Neural Component of the Tumor Microenvironment in Pancreatic Ductal Adenocarcinoma
Abstract
Simple Summary
Abstract
1. Introduction
2. Pancreas—Basic Relationships
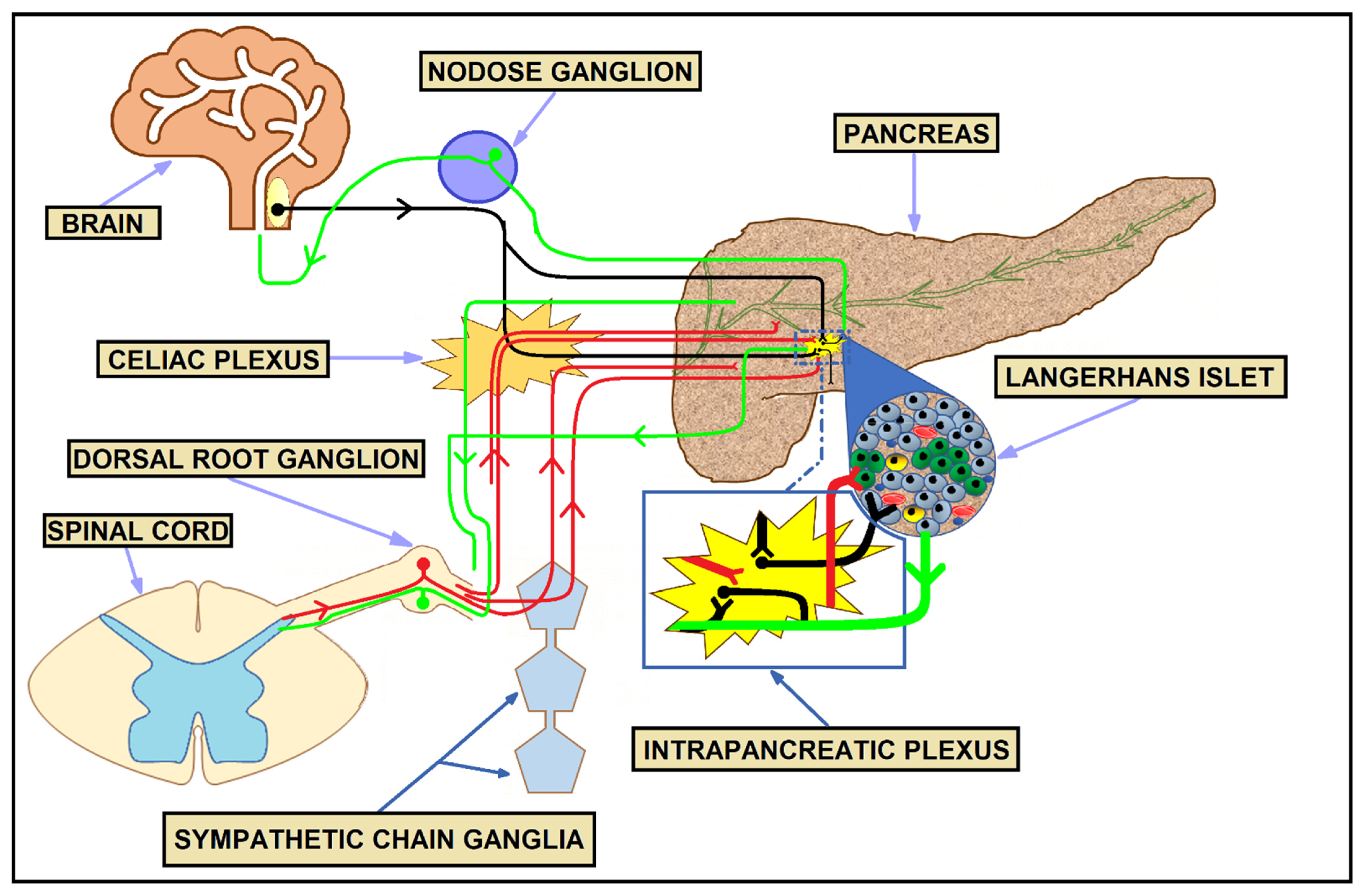
3. Physiologic Innervation of the Pancreas
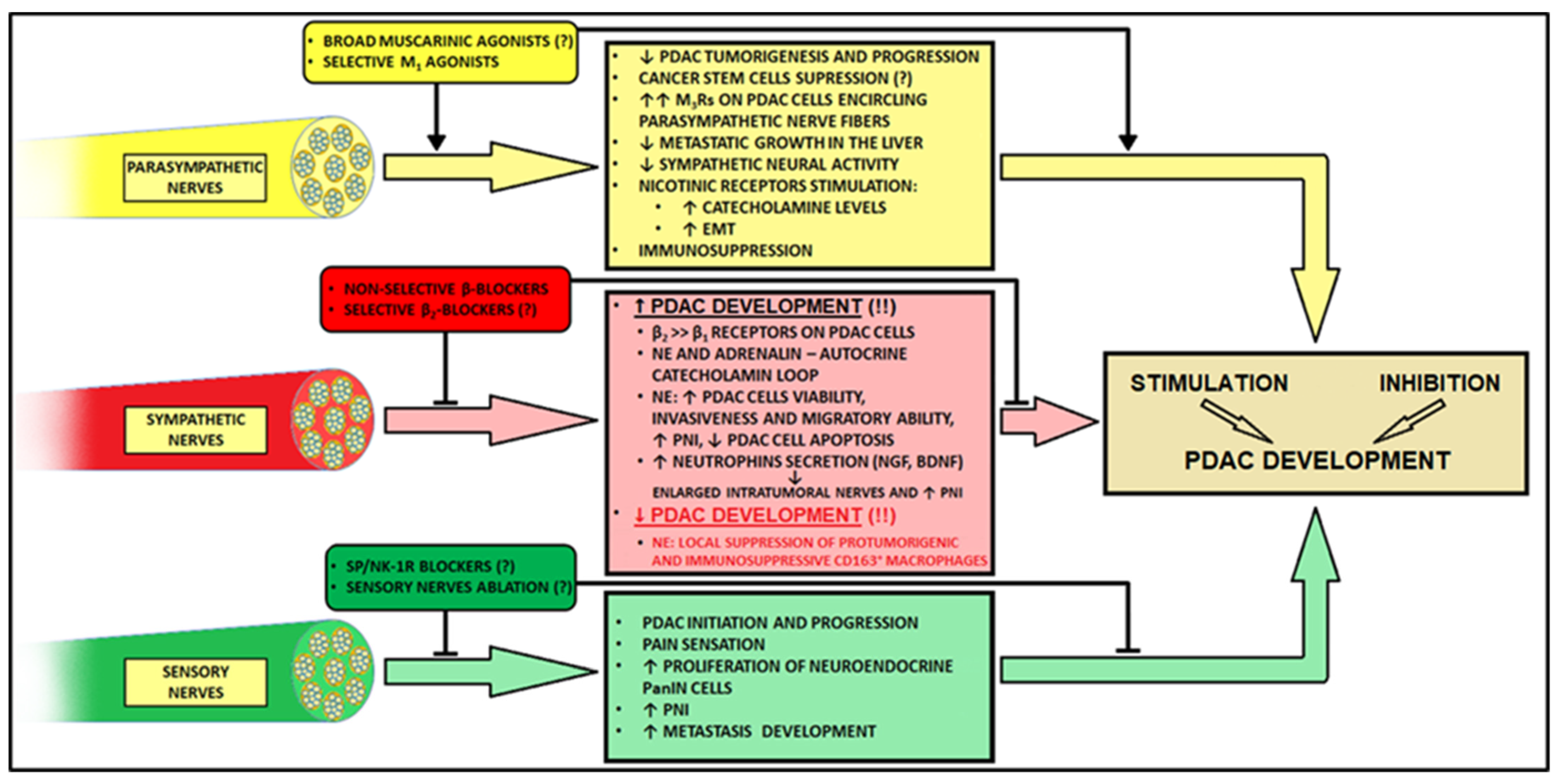
4. Innervation in PDAC
4.1. Parasympathetic Innervation
4.2. Sympathetic Innervation
4.3. Sensory Innervation
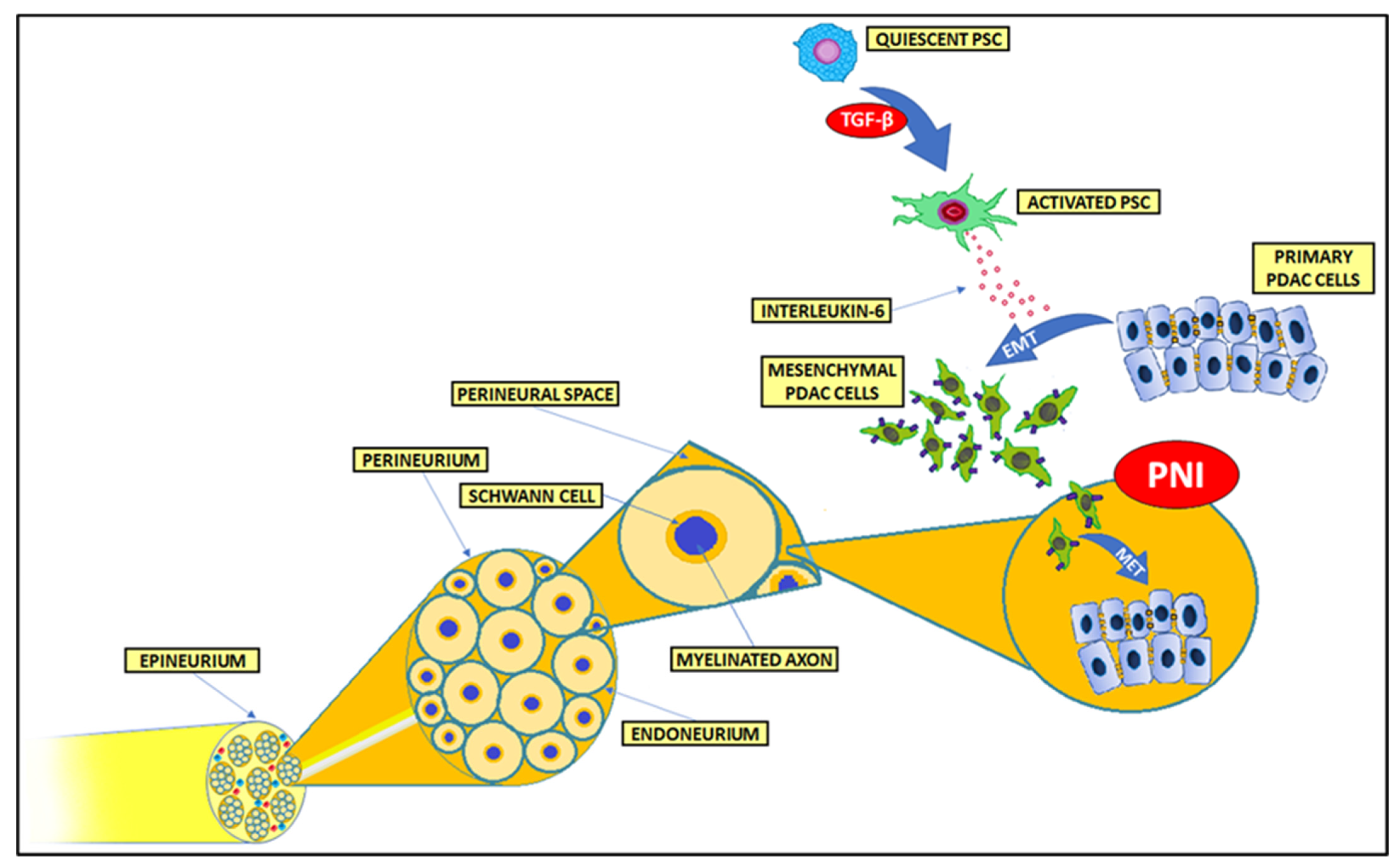
5. Perineural and Endoneural Invasion
6. Tumor–Nerves Bidirectional Interactions—Axonogenesis, Neurogenesis, and Nerve Reprogramming
7. Nerve Number and Neural Density in PDAC
8. Conclusions and Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Fouad, Y.A.; Aanei, C. Revisiting the hallmarks of cancer. Am. J. Cancer Res. 2017, 7, 1016–1036. [Google Scholar]
- Whiteside, T.L. The tumor microenvironment and its role in promoting tumor growth. Oncogene 2008, 27, 5904–5912. [Google Scholar] [CrossRef]
- Shurin, M.R.; Shurin, G.V.; Zlotnikov, S.B.; Bunimovich, Y.L. The Neuroimmune Axis in the Tumor Microenvironment. J. Immunol. 2020, 204, 280–285. [Google Scholar] [CrossRef]
- Arneth, B. Tumor Microenvironment. Medicina 2019, 56, 15. [Google Scholar] [CrossRef]
- Omary, M.B.; Lugea, A.; Lowe, A.W.; Pandol, S.J. The pancreatic stellate cell: A star on the rise in pancreatic diseases. J. Clin. Investig. 2007, 117, 50–59. [Google Scholar] [CrossRef]
- Schnittert, J.; Bansal, R.; Prakash, J. Targeting Pancreatic Stellate Cells in Cancer. Trends Cancer 2019, 5, 128–142. [Google Scholar] [CrossRef]
- Wang, S.; Li, Y.; Xing, C.; Ding, C.; Zhang, H.; Chen, L.; You, L.; Dai, M.; Zhao, Y. Tumor microenvironment in chemoresistance, metastasis and immunotherapy of pancreatic cancer. Am. J. Cancer Res. 2020, 10, 1937–1953. [Google Scholar]
- Du, J.; Gu, J.; Li, J. Mechanisms of drug resistance of pancreatic ductal adenocarcinoma at different levels. Biosci. Rep. 2020, 40, BSR20200401. [Google Scholar] [CrossRef]
- Apte, M.V.; Xu, Z.; Pothula, S.; Goldstein, D.; Pirola, R.C.; Wilson, J.S. Pancreatic cancer: The microenvironment needs attention too! Pancreatology 2015, 15, S32–S38. [Google Scholar] [CrossRef]
- Erkan, M.; Hausmann, S.; Michalski, C.W.; Fingerle, A.A.; Dobritz, M.; Kleeff, J.; Friess, H. The role of stroma in pancreatic cancer: Diagnostic and therapeutic implications. Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 454–467. [Google Scholar] [CrossRef]
- Erkan, M.; Kurtoglu, M.; Kleeff, J. The role of hypoxia in pancreatic cancer: A potential therapeutic target? Expert Rev. Gastroenterol. Hepatol. 2016, 10, 301–316. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef]
- Rahib, L.; Smith, B.D.; Aizenberg, R.; Rosenzweig, A.B.; Fleshman, J.M.; Matrisian, L.M. Projecting cancer incidence and deaths to 2030: The unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014, 74, 2913–2921, Erratum in: Cancer Res. 2014, 74, 4006. [Google Scholar] [CrossRef]
- Vincent, A.; Herman, J.; Schulick, R.; Hruban, R.H.; Goggins, M. Pancreatic cancer. Lancet 2011, 378, 607–620. [Google Scholar] [CrossRef]
- Bednar, F.; Pasca di Magliano, M. Chemotherapy and Tumor Evolution Shape Pancreatic Cancer Recurrence after Resection. Cancer Discov. 2020, 10, 762–764. [Google Scholar] [CrossRef]
- Loveday, B.P.T.; Lipton, L.; Thomson, B.N. Pancreatic cancer: An update on diagnosis and management. Aust. J. Gen. Pract. 2019, 48, 826–831. [Google Scholar] [CrossRef]
- Faulkner, S.; Jobling, P.; March, B.; Jiang, C.C.; Hondermarck, H. Tumor Neurobiology and the War of Nerves in Cancer. Cancer Discov. 2019, 9, 702–710. [Google Scholar] [CrossRef]
- Cole, S.W.; Nagaraja, A.S.; Lutgendorf, S.K.; Green, P.A.; Sood, A.K. Sympathetic nervous system regulation of the tumour microenvironment. Nat. Rev. Cancer 2015, 15, 563–572. [Google Scholar] [CrossRef]
- Tibensky, M.; Mravec, B. Role of the parasympathetic nervous system in cancer initiation and progression. Clin. Transl. Oncol. 2021, 23, 669–681. [Google Scholar] [CrossRef]
- Todd, T. On the process of reproduction of the members of the aquatic salamander. Q. J. Sci. Lit. Arts 1823, 16, 84–96. [Google Scholar]
- Zhang, X.; Zhang, Y.; He, Z.; Yin, K.; Li, B.; Zhang, L.; Xu, Z. Chronic stress promotes gastric cancer progression and metastasis: An essential role for ADRB2. Cell Death Dis. 2019, 10, 788. [Google Scholar] [CrossRef]
- Zhao, C.M.; Hayakawa, Y.; Kodama, Y.; Muthupalani, S.; Westphalen, C.B.; Andersen, G.T.; Flatberg, A.; Johannessen, H.; Friedman, R.A.; Renz, B.W. Denervation suppresses gastric tumorigenesis. Sci. Transl. Med. 2014, 6, 250ra115. [Google Scholar] [CrossRef]
- Wang, L.; Zhi, X.; Zhang, Q.; Wei, S.; Li, Z.; Zhou, J.; Jiang, J.; Zhu, Y.; Yang, L.; Xu, H.; et al. Muscarinic receptor M3 mediates cell proliferation induced by acetylcholine and contributes to apoptosis in gastric cancer. Tumour Biol. 2016, 37, 2105–2117. [Google Scholar] [CrossRef]
- Magnon, C.; Hall, S.J.; Lin, J.; Xue, X.; Gerber, L.; Freedland, S.J.; Frenette, P.S. Autonomic nerve development contributes to prostate cancer progression. Science 2013, 341, 1236361. [Google Scholar] [CrossRef]
