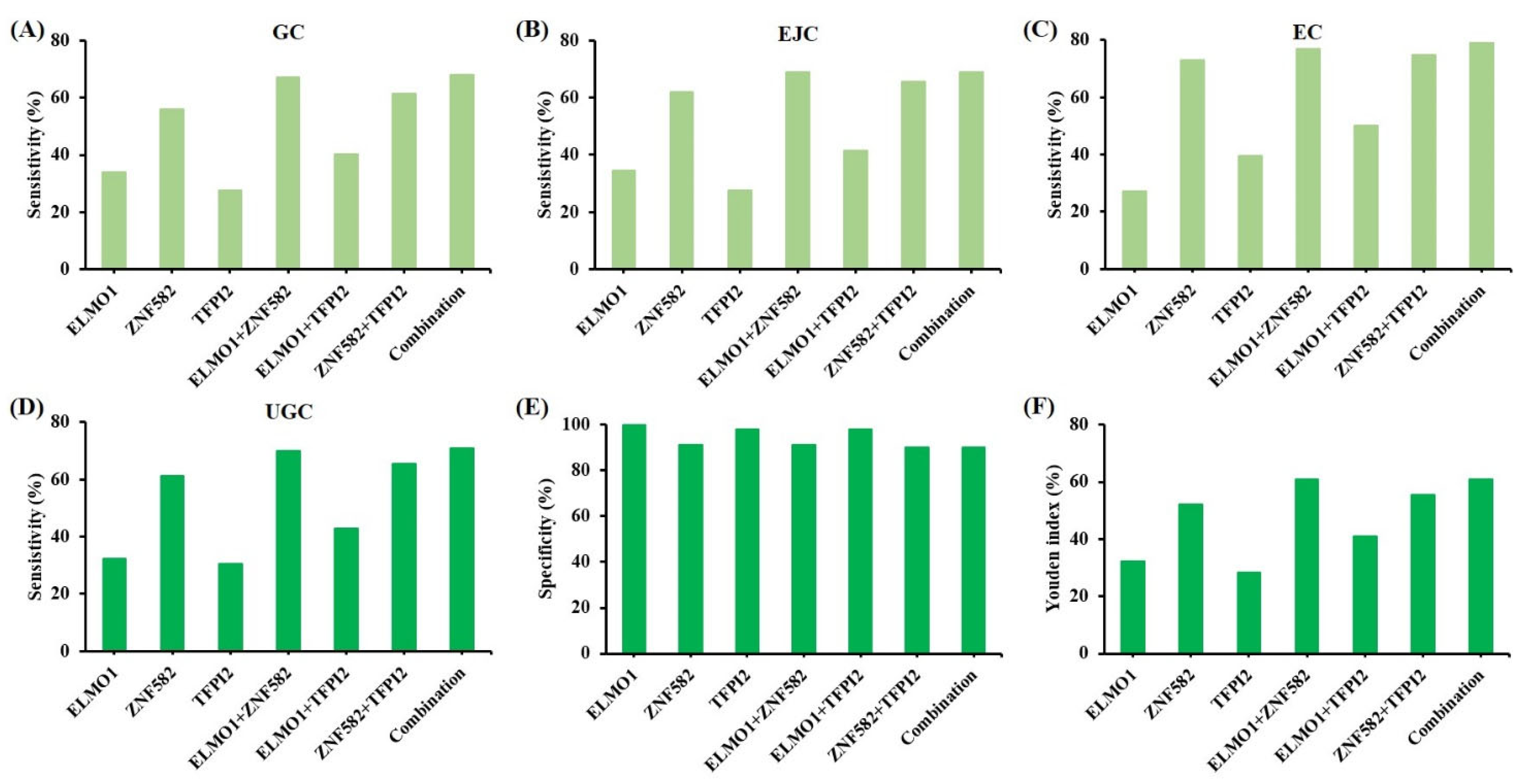
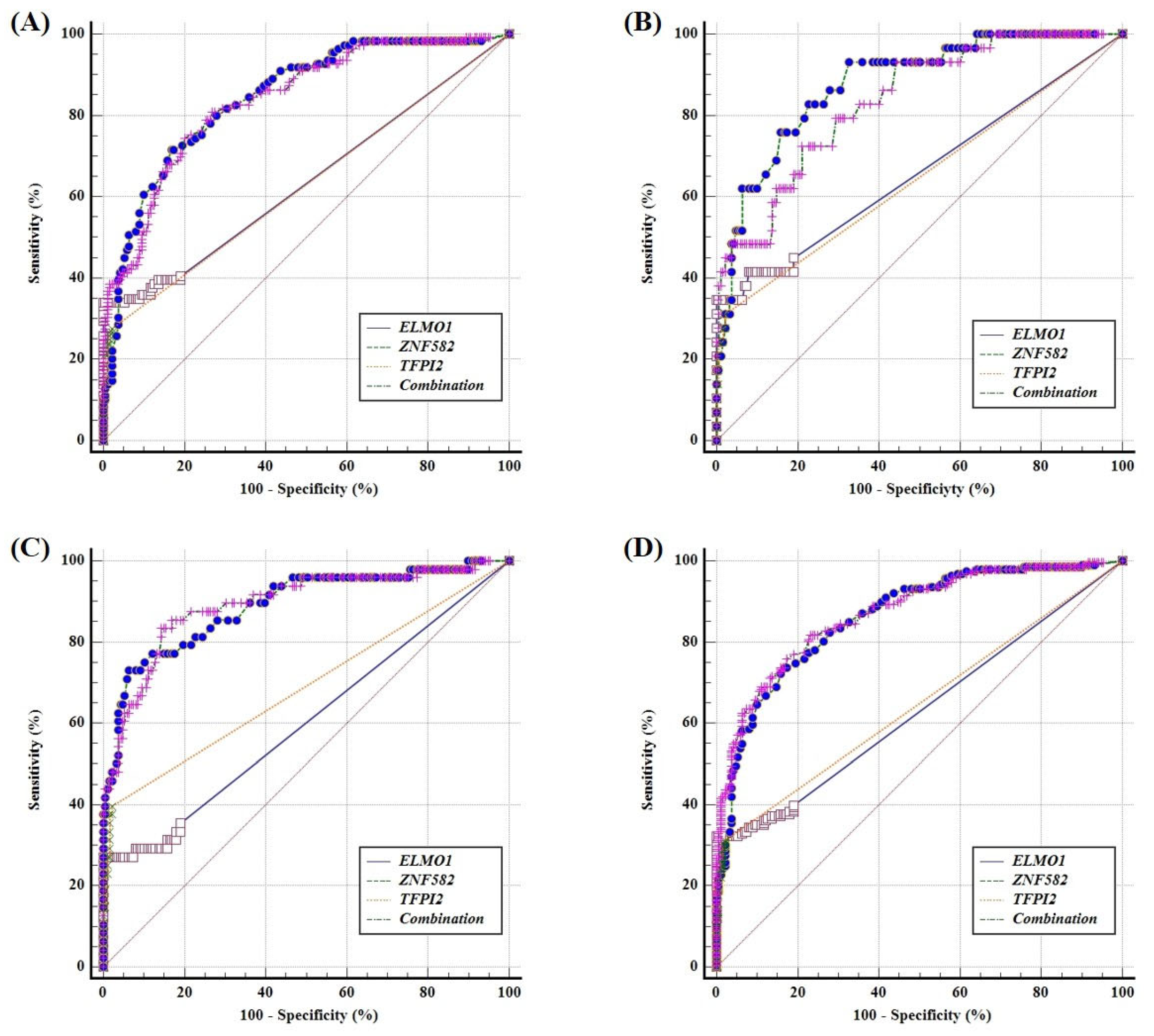
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Liu, X.M.; Ma, X.Y.; Liu, F.; Liu, Z.L.; Tang, X.Y.; Ji, M.Z.; Zheng, J.X. Gastric Cancer Screening Methods: A Comparative Study of the Chinese New Gastric Cancer Screening Score and Kyoto Classification of Gastritis. Gastroenterol. Res. Pract. 2022, 2022, 7639968. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.S.; Chen, X.L. Research on esophageal cancer: With personal perspectives from studies in China and Kenya. Int. J. Cancer 2021, 149, 264–276. [Google Scholar] [CrossRef] [PubMed]
- Miyoshi, J.; Zhu, Z.; Luo, A.; Toden, S.; Zhou, X.; Izumi, D.; Kanda, M.; Takayama, T.; Parker, I.M.; Wang, M.; et al. A microRNA-based liquid biopsy signature for the early detection of esophageal squamous cell carcinoma: A retrospective, prospective and multicenter study. Mol. Cancer 2022, 21, 44. [Google Scholar] [CrossRef] [PubMed]
- Sudo, K.; Kato, K.; Matsuzaki, J.; Boku, N.; Abe, S.; Saito, Y.; Daiko, H.; Takizawa, S.; Aoki, Y.; Sakamoto, H.; et al. Development and Validation of an Esophageal Squamous Cell Carcinoma Detection Model by Large-Scale MicroRNA Profiling. JAMA Netw. Open 2019, 2, e194573. [Google Scholar] [CrossRef]
- Sun, D.; Cao, M.; Li, H.; He, S.; Chen, W. Cancer burden and trends in China: A review and comparison with Japan and South Korea. Chin. J. Cancer Res. 2020, 32, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Miao, J.; Liu, Y.; Zhao, G.; Liu, X.; Ma, Y.; Li, H.; Li, S.; Zhu, Y.; Xiong, S.; Zheng, M.; et al. Feasibility of Plasma-Methylated SFRP2 for Early Detection of Gastric Cancer. Cancer Control. J. Moffitt Cancer Cent. 2020, 27, 1073274820922559. [Google Scholar] [CrossRef]
- Alix-Panabières, C.; Pantel, K. Liquid Biopsy: From Discovery to Clinical Application. Cancer Discov. 2021, 11, 858–873. [Google Scholar] [CrossRef]
