Securing Resection Margin Using Indocyanine Green Diffusion Range on Gastric Wall during NIR Fluorescence-Guided Surgery in Early Gastric Cancer Patients
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Preoperative Tumor Localization
2.3. Surgery
2.4. Specimen Examination for Margin Evaluation
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kim, W.; Kim, H.H.; Han, S.U.; Kim, M.C.; Hyung, W.J.; Ryu, S.W.; Cho, G.S.; Kim, C.Y.; Yang, H.K.; Park, D.J.; et al. Decreased Morbidity of Laparoscopic Distal Gastrectomy Compared with Open Distal Gastrectomy for Stage I Gastric Cancer: Short-Term Outcomes from a Multicenter Randomized Controlled Trial (klass-01). Ann. Surg. 2016, 263, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.H.; Han, S.U.; Kim, M.C.; Kim, W.; Lee, H.J.; Ryu, S.W.; Cho, G.S.; Kim, C.Y.; Yang, H.K.; Park, D.J.; et al. Effect of Laparoscopic Distal Gastrectomy vs Open Distal Gastrectomy on Long-Term Survival Among Patients with Stage I Gastric Cancer: The klass-01 Randomized Clinical Trial. JAMA Oncol. 2019, 5, 506–513. [Google Scholar] [CrossRef] [PubMed]
- Katai, H.; Sasako, M.; Fukuda, H.; Nakamura, K.; Hiki, N.; Saka, M.; Yamaue, H.; Yoshikawa, T.; Kojima, K.; JCOG Gastric Cancer Surgical Study Group. Safety and Feasibility of Laparoscopy-Assisted Distal Gastrectomy with Suprapancreatic Nodal Dissection for Clinical Stage I Gastric Cancer: A Multicenter Phase II Trial (JCOG 0703). Gastric Cancer 2010, 13, 238–244. [Google Scholar] [CrossRef]
- Katai, H.; Mizusawa, J.; Katayama, H.; Takagi, M.; Yoshikawa, T.; Fukagawa, T.; Terashima, M.; Misawa, K.; Teshima, S.; Koeda, K.; et al. Short-Term Surgical Outcomes from a Phase III Study of Laparoscopy-Assisted Versus Open Distal Gastrectomy with Nodal Dissection for Clinical Stage IA/IB Gastric Cancer: Japan Clinical Oncology Group Study JCOG0912. Gastric Cancer 2017, 20, 699–708. [Google Scholar] [CrossRef]
