Simple Summary
Immune related adverse events (irAEs) are a management challenge with an associated increased morbidity and mortality. The most common renal toxicity is acute interstitial nephritis (AIN), which may be analogous to kidney transplant rejection. In a retrospective cohort of 35 patients with biopsy confirmed AIN, a detailed pathological evaluation was performed using transplant rejection BANFF criteria. The study concluded that patients with increased interstitial fibrosis on kidney biopsies were less likely to have renal response compared to patients with less fibrosis, (p = 0.027). Interstitial inflammation, tubulitis, number of eosinophils, neutrophils, and immune subtype of cells had no impact on renal response. In addition, patients who received concurrent ICI and achieved renal response within 3 months had the best OS in comparison to patients who did not receive concurrent ICI nor achieved renal response.
Abstract
Background: Immune-related adverse events are a management challenge in patients receiving immune checkpoint inhibitors (ICIs). The most common renal immune-related adverse event, acute interstitial nephritis (AIN), is associated with patient morbidity and mortality. AIN, characterized by infiltration of renal tissue with immune cells, may be analogous to kidney transplant rejection. We evaluated clinical variables and pathologic findings to identify predictors of renal response and overall survival (OS) in patients with ICI-induced AIN. Design, setting, participants, and measurements: We reviewed the records and biopsy specimens of all 35 patients treated for ICI-induced AIN at our institution, between August 2007 and August 2020, who had biopsy specimens available. Two board-certified renal pathologists graded the severity of inflammation and chronicity using transplant rejection Banff criteria and performed immunohistochemistry analysis. Patients were categorized as renal responders if creatinine had any improvement or returned to baseline within 3 months of initiating treatment for AIN. Clinical and pathologic characteristics and OS were compared between responders and non-responders. Results: Patients with high levels of interstitial fibrosis were less likely to be responders than those with less fibrosis (p = 0.02). Inflammation, tubulitis, the number of eosinophils and neutrophils, and the clustering or presence of CD8+, CD4+, CD20+, or CD68+ cells were not associated with renal response. Responders had better OS than non-responders (12-month OS rate 77% compared with 27%, p = 0.025). Responders who received concurrent ICIs had the best OS, and non-responders who did not receive concurrent ICIs had the worst OS (12-month OS rate 100% for renal response and concurrent ICIs, 72% for renal response and no concurrent ICIs, and 27% for no renal response and no concurrent ICIs; p = 0.041). Conclusions: This is the first analysis of ICI induced nephritis where a detailed pathological and clinical evaluation was performed to predict renal response. Low levels of interstitial fibrosis in kidney tissue are associated with renal response to treatment for ICI-induced AIN, and the renal response and use of concurrent ICIs are associated with better OS in these patients. Our findings highlight the importance of the early diagnosis and treatment of ICI-AIN, while continuing concurrent ICI therapy.
1. Introduction
With the widespread use of immune checkpoint inhibitors (ICIs) in cancer treatment, unintended immune responses, termed immune-related adverse events (irAEs), have become an accepted challenge with these novel and life-saving drugs. Identifying predictors and early markers of irAEs has allowed the early intervention and treatment of irAEs without hindering the effectiveness of ICIs. An unintended immune response after ICI exposure can occur in any organ, but irAEs occur most often in the skin, gastrointestinal tract, and endocrine system [1,2].
Renal irAEs occur less frequently, are more difficult to diagnose (in part due to a lack of specific symptoms and diagnostic tests), and are associated with higher patient morbidity and mortality rates compared with other irAEs [1]. ICI-associated acute kidney injury (AKI) may occur in 1.4–16.5% of patients receiving ICI therapy, with median times to AKI diagnosis ranging from 1 to 3 months after ICI exposure [3,4,5,6]. However, data-driven guidance on the use of pathologic findings to treat or assess the prognosis of renal irAEs is lacking. Current societal guidelines for renal irAEs lack a grading system of risk for ICI-associated AKI or a biopsy-related approach to treatment. Although the incidence of ICI-induced AKI is low, ICIs are often used for adjuvant and neoadjuvant treatment of solitary kidney and chronic kidney disease, where progression to dialysis may be imminent, emphasizing the importance of a more informed approach to diagnosis and treatment of renal irAEs [7].
Several researchers have sought to evaluate predictors of AKI and other irAEs after ICI exposure [3,6,8]. Multiple studies have shown that achieving tumor response is associated with irAEs in general [9]. The combined use of anti–cytotoxic T-lymphocyte-associated antigen 4 and anti–programmed cell death protein 1/programmed death-ligand 1 agents has been shown to be an independent predictor of irAEs, including AKI [10,11]. Some authors have reported that low baseline estimated glomerular filtration rates were associated with AKI, but this finding was not consistent across studies [3,11]. Therefore, ICI therapy should not be withheld from patients with impaired kidney function or chronic kidney disease, particularly given the low incidence of AKI in these patients, but providers should nevertheless be aware of how to manage AKI should it arise.
AKI associated with ICIs has been predominately reported to be acute interstitial nephritis (AIN) with severe immune infiltration of the kidney parenchyma, which is often similar to acute cellular kidney transplant rejection. On rare occasions, glomerulonephritis could also be induced by an autoimmune process [12,13,14]. Despite the similarities of ICI-induced AIN to cellular kidney transplant rejection, the use of pathologic findings from biopsy specimens to assess renal irAEs has not been studied. In the current study, we evaluated available biopsy specimens from patients at our institution with ICI-induced AIN for immune cell subtypes, and using the Banff criteria for kidney transplant rejection, we analyzed the association of these characteristics and other clinical variables with renal response to treatment of AIN and survival outcomes.