- Renz, B.W.; Tanaka, T.; Sunagawa, M.; Takahashi, R.; Jiang, Z.; Macchini, M.; Dantes, Z.; Valenti, G.; White, R.A.; Middelhoff, M.A.; et al. Cholinergic Signaling via Muscarinic Receptors Directly and Indirectly Suppresses Pancreatic Tumorigenesis and Cancer Stemness. Cancer Discov. 2018, 8, 1458–1473. [Google Scholar] [CrossRef]
- Kamiya, A.; Hiyama, T.; Fujimura, A.; Yoshikawa, S. Sympathetic and parasympathetic innervation in cancer: Therapeutic implications. Clin. Auton. Res. 2021, 31, 165–178. [Google Scholar] [CrossRef]
- Mravec, B. Neurobiology of Cancer: Introduction of New Drugs in the Treatment and Prevention of Cancer. Int. J. Mol. Sci. 2021, 22, 6115. [Google Scholar] [CrossRef]
- Silverman, D.A.; Martinez, V.K.; Dougherty, P.M.; Myers, J.N.; Calin, G.A.; Amit, M. Cancer-Associated Neurogenesis and Nerve-Cancer Cross-talk. Cancer Res. 2021, 81, 1431–1440. [Google Scholar] [CrossRef]
- Madeo, M.; Colbert, P.L.; Vermeer, D.W.; Lucido, C.T.; Cain, J.T.; Vichaya, E.G.; Grossberg, A.J.; Muirhead, D.; Rickel, A.P.; Hong, Z.; et al. Cancer exosomes induce tumor innervation. Nat. Commun. 2018, 9, 4284. [Google Scholar] [CrossRef]
- Amit, M.; Takahashi, H.; Dragomir, M.P.; Lindemann, A.; Gleber-Netto, F.O.; Pickering, C.R.; Anfossi, S.; Osman, A.A.; Cai, Y.; Wang, R.; et al. Loss of p53 drives neuron reprogramming in head and neck cancer. Nature 2020, 578, 449–454. [Google Scholar] [CrossRef]
- Chen, S.H.; Zhang, B.Y.; Zhou, B.; Zhu, C.Z.; Sun, L.Q.; Feng, Y.J. Perineural invasion of cancer: A complex crosstalk between cells and molecules in the perineural niche. Am. J. Cancer Res. 2019, 9, 1–21. [Google Scholar]
- Lubig, S.; Thiesler, T.; Müller, S.; Vorreuther, R.; Leipner, N.; Kristiansen, G. Quantitative perineural invasion is a prognostic marker in prostate cancer. Pathology 2018, 50, 298–304. [Google Scholar] [CrossRef]
- Zhang, L.J.; Wu, B.; Zha, Z.L.; Qu, W.; Zhao, H.; Yuan, J.; Feng, Y.J. Perineural invasion as an independent predictor of biochemical recurrence in prostate cancer following radical prostatectomy or radiotherapy: A systematic review and meta-analysis. BMC Urol. 2018, 18, 5. [Google Scholar] [CrossRef]
- Shiratori, K.; Shimizu, K. Insulo–Acinar Relationship. In The Pancreas: An Integrated Textbook of Basic Science, Medicine, and Surgery, 3rd ed.; Beger, H.G., Warshaw, A.L., Hruban, R.H., Büchler, M.W., Lerch, M.M., Neoptolemos, J.P., Shimosegawa, T., Whitcomb, D.C., Groß, C., Eds.; Wiley-Blackwell: Hoboken, NJ, USA, 2018; pp. 123–131. ISBN 9781119188391. [Google Scholar]
- Barreto, S.G.; Carati, C.J.; Toouli, J.; Saccone, G.T. The islet-acinar axis of the pancreas: More than just insulin. Am. J. Physiol. Gastrointest. Liver Physiol. 2010, 299, G10–G22. [Google Scholar] [CrossRef]
- Hellman, B.; Wallgren, A.; Petersson, B. Cytological characteristics of the exocrine pancreatic cells with regard to their position in relation to the islets of Langerhans. A study in normal and obese-hyperglycaemic mice. Acta Endocrinol. (Copenh.) 1962, 39, 465–473. [Google Scholar] [CrossRef]
- Malaisse-Lagae, F.; Ravazzola, M.; Robberecht, P.; Vandermeers, A.; Malaisse, W.J.; Orci, L. Exocrine pancreas: Evidence for topographic partition of secretory function. Science 1975, 190, 795–797. [Google Scholar] [CrossRef]
- Nakagawa, A.; Stagner, J.I.; Samols, E. Suppressive role of the islet-acinar axis in the perfused rat pancreas. Gastroenterology 1993, 105, 868–875. [Google Scholar] [CrossRef]
- Adrian, T.E.; Besterman, H.S.; Mallinson, C.N.; Greenberg, G.R.; Bloom, S.R. Inhibition of secretin stimulated pancreatic secretion by pancreatic polypeptide. Gut 1979, 20, 37–40. [Google Scholar] [CrossRef]
- Bachem, M.G.; Schneider, E.; Gross, H.; Weidenbach, H.; Schmid, R.M.; Menke, A.; Siech, M.; Beger, H.; Grünert, A.; Adler, G. Identification, culture, and characterization of pancreatic stellate cells in rats and humans. Gastroenterology 1998, 115, 421–432. [Google Scholar] [CrossRef]
- Phillips, P.A.; McCarroll, J.A.; Park, S.; Wu, M.J.; Pirola, R.; Korsten, M.; Wilson, J.S.; Apte, M.V. Rat pancreatic stellate cells secrete matrix metalloproteinases: Implications for extracellular matrix turnover. Gut 2003, 52, 275–282. [Google Scholar] [CrossRef]
- Nagathihalli, N.S.; Castellanos, J.A.; VanSaun, M.N.; Dai, X.; Ambrose, M.; Guo, Q.; Xiong, Y.; Merchant, N.B. Pancreatic stellate cell secreted IL-6 stimulates STAT3 dependent invasiveness of pancreatic intraepithelial neoplasia and cancer cells. Oncotarget 2016, 7, 65982–65992. [Google Scholar] [CrossRef]
- Wu, Y.S.; Chung, I.; Wong, W.F.; Masamune, A.; Sim, M.S.; Looi, C.Y. Paracrine IL-6 signaling mediates the effects of pancreatic stellate cells on epithelial-mesenchymal transition via Stat3/Nrf2 pathway in pancreatic cancer cells. Biochim. Biophys. Acta Gen. Subj. 2017, 1861, 296–306. [Google Scholar] [CrossRef]
- Xu, Z.; Vonlaufen, A.; Phillips, P.A.; Fiala-Beer, E.; Zhang, X.; Yang, L.; Biankin, A.V.; Goldstein, D.; Pirola, R.C.; Wilson, J.S.; et al. Role of pancreatic stellate cells in pancreatic cancer metastasis. Am. J. Pathol. 2010, 177, 2585–2596. [Google Scholar] [CrossRef]
- Vonlaufen, A.; Joshi, S.; Qu, C.; Phillips, P.A.; Xu, Z.; Parker, N.R.; Toi, C.S.; Pirola, R.C.; Wilson, J.S.; Goldstein, D.; et al. Pancreatic stellate cells: Partners in crime with pancreatic cancer cells. Cancer Res. 2008, 68, 2085–2093. [Google Scholar] [CrossRef]