- So, J.B.Y.; Kapoor, R.; Zhu, F.; Koh, C.; Zhou, L.; Zou, R.; Tang, Y.C.; Goo, P.C.K.; Rha, S.Y.; Chung, H.C.; et al. Development and validation of a serum microRNA biomarker panel for detecting gastric cancer in a high-risk population. Gut 2021, 70, 829–837. [Google Scholar] [CrossRef]
- Kulis, M.; Esteller, M. 2—DNA Methylation and Cancer. In Advances in Genetics; Herceg, Z., Ushijima, T., Eds.; Academic Press: Cambridge, MA, USA, 2010; Volume 70, pp. 27–56. [Google Scholar]
- Yizhak, K.; Aguet, F.; Kim, J.; Hess, J.M.; Kübler, K.; Grimsby, J.; Frazer, R.; Zhang, H.; Haradhvala, N.J.; Rosebrock, D.; et al. RNA sequence analysis reveals macroscopic somatic clonal expansion across normal tissues. Science 2019, 364, eaaw0726. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, G.; Miao, J.; Li, H.; Ma, Y.; Liu, X.; Li, S.; Zhu, Y.; Xiong, S.; Zheng, M.; et al. Performance Comparison Between Plasma and Stool Methylated SEPT9 Tests for Detecting Colorectal Cancer. Front. Genet. 2020, 11, 324. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Li, H.; Yang, Z.; Wang, Z.; Xu, M.; Xiong, S.; Li, S.; Wu, X.; Liu, X.; Wang, Z.; et al. Multiplex methylated DNA testing in plasma with high sensitivity and specificity for colorectal cancer screening. Cancer Med. 2019, 8, 5619–5628. [Google Scholar] [CrossRef] [PubMed]
- Jensen, S.; Øgaard, N.; Ørntoft, M.W.; Rasmussen, M.H.; Bramsen, J.B.; Kristensen, H.; Mouritzen, P.; Madsen, M.R.; Madsen, A.H.; Sunesen, K.G.; et al. Novel DNA methylation biomarkers show high sensitivity and specificity for blood-based detection of colorectal cancer-a clinical biomarker discovery and validation study. Clin. Epigenetics 2019, 11, 158. [Google Scholar] [CrossRef] [PubMed]