2. Methods
2.1. Patient Data Collection
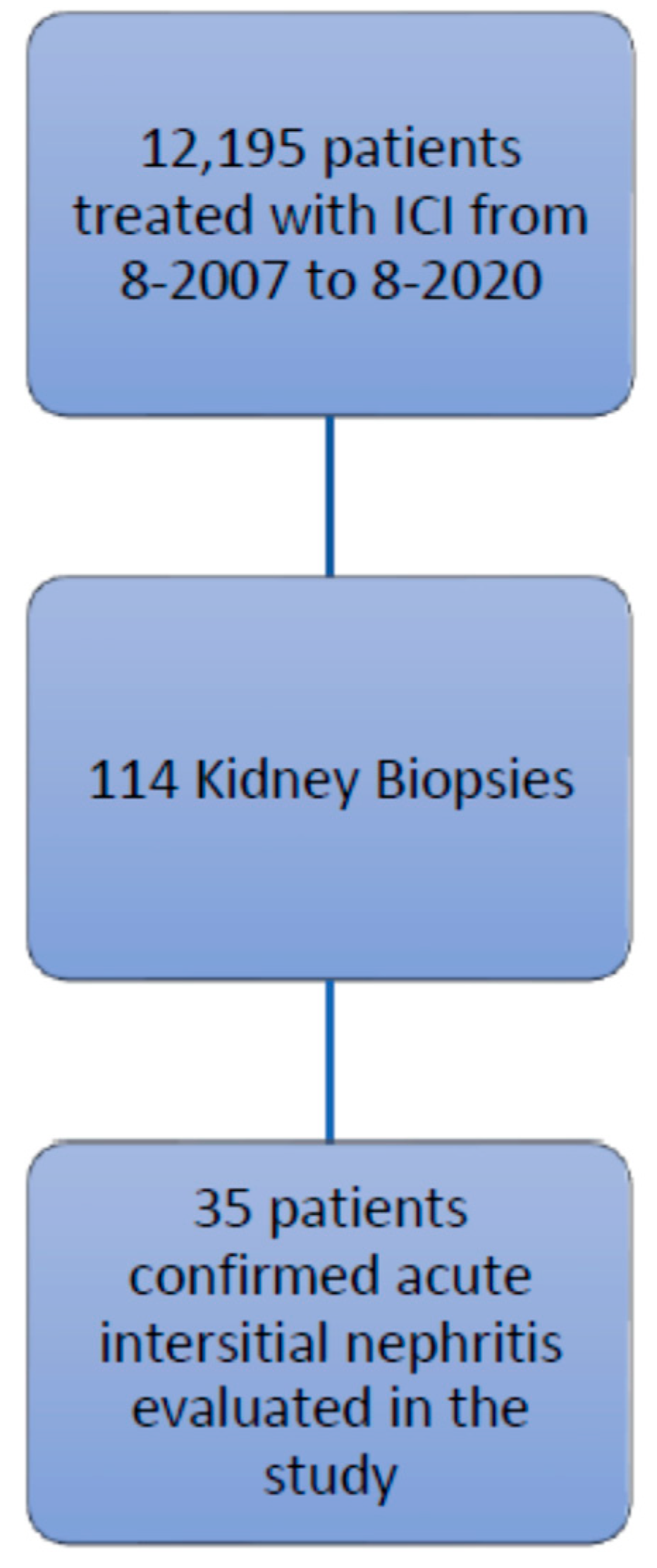
This retrospective study was approved by the Institutional Review Board at The University of Texas MD Anderson Cancer Center, and the procedures followed were in accordance with the principles of the Declaration of Helsinki. We identified patients listed in the MD Anderson pharmacy database who were treated with ipilimumab, nivolumab, pembrolizumab, atezolizumab, durvalumab, or avelumab, between August 2007 and August 2020. Among the 12,195 patients identified, 114 underwent a renal biopsy, and 35 of these had confirmed AIN and tissue specimen slides available for review (Figure 1). The records of these 35 patients were reviewed for baseline demographic characteristics, type of malignancy, comorbidities, number of cycles of ICIs, concurrent nephrotoxic chemotherapies, concurrent use of proton pump inhibitors and nonsteroidal anti-inflammatory drugs, urinalysis findings, the presence of other irAEs, and survival data.
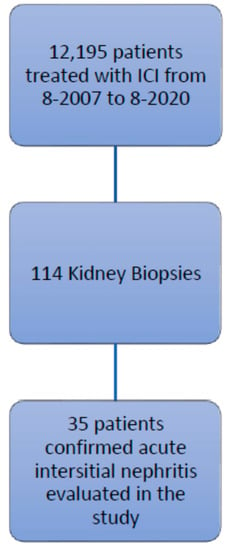
Figure 1.
Study flow chart of inclusion criteria.
2.2. Tissue Evaluation
All hematoxylin and eosin–stained tissue specimen slides were independently and blindly reviewed by two renal pathologists. The severity of inflammation and chronicity were graded using the Banff 2019 Kidney Meeting Report lesion grading system [15]. Quantitative criteria for inflammation (i score) were as follows: 0, no inflammation or inflammation in <10% of unscarred cortical parenchyma; 1, inflammation in 10–25% of unscarred cortical parenchyma; 2, inflammation in 26–50% of unscarred cortical parenchyma; and 3, inflammation in >50% of unscarred cortical parenchyma. Quantitative criteria for tubulitis (t score) were as follows: 0, no mononuclear cells in tubules; 1, foci with one to four cells per tubular cross-section or 10 tubular cells; 2, foci with 5–10 cells per tubular cross-section or 10 tubular cells; and 3, foci with >10 cells per tubular cross-section or the presence of two or more areas of tubular basement membrane destruction accompanied by i2/i3 inflammation and t2 elsewhere. Interstitial fibrosis with tubular atrophy (IFTA) was assessed as a percentage of the affected cortex. In addition, other pathologic findings were recorded: percentage of global glomerulosclerosis, presence of granuloma, and maximum number of interstitial neutrophils and eosinophils per 40× magnification. We also evaluated immune cell subtypes using immunohistochemistry staining for CD4, CD8, CD20, and CD68, for both presence and clustering of cells.
2.3. Renal Response and Survival
Upon diagnosis of AIN, all patients received prednisone at a starting dose of 60 mg, and the duration of treatment ranged from 1 week to 12 weeks. Three patients received infliximab in addition to steroids. Creatinine values were evaluated at diagnosis of AIN and at 3 months after initiation of therapy to assess response. The grades of renal toxicity were based on Common Terminology Criteria for Adverse Events: grade 1, creatinine 1.5 times baseline level; grade 2, creatinine >1.5 to 3 times baseline level; grade 3, creatinine >3 to 6 times baseline level; and grade 4, creatinine >6 times baseline level. Patients with any persistent creatinine improvement >0.35 mg/dL at 3 months after initiation of treatment for AIN were considered responders, and those with no improvement in creatinine at that time were considered non-responders.
Progression-free survival (PFS) was defined as the time interval from response evaluation (at 3 months after initiation of treatment for AIN) to progression or death, whichever occurred first. Overall survival (OS) was defined as the time interval from response evaluation to death. For events that had not occurred by the time of data analysis, times were censored at the last contact at which the patient was known to be alive or free of progression.