- Han, L.; Ma, J.; Duan, W.; Zhang, L.; Yu, S.; Xu, Q.; Lei, J.; Li, X.; Wang, Z.; Wu, Z.; et al. Pancreatic stellate cells contribute pancreatic cancer pain via activation of sHH signaling pathway. Oncotarget 2016, 7, 18146–18158. [Google Scholar] [CrossRef][Green Version]
- Li, X.; Wang, Z.; Ma, Q.; Xu, Q.; Liu, H.; Duan, W.; Lei, J.; Ma, J.; Wang, X.; Lv, S.; et al. Sonic hedgehog paracrine signaling activates stromal cells to promote perineural invasion in pancreatic cancer. Clin. Cancer Res. 2014, 20, 4326–4338. [Google Scholar] [CrossRef]
- Nan, L.; Qin, T.; Xiao, Y.; Qian, W.; Li, J.; Wang, Z.; Ma, J.; Ma, Q.; Wu, Z. Pancreatic Stellate Cells Facilitate Perineural Invasion of Pancreatic Cancer via HGF/c-Met Pathway. Cell Transplant. 2019, 28, 1289–1298. [Google Scholar] [CrossRef]
- Qin, T.; Xiao, Y.; Qian, W.; Wang, X.; Gong, M.; Wang, Q.; An, R.; Han, L.; Duan, W.; Ma, Q.; et al. HGF/c-Met pathway facilitates the perineural invasion of pancreatic cancer by activating the mTOR/NGF axis. Cell Death Dis. 2022, 13, 387. [Google Scholar] [CrossRef]
- Nicolescu, M.I.; Popescu, L.M. Telocytes in the interstitium of human exocrine pancreas: Ultrastructural evidence. Pancreas 2012, 41, 949–956. [Google Scholar] [CrossRef]
- Xu, Y.; Tian, H.; Luan, C.G.; Sun, K.; Bao, P.J.; Zhang, H.Y.; Zhang, N. Telocytes promote hepatocellular carcinoma by activating the ERK signaling pathway and miR-942-3p/MMP9 axis. Cell Death Discov. 2021, 7, 209. [Google Scholar] [CrossRef]
- Suzuki, A.; Naruse, S.; Kitagawa, M.; Ishiguro, H.; Yoshikawa, T.; Ko, S.B.; Yamamoto, A.; Hamada, H.; Hayakawa, T. 5-hydroxytryptamine strongly inhibits fluid secretion in guinea pig pancreatic duct cells. J. Clin. Investig. 2001, 108, 749–756. [Google Scholar] [CrossRef]
- Kalluri, R. The biology and function of exosomes in cancer. J. Clin. Investig. 2016, 126, 1208–1215. [Google Scholar] [CrossRef]
- Bettegowda, C.; Sausen, M.; Leary, R.J.; Kinde, I.; Wang, Y.; Agrawal, N.; Bartlett, B.R.; Wang, H.; Luber, B.; Alani, R.M.; et al. Detection of circulating tumor DNA in early- and late-stage human malignancies. Sci. Transl. Med. 2014, 6, 224ra24. [Google Scholar] [CrossRef]
- Lucido, C.T.; Wynja, E.; Madeo, M.; Williamson, C.S.; Schwartz, L.E.; Imblum, B.A.; Drapkin, R.; Vermeer, P.D. Innervation of cervical carcinoma is mediated by cancer-derived exosomes. Gynecol. Oncol. 2019, 154, 228–235. [Google Scholar] [CrossRef]
- Liu, M.T.; Kirchgessner, A.L. Guinea pig pancreatic neurons: Morphology, neurochemistry, electrical properties, and response to 5-HT. Am. J. Physiol. 1997, 273, G1273–G1289. [Google Scholar] [CrossRef]
- Sha, L.; Ou, L.L.; Miller, S.M.; Ma, R.; Szurszewski, J.H. Cat pancreatic neurons: Morphology, electrophysiological properties, and responses to 5-HT. Pancreas 1996, 13, 111–124. [Google Scholar] [CrossRef]
- Yi, E.; Love, J.A. Alpha-adrenergic modulation of synaptic transmission in rabbit pancreatic ganglia. Auton. Neurosci. 2005, 122, 45–57. [Google Scholar] [CrossRef]
- Berthoud, H.R.; Fox, E.A.; Powley, T.L. Localization of vagal preganglionics that stimulate insulin and glucagon secretion. Am. J. Physiol. 1990, 258, R160–R168. [Google Scholar] [CrossRef]
- Yi, E.; Smith, T.G.; Baker, R.C.; Love, J.A. Catecholamines and 5-hydroxytryptamine in tissues of the rabbit exocrine pancreas. Pancreas 2004, 29, 218–224. [Google Scholar] [CrossRef]
- Molina, J.; Rodriguez-Diaz, R.; Fachado, A.; Jacques-Silva, M.C.; Berggren, P.O.; Caicedo, A. Control of insulin secretion by cholinergic signaling in the human pancreatic islet. Diabetes 2014, 63, 2714–2726. [Google Scholar] [CrossRef]
- Niebergall-Roth, E.; Singer, M.V. Control of pancreatic exocrine secretion via muscarinic receptors: Which subtype(s) are involved? A review. Pancreatology 2003, 3, 284–292. [Google Scholar] [CrossRef]
- Singer, M.V.; Niebergall-Roth, E. Secretion from acinar cells of the exocrine pancreas: Role of enteropancreatic reflexes and cholecystokinin. Cell Biol. Int. 2009, 33, 1–9. [Google Scholar] [CrossRef]
- Rodriguez-Diaz, R.; Dando, R.; Jacques-Silva, M.C.; Fachado, A.; Molina, J.; Abdulreda, M.H.; Ricordi, C.; Roper, S.D.; Berggren, P.O.; Caicedo, A. Alpha cells secrete acetylcholine as a non-neuronal paracrine signal priming beta cell function in humans. Nat. Med. 2011, 17, 888–892. [Google Scholar] [CrossRef]
- Love, J.A.; Yi, E.; Smith, T.G. Autonomic pathways regulating pancreatic exocrine secretion. Auton. Neurosci. 2007, 133, 19–34. [Google Scholar] [CrossRef]
- Schmidt, W.E.; Seebeck, J.; Höcker, M.; Schwarzhoff, R.; Schäfer, H.; Fornefeld, H.; Morys-Wortmann, C.; Fölsch, U.R.; Creutzfeldt, W. PACAP and VIP stimulate enzyme secretion in rat pancreatic acini via interaction with VIP/PACAP-2 receptors: Additive augmentation of CCK/carbachol-induced enzyme release. Pancreas 1993, 8, 476–487. [Google Scholar] [CrossRef]
- Ahrén, B.; Taborsky, G.J., Jr. The mechanism of vagal nerve stimulation of glucagon and insulin secretion in the dog. Endocrinology 1986, 118, 1551–1557. [Google Scholar] [CrossRef]
- Brunicardi, F.C.; Shavelle, D.M.; Andersen, D.K. Neural regulation of the endocrine pancreas. Int. J. Pancreatol. 1995, 18, 177–195. [Google Scholar] [CrossRef]