- Imperiale, T.F.; Ransohoff, D.F.; Itzkowitz, S.H.; Levin, T.R.; Lavin, P.; Lidgard, G.P.; Ahlquist, D.A.; Berger, B.M. Multitarget stool DNA testing for colorectal-cancer screening. New Engl. J. Med. 2014, 370, 1287–1297. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Zhou, G.; Jin, P.; Zhu, J.; Li, S.; Wu, Q.; Wang, G.; Sheng, J.; Wang, J.; Song, L.; et al. Detection of Colorectal Cancer Using a Simplified SEPT9 Gene Methylation Assay Is a Reliable Method for Opportunistic Screening. J. Mol. Diagn. JMD 2016, 18, 535–545. [Google Scholar] [CrossRef] [PubMed]
- Jin, P.; You, P.; Fang, J.; Kang, Q.; Gu, F.; Cai, Y.; Zhai, H.; Wang, B.; Li, Y.; Xu, J.; et al. Comparison of Performance of Two Stool DNA Tests and a Fecal Immunochemical Test in Detecting Colorectal Neoplasm: A Multicenter Diagnostic Study. Cancer Epidemiol. Biomark. Prev. 2022, 31, 654–661. [Google Scholar] [CrossRef]
- Anderson, B.W.; Suh, Y.S.; Choi, B.; Lee, H.J.; Yab, T.C.; Taylor, W.R.; Dukek, B.A.; Berger, C.K.; Cao, X.; Foote, P.H.; et al. Detection of Gastric Cancer with Novel Methylated DNA Markers: Discovery, Tissue Validation, and Pilot Testing in Plasma. Clin. Cancer Res. 2018, 24, 5724–5734. [Google Scholar] [CrossRef]
- Ma, K.; Cao, B.; Guo, M. The detective, prognostic, and predictive value of DNA methylation in human esophageal squamous cell carcinoma. Clin. Epigenetics 2016, 8, 43. [Google Scholar] [CrossRef]
- Tang, L.; Liou, Y.L.; Wan, Z.R.; Tang, J.; Zhou, Y.; Zhuang, W.; Wang, G. Aberrant DNA methylation of PAX1, SOX1 and ZNF582 genes as potential biomarkers for esophageal squamous cell carcinoma. Biomed. Pharmacother. 2019, 120, 109488. [Google Scholar] [CrossRef]
- Zheng, R.; Zhang, S.; Zeng, H.; Wang, S.; Sun, K.; Chen, R.; Li, L.; Wei, W.; He, J. Cancer incidence and mortality in China, 2016. J. Natl. Cancer Cent. 2022, 2, 1–9. [Google Scholar] [CrossRef]
- Tocci, S.; Ibeawuchi, S.R.; Das, S.; Sayed, I.M. Role of ELMO1 in inflammation and cancer-clinical implications. Cell. Oncol. 2022, 45, 505–525. [Google Scholar] [CrossRef] [PubMed]
