Simple Summary
Melanoma of the vulva and vagina is a relatively rare neoplasm, unlike melanoma of the skin. Its prognosis is poor, and its pathogenesis is not fully understood. Immunotherapy is one of the rapidly developing cancer treatment methods. In this article, we focus on the pathogenesis of lower genital tract melanomas and related risk factors and compare the effectiveness of two groups of drugs—anti-PD-L1 and anti-CTLA4 antibodies—in the treatment of this condition. This type of immunotherapy is a relatively common treatment method for cutaneous melanoma but not for the rare vulvovaginal melanoma. For vulvovaginal melanoma, the effects of these treatments appear to be limited; however, this requires further research.
Abstract
Cutaneous melanoma is a relatively common neoplasm, with fairly well understood pathogenesis, risk factors, prognosis and therapeutic protocols. The incidence of this disease is increasing every year. The situation is different for rare malignancies such as vulvar melanomas and for the even rarer vaginal melanomas. The risk factors for vulvovaginal tumors are not fully understood. The basis of treatment in both cases is surgical resection; however, other types of treatments such as immunotherapy are available. This paper focuses on comparing the pathogenesis and risk factors associated with these neoplasms as well as the efficacy of two groups of drugs—anti-PD-L1 and anti-CTLA4 inhibitors—against both cutaneous melanoma and melanoma of the lower genital tract (vulva and vagina). In the case of cutaneous melanoma, the situation looks more optimistic than for vulvovaginal melanoma, which has a much worse prognosis and, as it turns out, shows a poorer response to immune therapy.
1. Introduction
Melanoma is a malignant skin neoplasm that derives from melanocytes. Melanocytes are a type of pigment cells located in the basal layer of the epidermis, responsible for the production of the pigment melanin. These specific cells, however, are not exclusively located in this tissue. Melanocytes can also be found in the mucous membranes of the genito-urinary tract, airway tract and even in the gastrointestinal track. Melanoma derived from this type of tissue is called mucosal melanoma. Every year, there are between 160,000 up to 230,000 new cases of melanoma worldwide. The mortality rate worldwide is between 48,000 and 55,000 yearly [1,2]. Since the 1970s, the number of new cases has increased both in women and in men [1]. The 5-year relative survival is 74% in eastern Europe, rising to 82% in southern Europe and up to 88% in Scandinavia and central Europe. For the United Kingdom and Ireland, the relative survival rate is 86% [3]. These data indicate a relatively good prognosis. The risk factors for melanoma include both extended and brief exposures to UV radiation (most probably including artificial UV sources) [4], a fair skin complexion and bright eye color, freckles developing before the age of 15, red or blonde hair [5], numerous skin lesions including atypical lesions [6], melanoma cases in family history [7], melanoma in patient’s history [8], a low socio-economic status [9]. Cutaneous melanoma can be divided into four distinctive main types: superficial—most commonly appearing on skin exposed to short-term, but intensive UV radiation—nodular melanoma—deriving from de novo lesions mostly on the torso, head and neck—lentigo maligna—commonly on skin exposed to long-term UV radiation—acral lentiginous—on hairless skin of feet, hands and underneath the nails. This neoplasm is known to easily create metastases, including satellite-type metastases, in-transit metastases, lymph nodes metastases and metastases spreading via the blood and becoming established mostly in the lungs, brain, liver and bone.
On the other hand, mucosal melanoma presents different features. It is a relatively rare form of neoplasm—only 1.5% of all melanoma cases, which amounts to around 0.03% of total neoplasm cases. It is mostly located in membranes of the head and neck area (55.4% of all mucosal melanomas), genital tract (18%), rectal area (23.8%) and urinary tract (2.8%) [10]. Its prognosis is unfavorable. The 5-year survival rate of patients with mucosal melanoma is 25% but drops to 11.4% in case of melanoma of the genital tract [10].
As it turns out, melanoma of the skin are detected faster than melanomas of the vulva and vagina (Table 1 and Table 2) [11,12,13].

Table 1.
Cutaneous melanoma; stage at diagnosis and 5-year survival.

Table 2.
Vulvar and vaginal melanoma; survival and diagnosis rate based on staging. Table designed and based on work of Wohlmuth et al. [12].
For cutaneous and genital melanomas, surgical resection remains a basic form of therapy. Depending on the stage of the disease, other forms of therapy such as radiotherapy, chemotherapy and immunotherapy are available. The main aim of this study was to compare the effectiveness of immunotherapy for cutaneous and genital tract melanoma.
2. Pathogenesis
About one half of skin melanomas arise from de novo lesions, and the other half from malignant pigmented nevi [14]. Various factors, both exo- and endogenous, influence the process of formation of tumor cells as well as their further expansion. The involvement of the immune system is also important. The proliferation rate of mutant melanocytes increases significantly [15]. The most prominent external factor that promotes such process is UVA and UVB radiation. Internal factors play a role in cutaneous melanoma pathogenesis, including mutations such as BRAF, NRAS, TP53, CDKN2A and PTEN [16,17]. A BRAF gene mutation (variant V600E) is found in roughly 50% of primary skin melanomas; however, the role of this mutation in metastatic melanoma is yet to be determined [18]. The BRAF600 mutation coexisting with mutations in the CDKN2A gene impairs the control over the entire cell-cycle. Additional mutations, e.g., in PTEN and TP53 favor a more aggressive growth and expansion of this neoplasm and promote the formation of metastases, including distant ones [14,16]. Another important mutation involves KIT. It is also common in acral melanoma [19]. The common changes regarding KIT are amplifications and missense mutations in the auto-inhibitory domain (coded by exon 11) and the tyrosine kinase domains (coded by exons 12–21). Those mutations are the most important non-synonymous KIT mutations [20].
The spliceosomal protein SF3B1 is a core component of the U2 snRNP. This protein’s main function is in the splicing process, during which non-coding pre-mRNA fragments are removed thus producing a target product, i.e., a proper mRNA. U2 snRNP targets the branch point sequence at the 3′ splice site of an exon–intron junction.
Mutations in SF31B result in alterations in the splicing process [21]. There are two possible outcomes for such scenario. The first outcome leads to a translation of a transcript, thus producing an aberrant protein. The second scenario leads to a nonsense-mediated decay (NMD), which results in mRNA and protein downregulation.