- Babic, T.; Travagli, R.A. Neural Control of the Pancreas. In The Pancreas: Biology and Physiology; Gorelick, F.S., Williams, J.A., Eds.; Michigan Publishing Services: Ann Arbor, MI, USA, 2021; pp. 259–269. ISBN 978-1-60785-716-7. [Google Scholar]
- Bishop, A.E.; Polak, J.M.; Green, I.C.; Bryant, M.G.; Bloom, S.R. The location of VIP in the pancreas of man and rat. Diabetologia 1980, 18, 73–78. [Google Scholar] [CrossRef]
- Ahrén, B.; Ar’Rajab, A.; Böttcher, G.; Sundler, F.; Dunning, B.E. Presence of galanin in human pancreatic nerves and inhibition of insulin secretion from isolated human islets. Cell Tissue Res. 1991, 264, 263–267. [Google Scholar] [CrossRef]
- Furlan, A.; La Manno, G.; Lübke, M.; Häring, M.; Abdo, H.; Hochgerner, H.; Kupari, J.; Usoskin, D.; Airaksinen, M.S.; Oliver, G.; et al. Visceral motor neuron diversity delineates a cellular basis for nipple- and pilo-erection muscle control. Nat. Neurosci. 2016, 19, 1331–1340. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Diaz, R.; Caicedo, A. Neural control of the endocrine pancreas. Best Pract. Res. Clin. Endocrinol. Metab. 2014, 28, 745–756. [Google Scholar] [CrossRef] [PubMed]
- Gilliam, L.K.; Palmer, J.P.; Taborsky, G.J., Jr. Tyramine-mediated activation of sympathetic nerves inhibits insulin secretion in humans. J. Clin. Endocrinol. Metab. 2007, 92, 4035–4038. [Google Scholar] [CrossRef] [PubMed]
- Liddle, R.A. The role of Transient Receptor Potential Vanilloid 1 (TRPV1) channels in pancreatitis. Biochim. Biophys. Acta 2007, 1772, 869–878. [Google Scholar] [CrossRef]
- De Couc, M.; Maréchal, R.; Moorthamers, S.; Van Laethem, J.L.; Gidron, Y. Vagal nerve activity predicts overall survival in metastatic pancreatic cancer, mediated by inflammation. Cancer Epidemiol. 2016, 40, 47–51. [Google Scholar] [CrossRef]
- Yang, M.W.; Tao, L.Y.; Jiang, Y.S.; Yang, J.Y.; Huo, Y.M.; Liu, D.J.; Li, J.; Fu, X.L.; He, R.; Lin, C.; et al. Perineural Invasion Reprograms the Immune Microenvironment through Cholinergic Signaling in Pancreatic Ductal Adenocarcinoma. Cancer Res. 2020, 80, 1991–2003. [Google Scholar] [CrossRef]
- Al-Wadei, M.H.; Al-Wadei, H.A.; Schuller, H.M. Pancreatic cancer cells and normal pancreatic duct epithelial cells express an autocrine catecholamine loop that is activated by nicotinic acetylcholine receptors α3, α5, and α7. Mol. Cancer Res. 2012, 10, 239–249. [Google Scholar] [CrossRef]
- Ben, Q.; An, W.; Sun, Y.; Qian, A.; Liu, J.; Zou, D.; Yuan, Y. A nicotine-induced positive feedback loop between HIF1A and YAP1 contributes to epithelial-to-mesenchymal transition in pancreatic ductal adenocarcinoma. J. Exp. Clin. Cancer Res. 2020, 39, 181. [Google Scholar] [CrossRef]
- Mravec, B.; Ondicova, K.; Tillinger, A.; Pecenak, J. Subdiaphragmatic vagotomy enhances stress-induced epinephrine release in rats. Auton. Neurosci. 2015, 190, 20–25. [Google Scholar] [CrossRef]
- Khasar, S.G.; Green, P.G.; Miao, F.J.; Levine, J.D. Vagal modulation of nociception is mediated by adrenomedullary epinephrine in the rat. Eur. J. Neurosci. 2003, 17, 909–915. [Google Scholar] [CrossRef]
- Weddle, D.L.; Tithoff, P.; Williams, M.; Schuller, H.M. Beta-adrenergic growth regulation of human cancer cell lines derived from pancreatic ductal carcinomas. Carcinogenesis 2001, 22, 473–479. [Google Scholar] [CrossRef] [PubMed]
- Kim-Fuchs, C.; Le, C.P.; Pimentel, M.A.; Shackleford, D.; Ferrari, D.; Angst, E.; Hollande, F.; Sloan, E.K. Chronic stress accelerates pancreatic cancer growth and invasion: A critical role for beta-adrenergic signaling in the pancreatic microenvironment. Brain Behav. Immun. 2014, 40, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Gan, Y.; Wang, Q.; Meng, Z.; Li, G.; Shen, Y.; Wu, Y.; Li, P.; Yao, M.; Gu, J.; et al. Enriching the Housing Environment for Mice Enhances Their NK Cell Antitumor Immunity via Sympathetic Nerve-Dependent Regulation of NKG2D and CCR5. Cancer Res. 2017, 77, 1611–1622. [Google Scholar] [CrossRef] [PubMed]
- Guillot, J.; Dominici, C.; Lucchesi, A.; Nguyen, H.T.T.; Puget, A.; Hocine, M.; Rangel-Sosa, M.M.; Simic, M.; Nigri, J.; Guillaumond, F.; et al. Sympathetic axonal sprouting induces changes in macrophage populations and protects against pancreatic cancer. Nat. Commun. 2022, 13, 1985. [Google Scholar] [CrossRef]
- Mu, W.; Wang, Z.; Zöller, M. Ping-Pong-Tumor and Host in Pancreatic Cancer Progression. Front. Oncol. 2019, 9, 1359. [Google Scholar] [CrossRef]
- Quốc Lu’o’ng, K.V.; Nguyễn, L.T. The roles of beta-adrenergic receptors in tumorigenesis and the possible use of beta-adrenergic blockers for cancer treatment: Possible genetic and cell-signaling mechanisms. Cancer Manag. Res. 2012, 4, 431–445. [Google Scholar] [CrossRef]
- Schuller, H.M.; Al-Wadei, H.A.; Majidi, M. GABA B receptor is a novel drug target for pancreatic cancer. Cancer 2008, 112, 767–778. [Google Scholar] [CrossRef]
- Renz, B.W.; Takahashi, R.; Tanaka, T.; Macchini, M.; Hayakawa, Y.; Dantes, Z.; Maurer, H.C.; Chen, X.; Jiang, Z.; Westphalen, C.B.; et al. β2 Adrenergic-Neurotrophin Feedforward Loop Promotes Pancreatic Cancer. Cancer Cell 2018, 34, 863–867, Erratum for: Cancer Cell 2018, 33, 75–90.e7. [Google Scholar] [CrossRef]