- Hiki, N.; Katai, H.; Mizusawa, J.; Nakamura, K.; Nakamori, M.; Yoshikawa, T.; Kojima, K.; Imamoto, H.; Ninomiya, M.; Kitano, S.; et al. Long-Term Outcomes of Laparoscopy-Assisted Distal Gastrectomy with Suprapancreatic Nodal Dissection for Clinical Stage I Gastric Cancer: A Multicenter Phase II Trial (JCOG0703). Gastric Cancer 2018, 21, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Katai, H.; Mizusawa, J.; Katayama, H.; Morita, S.; Yamada, T.; Bando, E.; Ito, S.; Takagi, M.; Takagane, A.; Teshima, S.; et al. Survival Outcomes After Laparoscopy-Assisted Distal Gastrectomy Versus Open Distal Gastrectomy with Nodal Dissection for Clinical Stage IA or IB Gastric Cancer (JCOG0912): A Multicentre, Non-inferiority, Phase 3 Randomised Controlled Trial. Lancet Gastroenterol. Hepatol. 2020, 5, 142–151. [Google Scholar] [CrossRef]
- Guideline Committee of the Korean Gastric Cancer Association (KGCA); Development Working Group; Review Panel. Korean Practice Guideline for Gastric Cancer 2018: An Evidence-Based, Multidisciplinary Approach. J. Gastric Cancer 2019, 19, 1–48. [Google Scholar] [CrossRef]
- Japanese Gastric Cancer Association. Japanese Gastric Cancer Treatment Guidelines 2018 (5th edition). Gastric Cancer 2021, 24, 1–21. [Google Scholar] [CrossRef]
- Information Committee of the Korean Gastric Cancer Association. Korean Gastric Cancer Association-Led Nationwide Survey on Surgically Treated Gastric Cancers in 2019. J. Gastric Cancer 2021, 21, 221–235. [Google Scholar] [CrossRef]
- Kakeji, Y.; Ishikawa, T.; Suzuki, S.; Akazawa, K.; Irino, T.; Miyashiro, I.; Ono, H.; Suzuki, H.; Tanabe, S.; Kadowaki, S.; et al. A Retrospective 5-Year Survival Analysis of Surgically Resected Gastric Cancer Cases from the Japanese Gastric Cancer Association Nationwide Registry (2001–2013). Gastric Cancer 2022, 25, 1082–1093. [Google Scholar] [CrossRef]
- Ryu, K.W.; Lee, J.H.; Choi, I.J.; Bae, J.M. Preoperative Endoscopic Clipping: Localizing Technique of Early Gastric Cancer. J. Surg. Oncol. 2003, 82, 75–77. [Google Scholar] [CrossRef] [PubMed]
- Hyung, W.J.; Lim, J.S.; Cheong, J.H.; Kim, J.; Choi, S.H.; Song, S.Y.; Noh, S.H. Intraoperative Tumor Localization Using Laparoscopic Ultrasonography in Laparoscopic-Assisted Gastrectomy. Surg. Endosc. Other Interv. Tech. 2005, 19, 1353–1357. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.I.; Hyung, W.J.; Lee, C.R.; Lim, J.S.; An, J.Y.; Cheong, J.H.; Choi, S.H.; Noh, S.H. Intraoperative Portable Abdominal Radiograph for Tumor Localization: A Simple and Accurate Method for Laparoscopic Gastrectomy. Surg. Endosc. 2011, 25, 958–963. [Google Scholar] [CrossRef] [PubMed]