Across neoplasm types, SF3B1 mutations have been identified as heterozygous and regard, in particular, R625, K666 and K700 residues. Mutations that are characteristic for mucosal and uveal melanomas almost exclusively occur at R625.
Hintzsche et al. [22] documented the presence of SF3B1 mutations in 35% (7/19) of mucosal melanoma cases. They were most commonly observed in anorectal melanomas (3/5, amounting to 60%) and in the vulvovaginal melanomas (4/9, amounting to 44.4%).
Quek et al. [23] provided similar results by showing that SF3B1 mutations mostly occurred at SF3B1-R625 (5/6, amounting to 83%) and were almost exclusively found in vulvovaginal (5/19, amounting to 26%) and the anorectal melanomas (3/5, amounting to 60%). The following graphic (Figure 1) shows collectively the most common mutations leading to melanoma development.
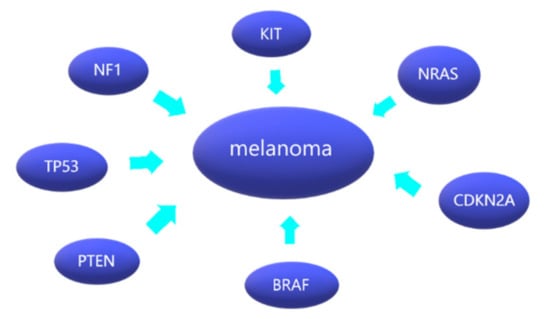
Figure 1.
Melanoma and its most common mutations.
Mutations in specific proteins disrupt the function of cellular pathways, such as the MAP kinase, B cell kinase (AKT), PI3K–mTOR kinase, or the PTEN pathway [24,25,26]. In addition, impaired immune response mechanisms are involved in the pathogenesis of cutaneous melanoma. Under the influence of interferons (including γ, as well as α or β), there is excessive activation of the JAK1/2 and STAT kinase pathways, which stimulate the production of the PD-L1 and PD-L2 ligands. These ligands are transported to the cell membrane and are presented to T lymphocytes. On the surface of these lymphocytes are PD-1 (programmed-cell-death protein-1) checkpoints, which protect the cells from an excessive immune system response which would destroy them. In this way, cutaneous melanoma cells escape the control of T cells [27,28]. Interferons may have different functions, such as anti-angiogenic, anti-proliferative as well as immunomodulatory effects, which may be crucial in the development of melanoma [29]. The following graphic illustrates the effect of anti-PD-L1 antibodies that prevent PD-1 from binding to its ligand (Figure 2).
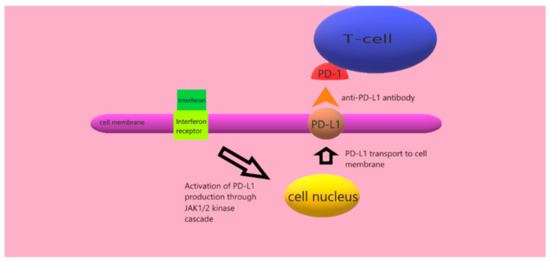
Figure 2.
Effect of anti-PD-L1 antibody.
In the case of genital tract melanoma, in particular of the vulva and vagina, the situation is different. In fact, the risk factors are not fully understood, and, in comparison to skin melanoma, this neoplasm occurs much less frequently—it accounts for 3 to 7% of all melanomas [30] and only for 10% of all vulvar malignant neoplasms [31]. Among the risk factors is advanced age (average age for this melanoma is over 60 years) and white race [32,33,34]. The vulva area is not exposed to UV radiation, so this exogenous risk factor can be ruled out [33].
Vulvar melanoma is most commonly located on the labia majora (around 51%) and labia minora (around 38%); the least common location is the clitoris (around 12%) [33].
The genetic profiles of cutaneous melanoma and vulvar and vaginal melanoma are different in terms of mutations. The KIT gene mutation, which rarely appears in cutaneous melanoma patients [35,36], appears to be commonly occurring in vulvar melanoma (from 22 up to 31% of cases). It is more common in vulvar melanoma (27%) and less common in vaginal melanoma (8%) [37,38]. On the other hand, NRAS mutation occurs in around 5.3% to 12% of genital tract melanomas [38,39], with a higher incidence in vaginal melanomas [40].
There are studies suggesting that the occurrence of the NRAS mutation might even be more common than that of the KIT mutation [41]. The BRAF mutation seems to be less frequent in vulvar melanoma [37,38] than in cutaneous melanoma (where it is present in 60–63% of cases)) [16,18]. As regards protection against T cell activation, the defense mechanism in genital tract melanoma is similar to that in cutaneous melanoma: the PD-L1 ligand is produced and binds to PD-1 T cell checkpoints on the surface of the tumor cells; this mechanism is also relatively common in vulvar melanoma [29,42,43]. In addition to the previously described mechanism, cells have also a CTLA-4 checkpoint which, upon associating with specific ligands (e.g., PD-L1) blocks the immune reaction mediated by T cell lymphocytes [44].
3. Immunotherapy of Cutaneous Melanoma
The primary treatment for both cutaneous and vulvar melanoma is surgical resection. Other alternative treatments include radiation therapy, chemotherapy and immunotherapy. Different drugs are used in immunotherapy for cutaneous melanoma, but the focus still remains on two groups, i.e., anti-PD-1 antibodies (nivolumab and pembrolizumab) and anti-CTLA-4 antibodies (ipilimumab) (Table 3). According to different studies from different medical centers, the effectiveness of treatment varies. Larkin J. et al. [45] conducted an analysis of the treatment efficacy of nivolumab and ipilimumab in monotherapy and in combination therapy in patients with stage III and IV disease. With the combination therapy, the median disease progression-free period averaged 11.5 months compared to 6.9 for nivolumab monotherapy and 2.9 for ipilimumab monotherapy. In the presence of the PD-L1 ligands from the combination therapy and the nivolumab monotherapy, the median progression-free period was 14 months, while in the absence of these ligands, it was 11.2 months (combination therapy) and 5.3 months (nivolumab). The response rate for the combination therapy was 57.6%, 43.7% for the nivolumab group and 19% when ipilimumab was administered. Another study (Wolchok et al.) [46] examined the response in patients with advanced melanoma receiving different doses of a combination therapy with nivolumab and ipilimumab. The largest reduction in tumor volume after 12 weeks of treatment occurred at the doses of nivolumab of 1 mg/kg and of ipilimumab of 3 mg/kg (41%): these doses also promoted the largest partial and objective response (53%) to treatment. The lowest partial and objective (21%) response to treatment occurred with nivolumab at a dose of 0.3 mg/kg and ipilimumab at a dose of 3 mg/kg. In conclusion, the combined therapy and nivolumab are more effective than ipilimumab alone.