2.4. Statistical Analysis
Descriptive statistics (frequency distribution, mean ± standard deviation, and median and range) were used to summarize patient characteristics. The Fisher exact test was used to compare categorical variables between responders and non-responders, and the t test or analysis of variance or their counterparts for nonparametric data (Wilcoxon rank-sum or Kruskal-Wallis test) were used to compare continuous variables between the groups. The distributions of OS and PFS were estimated using the Kaplan-Meier method [16], and the log-rank test [17], was performed to identify differences in survival between groups.
3. Results
The characteristics of the 35 patients with confirmed AIN during the period studied are summarized in Table 1. The median follow-up time was 12.2 months, and the median time from ICI therapy initiation to diagnosis of AIN was 123 days. Twenty-two patients (63%) were male with a median age of 67 years at diagnosis of AIN. The most common malignancy was melanoma (n = 10, 29%), followed by lung cancer (n = 7, 20%) and urothelial cancer (n = 3, 9%).

Table 1.
Baseline (i.e., at diagnosis of acute interstitial nephritis) demographic and clinical characteristics of patients who developed acute interstitial nephritis after treatment with immune checkpoint inhibitors (n = 35).
A total of 29 patients (83%) received single-agent immunotherapy; nivolumab (n = 17, 49%) and pembrolizumab (n = 13, 37%) were the most commonly used ICIs (Table 1). Six patients (17%) received two ICIs. The median number of ICI cycles was six (range 1–123 cycles), with a median length of steroid treatment of 4 weeks.
Thirteen patients (37%) were exposed to nonsteroidal anti-inflammatory drugs or proton pump inhibitors. Ten patients (29%) were treated with steroids prior to the kidney biopsy and four of the 10 patients had Banff inflammation scores of 0 or 1. Grade III or IV renal toxicity was noted in 22 (63%) patients; six had to undergo dialysis. 29% of the patients were on concurrent nephrotoxic agents such as carboplatin, pemetrexed, Dabrafenib, trametinib, Sitravatinib, and Axitinib.
Renal toxicity at the time of AIN diagnosis was categorized as grade II in 13 patients (37%), grade III in 10 patients (29%), and grade IV in 12 patients (34%; Table 1). Urinalysis showed that 25 patients (71%) had red or white blood cells present in their urine. Other irAEs were reported in 15 patients (43%); the most common of these were colitis in three patients (9%) and arthritis in two patients (6%).
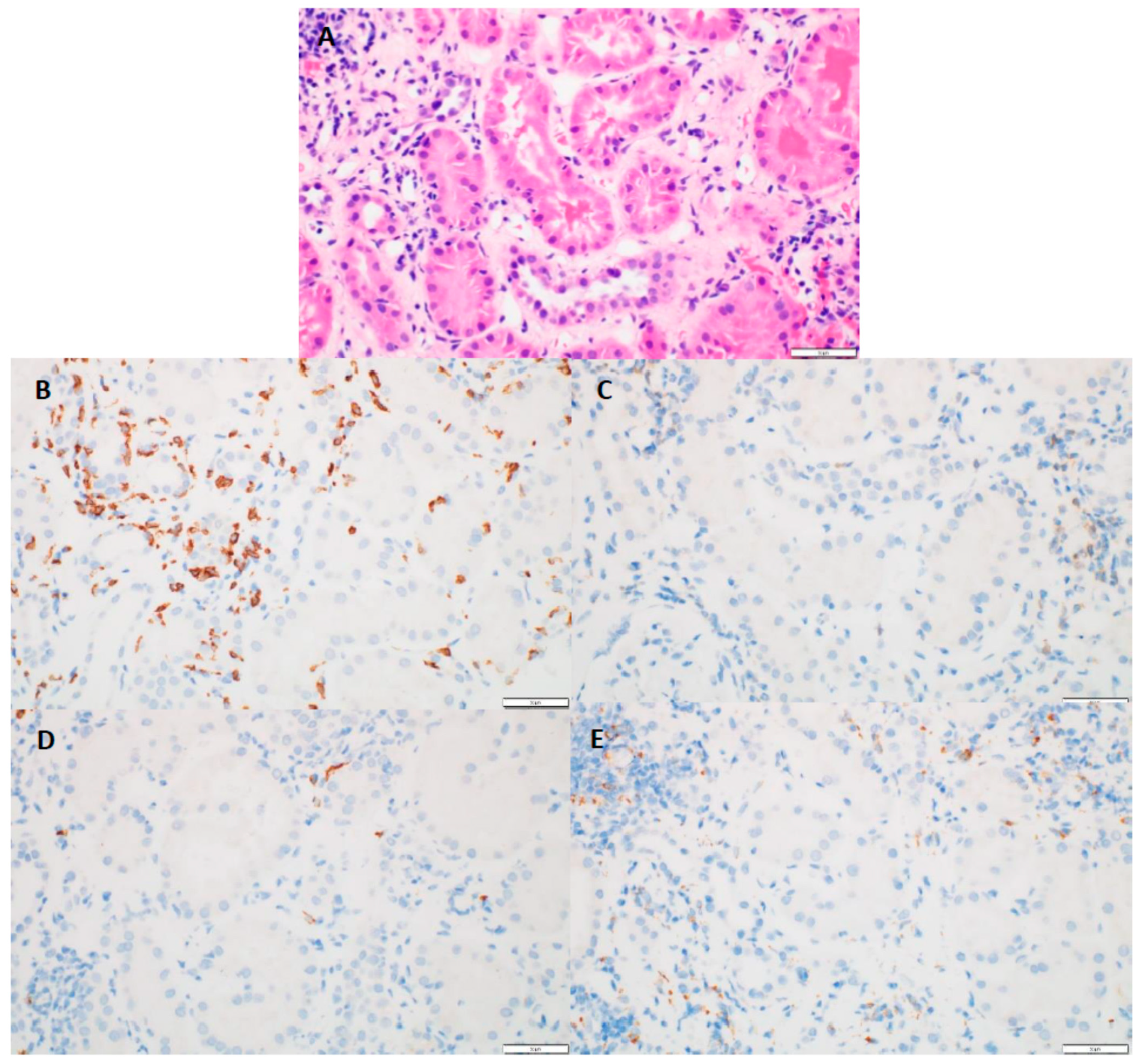
Kidney biopsy results showed that 31 patients (89%) had chronic interstitial nephritis and 26 (83%) had acute tubular necrosis in addition to AIN (Table 1). In addition, five patients (14%) had granuloma in conjunction with AIN. The median interstitial fibrosis noted was 10% (range 0–70%). The median global glomerulosclerosis observed was 14% (range 0–55%). The median Banff inflammation score was 2 (range 0–3), and the median tubulitis score was 2 (range 0–3). The maximum number of eosinophils observed was 101, with a median of two cells per high-power field. The maximum number of neutrophils observed was 89 cells, with a median of seven cells per high-power field. CD4+, CD8+, CD20+, and CD68+ immune cell subtypes were each observed in more than two-thirds of the biopsy specimens, and CD8+ cells were most often associated with tubulitis (Figure 2).