- Huang, X.Y.; Wang, H.C.; Yuan, Z.; Huang, J.; Zheng, Q. Norepinephrine stimulates pancreatic cancer cell proliferation, migration and invasion via β-adrenergic receptor-dependent activation of P38/MAPK pathway. Hepatogastroenterology 2012, 59, 1889–1893. [Google Scholar] [CrossRef]
- Qian, W.; Lv, S.; Li, J.; Chen, K.; Jiang, Z.; Cheng, L.; Zhou, C.; Yan, B.; Cao, J.; Ma, Q.; et al. Norepinephrine enhances cell viability and invasion, and inhibits apoptosis of pancreatic cancer cells in a Notch-1-dependent manner. Oncol. Rep. 2018, 40, 3015–3023. [Google Scholar] [CrossRef]
- Zhang, D.; Ma, Q.; Wang, Z.; Zhang, M.; Guo, K.; Wang, F.; Wu, E. β2-adrenoceptor blockage induces G1/S phase arrest and apoptosis in pancreatic cancer cells via Ras/Akt/NFκB pathway. Mol. Cancer 2011, 10, 146. [Google Scholar] [CrossRef] [PubMed]
- Guo, K.; Ma, Q.; Li, J.; Wang, Z.; Shan, T.; Li, W.; Xu, Q.; Xie, K. Interaction of the sympathetic nerve with pancreatic cancer cells promotes perineural invasion through the activation of STAT3 signaling. Mol. Cancer Ther. 2013, 12, 264–273. [Google Scholar] [CrossRef]
- Zhang, D.; Ma, Q.Y.; Hu, H.T.; Zhang, M. β2-adrenergic antagonists suppress pancreatic cancer cell invasion by inhibiting CREB, NFκB and AP-1. Cancer Biol. Ther. 2010, 10, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Saad, A.; Goldstein, J.; Margalit, O.; Shacham-Shmueli, E.; Lawrence, Y.R.; Yang, Y.X.; Reiss, K.A.; Golan, T.; Mamtani, R.; Halpern, N.; et al. Assessing the effects of beta-blockers on pancreatic cancer risk: A nested case-control study. Pharmacoepidemiol. Drug Saf. 2020, 29, 599–604. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; White, D.L.; Hoogeveen, R.; Chen, L.; Whitsel, E.A.; Richardson, P.A.; Virani, S.S.; Garcia, J.M.; El-Serag, H.B.; Jiao, L. Anti-Hypertensive Medication Use, Soluble Receptor for Glycation End Products and Risk of Pancreatic Cancer in the Women’s Health Initiative Study. J. Clin. Med. 2018, 7, 197. [Google Scholar] [CrossRef]
- Udumyan, R.; Montgomery, S.; Fang, F.; Almroth, H.; Valdimarsdottir, U.; Ekbom, A.; Smedby, K.E.; Fall, K. Beta-Blocker Drug Use and Survival among Patients with Pancreatic Adenocarcinoma. Cancer Res. 2017, 77, 3700–3707. [Google Scholar] [CrossRef]
- Yang, A.; Zylberberg, H.M.; Rustgi, S.D.; Amin, S.P.; Bar-Mashiah, A.; Boffetta, P.; Lucas, A.L. Beta-blockers have no impact on survival in pancreatic ductal adenocarcinoma prior to cancer diagnosis. Sci. Rep. 2021, 11, 1038. [Google Scholar] [CrossRef]
- Saloman, J.L.; Albers, K.M.; Li, D.; Hartman, D.J.; Crawford, H.C.; Muha, E.A.; Rhim, A.D.; Davis, B.M. Ablation of sensory neurons in a genetic model of pancreatic ductal adenocarcinoma slows initiation and progression of cancer. Proc. Natl. Acad. Sci. USA 2016, 113, 3078–3083. [Google Scholar] [CrossRef]
- Li, X.; Ma, G.; Ma, Q.; Li, W.; Liu, J.; Han, L.; Duan, W.; Xu, Q.; Liu, H.; Wang, Z.; et al. Neurotransmitter substance P mediates pancreatic cancer perineural invasion via NK-1R in cancer cells. Mol. Cancer Res. 2013, 11, 294–302. [Google Scholar] [CrossRef]
- Han, L.; Jiang, J.; Xue, M.; Qin, T.; Xiao, Y.; Wu, E.; Shen, X.; Ma, Q.; Ma, J. Sonic hedgehog signaling pathway promotes pancreatic cancer pain via nerve growth factor. Reg. Anesth. Pain Med. 2020, 45, 137–144. [Google Scholar] [CrossRef]
- Stopczynski, R.E.; Normolle, D.P.; Hartman, D.J.; Ying, H.; DeBerry, J.J.; Bielefeldt, K.; Rhim, A.D.; DePinho, R.A.; Albers, K.M.; Davis, B.M. Neuroplastic changes occur early in the development of pancreatic ductal adenocarcinoma. Cancer Res. 2014, 74, 1718–1727. [Google Scholar] [CrossRef] [PubMed]
- Sinha, S.; Fu, Y.Y.; Grimont, A.; Ketcham, M.; Lafaro, K.; Saglimbeni, J.A.; Askan, G.; Bailey, J.M.; Melchor, J.P.; Zhong, Y.; et al. PanIN Neuroendocrine Cells Promote Tumorigenesis via Neuronal Cross-talk. Cancer Res. 2017, 77, 1868–1879. [Google Scholar] [CrossRef] [PubMed]
- Zareba, P.; Flavin, R.; Isikbay, M.; Rider, J.R.; Gerke, T.A.; Finn, S.; Pettersson, A.; Giunchi, F.; Unger, R.H.; Tinianow, A.M.; et al. Perineural Invasion and Risk of Lethal Prostate Cancer. Cancer Epidemiol. Biomark. Prev. 2017, 26, 719–726. [Google Scholar] [CrossRef] [PubMed]
- Bakst, R.L.; Glastonbury, C.M.; Parvathaneni, U.; Katabi, N.; Hu, K.S.; Yom, S.S. Perineural Invasion and Perineural Tumor Spread in Head and Neck Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2019, 103, 1109–1124. [Google Scholar] [CrossRef]
- Oven Ustaalioglu, B.B.; Bilici, A.; Seker, M.; Kefeli, U.; Aydin, D.; Celik, S.; Demir, T.; Erkol, B. Prognostic Factors for Operated Gallbladder Cancer. J. Gastrointest. Cancer 2019, 50, 451–457. [Google Scholar] [CrossRef]
- Murakami, Y.; Uemura, K.; Sudo, T.; Hashimoto, Y.; Kondo, N.; Nakagawa, N.; Muto, T.; Sasaki, H.; Urabe, K.; Sueda, T. Perineural invasion in extrahepatic cholangiocarcinoma: Prognostic impact and treatment strategies. J. Gastrointest. Surg. 2013, 17, 1429–1439. [Google Scholar] [CrossRef]
- Schorn, S.; Demir, I.E.; Haller, B.; Scheufele, F.; Reyes, C.M.; Tieftrunk, E.; Sargut, M.; Goess, R.; Friess, H.; Ceyhan, G.O. The influence of neural invasion on survival and tumor recurrence in pancreatic ductal adenocarcinoma—A systematic review and meta-analysis. Surg. Oncol. 2017, 26, 105–115. [Google Scholar] [CrossRef]