Table 3.
Effects of immunotherapy in patients with cutaneous melanoma. Nd—no data; WT—wild-type; T-N—treatment-naive; P-T—previously treated.
A team under the same leadership [47] performed another analysis of the efficacy of nivolumab and ipilimumab in patients depending on the presence or absence of BRAF mutations in previously untreated advanced melanoma. The patients were divided into three groups: one receiving the combination therapy (group 1), the second receiving the nivolumab monotherapy (group 2), and the third administered the ipilimumab monotherapy (group 3). The period without disease progression (assumed to be a cutoff point at 36 months) was the longest for group 1 (39%), followed by group 2 (32%) and group 3 (10%). Objective response to treatment was the highest in group 1 (58%), followed by group 2 (44%) and finally group 3 (19%). The overall survival rate was analogous: the rate was the highest in group 1 (58%) and the lowest in group 3 (34%). Based on this study sample, it can be concluded that nivolumab is more effective in the treatment of cutaneous melanoma than ipilimumab. Larkin et al. [48] analyzed the 5-year follow-up of patients with advanced melanoma (previously untreated) undergoing immunotherapy and divided in three groups: group 1 with combination therapy, group 2 with nivolumab monotherapy and group 3 with ipilimumab monotherapy. The presence or absence of BRAF mutations was also considered. The overall survival rate at 60 months was the highest in group 1 (60%) and the lowest in group 3 (26%). The response to treatment was the highest in group 1 (58%), followed by group 2 (45%) and finally group 3 (19%). The presence of a BRAF mutation was not insignificant: in group 1, the survival rate of patients with this mutation was 60%, versus 48% for patients without the mutation. A smaller difference was noted in group 3, i.e., 30 versus 25%. In conclusion, the combined therapy seems to be more effective than the monotherapy.
A comparative analysis of the efficacy and overall survival of patients with advanced-stage (metastatic) melanoma treated with either nivolumab or dacarbazine was also performed. Patients with BRAF mutation were excluded [49]. After one year of treatment, the overall survival rate was 72% for patients receiving nivolumab and 42% for those receiving dacarbazine. The response to treatment differed significantly, being 40% for nivolumab-treated patients and only 13.9% for dacarbazine-treated patients. The median survival also differed significantly: 5.1 for patients treated with immunotherapy and 2.2 for those receiving dacarbazine. The presence of PD-L1 ligands was nearly the same in the two groups. i.e., 35.4% of patients revealed the presence of these ligands. This study indicates a higher efficacy of nivolumab in comparison to dacarbazine.
The effectiveness of immunotherapy in melanoma patients carrying the CDKN2A mutation was investigated [50]. All patients had advanced-stage cancer, and some of them also carried BRAF mutations (V600E or V600K variant, 73%). It was found that 58% of the patients achieved a partial response to treatment, and 32% achieved a complete response. This is a very good result; however, the relatively small study group of 19 patients should be taken into account. Compared to other treatments, partial and complete response rates were higher in patients with this mutation; however, the mechanisms responsible for this are not fully understood.
The efficacy of immunotherapy against melanoma and other cancers was examined in a larger group of patients carrying various CDKN2A major loss-of-function (LOF) mutations. The presence of the wild-type gene was also investigated [51]. The results showed that patients with LOF mutations in bladder cancer had a lower median survival and overall survival rate compared to patients with the wild-type gene. However, there was no association between the LOF mutation and the efficacy of immunotherapy for melanoma and esophageal or lung cancers. Horn et al. investigated the presence not only of CDKN2A mutations, but also of abnormal JAK2 kinase function. This study showed that tumors with these two concomitant abnormalities, may be susceptible to developing resistance to treatment with interferon and immunotherapy [52]. On the other hand, a study showed that the presence of CDKN2A and TP53 mutations did not affect the efficacy of immunotherapy. The response rates of patients with a TP53 mutation or the wild-type gene were 47.4% and 34.3%, respectively, at p = 0.15; for patients with a CDKN2A mutation or the wild-type gene, the response rates were 45.7% and 36%, respectively, at p = 0.54 [53]. Patients with cutaneous melanoma or melanomas of unknown origin were included in this study.
An analysis of the efficacy of treatment in patients with or without NRAS mutation was performed [54]. The response to treatment in both cases was similar, i.e., 13 to 15% for patients treated with ipilimumab, 21% to 13% (wild type) with for patients receiving anti-PD-L1 antibodies and 40% to 39% (wild-type) for patients treated with combination therapy, but the median survival was higher for patients without an NRAS mutation (33 months versus 21 months). The study showed that a prior treatment of melanoma with MEK inhibitors likely has a favorable effect on patient prognosis. Hu-Lieskovian et al. [55] investigated in vivo the treatment efficacy of anti-CTLA4 antibodies with the simultaneous administration of BRAF inhibitors and/or MEK inhibitors in melanoma patients with the BRAF (V600E) mutation. The highest efficacy was measured for the triple-drug therapy: within 30 days, the tumor area did not exceed 25 mm2; however, this was a preclinical experiment that needs to be repeated in a clinical trial.