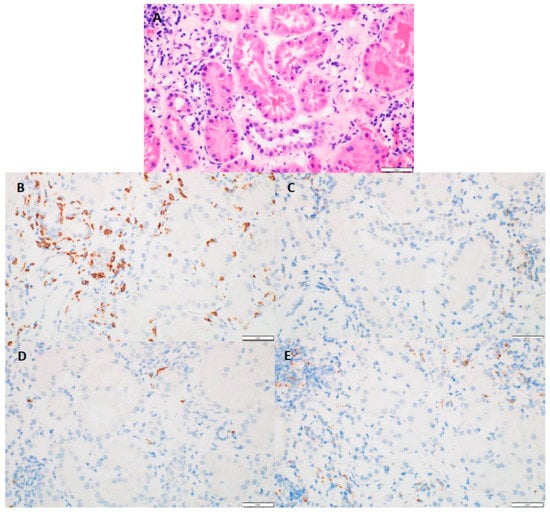
Figure 2.
(A) (H&E, 40×)-tubulitis present in non-atrophic tubules; (B) (CD8, 40×)-numerous positive cells present with the areas of tubulitis; (C) (CD4, 40×)-absence of positive staining cells within tubulitis; (D) (C20, 40×)-absence of positive staining cells within tubulitis; (E) (CD68, 40×)-absence of positive staining cells within tubulitis.
3.1. Renal Response
Twenty-nine patients (83%) achieved complete or partial renal response at 3 months after initiation of treatment for AIN (i.e., responders), and six patients (17%) did not (i.e., non-responders). Eleven patients (31%) had renal relapse after treatment for AIN.
Tumor responses to ICIs at the time of renal response assessment were as follows: 18 patients (51%) had tumor progression, 14 (40%) were in remission, and three (9%) had stable disease. Six patients (17%) had ICI re-challenge after treatment for AIN; five had AIN relapse and two had progression of their cancer.
3.2. Predictors of Renal Response
None of the baseline clinical characteristics evaluated, including comorbidities, exposure to nephrotoxic agents, use of nonsteroidal anti-inflammatory drugs or proton pump inhibitors, urine analysis findings, or the presence of acute tubular necrosis, were associated with renal response (Table 2). Pathologic features such as intensity of inflammation and tubulitis were not associated with renal response, but the presence of IFTA was associated with renal response (p = 0.02; Table 3). Higher numbers of CD8+ cells (p = 0.05), and CD8+ cell density (p = 0.07), were observed in responders. CD68 clustering was not observed in any of the samples.

Table 2.
Association of baseline clinical characteristics with response to treatment for immune checkpoint inhibitor–induced acute interstitial nephritis (at 3 months after initiation of treatment).

Table 3.
Association of pathologic markers with response (n = 26) or non-response (n = 9) to treatment for immune checkpoint inhibitor–induced acute interstitial nephritis at 3 months after initiation of treatment.
3.3. OS and PFS
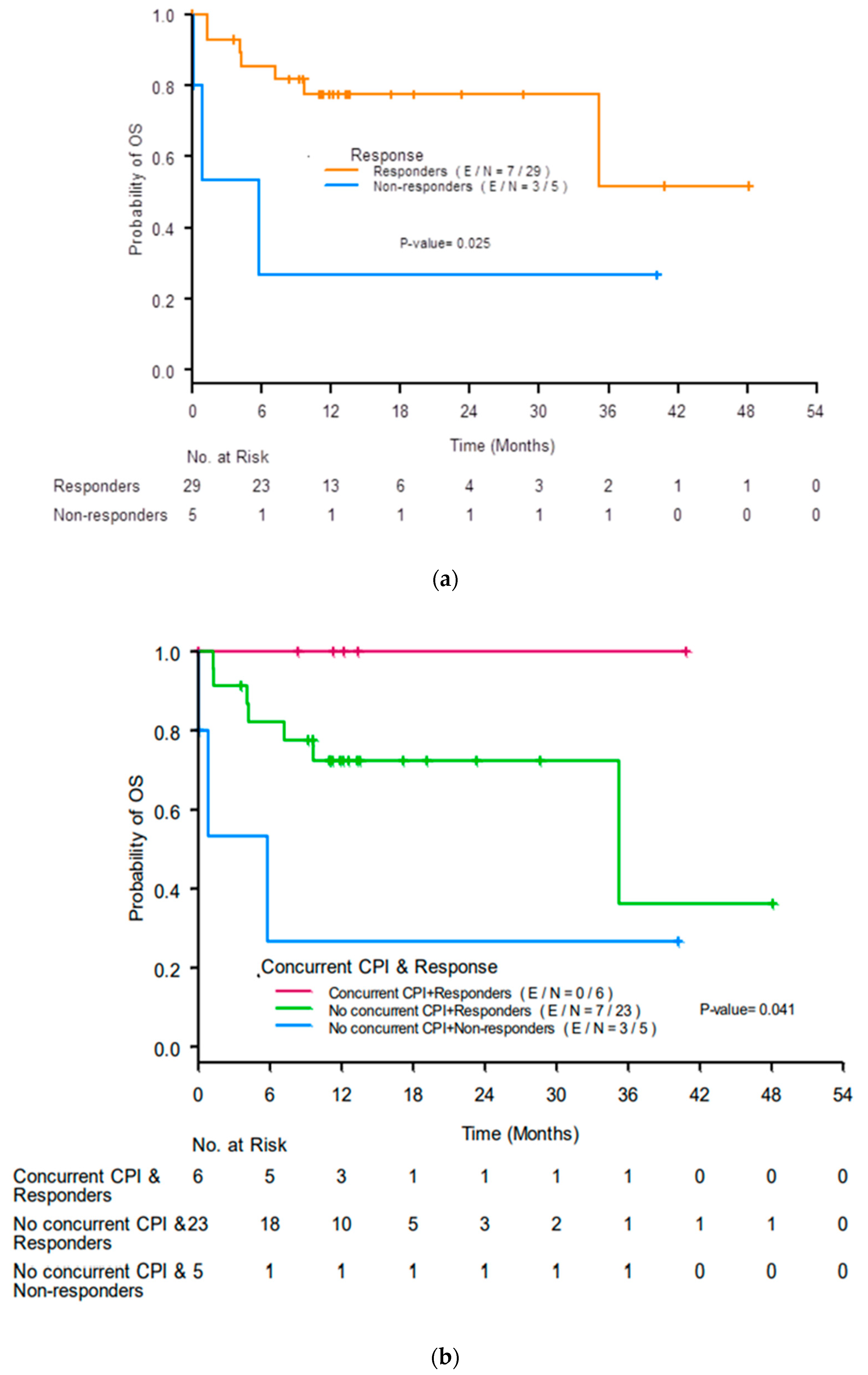
The median OS was not reached (NR) (95% confidence interval (CI) 35.22-NR). Responders had better 12-month OS rates than non-responders (77% (95% CI 56–89%) compared with 27% (95% CI 1–69%), p = 0.025; Figure 3a). Responders who received concurrent ICIs had the best 12-month OS rate (100%), responders who did not receive concurrent ICIs had a lower 12-month OS rate (72%, 95% CI 48–87%), and non-responders who did not receive concurrent ICIs had the worst 12-month OS rate (27%, 95% CI 1–69%; p = 0.041; Figure 3b).