- Kwon, I.G.; Son, T.; Kim, H.I.; Hyung, W.J. Fluorescent Lymphography-Guided Lymphadenectomy During Robotic Radical Gastrectomy for Gastric Cancer. JAMA Surg. 2019, 154, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Herrera-Almario, G.; Patane, M.; Sarkaria, I.; Strong, V.E. Initial Report of Near-Infrared Fluorescence Imaging as an Intraoperative Adjunct for Lymph Node Harvesting During Robot-Assisted Laparoscopic Gastrectomy. J. Surg. Oncol. 2016, 113, 768–770. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.Y.; Xie, J.W.; Zhong, Q.; Wang, J.B.; Lin, J.X.; Lu, J.; Cao, L.L.; Lin, M.; Tu, R.H.; Huang, Z.N.; et al. Safety and Efficacy of Indocyanine Green Tracer-Guided Lymph Node Dissection During Laparoscopic Radical Gastrectomy in Patients with Gastric Cancer: A Randomized Clinical Trial. JAMA Surg. 2020, 155, 300–311. [Google Scholar] [CrossRef]
- Baiocchi, G.L.; Molfino, S.; Molteni, B.; Quarti, L.; Arcangeli, G.; Manenti, S.; Arru, L.; Botticini, M.; Gheza, F. Fluorescence-Guided Lymphadenectomy in Gastric Cancer: A Prospective Western Series. Updates Surg. 2020, 72, 761–772. [Google Scholar] [CrossRef]
- Romanzi, A.; Mancini, R.; Ioni, L.; Picconi, T.; Pernazza, G. ICG-NIR-guided Lymph Node Dissection During Robotic Subtotal Gastrectomy for Gastric Cancer. A Single-Centre Experience. Int. J. Med. Robot. 2021, 17, e2213. [Google Scholar] [CrossRef]
- Jung, M.K.; Cho, M.; Roh, C.K.; Seo, W.J.; Choi, S.; Son, T.; Kim, H.I.; Hyung, W.J. Assessment of Diagnostic Value of Fluorescent Lymphography-Guided Lymphadenectomy for Gastric Cancer. Gastric Cancer 2021, 24, 515–525. [Google Scholar] [CrossRef]
- Maruri, I.; Pardellas, M.H.; Cano-Valderrama, O.; Jove, P.; López-Otero, M.; Otero, I.; Campo, V.; Fernández, R.; Fernández-Fernández, N.; Sánchez-Santos, R. Retrospective Cohort Study of Laparoscopic ICG-Guided Lymphadenectomy in Gastric Cancer from a Western Country Center. Surg. Endosc. 2022. [Google Scholar] [CrossRef]
- Brierley, J.D.; Gospodarowicz, M.K.; Wittekind, C. TNM Classification of Malignant Tumours; John Wiley & Sons: Hoboken, NJ, USA, 2017. [Google Scholar]