- Liebl, F.; Demir, I.E.; Mayer, K.; Schuster, T.; DʼHaese, J.G.; Becker, K.; Langer, R.; Bergmann, F.; Wang, K.; Rosenberg, R.; et al. The impact of neural invasion severity in gastrointestinal malignancies: A clinicopathological study. Ann. Surg. 2014, 260, 900–908. [Google Scholar] [CrossRef]
- Batsakis, J.G. Nerves and neurotropic carcinomas. Ann. Otol. Rhinol. Laryngol. 1985, 94, 426–427. [Google Scholar] [CrossRef]
- Drapiewski, J.F. Carcinoma of the pancreas: A study of neoplastic invasion of nerves and its possible clinical significance. Am. J. Clin. Pathol. 1944, 14, 549–556. [Google Scholar] [CrossRef]
- Bockman, D.E.; Büchler, M.; Beger, H.G. Interaction of pancreatic ductal carcinoma with nerves leads to nerve damage. Gastroenterology 1994, 107, 219–230. [Google Scholar] [CrossRef]
- Fagan, J.J.; Collins, B.; Barnes, L.; D’Amico, F.; Myers, E.N.; Johnson, J.T. Perineural invasion in squamous cell carcinoma of the head and neck. Arch. Otolaryngol. Head Neck Surg. 1998, 124, 637–640. [Google Scholar] [CrossRef] [PubMed]
- Liebig, C.; Ayala, G.; Wilks, J.A.; Berger, D.H.; Albo, D. Perineural invasion in cancer: A review of the literature. Cancer 2009, 115, 3379–3391. [Google Scholar] [CrossRef] [PubMed]
- Dwivedi, S.; Krishnan, A. Neural invasion: A scenic trail for the nervous tumor and hidden therapeutic opportunity. Am. J. Cancer Res. 2020, 10, 2258–2270. [Google Scholar]
- Demir, I.E.; Ceyhan, G.O.; Liebl, F.; D’Haese, J.G.; Maak, M.; Friess, H. Neural invasion in pancreatic cancer: The past, present and future. Cancers 2010, 2, 1513–1527. [Google Scholar] [CrossRef] [PubMed]
- Ceyhan, G.O.; Bergmann, F.; Kadihasanoglu, M.; Altintas, B.; Demir, I.E.; Hinz, U.; Müller, M.W.; Giese, T.; Büchler, M.W.; Giese, N.A.; et al. Pancreatic neuropathy and neuropathic pain--a comprehensive pathomorphological study of 546 cases. Gastroenterology 2009, 136, 177–186. [Google Scholar] [CrossRef]
- Amit, M.; Binenbaum, Y.; Trejo-Leider, L.; Sharma, K.; Ramer, N.; Ramer, I.; Agbetoba, A.; Miles, B.; Yang, X.; Lei, D.; et al. International collaborative validation of intraneural invasion as a prognostic marker in adenoid cystic carcinoma of the head and neck. Head Neck 2015, 37, 1038–1045. [Google Scholar] [CrossRef]
- Sroka, I.C.; Anderson, T.A.; McDaniel, K.M.; Nagle, R.B.; Gretzer, M.B.; Cress, A.E. The laminin binding integrin alpha6beta1 in prostate cancer perineural invasion. J. Cell. Physiol. 2010, 224, 283–288. [Google Scholar] [CrossRef]
- Mirkin, K.A.; Hollenbeak, C.S.; Mohamed, A.; Jia, Y.; El-Deiry, W.S.; Messaris, E. Impact of perineural invasion on survival in node negative colon cancer. Cancer Biol. Ther. 2017, 18, 740–745. [Google Scholar] [CrossRef]
- Huang, Y.; He, L.; Dong, D.; Yang, C.; Liang, C.; Chen, X.; Ma, Z.; Huang, X.; Yao, S.; Liang, C.; et al. Individualized prediction of perineural invasion in colorectal cancer: Development and validation of a radiomics prediction model. Chin. J. Cancer Res. 2018, 30, 40–50. [Google Scholar] [CrossRef]
- Kinugasa, T.; Mizobe, T.; Shiraiwa, S.; Akagi, Y.; Shirouzu, K. Perineural Invasion Is a Prognostic Factor and Treatment Indicator in Patients with Rectal Cancer Undergoing Curative Surgery: 2000–2011 Data from a Single-center Study. Anticancer Res. 2017, 37, 3961–3968. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Deng, J.; You, Q.; Gao, Y.; Yu, Q.; Zhao, P.; Zheng, Y.; Fang, W.; Xu, N.; Teng, L. Prognostic value of perineural invasion in gastric cancer: A systematic review and meta-analysis. PLoS ONE 2014, 9, e88907. [Google Scholar] [CrossRef] [PubMed]
- Sejda, A.; Sigorski, D.; Gulczyński, J.; Wesołowski, W.; Kitlińska, J.; Iżycka-Świeszewska, E. Complexity of Neural Component of Tumor Microenvironment in Prostate Cancer. Pathobiology 2020, 87, 87–99. [Google Scholar] [CrossRef] [PubMed]
- Lee, L.Y.; De Paz, D.; Lin, C.Y.; Fan, K.H.; Wang, H.M.; Hsieh, C.H.; Lee, L.A.; Yen, T.C.; Liao, C.T.; Yeh, C.H.; et al. Prognostic impact of extratumoral perineural invasion in patients with oral cavity squamous cell carcinoma. Cancer Med. 2019, 8, 6185–6194. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, D.; Katz, M.H.; Rashid, A.; Wang, H.; Iuga, A.C.; Varadhachary, G.R.; Wolff, R.A.; Lee, J.E.; Pisters, P.W.; Crane, C.H.; et al. Perineural and intraneural invasion in posttherapy pancreaticoduodenectomy specimens predicts poor prognosis in patients with pancreatic ductal adenocarcinoma. Am. J. Surg. Pathol. 2012, 36, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Shimada, K.; Nara, S.; Esaki, M.; Sakamoto, Y.; Kosuge, T.; Hiraoka, N. Intrapancreatic nerve invasion as a predictor for recurrence after pancreaticoduodenectomy in patients with invasive ductal carcinoma of the pancreas. Pancreas 2011, 40, 464–468. [Google Scholar] [CrossRef]
- Barbier, L.; Turrini, O.; Grégoire, E.; Viret, F.; Le Treut, Y.P.; Delpero, J.R. Pancreatic head resectable adenocarcinoma: Preoperative chemoradiation improves local control but does not affect survival. HPB (Oxf.) 2011, 13, 64–69, Erratum in HPB (Oxf.) 2011, 13, 899. [Google Scholar] [CrossRef]
- Tanaka, M.; Mihaljevic, A.L.; Probst, P.; Heckler, M.; Klaiber, U.; Heger, U.; Büchler, M.W.; Hackert, T. Meta-analysis of recurrence pattern after resection for pancreatic cancer. Br. J. Surg. 2019, 106, 1590–1601. [Google Scholar] [CrossRef]