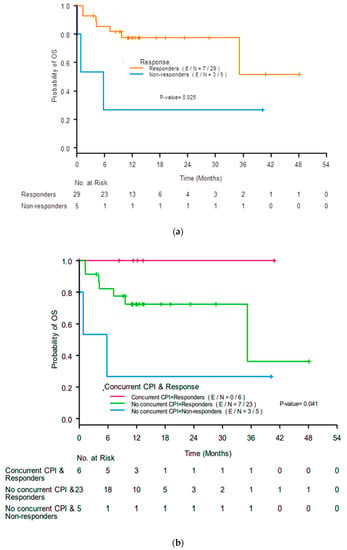
Figure 3.
Kaplan-Meier curves for overall survival (OS) by (a) response to treatment for acute interstitial nephritis at 3 months after initiation of treatment and (b) renal response and use of concurrent immune checkpoint inhibitors (ICIs). CR, complete response; PR, partial response; NR, no response.
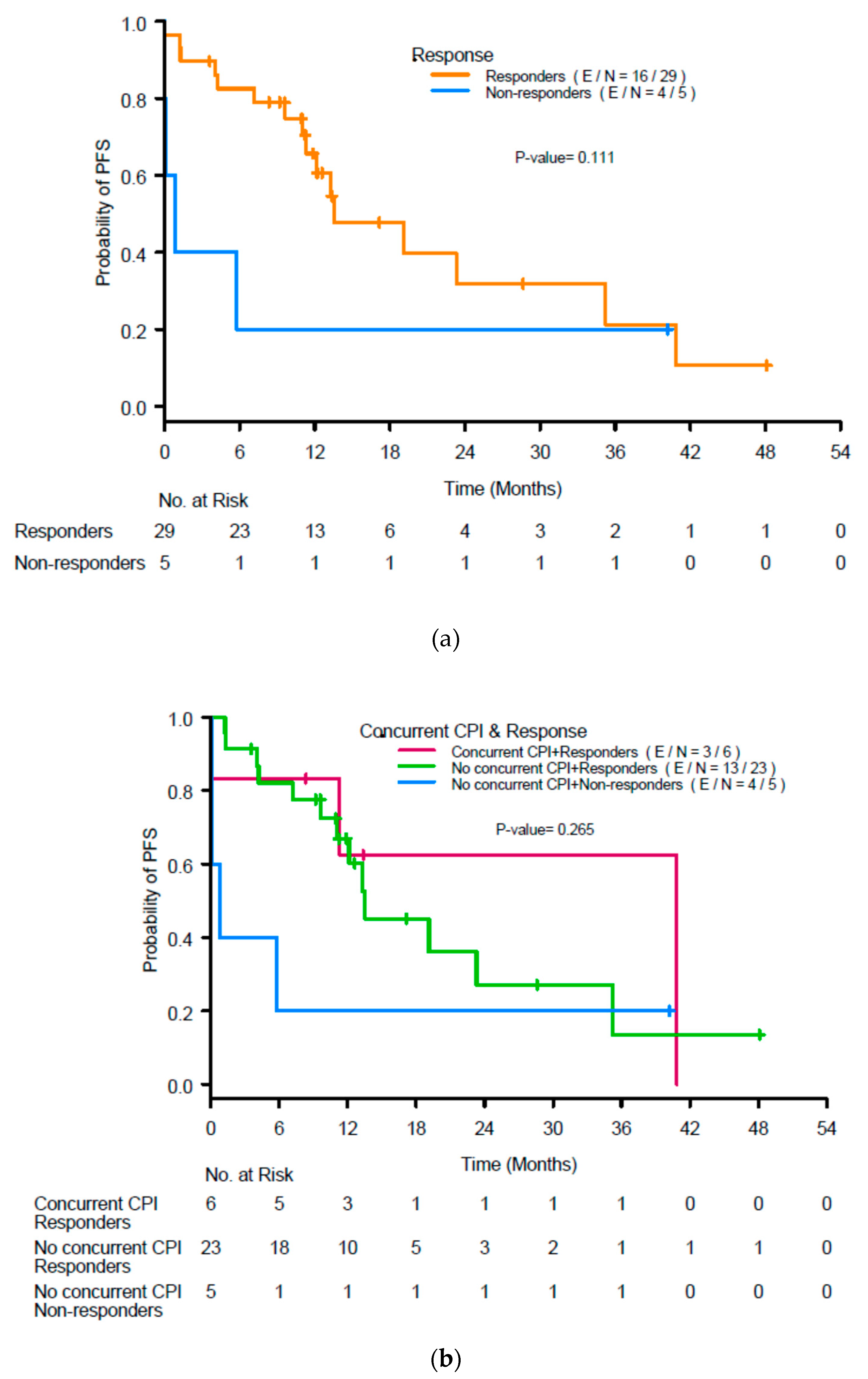
The median PFS in the population was 13.1 months. Renal response was not associated with PFS (p = 0.11; Figure 4a), nor was use of concurrent ICIs (p = 0.27; Figure 4b).
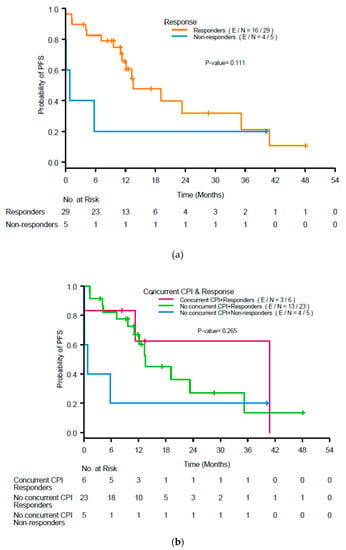
Figure 4.