The large clinical trial KEYNOTE-054 [56] compared the survival of patients with stage III cutaneous melanoma (IIIA, B or C) treated with pembrolizumab to that of patients receiving a placebo. All patients included in this study had histologically confirmed metastases in regional lymph nodes. It was required to check PD-L1 expression in tumor sample. After three years, the survival rate of patients in the pembrolizumab group was 63.7%, and that of patients in the placebo group was 44.1%. The KEYNOTE-006 study [57] compared the efficacy of pembrolizumab (different doses and dosing regimens) to that of ipilimumab. The patients included in this study had ipilimumab-naive, unresectable stage III or IV melanoma. The results showed that pembrolizumab was more effective than ipilimumab (OS = 32.7 months vs. 15.9 months) and was also associated with a better quality of life after 3 months of treatment [58]. The survival rates after 24 months of therapy with pembrolizumab and ipilimumab were also examined. The survival rate in patients previously untreated was higher in the pembrolizumab group (31%) than in the ipilimumab one (14.6%). In relation to the presence of PD-L1 ligands, similar results were obtained, i.e., 33.2% to 13.1% [59]. The KEYNOTE-002 trial compared the efficacy of pembrolizumab to that of chemotherapy in patients with ipilimumab-resistant melanomas. The patients included in this clinical trial had histologically or cytologically confirmed stage III or IV melanomas, which were unresectable. The overall survival was higher for patients treated with pembrolizumab than for those who received standard chemotherapy [60,61,62].
4. Immunotherapy of Vulvar and Vaginal Melanoma
As it is a relatively rare cancer mainly treated by surgical resection, large clinical trials as those for cutaneous melanoma (KEYNOTE type) have not been conducted to date (Table 4). Hou et al. [37] conducted an analysis of different genes expression variants in vulvar melanoma and cutaneous melanoma. The vulvovaginal melanoma study group was much smaller than the cutaneous melanoma group. It turned out that the BRAF mutation rate was higher than that reported in the literature (in this study BRAF rate was 26%). The KIT mutation rate was 22%. What is interesting is that the KIT mutation rate was higher in vulvar melanoma (31.4%) than in vaginal melanoma (6.2%). The authors demonstrated that 75% of the patients showed PD-1 expression, and 56% showed PD-L1 expression, which suggests a therapeutic option in the form of immunotherapy for these patients.

Table 4.
Effects of immunotherapy in patients with vulvar and vaginal melanoma. L—localized; R—regional; D—distant; nd—no data; VVM—vaginal/vulvar melanoma, CM—cutaneous melanoma.
Albert et al. [63] took into account the contribution of immunotherapy to treatment. The overall survival rate was higher for patients who received the monoclonal antibodies but statistically insignificant. In this study of more than 1900 patients with melanoma in the genital tract, patients with in situ tumors were excluded.
In a combined analysis of six clinical trials [64], patients with vulvar melanoma received nivolumab as monotherapy (86 patients) or in combination with ipilimumab (35 patients). The progression-free period was shown to be similar to that of patients with other mucosal melanomas, but the response rate was lower (37%) than that observed in patients with cutaneous melanoma (60%). An even lower treatment effect (19% response rate) was shown for patients with mucosal melanoma in the KEYNOTE 001, 002 and 006 clinical trials [65].
Egger et al. [66] studied 20 patients with melanoma of the lower genital tract (14 vulvar and 6 vaginal). Nine patients received immunotherapy (the average response time was 4 months), and three received immunotherapy in combination with radiation therapy (the response appeared after an average of 5 months). The treatment of five cases of vaginal melanoma [67] in which nivolumab was administered at recurrence (in one patient undergoing monotherapy, in the other in combination with dacarbazine) was described retrospectively. When comparing the overall survival of these patients to that of patients who refused further treatment at recurrence, the treatments administered likely prolonged patients’ survival. Wilhite et al. [68] reported that vulvar and vaginal melanomas showed a lower expression of adaptive immunity genes and a lower expression of PD-L1 ligands compared to cutaneous melanoma, which probably caused a worse prognosis after immunotherapy.
Skovsted et al. [69] described a case series of vulvar and vaginal melanomas; however, the number of patients was too small to assess the efficacy of the treatment, which, perhaps in combination with resection, could have a significant impact on patient survival. Boer et al. [70] described 198 cases of vulvar melanomas. The response rate to anti-PD-L1 antibodies was 18% and increased to 20% when this treatment was combined with anti-CTLA-4 antibodies; however, the study group was too small for far-reaching conclusions.
In a small study group of six patients, ipilimumab was used as therapy. The survival rate after one year was 33% in this group of patients [71].
Chlopik et al. [72] examined in their study the prognostic role of the expression of the PD-L1 in the tumor and of CD8+ and FoxP3+ in lymphocytes in vulvar melanoma patients. The study group included 75 patients with primary vulvar melanoma. The authors found that the peritumoral expression of CD8+ and the tumoral expression of FoxP3+ lymphocytes were associated with better overall survival (p = 0.021 and p = 0.0055 respectively). The same results were reported in regard to PD-L1 expression > 5% (p = 0.03).
Indini et al. [73] investigated the results of immunotherapy against tumors in the lower genital tract. In this study, 29% of the patients had metastatic disease from the start, and the rest developed metastases during the treatment. As for the treatment, 57% of the patients received ipilimumab, while 43% were administered anti-PD-L1 antibodies. The response rate reached 28.5%. The patients in the anti-PD-L1 antibodies group had a better progression-free survival.
Several cases have also been published in which immunotherapy likely prolonged patients’ lives, but the use of this type of treatment requires further research in the future [74,75,76].
5. Conclusions
Unlike skin melanoma, melanoma of the lower genital tract is a rare and therefore little understood cancer. A comparison of the effectiveness of common treatments against vulvovaginal melanoma and cutaneous melanoma, it appears that both survival and recurrence-free period are shorter for patients with melanoma of the lower genital tract. Not all molecular mechanisms involved in the pathogenesis of vulvar melanoma have been understood. This pathogenesis of this tumor as well as the efficacy of immunotherapy against it, require further study.