- Maeda, M.; Yamashita, S.; Shimazu, T.; Iida, N.; Takeshima, H.; Nakajima, T.; Oda, I.; Nanjo, S.; Kusano, C.; Mori, A.; et al. Novel epigenetic markers for gastric cancer risk stratification in individuals after Helicobacter pylori eradication. Gastric Cancer 2018, 21, 745–755. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Wu, C.W.; Taylor, W.R.; Sawas, T.; Burger, K.N.; Mahoney, D.W.; Sun, Z.; Yab, T.C.; Lidgard, G.P.; Allawi, H.T.; et al. Discovery, Validation, and Application of Novel Methylated DNA Markers for Detection of Esophageal Cancer in Plasma. Clin. Cancer Res. 2019, 25, 7396–7404. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Wang, Y.; Hu, X. Identification and Comprehensive Validation of a DNA Methylation-Driven Gene-Based Prognostic Model for Clear Cell Renal Cell Carcinoma. DNA Cell Biol. 2020, 39, 1799–1812. [Google Scholar] [CrossRef]
- Li, N.; He, Y.; Mi, P.; Hu, Y. ZNF582 methylation as a potential biomarker to predict cervical intraepithelial neoplasia type III/worse: A meta-analysis of related studies in Chinese population. Medicine 2019, 98, e14297. [Google Scholar] [CrossRef]
- Huang, J.; Wang, G.; Tang, J.; Zhuang, W.; Wang, L.P.; Liou, Y.L.; Liu, Y.Z.; Zhou, H.H.; Zhu, Y.S. DNA Methylation Status of PAX1 and ZNF582 in Esophageal Squamous Cell Carcinoma. Int. J. Environ. Res. Public Health 2017, 14, 216. [Google Scholar] [CrossRef]
- Sierko, E.; Wojtukiewicz, M.Z.; Kisiel, W. The role of tissue factor pathway inhibitor-2 in cancer biology. Semin. Thromb. Hemost. 2007, 33, 653–659. [Google Scholar] [CrossRef]
- Jia, Y.; Yang, Y.; Brock, M.V.; Cao, B.; Zhan, Q.; Li, Y.; Yu, Y.; Herman, J.G.; Guo, M. Methylation of TFPI-2 is an early event of esophageal carcinogenesis. Epigenomics 2012, 4, 135–146. [Google Scholar] [CrossRef]