(a) Kaplan-Meier curves for progression-free survival (PFS) by response to treatment for acute interstitial nephritis at 3 months after initiation of treatment and (b) renal response and use of concurrent immune checkpoint inhibitors (ICIs). CR, complete response; PR, partial response; NR, no response.
4. Discussion
Our findings indicate that in patients with ICI-induced AIN, low levels of IFTA in kidney tissue specimens are associated with renal response to treatment for AIN within 3 months, and that renal response and use of concurrent ICIs are associated with better OS in these patients.
ICI-induced AIN is a rare irAE, and its incidence and predictors have been established primarily through retrospective analysis [2,4,12,13]. However, there is a paucity of data to support an evidence-based approach to the management and treatment of ICI-induced AIN, with guidelines based on expert opinion [18,19,20,21]. Several review papers have highlighted the importance of the kidney biopsy as a gold standard in diagnosing ICI induced nephritis, but evaluating the pathological features of AIN to help guide treatment further is yet to be elucidated [22].
The data on the use of a pathologic approach to evaluate irAE response has also been limited. Cortazar et al. reported that in 12 of 13 patients who developed AIN after treatment with ICIs, the infiltrates were composed predominantly of lymphocytes, with varying degrees of plasma cells and eosinophils. CD3 and CD20 staining revealed a predominance of CD3+ cells in only three patients [2]. The authors did not study biopsy findings and renal response, but they did note that their two patients with AIN who achieved a complete renal response to treatment of AIN had minimal to no fibrosis, whereas patients with no renal response had more fibrosis. Our findings confirm in a more robust statistical and pathologic analysis that the presence of high levels of fibrosis (i.e., IFTA) is a predictor of poor renal response. Therefore, the preservation of kidney function may be best achieved by early recognition of injury and the prompt treatment of ICI-induced AIN. This is further highlighted by our finding that patients who had renal response within 3 months of initiating treatment for AIN had better OS than non-responders.
Our study also evaluated immune subtypes CD4+, CD8+, CD20+, and CD68+. CD4+ and CD8+ cells are often found in irAEs in other organ systems, such as ICI-induced colitis, in which CD8+ cells are more predominant than in other inflammatory bowel disease [23]. The increased presence of B cells (CD20+) in irAEs and other autoimmune disease occurs as a result of activated T cell–B cell interaction, which can lead to autoantibody production. Therefore, the use of anti-CD20 rituximab (chimeric monoclonal antibody against the protein CD20) has been successfully used to treat hematologic, dermatologic, renal, neurologic, and rheumatologic irAEs [14,24,25,26,27]. Increasing evidence of CD68+ staining in myositis has been reported in studies showing that both CD4+/CD8+ and CD68+ macrophages are present in muscle biopsy specimens from patients with ICI-induced myositis and myasthenia gravis [28]. The current study did not show any association of renal response with the number of immune subtype cells or their density, clustering, or tubulitis, but we believe this is due to the small sample size.
An interesting aspect of ICI-induced AIN is its pathologic resemblance with kidney transplant rejection, in which lymphocyte-predominant tubulointerstitial infiltrate is present. This led us to evaluate our tissue specimens using the well-established Banff criteria for evaluation of kidney transplant rejection. The resemblance of ICI-induced AIN to kidney transplant rejection has been further validated in a study using NanoString technology, in which tissue samples from 15 patients with kidney transplant rejection were compared with those from 10 patients with ICI-induced AIN. In that study, almost all genes evaluated were not significantly differentially expressed between ICI-induced AIN and kidney transplant rejection, except for one gene, IFI27, which is inducible by interferon-alpha [29]. Similar to organ rejection, graft vs. host disease in liver tissue had similar immune expression to hepatis irAEs with infiltration of CD8+ T cells and defective accumulation of regulatory T cells expressing forkhead box p3 (FOXP3), but not in those from patients with autoimmune hepatitis [30].
Although our study is one of the largest detailed pathological evaluations of irAE induced nephritis we were limited in making more definitive associations, especially with the immune variables, due to the small sample size. However, what is evident is that there are features that defer in the biopsies such as presence or absence of eosinophils and granulomas, which suggest different pathways of injury. More work is needed to evaluate the pathophysiology of ICI induced nephritis and its distinct entity in comparison to drug induced nephritis, where simply stopping offending agent or treating with a short dose of steroids is not always effective. IrAE’s in other organ systems have been evaluated further and the use of steroid sparing agents has become the forefront of treatment in such scenarios, such as immune mediated colitis and arthritis [31,32]. In our experience, the use of infliximab has been successful in treating relapsed AIN induced by ICI [33]. The move towards steroid sparing agent has been as a result of concern to inhibiting tumor responses [34]. However, with the majority of the research in IrAE’s’s being clinical, there is a lack of understanding of the pathophysiology, genetic predisposition, and findings of the pathology, that would give a more guided approach and support the use of biologics in ICI induced nephritis.
5. Conclusions
Our detailed pathologic evaluation of ICI-induced AIN showed that the degree of fibrosis (i.e., IFTA) is associated with renal response within 3 months of initiating treatment, and that renal response and the use of concurrent ICIs was associated with better OS in these patients. Given the widespread use of ICIs in cancer treatment, further studies are needed to identify the novel biomarkers of risk for, and the effective early intervention and treatment of ICI-induced AIN, to improve both renal outcomes and OS in these patients.
Author Contributions
A.A.: concept/design, interpretation, drafting article, critical revision of article, approval of article, statistics interpretation, data collection, and secured funding. L.S.: blinded tissue evaluation, interpretation, critical revision of article, approval of article, and pathologic data collection. H.L.: concept/design, data analysis/interpretation, drafting article, critical revision of article, approval of article, and statistics. O.M.: data collection, critical revision of article, and approval of article. N.A.-W.: concept development, critical revision of article, and approval of article. A.T.: blinded tissue evaluation, concept/design, interpretation, drafting article, critical revision of article, approval of article, statistics interpretation, and pathologic data collection. All authors have read and agreed to the published version of the manuscript.
Funding
Supported by the National Institutes of Health/National Cancer Institute under award number P30CA016672 (used the Clinical Trials Office and Biostatistics Resource Group). Abdel-Wahab is supported by National Institute of Health (K01AI163412), and reports receiving the University of Texas MD Anderson Cancer Center Division of Internal Medicine Research and Quality Improvement Development Award, Division of Internal Medicine Bridge Funding Award, Survivorship Seed Money Award, and Institutional Research Grant.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board at The University of Texas MD Anderson Cancer Center (PA16-1016).