Author Contributions
Conceptualization, A.C.-P.; methodology, A.C.-P.; software, S.L.; validation, S.K. and M.K.; formal analysis, M.K.; investigation, A.L.; resources, A.L.; data curation, A.L.; writing—original draft preparation, S.L.; writing—review and editing, S.K.; visualization, M.K.; supervision, A.C.-P.; project administration, A.C.-P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Schadendorf, D.; van Akkooi, A.C.J.; Berking, C.; Griewank, K.G.; Gutzmer, R.; Hauschild, A.; Stang, A.; Roesch, A.; Ugurel, S. Melanoma. Lancet 2018, 392, 971–984. [Google Scholar] [CrossRef]
- Eggermont, A.M.; Spatz, A.; Robert, C. Cutaneous melanoma. Lancet 2014, 383, 816–827. [Google Scholar] [CrossRef]
- Crocetti, E.; Mallone, S.; Robsahm, T.E.; Gavin, A.; Agius, D.; Ardanaz, E.; Lopez, M.-D.C.; Innos, K.; Minicozzi, P.; Borgognoni, L.; et al. Survival of patients with skin melanoma in Europe increases further: Results of the EUROCARE-5 study. Eur. J. Cancer 2015, 51, 2179–2190. [Google Scholar] [CrossRef]
- Bataille, V.; Winnett, A.; Sasieni, P.; Bishop, J.N.; Cuzick, J. Exposure to the sun and sunbeds and the risk of cutaneous melanoma in the UK: A case–control study. Eur. J. Cancer 2004, 40, 429–435. [Google Scholar] [CrossRef]
- Titus-Ernstoff, L.; Perry, A.E.; Spencer, S.K.; Gibson, J.J.; Cole, B.F.; Ernstoff, M.S. Pigmentary characteristics and moles in relation to melanoma risk. Int. J. Cancer 2005, 116, 144–149. [Google Scholar] [CrossRef] [PubMed]
- JGrob, J.-J.; Gouvernet, J.; Aymar, D.; Mostaque, A.; Romano, M.H.; Collet, A.M.; Noe, M.C.; DiConstanzo, M.P.; Bonerandi, J.J. Count of benign melanocytic nevi as a major indicator of risk for nonfamilial nodular and superficial spreading melanoma. Cancer 1990, 66, 387–395. [Google Scholar] [CrossRef]
- Ford, D.; Bliss, J.M.; Swerdlow, A.J.; Armstrong, B.K.; Franceschi, S.; Green, A.; Holly, E.A.; Mack, T.; Mackie, R.M.; Østerlind, A.; et al. Risk of cutaneous melanoma associated with a family history of the disease. Int. J. Cancer 1995, 62, 377–381. [Google Scholar] [CrossRef] [PubMed]
- Spanogle, J.P.; Clarke, C.A.; Aroner, S.; Swetter, S.M. Risk of second primary malignancies following cutaneous melanoma diagnosis: A population-based study. J. Am. Acad. Dermatol. 2010, 62, 757–767. [Google Scholar] [CrossRef]
- Sitenga, J.L.; Aird, G.; Ahmed, A.; Walters, R.; Silberstein, P.T. Socioeconomic status and survival for patients with melanoma in the United States: An NCDB analysis. Int. J. Dermatol. 2018, 57, 1149–1156. [Google Scholar] [CrossRef]
- Chang, E.; Karnell, L.H.; Menck, H.R. The National Cancer Data Base report on cutaneous and noncutaneous melanoma. Cancer 1998, 83, 1664–1678. [Google Scholar] [CrossRef]
- Rachidi, S.; Deng, Z.; Sullivan, D.Y.; Lipson, E.J. Shorter survival and later stage at diagnosis among unmarried patients with cutaneous melanoma: A US national and tertiary care center study. J. Am. Acad. Dermatol. 2020, 83, 1012–1020. [Google Scholar] [CrossRef] [PubMed]
- Wohlmuth, C.; Wohlmuth-Wieser, I.; May, T.; Vicus, D.; Gien, L.T.; Laframboise, S. Malignant Melanoma of the Vulva and Vagina: A US Population-Based Study of 1863 Patients. Am. J. Clin. Dermatol. 2020, 21, 285–295. [Google Scholar] [CrossRef] [PubMed]
- Balch, C.M.; Soong, S.-J.; Atkins, M.B.; Buzaid, A.C.; Cascinelli, N.; Coit, D.G.; Fleming, I.D.; Gershenwald, J.E.; Houghton, A.; Kirkwood, J.M.; et al. An evidence-based staging system for cutaneous melanoma. CA A Cancer J. Clin. 2004, 54, 131–149. [Google Scholar] [CrossRef] [PubMed]
- Goodson, A.G.; Grossman, D. Strategies for early melanoma detection: Approaches to the patient with nevi. J. Am. Acad. Dermatol. 2009, 60, 719–735. [Google Scholar] [CrossRef]
- September, P. Mitotic activity in non-neoplastic melanocytes in vivo. Prospects 1975, 66, 23. [Google Scholar]
- Hodis, E.; Watson, I.R.; Kryukov, G.V.; Arold, S.T.; Imielinski, M.; Theurillat, J.-P.; Nickerson, E.; Auclair, D.; Li, L.; Place, C.; et al. A Landscape of Driver Mutations in Melanoma. Cell 2012, 150, 251–263. [Google Scholar] [CrossRef] [PubMed]
- The Cancer Genome Atlas Network. Genomic Classification of Cutaneous Melanoma. Cell 2015, 161, 1681–1696. [Google Scholar] [CrossRef]
- Libra, M.; Malaponte, G.; Navolanic, P.M.; Gangemi, P.; Bevelacqua, V.; Proietti, L.; Bruni, B.; Stivala, F.; Mazzarino, M.C.; Travali, S.; et al. Analysis of BRAF Mutation in Primary and Metastatic Melanoma Brief Report ABBREVIATIONS ND KEY WORDS ES CE INTRODUCTION RIB. Cell Cycle 2005, 4, 1382–1384. [Google Scholar] [CrossRef] [PubMed]
- Yun, J.; Lee, J.; Jang, J.; Lee, E.J.; Jang, K.T.; Kim, J.H.; Kim, K.-M. KIT amplification and gene mutations in acral/mucosal melanoma in Korea. APMIS 2011, 119, 330–335. [Google Scholar] [CrossRef] [PubMed]