- Lauren, P. The Two Histological Main Types of Gastric Carcinoma: Diffuse and So-Called Intestinal-Type Carcinoma. An Attempt at a Histo-Clinical Classification. Acta Pathol. Microbiol. Scand. 1965, 64, 31–49. [Google Scholar] [CrossRef] [PubMed]
- Bosman, F.T.; World Health Organization; Carneiro, F.; Cancer, I.A.f.R.o.; Hruban, R.H. WHO Classification of Tumours of the Digestive System: WHO Classification of Tumours; World Health Organization: Geneva, Switzerland, 2010; Volume 3. [Google Scholar]
- Roh, C.K.; Choi, S.; Seo, W.J.; Cho, M.; Son, T.; Kim, H.I.; Hyung, W.J. Indocyanine Green Fluorescence Lymphography During Gastrectomy After Initial Endoscopic Submucosal Dissection for Early Gastric Cancer. Br. J. Surg. 2020, 107, 712–719. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Song, J.H.; Choi, S.; Cho, M.; Kim, Y.M.; Kim, H.I.; Hyung, W.J. Fluorescent Lymphography During Minimally Invasive Total Gastrectomy for Gastric Cancer: An Effective Technique for Splenic Hilar Lymph Node Dissection. Surg. Endosc. 2022, 36, 2914–2924. [Google Scholar] [CrossRef] [PubMed]
- Japanese Gastric Cancer Association. Japanese Classification of Gastric Carcinoma: 3rd English Edition. Gastric Cancer 2011, 14, 101–112. [Google Scholar] [CrossRef]
- Lee, J.H.; Hyung, W.J.; Kim, H.I.; Kim, Y.M.; Son, T.; Okumura, N.; Hu, Y.; Kim, C.B.; Noh, S.H. Method of Reconstruction Governs Iron Metabolism After Gastrectomy for Patients with Gastric Cancer. Ann. Surg. 2013, 258, 964–969. [Google Scholar] [CrossRef]
- Kim, A.R.; Cho, J.; Hsu, Y.J.; Choi, M.G.; Noh, J.H.; Sohn, T.S.; Bae, J.M.; Yun, Y.H.; Kim, S. Changes of Quality of Life in Gastric Cancer Patients After Curative Resection: A Longitudinal Cohort Study in Korea. Ann. Surg. 2012, 256, 1008–1013. [Google Scholar] [CrossRef]
- Kobayashi, D.; Kodera, Y.; Fujiwara, M.; Koike, M.; Nakayama, G.; Nakao, A. Assessment of Quality of Life After Gastrectomy Using EORTC QLQ-C30 and STO22. World J. Surg. 2011, 35, 357–364. [Google Scholar] [CrossRef]
- Davies, J.; Johnston, D.; Sue-Ling, H.; Young, S.; May, J.; Griffith, J.; Miller, G.; Martin, I. Total or Subtotal Gastrectomy for Gastric Carcinoma? A Study of Quality of Life. World J. Surg. 1998, 22, 1048–1055. [Google Scholar] [CrossRef]