Informed Consent Statement
Not applicable.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.
Acknowledgments
Editorial support was provided by Erica Goodoff in the Research Medical Library at The University of Texas MD Anderson Cancer Center.
Conflicts of Interest
The authors declare that they have no competing interests.
References
- Postow, M.A.; Sidlow, R.; Hellmann, M.D. Immune-Related Adverse Events Associated with Immune Checkpoint Blockade. N. Engl. J. Med. 2018, 378, 158–168. [Google Scholar] [CrossRef]
- Cortazar, F.B.; Kibbelaar, Z.A.; Glezerman, I.G.; Abudayyeh, A.; Mamlouk, O.; Motwani, S.S.; Murakami, N.; Herrmann, S.M.; Manohar, S.; Shirali, A.C.; et al. Clinical Features and Outcomes of Immune Checkpoint Inhibitor-Associated AKI: A Multicenter Study. J. Am. Soc. Nephrol. 2020, 31, 435–446. [Google Scholar] [CrossRef] [PubMed]
- Seethapathy, H.; Zhao, S.; Chute, D.F.; Zubiri, L.; Oppong, Y.; Strohbehn, I.; Cortazar, F.B.; Leaf, D.E.; Mooradian, M.J.; Villani, A.C.; et al. The Incidence, Causes, and Risk Factors of Acute Kidney Injury in Patients Receiving Immune Checkpoint Inhibitors. Clin. J. Am. Soc. Nephrol. 2019, 14, 1692–1700. [Google Scholar] [CrossRef] [PubMed]
- Meraz-Munoz, A.; Amir, E.; Ng, P.; Avila-Casado, C.; Ragobar, C.; Chan, C.; Kim, J.; Wald, R.; Kitchlu, A. Acute kidney injury associated with immune checkpoint inhibitor therapy: Incidence, risk factors and outcomes. J. Immunother. Cancer 2020, 8, e000467. [Google Scholar] [CrossRef] [PubMed]
- García-Carro, C.; Bolufer, M.; Bury, R.; Catañeda, Z.; Muñoz, E.; Felip, E.; Lorente, D.; Carreras, M.J.; Gabaldon, A.; Agraz, I.; et al. Acute kidney injury as a risk factor for mortality in oncological patients receiving check-point inhibitors. Nephrol. Dial. Transplant. 2021, 37, 887–894. [Google Scholar] [CrossRef]
- Abdelrahim, M.; Mamlouk, O.; Lin, H.; Lin, J.; Page, V.; Abdel-Wahab, N.; Swan, J.; Selamet, U.; Yee, C.; Diab, A.; et al. Incidence, predictors, and survival impact of acute kidney injury in patients with melanoma treated with immune checkpoint inhibitors: A 10-year single-institution analysis. Oncoimmunology 2021, 10, 1927313. [Google Scholar] [CrossRef] [PubMed]
- Haslam, A.; Prasad, V. Estimation of the Percentage of US Patients with Cancer Who Are Eligible for and Respond to Checkpoint Inhibitor Immunotherapy Drugs. JAMA Netw. Open 2019, 2, e192535. [Google Scholar] [CrossRef]
- Gupta, S.; Short, S.A.P.; Sise, M.E.; Prosek, J.M.; Madhavan, S.M.; Soler, M.J.; Ostermann, M.; Herrmann, S.M.; Abudayyeh, A.; Anand, S.; et al. Acute kidney injury in patients treated with immune checkpoint inhibitors. J. Immunother. Cancer 2021, 9, e003467. [Google Scholar] [CrossRef]
- Robert, C.; Ribas, A.; Hamid, O.; Daud, A.; Wolchok, J.D.; Joshua, A.M.; Hwu, W.J.; Weber, J.S.; Gangadhar, T.C.; Joseph, R.W.; et al. Durable Complete Response After Discontinuation of Pembrolizumab in Patients with Metastatic Melanoma. J. Clin. Oncol. 2018, 36, 1668–1674. [Google Scholar] [CrossRef]
- Shoushtari, A.N.; Friedman, C.F.; Navid-Azarbaijani, P.; Postow, M.A.; Callahan, M.K.; Momtaz, P.; Panageas, K.S.; Wolchok, J.D.; Chapman, P.B. Measuring Toxic Effects and Time to Treatment Failure for Nivolumab Plus Ipilimumab in Melanoma. JAMA Oncol. 2018, 4, 98–101. [Google Scholar] [CrossRef]
- Cortazar, F.B.; Marrone, K.A.; Troxell, M.L.; Ralto, K.M.; Hoenig, M.P.; Brahmer, J.R.; Le, D.T.; Lipson, E.J.; Glezerman, I.G.; Wolchok, J.; et al. Clinicopathological features of acute kidney injury associated with immune checkpoint inhibitors. Kidney Int. 2016, 90, 638–647. [Google Scholar] [CrossRef] [PubMed]
- Mamlouk, O.; Selamet, U.; Machado, S.; Abdelrahim, M.; Glass, W.F.; Tchakarov, A.; Gaber, L.; Lahoti, A.; Workeneh, B.; Chen, S.; et al. Nephrotoxicity of immune checkpoint inhibitors beyond tubulointerstitial nephritis: Single-center experience. J. Immunother. Cancer 2019, 7, 2. [Google Scholar] [CrossRef] [PubMed]
- Kitchlu, A.; Jhaveri, K.D.; Wadhwani, S.; Deshpande, P.; Harel, Z.; Kishibe, T.; Henriksen, K.; Wanchoo, R. A Systematic Review of Immune Checkpoint Inhibitor-Associated Glomerular Disease. Kidney Int. Rep. 2021, 6, 66–77. [Google Scholar] [CrossRef] [PubMed]
- Mamlouk, O.; Lin, J.S.; Abdelrahim, M.; Tchakarov, A.S.; Glass, W.F.; Selamet, U.; Buni, M.; Abdel-Wahab, N.; Abudayyeh, A. Checkpoint inhibitor-related renal vasculitis and use of rituximab. J. Immunother. Cancer 2020, 8, e000750. [Google Scholar] [CrossRef] [PubMed]
- Loupy, A.; Haas, M.; Roufosse, C.; Naesens, M.; Adam, B.; Afrouzian, M.; Akalin, E.; Alachkar, N.; Bagnasco, S.; Becker, J.U.; et al. The Banff 2019 Kidney Meeting Report (I): Updates on and clarification of criteria for T cell- and antibody-mediated rejection. Am. J. Transplant. 2020, 20, 2318–2331. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, E.L.; Meier, P. Nonparametric-Estimation from Incomplete Observations. J. Am. Stat. Assoc. 1958, 53, 457–481. [Google Scholar] [CrossRef]