- Nassar, K.; Tan, A.C. The mutational landscape of mucosal melanoma. Semin. Cancer Biol. 2020, 61, 139–148. [Google Scholar] [CrossRef]
- Darman, R.B.; Seiler, M.; Agrawal, A.A.; Lim, K.H.; Peng, S.; Aird, D.; Bailey, S.L.; Bhavsar, E.B.; Chan, B.; Colla, S.; et al. Cancer-Associated SF3B1 Hotspot Mutations Induce Cryptic 3′ Splice Site Selection through Use of a Different Branch Point. Cell Rep. 2015, 13, 1033–1045. [Google Scholar] [CrossRef] [PubMed]
- Hintzsche, J.D.; Gorden, N.T.; Amato, C.M.; Kim, J.; Wuensch, K.E.; Robinson, S.E.; Applegate, A.J.; Couts, K.L.; Medina, T.M.; Wells, K.R.; et al. Whole-exome sequencing identifies recurrent SF3B1 R625 mutation and comutation of NF1 and KIT in mucosal melanoma. Melanoma Res. 2017, 27, 189–199. [Google Scholar] [CrossRef] [PubMed]
- Quek, C.; Rawson, R.V.; Ferguson, P.M.; Shang, P.; Silva, I.; Saw, R.P.; Shannon, K.; Thompson, J.F.; Hayward, N.K.; Long, G.V.; et al. Recurrent hotspot SF3B1 mutations at codon 625 in vulvovaginal mucosal melanoma identified in a study of 27 Australian mucosal melanomas. Oncotarget 2019, 10, 930–941. [Google Scholar] [CrossRef] [PubMed]
- Dhawan, P.; Singh, A.B.; Ellis, D.L.; Richmond, A. Constitutive activation of Akt/protein kinase B in melanoma leads to up-regulation of nuclear factor-κB and tumor progression. Cancer Res. 2002, 62, 7335–7342. [Google Scholar] [PubMed]
- Kong, Y.; Si, L.; Li, Y.; Wu, X.; Xu, X.; Dai, J.; Tang, H.; Ma, M.; Chi, Z.; Sheng, X.; et al. Analysis of mTOR Gene Aberrations in Melanoma Patients and Evaluation of Their Sensitivity to PI3K–AKT–mTOR Pathway Inhibitors. Clin. Cancer Res. 2016, 22, 1018–1027. [Google Scholar] [CrossRef]
- Wu, H.; Goel, V.; Haluska, F.G. PTEN signaling pathways in melanoma. Oncogene 2003, 22, 3113–3122. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Diaz, A.; Shin, D.S.; Moreno, B.H.; Saco, J.; Escuin-Ordinas, H.; Rodriguez, G.A.; Zaretsky, J.M.; Sun, L.; Hugo, W.; Wang, X.; et al. Interferon Receptor Signaling Pathways Regulating PD-L1 and PD-L2 Expression. Cell Rep. 2017, 19, 1189–1201. [Google Scholar] [CrossRef] [PubMed]
- Freeman, G.J.; Long, A.J.; Iwai, Y.; Bourque, K.; Chernova, T.; Nishimura, H.; Fitz, L.J.; Malenkovich, N.; Okazaki, T.; Byrne, M.C.; et al. Engagement of the Pd-1 Immunoinhibitory Receptor by a Novel B7 Family Member Leads to Negative Regulation of Lymphocyte Activation. J. Exp. Med. 2000, 192, 1027–1034. [Google Scholar] [CrossRef] [PubMed]
- Kirkwood, J.M.; Richards, T.; Zarour, H.M.; Sosman, J.; Ernstoff, M.; Whiteside, T.L.; Ibrahim, J.; Blum, R.; Wieand, S.; Mascari, R. Immunomodulatory effects of high-dose and low-dose interferon ?2b in patients with high-risk resected melanoma. Cancer 2002, 95, 1101–1112. [Google Scholar] [CrossRef] [PubMed]
- Tasseron, E.W.; Van Der Esch, E.P.; Hart, A.; De La Rivière, G.B.; Aartsen, E.J. A clinicopathological study of 30 melanomas of the vulva. Gynecol. Oncol. 1992, 46, 170–175. [Google Scholar] [CrossRef]
- Moxley, K.; Fader, A.; Rose, P.; Case, A.; Mutch, D.; Berry, E.; Schink, J.; Kim, C.; Chi, D.; Moore, K. Malignant melanoma of the vulva: An extension of cutaneous melanoma? Gynecol. Oncol. 2011, 122, 612–617. [Google Scholar] [CrossRef] [PubMed]
- Cancer-1 March 1993-Ragnarsson-Olding-Malignant Melanoma of the Vulva and Vagina Trends in Incidence Age.pdf. Available online: https://acsjournals.onlinelibrary.wiley.com/doi/abs/10.1002/1097-0142(19930301)71:5%3C1893::AID-CNCR2820710528%3E3.0.CO;2-7 (accessed on 30 August 2022).
- Weinstock, M.A. Malignant melanoma of the vulva and vagina in the United States: Patterns of incidence and popu lation-based estimates of survival. Am. J. Obstet. Gynecol. 1994, 171, 1225–1230. [Google Scholar] [CrossRef]
- Mert, I.; Semaan, A.; Winer, I.; Morris, R.T.; Ali-Fehmi, R. Vulvar/Vaginal Melanoma. Int. J. Gynecol. Cancer 2013, 23, 1118–1126. [Google Scholar] [CrossRef]
- Curtin, J.A.; Busam, K.; Pinkel, D.; Bastian, B.C. Somatic Activation of KIT in Distinct Subtypes of Melanoma. J. Clin. Oncol. 2006, 24, 4340–4346. [Google Scholar] [CrossRef]
- Willmore-Payne, C.; Holden, J.A.; Tripp, S.; Layfield, L.J. Human malignant melanoma: Detection of BRAF- and c-kit–activating mutations by high-resolution amplicon melting analysis. Hum. Pathol. 2005, 36, 486–493. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.Y.; Baptiste, C.; Mbbs, R.B.H.; Tergas, A.I.; Feldman, R.; Jones, N.L.; Chatterjee-Paer, S.; Bus-Kwolfski, A.; Wright, J.D.; Burke, W.M. Vulvar and vaginal melanoma: A unique subclass of mucosal melanoma based on a comprehensive molecular analysis of 51 cases compared with 2253 cases of nongynecologic melanoma. Cancer 2017, 123, 1333–1344. [Google Scholar] [CrossRef]