- Hibi, K.; Goto, T.; Kitamura, Y.H.; Sakuraba, K.; Shirahata, A.; Mizukami, H.; Saito, M.; Ishibashi, K.; Kigawa, G.; Nemoto, H.; et al. Methylation of the TFPI2 gene is frequently detected in advanced gastric carcinoma. Anticancer. Res. 2010, 30, 4131–4133. [Google Scholar]
- Hibi, K.; Goto, T.; Shirahata, A.; Saito, M.; Kigawa, G.; Nemoto, H.; Sanada, Y. Detection of TFPI2 methylation in the serum of gastric cancer patients. Anticancer. Res. 2011, 31, 3835–3838. [Google Scholar]
- Lin, Z.; Luo, M.; Chen, X.; He, X.; Qian, Y.; Lai, S.; Si, J.; Chen, S. Combined Detection of Plasma ZIC1, HOXD10 and RUNX3 Methylation is a Promising Strategy for Early Detection of Gastric Cancer and Precancerous Lesions. J. Cancer 2017, 8, 1038–1044. [Google Scholar] [CrossRef] [PubMed]
- Salta, S.; Macedo-Silva, C.; Miranda-Gonçalves, V.; Lopes, N.; Gigliano, D.; Guimarães, R.; Farinha, M.; Sousa, O.; Henrique, R.; Jerónimo, C. A DNA methylation-based test for esophageal cancer detection. Biomark. Res. 2020, 8, 68. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Zhao, G.; Cao, Y.; Chen, Z.; Fei, S. Feasibility of Methylated CLIP4 in Stool for Early Detection of Colorectal Cancer: A Training Study in Chinese Population. Front. Oncol. 2021, 11, 647066. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Song, J.; Wang, T.; Zhu, W.; Zuo, L.; Wu, J.; Guo, J.; Yang, X. A combination of methylation and protein markers is capable of detecting gastric cancer detection by combined markers. Epigenomics 2021, 13, 1557–1570. [Google Scholar] [CrossRef] [PubMed]
| Total Number | Gender | Age | |||
|---|---|---|---|---|---|
| Male (n, %) | Female (n, %) | Min–Max | Median | ||
| UGC | 186 | 136 (73.1) | 50 (26.9) | 28–90 | 68 |
| EC | 48 | 42 (87.5) | 6 (12.5) | 52–90 | 68 |
| EJC | 29 | 22 (75.9) | 7 (24.1) | 50–87 | 71 |
| GC | 109 | 72 (66.1) | 37 (33.9) | 28–86 | 64 |
| Control | 190 | 74 (38.9) | 116 (61.1) | 23–79 | 45 |
| Characteristics | Total Number | Positive Number | Sensitivity (%) | p-Value | |