- Ceyhan, G.O.; Demir, I.E.; Rauch, U.; Bergmann, F.; Müller, M.W.; Büchler, M.W.; Friess, H.; Schäfer, K.H. Pancreatic neuropathy results in “neural remodeling” and altered pancreatic innervation in chronic pancreatitis and pancreatic cancer. Am. J. Gastroenterol. 2009, 104, 2555–2565. [Google Scholar] [CrossRef]
- Wang, J.; Chen, Y.; Li, X.; Zou, X. Perineural Invasion and Associated Pain Transmission in Pancreatic Cancer. Cancers 2021, 13, 4594. [Google Scholar] [CrossRef]
- Hirth, M.; Gandla, J.; Höper, C.; Gaida, M.M.; Agarwal, N.; Simonetti, M.; Demir, A.; Xie, Y.; Weiss, C.; Michalski, C.W.; et al. CXCL10 and CCL21 Promote Migration of Pancreatic Cancer Cells Toward Sensory Neurons and Neural Remodeling in Tumors in Mice, Associated With Pain in Patients. Gastroenterology 2020, 159, 665–681. [Google Scholar] [CrossRef] [PubMed]
- Jurcak, N.R.; Rucki, A.A.; Muth, S.; Thompson, E.; Sharma, R.; Ding, D.; Zhu, Q.; Eshleman, J.R.; Anders, R.A.; Jaffee, E.M.; et al. Axon Guidance Molecules Promote Perineural Invasion and Metastasis of Orthotopic Pancreatic Tumors in Mice. Gastroenterology 2019, 157, 838–850. [Google Scholar] [CrossRef] [PubMed]
- Ding, C.; Li, Y.; Xing, C.; Zhang, H.; Wang, S.; Dai, M. Research Progress on Slit/Robo Pathway in Pancreatic Cancer: Emerging and Promising. J. Oncol. 2020, 2020, 2845906. [Google Scholar] [CrossRef]
- Fujioka, S.; Sclabas, G.M.; Schmidt, C.; Niu, J.; Frederick, W.A.; Dong, Q.G.; Abbruzzese, J.L.; Evans, D.B.; Baker, C.; Chiao, P.J. Inhibition of constitutive NF-kappa B activity by I kappa B alpha M suppresses tumorigenesis. Oncogene 2003, 22, 1365–1370. [Google Scholar] [CrossRef]
- Wang, W.; Abbruzzese, J.L.; Evans, D.B.; Larry, L.; Cleary, K.R.; Chiao, P.J. The nuclear factor-kappa B RelA transcription factor is constitutively activated in human pancreatic adenocarcinoma cells. Clin. Cancer Res. 1999, 5, 119–127. [Google Scholar] [PubMed]
- Nomura, A.; Majumder, K.; Giri, B.; Dauer, P.; Dudeja, V.; Roy, S.; Banerjee, S.; Saluja, A.K. Inhibition of NF-kappa B pathway leads to deregulation of epithelial-mesenchymal transition and neural invasion in pancreatic cancer. Lab. Investig. 2016, 96, 1268–1278. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.; Mitsunaga, S.; Ikeda, M.; Aoyama, T.; Yoshizawa, K.; Yamaguchi, M.; Suzuki, M.; Narita, M.; Kawasaki, T.; Ochiai, A. Interleukin 6/gp130 axis promotes neural invasion in pancreatic cancer. Cancer Med. 2022, 16. [Google Scholar] [CrossRef]
- Bai, J.; Zhang, X.; Shi, D.; Xiang, Z.; Wang, S.; Yang, C.; Liu, Q.; Huang, S.; Fang, Y.; Zhang, W.; et al. Exosomal miR-128-3p Promotes Epithelial-to-Mesenchymal Transition in Colorectal Cancer Cells by Targeting FOXO4 via TGF-β/SMAD and JAK/STAT3 Signaling. Front. Cell Dev. Biol. 2021, 9, 568738. [Google Scholar] [CrossRef]
- Lu, R.; Fan, C.; Shangguan, W.; Liu, Y.; Li, Y.; Shang, Y.; Yin, D.; Zhang, S.; Huang, Q.; Li, X.; et al. Neurons generated from carcinoma stem cells support cancer progression. Signal Transduct. Target. Ther. 2017, 2, 16036. [Google Scholar] [CrossRef]
- Mauffrey, P.; Tchitchek, N.; Barroca, V.; Bemelmans, A.P.; Firlej, V.; Allory, Y.; Roméo, P.H.; Magnon, C. Progenitors from the central nervous system drive neurogenesis in cancer. Nature 2019, 569, 672–678, Erratum in: Nature 2020, 577, E10. [Google Scholar]
- Findlay, Q.; Yap, K.K.; Bergner, A.J.; Young, H.M.; Stamp, L.A. Enteric neural progenitors are more efficient than brain-derived progenitors at generating neurons in the colon. Am. J. Physiol. Gastrointest. Liver Physiol. 2014, 307, G741–G748. [Google Scholar] [CrossRef] [PubMed]
- Scuteri, A.; Miloso, M.; Foudah, D.; Orciani, M.; Cavaletti, G.; Tredici, G. Mesenchymal stem cells neuronal differentiation ability: A real perspective for nervous system repair? Curr. Stem Cell Res. Ther. 2011, 6, 82–92. [Google Scholar] [CrossRef] [PubMed]
- Karakaş, N.; Bay, S.; Türkel, N.; Öztunç, N.; Öncül, M.; Bilgen, H.; Shah, K.; Şahin, F.; Öztürk, G. Neurons from human mesenchymal stem cells display both spontaneous and stimuli responsive activity. PLoS ONE 2020, 15, e0228510. [Google Scholar] [CrossRef]
- Ayala, G.E.; Dai, H.; Powell, M.; Li, R.; Ding, Y.; Wheeler, T.M.; Shine, D.; Kadmon, D.; Thompson, T.; Miles, B.J.; et al. Cancer-related axonogenesis and neurogenesis in prostate cancer. Clin. Cancer Res. 2008, 14, 7593–7603. [Google Scholar] [CrossRef] [PubMed]
- Olar, A.; He, D.; Florentin, D.; Ding, Y.; Ayala, G. Biologic correlates and significance of axonogenesis in prostate cancer. Hum. Pathol. 2014, 45, 1358–1364. [Google Scholar] [CrossRef]
- Albo, D.; Akay, C.L.; Marshall, C.L.; Wilks, J.A.; Verstovsek, G.; Liu, H.; Agarwal, N.; Berger, D.H.; Ayala, G.E. Neurogenesis in colorectal cancer is a marker of aggressive tumor behavior and poor outcomes. Cancer 2011, 117, 4834–4845. [Google Scholar] [CrossRef] [PubMed]
- Dobrenis, K.; Gauthier, L.R.; Barroca, V.; Magnon, C. Granulocyte colony-stimulating factor off-target effect on nerve outgrowth promotes prostate cancer development. Int. J. Cancer 2015, 136, 982–988. [Google Scholar] [CrossRef] [PubMed]
- Demir, I.E.; Tieftrunk, E.; Schorn, S.; Saricaoglu, Ö.C.; Pfitzinger, P.L.; Teller, S.; Wang, K.; Waldbaur, C.; Kurkowski, M.U.; Wörmann, S.M.; et al. Activated Schwann cells in pancreatic cancer are linked to analgesia via suppression of spinal astroglia and microglia. Gut 2016, 65, 1001–1014. [Google Scholar] [CrossRef]