- Udager, A.M.; Frisch, N.K.; Hong, L.J.; Stasenko, M.; Johnston, C.M.; Liu, J.R.; Chan, M.; Harms, P.; Fullen, D.R.; Orsini, A.; et al. Gynecologic melanomas: A clinicopathologic and molecular analysis. Gynecol. Oncol. 2017, 147, 351–357. [Google Scholar] [CrossRef]
- Aulmann, S.; Sinn, H.-P.; Penzel, R.; Gilks, C.B.; Schott, S.; Hassel, J.C.; Schmidt, D.; Kommoss, F.; Schirmacher, P.; Kommoss, S. Comparison of molecular abnormalities in vulvar and vaginal melanomas. Mod. Pathol. 2014, 27, 1386–1393. [Google Scholar] [CrossRef]
- Wohlmuth, C.; Wohlmuth-Wieser, I. Vulvar Melanoma: Molecular Characteristics, Diagnosis, Surgical Management, and Medical Treatment. Am. J. Clin. Dermatol. 2021, 22, 639–651. [Google Scholar] [CrossRef]
- Grunsven, A.C.V.E.-V.; Küsters-Vandevelde, H.V.; De Hullu, J.; van Duijn, L.M.; Rijntjes, J.; Bovée, J.V.; Groenen, P.J.; Blokx, W.A. NRAS mutations are more prevalent than KIT mutations in melanoma of the female urogenital tract—A study of 24 cases from the Netherlands. Gynecol. Oncol. 2014, 134, 10–14. [Google Scholar] [CrossRef]
- Saleh, B.; Kriegsmann, J.; Falk, S.; Aulmann, S. Frequent PD-L1 Expression in Malignant Melanomas of the Vulva. Int. J. Gynecol. Pathol. 2018, 37, 477–481. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.-Y.; Wu, X.-Y.; Zhang, X.; Yang, X.-H.; Long, Y.-K.; Feng, Y.-F.; Wang, F. Prevalence of NRAS Mutation, PD-L1 Expression and Amplification, and Overall Survival Analysis in 36 Primary Vaginal Melanomas. Oncologist 2020, 25, e291–e301. [Google Scholar] [CrossRef] [PubMed]
- Tsai, K.K.; Zarzoso, I.; Daud, A.I. PD-1 and PD-L1 antibodies for melanoma. Hum. Vaccines Immunother. 2014, 10, 3111–3116. [Google Scholar] [CrossRef] [PubMed]
- Larkin, J.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.-J.; Cowey, C.L.; Lao, C.D.; Schadendorf, D.; Dummer, R.; Smylie, M.; Rutkowski, P.; et al. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N. Engl. J. Med. 2015, 373, 23–34. [Google Scholar] [CrossRef]
- Wolchok, J.D.; Kluger, H.; Callahan, M.K.; Postow, M.A.; Rizvi, N.A.; Lesokhin, A.M.; Segal, N.H.; Ariyan, C.E.; Gordon, R.-A.; Reed, K.; et al. Nivolumab plus Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 2013, 369, 122–133. [Google Scholar] [CrossRef]
- Wolchok, J.D.; Chiarion-Sileni, V.; Gonzalez, R.; Rutkowski, P.; Grob, J.-J.; Cowey, C.L.; Lao, C.D.; Wagstaff, J.; Schadendorf, D.; Ferrucci, P.F.; et al. Overall Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 2017, 377, 1345–1356. [Google Scholar] [CrossRef]
- Larkin, J.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.-J.; Rutkowski, P.; Lao, C.D.; Cowey, C.L.; Schadendorf, D.; Wagstaff, J.; Dummer, R.; et al. Five-Year Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 2019, 381, 1535–1546. [Google Scholar] [CrossRef]
- Robert, C.; Long, G.V.; Brady, B.; Dutriaux, C.; Maio, M.; Mortier, L.; Hassel, J.C.; Rutkowski, P.; McNeil, C.; Kalinka-Warzocha, E.; et al. Nivolumab in Previously Untreated Melanoma without BRAF Mutation. N. Engl. J. Med. 2015, 372, 320–330. [Google Scholar] [CrossRef]
- Helgadottir, H.; Ghiorzo, P.; van Doorn, R.; Puig, S.; Levin, M.; Kefford, R.; Lauss, M.; Queirolo, P.; Pastorino, L.; Kapiteijn, E.; et al. Efficacy of novel immunotherapy regimens in patients with metastatic melanoma with germline CDKN2A mutations. J. Med. Genet. 2020, 57, 316–321. [Google Scholar] [CrossRef]
- Adib, E.; Nassar, A.H.; Akl, E.W.; Alaiwi, S.A.; Nuzzo, P.V.; Mouhieddine, T.H.; Sonpavde, G.; Haddad, R.I.; Mouw, K.W.; Giannakis, M.; et al. CDKN2A Alterations and Response to Immunotherapy in Solid Tumors. Clin. Cancer Res. 2021, 27, 4025–4035. [Google Scholar] [CrossRef]
- Horn, S.; Leonardelli, S.; Sucker, A.; Schadendorf, D.; Griewank, K.G.; Paschen, A. Tumor CDKN2A-Associated JAK2 Loss and Susceptibility to Immunotherapy Resistance. JNCI: J. Natl. Cancer Inst. 2018, 110, 677–681. [Google Scholar] [CrossRef] [PubMed]
- DeLeon, T.T.; Almquist, D.R.; Kipp, B.R.; Langlais, B.T.; Mangold, A.; Winters, J.L.; Kosiorek, H.E.; Joseph, R.W.; Dronca, R.S.; Block, M.S.; et al. Assessment of clinical outcomes with immune checkpoint inhibitor therapy in melanoma patients with CDKN2A and TP53 pathogenic mutations. PLoS ONE 2020, 15, e0230306. [Google Scholar] [CrossRef] [PubMed]
- Kirchberger, M.C.; Ugurel, S.; Mangana, J.; Heppt, M.V.; Eigentler, T.K.; Berking, C.; Schadendorf, D.; Schuler, G.; Dummer, R.; Heinzerling, L. MEK inhibition may increase survival of NRAS-mutated melanoma patients treated with checkpoint blockade: Results of a retrospective multicentre analysis of 364 patients. Eur. J. Cancer 2018, 98, 10–16. [Google Scholar] [CrossRef]
- Hu-Lieskovan, S. Improved antitumor activity of immunotherapy with BRAF and MEK inhibitors in BRAFV600E melanoma. Cancer 2012, 17, 1310–1314. [Google Scholar]