|---|---|---|---|---|---|
| UGC | |||||
| Gender | Male | 136 | 92 | 67.6 | 0.100 |
| Female | 50 | 40 | 80.0 | ||
| Age | <60 | 47 | 31 | 66.0 | 0.629 |
| 60–70 | 77 | 57 | 74.0 | ||
| >70 | 62 | 45 | 72.6 | ||
| GC | |||||
| Gender | Male | 72 | 47 | 65.3 | 0.415 |
| Female | 37 | 27 | 73.0 | ||
| Age | <60 | 39 | 24 | 61.5 | 0.376 |
| 60–70 | 41 | 31 | 75.6 | ||
| >70 | 29 | 19 | 65.5 | ||
| EJC | |||||
| Gender | Male | 22 | 13 | 59.1 | 0.042 |
| Female | 7 | 7 | 100.0 | ||
| Age | <60 | 2 | 2 | 100.0 | 0.050 |
| 60–70 | 11 | 5 | 45.5 | ||
| >70 | 16 | 14 | 87.5 | ||
| EC | |||||
| Gender | Male | 42 | 32 | 76.2 | 0.179 |
| Female | 6 | 6 | 100.0 | ||
| Age | <60 | 6 | 5 | 83.3 | 0.622 |
| 60–70 | 25 | 21 | 84.0 | ||
| >70 | 17 | 12 | 70.6 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, C.; Zhao, G.; Pei, B.; Wang, K.; Li, H.; Fei, S.; Song, L.; Wang, C.; Xiong, S.; Xue, Y.; et al. A Novel Plasma-Based Methylation Panel for Upper Gastrointestinal Cancer Early Detection. Cancers 2022, 14, 5282. https://doi.org/10.3390/cancers14215282
Peng C, Zhao G, Pei B, Wang K, Li H, Fei S, Song L, Wang C, Xiong S, Xue Y, et al. A Novel Plasma-Based Methylation Panel for Upper Gastrointestinal Cancer Early Detection. Cancers. 2022; 14(21):5282. https://doi.org/10.3390/cancers14215282
Chicago/Turabian StylePeng, Cheng, Guodong Zhao, Bing Pei, Kai Wang, Hui Li, Sujuan Fei, Lishuang Song, Chunkai Wang, Shangmin Xiong, Ying Xue, and et al. 2022. "A Novel Plasma-Based Methylation Panel for Upper Gastrointestinal Cancer Early Detection" Cancers 14, no. 21: 5282. https://doi.org/10.3390/cancers14215282
APA StylePeng, C., Zhao, G., Pei, B., Wang, K., Li, H., Fei, S., Song, L., Wang, C., Xiong, S., Xue, Y., He, Q., & Zheng, M. (2022). A Novel Plasma-Based Methylation Panel for Upper Gastrointestinal Cancer Early Detection. Cancers, 14(21), 5282. https://doi.org/10.3390/cancers14215282