- Bressy, C.; Lac, S.; Nigri, J.; Leca, J.; Roques, J.; Lavaut, M.N.; Secq, V.; Guillaumond, F.; Bui, T.T.; Pietrasz, D.; et al. LIF Drives Neural Remodeling in Pancreatic Cancer and Offers a New Candidate Biomarker. Cancer Res. 2018, 78, 909–921. [Google Scholar] [CrossRef]
- Iwasaki, T.; Hiraoka, N.; Ino, Y.; Nakajima, K.; Kishi, Y.; Nara, S.; Esaki, M.; Shimada, K.; Katai, H. Reduction of intrapancreatic neural density in cancer tissue predicts poorer outcome in pancreatic ductal carcinoma. Cancer Sci. 2019, 110, 1491–1502. [Google Scholar] [CrossRef]
- Samkharadze, T.; Erkan, M.; Reiser-Erkan, C.; Demir, I.E.; Kong, B.; Ceyhan, G.O.; Michalski, C.W.; Esposito, I.; Friess, H.; Kleeff, J. Pigment epithelium-derived factor associates with neuropathy and fibrosis in pancreatic cancer. Am. J. Gastroenterol. 2011, 106, 968–980. [Google Scholar] [CrossRef] [PubMed]
- Ceyhan, G.O.; Schäfer, K.H.; Kerscher, A.G.; Rauch, U.; Demir, I.E.; Kadihasanoglu, M.; Böhm, C.; Müller, M.W.; Büchler, M.W.; Giese, N.A.; et al. Nerve growth factor and artemin are paracrine mediators of pancreatic neuropathy in pancreatic adenocarcinoma. Ann. Surg. 2010, 251, 923–931. [Google Scholar] [CrossRef] [PubMed]
- Sigorski, D.; Gulczyński, J.; Sejda, A.; Rogowski, W.; Iżycka-Świeszewska, E. Investigation of Neural Microenvironment in Prostate Cancer in Context of Neural Density, Perineural Invasion, and Neuroendocrine Profile of Tumors. Front. Oncol. 2021, 11, 710899. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Guo, L.; Tao, M.; Fu, W.; Xiu, D. Parasympathetic neurogenesis is strongly associated with tumor budding and correlates with an adverse prognosis in pancreatic ductal adenocarcinoma. Chin. J. Cancer Res. 2016, 28, 180–186. [Google Scholar] [CrossRef]
- Lin, G.; Sun, L.; Wang, R.; Guo, Y.; Xie, C. Overexpression of muscarinic receptor 3 promotes metastasis and predicts poor prognosis in non-small-cell lung cancer. J. Thorac. Oncol. 2014, 9, 170–178. [Google Scholar] [CrossRef]
- Von Rosenvinge, E.C.; Raufman, J.P. Muscarinic receptor signaling in colon cancer. Cancers 2011, 3, 971–981. [Google Scholar] [CrossRef]
- Kodaira, M.; Kajimura, M.; Takeuchi, K.; Lin, S.; Hanai, H.; Kaneko, E. Functional muscarinic m3 receptor expressed in gastric cancer cells stimulates tyrosine phosphorylation and MAP kinase. J. Gastroenterol. 1999, 34, 63–71. [Google Scholar] [CrossRef]
- Zhang, L.; Xiu, D.; Zhan, J.; He, X.; Guo, L.; Wang, J.; Tao, M.; Fu, W.; Zhang, H. High expression of muscarinic acetylcholine receptor 3 predicts poor prognosis in patients with pancreatic ductal adenocarcinoma. Onco Targets Ther. 2016, 9, 6719–6726. [Google Scholar] [CrossRef]
- Hayakawa, Y.; Sakitani, K.; Konishi, M.; Asfaha, S.; Niikura, R.; Tomita, H.; Renz, B.W.; Tailor, Y.; Macchini, M.; Middelhoff, M.; et al. Nerve Growth Factor Promotes Gastric Tumorigenesis through Aberrant Cholinergic Signaling. Cancer Cell 2017, 31, 21–34. [Google Scholar] [CrossRef]
- Bapat, A.A.; Munoz, R.M.; Von Hoff, D.D.; Han, H. Blocking Nerve Growth Factor Signaling Reduces the Neural Invasion Potential of Pancreatic Cancer Cells. PLoS ONE 2016, 11, e0165586. [Google Scholar] [CrossRef]
- Lu, M.; Xiu, D.R.; Guo, L.M.; Yuan, C.H.; Zhang, L.F.; Tao, L.Y. Extrapancreatic Neuropathy Correlates with Early Liver Metastasis in Pancreatic Head Adenocarcinoma. Onco Targets Ther. 2019, 12, 11083–11095. [Google Scholar] [CrossRef] [PubMed]
- Hwang, R.F.; Gordon, E.M.; Anderson, W.F.; Parekh, D. Gene therapy for primary and metastatic pancreatic cancer with intraperitoneal retroviral vector bearing the wild-type p53 gene. Surgery 1998, 124, 143–150. [Google Scholar] [CrossRef]
- Klein, A.P. Pancreatic cancer epidemiology: Understanding the role of lifestyle and inherited risk factors. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 493–502. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Ahuja, N.; Makary, M.A.; Cameron, J.L.; Eckhauser, F.E.; Choti, M.A.; Hruban, R.H.; Pawlik, T.M.; Wolfgang, C.L. 2564 resected periampullary adenocarcinomas at a single institution: Trends over three decades. HPB (Oxf.) 2014, 16, 83–90. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gola, M.; Sejda, A.; Godlewski, J.; Cieślak, M.; Starzyńska, A. Neural Component of the Tumor Microenvironment in Pancreatic Ductal Adenocarcinoma. Cancers 2022, 14, 5246. https://doi.org/10.3390/cancers14215246
Gola M, Sejda A, Godlewski J, Cieślak M, Starzyńska A. Neural Component of the Tumor Microenvironment in Pancreatic Ductal Adenocarcinoma. Cancers. 2022; 14(21):5246. https://doi.org/10.3390/cancers14215246
Chicago/Turabian StyleGola, Michał, Aleksandra Sejda, Janusz Godlewski, Małgorzata Cieślak, and Anna Starzyńska. 2022. "Neural Component of the Tumor Microenvironment in Pancreatic Ductal Adenocarcinoma" Cancers 14, no. 21: 5246. https://doi.org/10.3390/cancers14215246
APA StyleGola, M., Sejda, A., Godlewski, J., Cieślak, M., & Starzyńska, A. (2022). Neural Component of the Tumor Microenvironment in Pancreatic Ductal Adenocarcinoma. Cancers, 14(21), 5246. https://doi.org/10.3390/cancers14215246