- Mantel, N. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother. Rep. 1966, 50, 163–170. [Google Scholar]
- Haanen, J.; Carbonnel, F.; Robert, C.; Kerr, K.M.; Peters, S.; Larkin, J.; Jordan, K.; Committee, E.G. Management of toxicities from immunotherapy: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2018, 29, iv264–iv266. [Google Scholar] [CrossRef]
- Brahmer, J.R.; Abu-Sbeih, H.; Ascierto, P.A.; Brufsky, J.; Cappelli, L.C.; Cortazar, F.B.; Gerber, D.E.; Hamad, L.; Hansen, E.; Johnson, D.B.; et al. Society for Immunotherapy of Cancer (SITC) clinical practice guideline on immune checkpoint inhibitor-related adverse events. J. Immunother. Cancer 2021, 9, e002435. [Google Scholar] [CrossRef]
- Brahmer, J.R.; Lacchetti, C.; Thompson, J.A. Management of Immune-Related Adverse Events in Patients Treated With Immune Checkpoint Inhibitor Therapy: American Society of Clinical Oncology Clinical Practice Guideline Summary. J. Oncol. Pract. 2018, 14, 247–249. [Google Scholar] [CrossRef]
- Thompson, J.A.; Schneider, B.J.; Brahmer, J.; Andrews, S.; Armand, P.; Bhatia, S.; Budde, L.E.; Costa, L.; Davies, M.; Dunnington, D.; et al. NCCN Guidelines Insights: Management of Immunotherapy-Related Toxicities, Version 1.2020. J. Natl. Compr. Cancer Netw. 2020, 18, 230–241. [Google Scholar] [CrossRef]
- Perazella, M.A.; Sprangers, B. Checkpoint inhibitor therapy-associated acute kidney injury: Time to move on to evidence-based recommendations. Clin. Kidney J. 2021, 14, 1301–1306. [Google Scholar] [CrossRef]
- Takahashi, Y.; Nagaya, T.; Iwaya, Y.; Okamura, T.; Hirayama, A.; Iwaya, M.; Uehara, T.; Umemura, T. CD8(+) Lymphocyte Infiltration Is a Specific Feature of Colitis Induced by Immune Checkpoint Inhibitors. Dig. Dis. Sci. 2022; Online ahead of print. [Google Scholar] [CrossRef]
- Dutertre, M.; de Menthon, M.; Noel, N.; Albiges, L.; Lambotte, O. Cold agglutinin disease as a new immune-related adverse event associated with anti-PD-L1s and its treatment with rituximab. Eur. J. Cancer 2019, 110, 21–23. [Google Scholar] [CrossRef]
- Deftereos, S.N.; Georgonikou, D. Effectiveness of rituximab in treating immune-checkpoint-inhibitor-induced immune-related adverse events: Results of a systematic review. Ann. Oncol. 2021, 32, 282–283. [Google Scholar] [CrossRef]
- Sowerby, L.; Dewan, A.K.; Granter, S.; Gandhi, L.; LeBoeuf, N.R. Rituximab Treatment of Nivolumab-Induced Bullous Pemphigoid. JAMA Dermatol. 2017, 153, 603–605. [Google Scholar] [CrossRef]
- Lin, J.S.; Wang, D.Y.; Mamlouk, O.; Glass, W.F.; Abdelrahim, M.; Yee, C.; Abudayyeh, A. Immune checkpoint inhibitor associated reactivation of primary membranous nephropathy responsive to rituximab. J. Immunother. Cancer 2020, 8, e001287. [Google Scholar] [CrossRef] [PubMed]
- Vermeulen, L.; Depuydt, C.E.; Weckx, P.; Bechter, O.; Van Damme, P.; Thal, D.R.; Claeys, K.G. Myositis as a neuromuscular complication of immune checkpoint inhibitors. Acta Neurol. Belg. 2020, 120, 355–364. [Google Scholar] [CrossRef]
- Adam, B.A.; Murakami, N.; Reid, G.; Du, K.; Jasim, R.; Boils, C.L.; Bu, L.; Hill, P.D.; Murray, A.G.; Renaudin, K.; et al. Gene Expression Profiling in Kidney Transplants with Immune Checkpoint Inhibitor-Associated Adverse Events. Clin. J. Am. Soc. Nephrol. 2021, 16, 1376–1386. [Google Scholar] [CrossRef] [PubMed]
- Hagiwara, S.; Watanabe, T.; Kudo, M.; Minaga, K.; Komeda, Y.; Kamata, K.; Kimura, M.; Hayashi, H.; Nakagawa, K.; Ueshima, K.; et al. Clinicopathological analysis of hepatic immune-related adverse events in comparison with autoimmune hepatitis and graft-versus host disease. Sci. Rep. 2021, 11, 9242. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.H.; Zobniw, C.M.; Trinh, V.A.; Ma, J.; Bassett, R.L., Jr.; Abdel-Wahab, N.; Anderson, J.; Davis, J.E.; Joseph, J.; Uemura, M.; et al. Correction to: Infliximab associated with faster symptom resolution compared with corticosteroids alone for the management of immune-related enterocolitis. J. Immunother. Cancer 2019, 7, 107. [Google Scholar] [CrossRef] [PubMed]
- Abu-Sbeih, H.; Ali, F.S.; Alsaadi, D.; Jennings, J.; Luo, W.; Gong, Z.; Richards, D.M.; Charabaty, A.; Wang, Y. Outcomes of vedolizumab therapy in patients with immune checkpoint inhibitor-induced colitis: A multi-center study. J. Immunother. Cancer 2018, 6, 142. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.S.; Mamlouk, O.; Selamet, U.; Tchakarov, A.; Glass, W.F.; Sheth, R.A.; Layman, R.M.; Dadu, R.; Abdelwahab, N.; Abdelrahim, M.; et al. Infliximab for the treatment of patients with checkpoint inhibitor-associated acute tubular interstitial nephritis. Oncoimmunology 2021, 10, 1877415. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Hu, J.; Betof Warner, A.; Quach, H.T.; Cann, C.G.; Zhang, M.Z.; Si, L.; Tang, B.; Cui, C.; Yang, X.; et al. Early use of high-dose-glucocorticoid for the management of irAE is associated with poorer survival in patients with advanced melanoma treated with anti-PD-1 monotherapy. Clin. Cancer Res. 2021. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).