- Yamazaki, Y.; Kanaji, S.; Takiguchi, G.; Urakawa, N.; Hasegawa, H.; Yamamoto, M.; Matsuda, Y.; Yamashita, K.; Matsuda, T.; Oshikiri, T.; et al. Preoperative Endoscopic Tattooing Using India Ink to Determine the Resection Margins During Totally Laparoscopic Distal Gastrectomy for Gastric Cancer. Surg. Today 2021, 51, 111–117. [Google Scholar] [CrossRef]
- Jeong, O.; Cho, S.B.; Joo, Y.E.; Ryu, S.Y.; Park, Y.K. Novel Technique for Intraoperative Tumor Localization During Totally Laparoscopic Distal Gastrectomy: Endoscopic Autologous Blood Tattooing. Surg. Endosc. 2012, 26, 1778–1783. [Google Scholar] [CrossRef]
- Miyoshi, N.; Ohue, M.; Noura, S.; Yano, M.; Sasaki, Y.; Kishi, K.; Yamada, T.; Miyashiro, I.; Ohigashi, H.; Iishi, H.; et al. Surgical Usefulness of Indocyanine Green as an Alternative to India Ink for Endoscopic Marking. Surg. Endosc. 2009, 23, 347–351. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, C.; Kanda, M.; Funasaka, K.; Miyahara, R.; Murotani, K.; Tanaka, Y.; Takeda, S.; Kobayashi, D.; Hirooka, Y.; Fujiwara, M.; et al. Detection of Indocyanine Green Fluorescence to Determine Tumor Location During Laparoscopic Gastrectomy for Gastric Cancer: Results of a Prospective Study. Asian J. Endosc. Surg. 2020, 13, 160–167. [Google Scholar] [CrossRef] [PubMed]

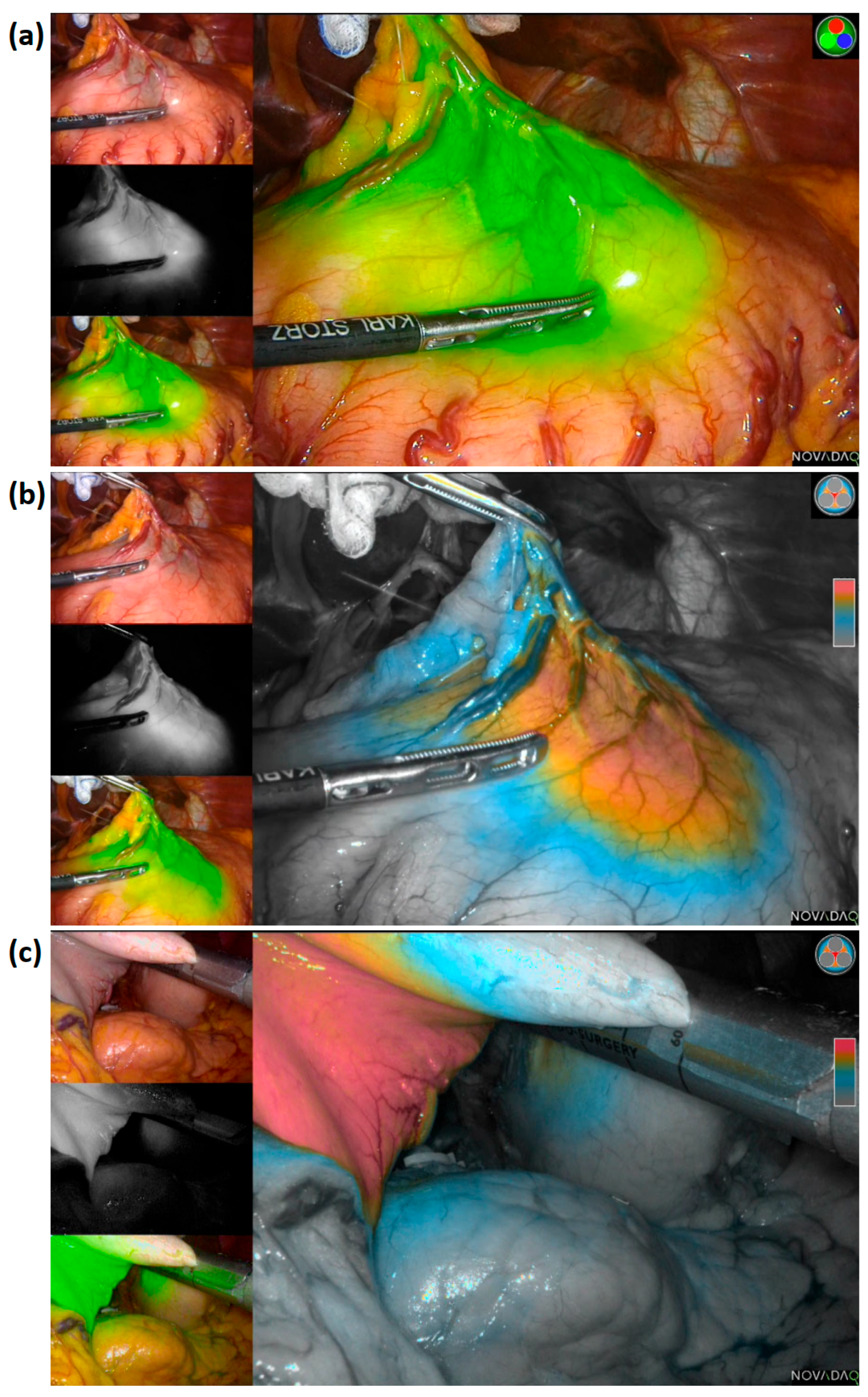
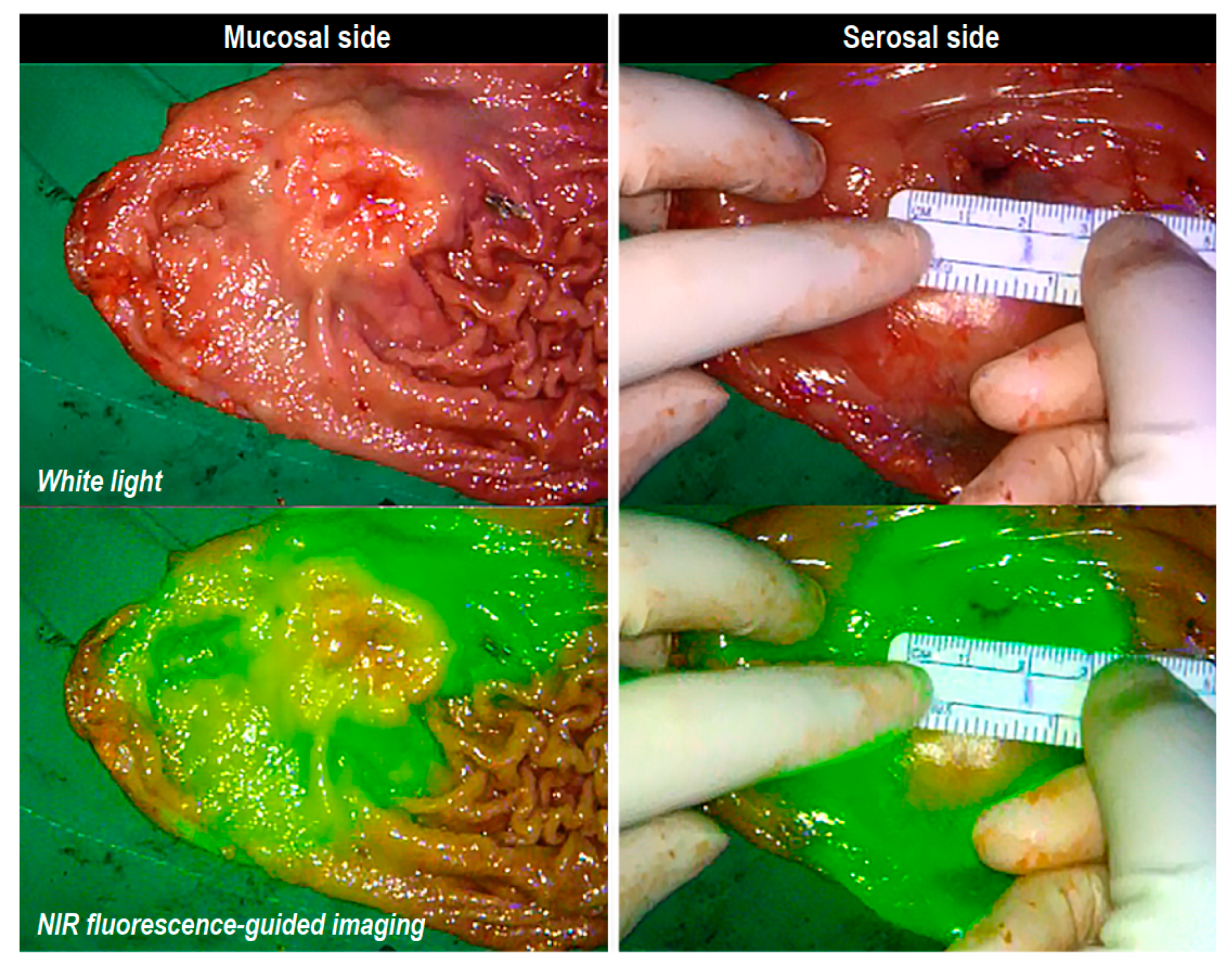
| Variable | Total (n = 503) |
|---|---|
| Age (years) | 57.0 ± 11.3 |
| Sex (n, %) | |
| Male | 220 (43.7%) |
| Female | 283 (56.3%) |
| BMI | 23.3 ± 2.9 |
| ASA-PS classification (n, %) | |
| ASA I | 78 (15.5%) |
| ASA II | 315 (62.6%) |
| ASA III | 107 (21.3%) |
| ASA IV | 3 (0.6%) |
| Tumor location, Tubular (n, %) | |
| Lower body | 262 (52.1%) |
| Midbody | 153 (30.4%) |
| Upper body | 86 (17.1%) |
| Lower body~Midbody | 1 (0.2%) |
| Midbody~Upper body | 1 (0.2%) |
| Tumor location, Circular (n, %) | |
| Lesser curvature | 141 (28.0%) |
| Greater curvature | 102 (20.3%) |
| Anterior wall | 101 (20.1%) |
| Posterior wall | 159 (31.6%) |
| Operation method (n, %) | |
| Laparoscopic | 235 (46.7%) |
| Robotic | 268 (53.3%) |
| Gastrectomy type (n, %) | |
| Subtotal gastrectomy | 404 (80.3%) |
| Proximal gastrectomy | 68 (13.5%) |
| Total gastrectomy | 31 (6.2%) |
| Reconstruction (n, %) | |
| Gastroduodenostomy | 275 (54.7%) |
| Gastrojejunostomy, loop | 66 (13.1%) |
| Roux-en-Y gastrojejunostomy | 63 (12.5%) |
| Double tract reconstruction | 68 (13.5%) |
| Roux-en-Y esophagojejunostomy | 31 (6.2%) |
| LN dissection extent (n, %) | |
| D1+ | 483 (96.0%) |
| D2 | 20 (4.0%) |
| Blood loss (mL) | 63.0 ± 197.0 |
| Operation time (min) | 177.1 ± 51.5 |
| Length of hospital stay (days) | 5.7 ± 3.9 |
| Variable | Total (n = 503) |
|---|---|
| Histology, Lauren classification (n, %) | |
| Intestinal | 338 (67.2%) |
| Diffuse | 112 (22.3%) |
| Mixed | 34 (6.8%) |
| Other | 19 (3.8%) |
| Histology, WHO classification (n, %) | |
| Tubular denocarcinoma | |
| - Well differentiated | 26 (5.2%) |
| - Moderately differentiated | 101 (20.1%) |
| - Poorly differentiated | 121 (24.1%) |
| Poorly cohesive carcinoma | |
| - Signet-ring cell carcinoma | 111 (22.1%) |
| - Other | 133 (26.4%) |
| Mucinous adenocarcinoma | 2 (0.4%) |
| Mixed adenocarcinoma | 2 (0.4%) |
| Carcinoma with lymphoid stroma | 4 (0.8%) |
| Others | 3 (0.6%) |
| Depth of invasion (n, %) | |
| T1a (mucosa) | 291 (57.9%) |
| T1b (submucosa) | 164 (32.6%) |
| T2 (muscularis propria) | 29 (5.8%) |
| T3 (subserosa) | 17 (3.4%) |
| T4a (serosa) | 1 (0.2%) |
| Tx (cannot be assessed) | 1 (0.2%) |
| Regional nodal involvement (n, %) | |
| N0 (none) | 458 (91.1%) |
| N1 (1 or 2 lymph nodes) | 20 (4.0%) |
| N2 (3 to 6 lymph nodes) | 15 (3.0%) |
| N3a (7 to 15 lymph nodes) | 7 (1.4%) |
| N3b (16 or more lymph nodes) | 2 (0.4%) |
| Nx (cannot be assessed) * | 1 (0.2%) * |
| No. of metastatic LN (n, %) | 0.5 ± 4.0 |
| No. of total LN (n, %) | 44.6 ± 16.3 |
| Tumor size (mm) | 26.9 ± 17.0 |
| Length of proximal margin (mm) | 35.0 ± 20.6 |
| Involvement of proximal margin (n, %) | 3 (0.6%) |
| Length of distal margin (mm) | 88.1 ± 38.4 |
| Involvement of distal margin (n, %) | 0 (0%) |
| Variable | Case #1 | Case #2 | Case #3 |
|---|---|---|---|
| Sex/Age | F/66 | M/54 | M/48 |
| Tumor location | LB/LC | LB/GC | LB/LC |
| Endoscopic finding | EGC, type IIc, 13 mm | EGC, type IIc, 30 mm | EGC, type IIa + IIc, 18 mm |
| Endoscopic image | 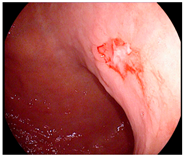 | 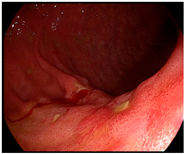 |  |
| EUS | Submucosa+, 13 mm, LN (-) | N/A | Submucosa+, 21 mm, LN (-) |
| Helicobacter pylori | Positive | N/A | Positive |
| Preoperative histology | Signet ring cell carcinoma | Adenoca., moderate diff. | Adenoca., moderate diff. |
| Surgery | Robot STG BI | Robot-attempted STG BII | Robot STG BI |
| Specimen gross evaluation by the surgeon | Lesion 20 × 20 mm | Lesion 15 × 10 mm | Lesion 20 × 20 mm |
| Proximal margin 10 mm | Proximal margin 40 mm | Proximal margin 30 mm | |
| Distal margin enough | Distal margin 80 mm | Distal margin 60 mm | |
| ICG (mucosa) 70 × 60 mm | ICG (mucosa) 90 × 80 mm | ICG (mucosa) 60 × 60 mm | |
| ICG (serosa) 80 × 80 mm | ICG (serosa) 80 × 70 mm | ICG (serosa) 70 × 70 mm | |
| Specimen gross evaluation by the pathologist | Lesion 30 × 15 mm | Lesion 15 × 10 mm | Lesion 65 × 55 mm |
| Proximal margin 8 mm | Proximal margin 25 mm | Proximal margin 5 mm | |
| Distal margin 92 mm | Distal margin 90 mm | Distal margin 30 mm | |
| Pathological result | Diffuse type (PCC:SRC) Invades mucosa (pT1a) Tumor size 63 × 43 mm Proximal margin involved Distal margin 66 mm Lymph node 0/33 (pN0) | Intestinal type (AMD with crawling features) Invades submucosa, sm1 (pT1b) Tumor size 90 × 50 mm Proximal margin involved Distal margin 90 mm Lymph node 0/28 (pN0) | Intestinal type (AMD) Invades submucosa, sm1 (pT1b) Tumor size 65 × 55 mm Proximal margin involved Distal margin 30 mm Lymph node 0/38 (pN0) |
| Variable | Total (n = 503) | Tumor < 2 cm (n = 187) | Tumor, 2–4 cm (n = 225) | Tumor ≥ 4 cm (n = 91) |
|---|---|---|---|---|
| Tumor size (mm) | 26.9 ± 17.0 × 19.0 ± 12.8 | 12.4 ± 4.2 × 9.0 ± 3.7 | 27.1 ± 5.5 × 19.0 ± 6.0 | 55.3 ± 15.5 × 39.1 ± 13.0 |
| ICG diffusion range, mucosal side (mm) | 82.7 ± 23.8 × 75.3 ± 21.2 | 81.1 ± 23.9 × 73.9 ± 21.2 | 82.7 ± 24.2 × 76.0 ± 20.3 | 85.9 ± 22.4 × 76.5 ± 23.5 |
| ICG diffusion range, serosal side (mm) | 86.7 ± 23.2 × 80.2 ± 21.6 | 85.7 ± 23.8 × 77.9 ± 21.1 | 86.2 ± 22.3 × 81.8 ± 21.1 | 89.6 ± 23.9 × 80.9 ± 23.5 |
| Variable | n (%) | Proximal Margin (mm) | Distal Margin (mm) | Tumor Size (mm) | ICG Diffusion Range, Mucosal Side (mm) | ICG Diffusion Range, Serosal Side (mm) |
|---|---|---|---|---|---|---|
| Total gastrectomy | 31 (6.2%) | 34.9 ± 24.5 | 124.8 ± 33.9 | 41.2 ± 24.2 × 29.8 ± 18.4 | 98.5 ± 36.5 × 82.6 ± 32.3 | 101.8 ± 30.6 × 87.1 ± 33.1 |
| Proximal gastrectomy | 68 (13.5%) | 29.7 ± 19.6 | 50.8 ± 33.8 | 27.3 ± 18.5 × 20.3 ± 13.4 | 78.8 ± 26.8 × 72.6 ± 20.5 | 85.4 ± 27.3 × 87.1 ± 33.1 |
| Subtotal gastrectomy | 404 (80.3%) | 35.9 ± 20.3 | 91.4 ± 34.9 | 25.7 ± 15.5 × 18.0 ± 11.7 | 82.1 ± 21.5 × 75.2 ± 20.2 | 85.7 ± 21.4 × 79.8 ± 20.2 |
| Gastroduodenostomy | 275 | 38.1 ± 20.1 | 79.5 ± 27.6 | 24.4 ± 14.1 × 17.0 ± 10.9 | 79.8 ± 21.2 × 74.8 ± 19.4 | 83.0 ± 20.4 × 78.9 ± 19.1 |
| Loop gastrojejunostomy | 66 | 33.4 ± 22.2 | 108.5 ± 36.9 | 26.8 ± 19.0 × 18.8 ± 12.1 | 84.8 ± 23.1 × 71.9 ± 21.1 | 88.3 ± 20.7 × 80.2 ± 23.2 |
| Roux-en-Y gastrojejunostomy | 63 | 28.7 ± 17.2 | 125.5 ± 31.7 | 30.0 ± 16.9 × 21.4 ± 14.2 | 89.4 ± 19.5 × 80.5 ± 21.9 | 94.9 ± 23.6 × 83.6 ± 21.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, M.; Kim, K.-Y.; Park, S.H.; Kim, Y.M.; Kim, H.-I.; Hyung, W.J. Securing Resection Margin Using Indocyanine Green Diffusion Range on Gastric Wall during NIR Fluorescence-Guided Surgery in Early Gastric Cancer Patients. Cancers 2022, 14, 5223. https://doi.org/10.3390/cancers14215223
Cho M, Kim K-Y, Park SH, Kim YM, Kim H-I, Hyung WJ. Securing Resection Margin Using Indocyanine Green Diffusion Range on Gastric Wall during NIR Fluorescence-Guided Surgery in Early Gastric Cancer Patients. Cancers. 2022; 14(21):5223. https://doi.org/10.3390/cancers14215223
Chicago/Turabian StyleCho, Minah, Ki-Yoon Kim, Sung Hyun Park, Yoo Min Kim, Hyoung-Il Kim, and Woo Jin Hyung. 2022. "Securing Resection Margin Using Indocyanine Green Diffusion Range on Gastric Wall during NIR Fluorescence-Guided Surgery in Early Gastric Cancer Patients" Cancers 14, no. 21: 5223. https://doi.org/10.3390/cancers14215223
APA StyleCho, M., Kim, K.-Y., Park, S. H., Kim, Y. M., Kim, H.-I., & Hyung, W. J. (2022). Securing Resection Margin Using Indocyanine Green Diffusion Range on Gastric Wall during NIR Fluorescence-Guided Surgery in Early Gastric Cancer Patients. Cancers, 14(21), 5223. https://doi.org/10.3390/cancers14215223