- Eggermont, A.M.M.; Blank, C.U.; Mandala, M.; Long, G.V.; Atkinson, V.G.; Dalle, S.; Haydon, A.M.; Meshcheryakov, A.; Khattak, A.; Carlino, M.S.; et al. Longer Follow-Up Confirms Recurrence-Free Survival Benefit of Adjuvant Pembrolizumab in High-Risk Stage III Melanoma: Updated Results From the EORTC 1325-MG/KEYNOTE-054 Trial. J. Clin. Oncol. 2020, 38, 3925–3936. [Google Scholar] [CrossRef] [PubMed]
- Robert, C.; Ribas, A.; Schachter, J.; Arance, A.; Grob, J.-J.; Mortier, L.; Daud, A.; Carlino, M.S.; McNeil, C.M.; Lotem, M.; et al. Pembrolizumab versus ipilimumab in advanced melanoma (KEYNOTE-006): Post-hoc 5-year results from an open-label, multicentre, randomised, controlled, phase 3 study. Lancet Oncol. 2019, 20, 1239–1251. [Google Scholar] [CrossRef]
- Petrella, T.M.; Robert, C.; Richtig, E.; Miller, W.H.; Masucci, G.V.; Walpole, E.; Lebbe, C.; Steven, N.; Middleton, M.R.; Hille, D.; et al. Patient-reported outcomes in KEYNOTE-006, a randomised study of pembrolizumab versus ipilimumab in patients with advanced melanoma. Eur. J. Cancer 2017, 86, 115–124. [Google Scholar] [CrossRef]
- Carlino, M.S.; Long, G.V.; Schadendorf, D.; Robert, C.; Ribas, A.; Richtig, E.; Nyakas, M.; Caglevic, C.; Tarhini, A.; Blank, C.; et al. Outcomes by line of therapy and programmed death ligand 1 expression in patients with advanced melanoma treated with pembrolizumab or ipilimumab in KEYNOTE-006: A randomised clinical trial. Eur. J. Cancer 2018, 101, 236–243. [Google Scholar] [CrossRef]
- Daud, A.; Puzanov, I.; Dummer, R.; Schadendorf, D.; Hamid, O.; Robert, C.; Hodi, F.; Schachter, J.; Sosman, J.; Pavlick, A.; et al. Analysis of response and survival in patients (pts) with ipilimumab (ipi)-refractory melanoma treated with pembrolizumab (pembro) in KEYNOTE-002. Ann. Oncol. 2017, 28, v434. [Google Scholar] [CrossRef]
- Schadendorf, D.; Dummer, R.; Hauschild, A.; Robert, C.; Hamid, O.; Daud, A.; Eertwegh, A.V.D.; Cranmer, L.; O’Day, S.; Puzanov, I.; et al. Health-related quality of life in the randomised KEYNOTE-002 study of pembrolizumab versus chemotherapy in patients with ipilimumab-refractory melanoma. Eur. J. Cancer 2016, 67, 46–54. [Google Scholar] [CrossRef]
- Ribas, A.; Puzanov, I.; Dummer, R.; Schadendorf, D.; Hamid, O.; Robert, C.; Hodi, F.S.; Schachter, J.; Pavlick, A.C.; Lewis, K.D.; et al. Pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory melanoma (KEYNOTE-002): A randomised, controlled, phase 2 trial. Lancet Oncol. 2015, 16, 908–918. [Google Scholar] [CrossRef]
- Albert, A.; Lee, A.; Allbright, R.; Vijayakumar, S. Vulvar melanoma: An analysis of prognostic factors and treatment patterns. J. Gynecol. Oncol. 2020, 31, e66. [Google Scholar] [CrossRef] [PubMed]
- D’Angelo, S.P.; Larkin, J.; Sosman, J.A.; Lebbé, C.; Brady, B.; Neyns, B.; Schmidt, H.; Hassel, J.C.; Hodi, F.S.; Lorigan, P.; et al. Efficacy and Safety of Nivolumab Alone or in Combination With Ipilimumab in Patients With Mucosal Melanoma: A Pooled Analysis. J. Clin. Oncol. 2017, 35, 226–235. [Google Scholar] [CrossRef]
- Hamid, O.; Robert, C.; Ribas, A.; Hodi, F.S.; Walpole, E.; Daud, A.; Arance, A.S.; Brown, E.; Hoeller, C.; Mortier, L.; et al. Antitumour activity of pembrolizumab in advanced mucosal melanoma: A post-hoc analysis of KEYNOTE-001, 002, 006. Br. J. Cancer 2018, 119, 670–674. [Google Scholar] [CrossRef] [PubMed]
- Egger, E.K.; Stope, M.B.; Recker, F.; Konsgen, D.; Landsberg, J.; Frohlich, A.; Abramian, A.; Mustea, A. Lower Genital Tract Melanomas: Staging, Predictors of Outcome, and New Therapeutic Options. Anticancer Res. 2021, 41, 999–1004. [Google Scholar] [CrossRef]
- Tasaka, R.; Fukuda, T.; Wada, T.; Kawanishi, M.; Imai, K.; Kasai, M.; Hashiguchi, Y.; Ichimura, T.; Yasui, T.; Sumi, T. A retrospective clinical analysis of 5 cases of vaginal melanoma. Mol. Clin. Oncol. 2017, 6, 373–376. [Google Scholar] [CrossRef]
- Wilhite, A.; Wu, S.; Xiu, J.; Korn, W.M.; Phung, T.; Herzog, T.; In, G.; Gibney, G.; Brown, J.; Rocconi, R.; et al. Too much skin in the game? A paradigm shift in our understanding of vulvar and vaginal melanomas as distinct tumor types compared with cutaneous melanomas. Gynecol. Oncol. 2021, 162, S33–S34. [Google Scholar] [CrossRef]
- Skovsted, S.; Nielsen, K.; Blaakær, J. Melanomas of the vulva and vagina. Dan. Med. J. 2017, 64. [Google Scholar]
- Boer, F.L.; Eikelder, M.L.T.; van Geloven, N.; Kapiteijn, E.H.; Gaarenstroom, K.N.; Hughes, G.; Nooij, L.S.; Jozwiak, M.; Tjiong, M.Y.; de Hullu, J.M.; et al. Evaluation of treatment, prognostic factors, and survival in 198 vulvar melanoma patients: Implications for clinical practice. Gynecol. Oncol. 2021, 161, 202–210. [Google Scholar] [CrossRef]
- Palareti, G.; Legnani, C.; Cosmi, B.; Antonucci, E.; Erba, N.; Poli, D.; Testa, S. The DULCIS (D-Dimer-ULtrasonography In Combination Italian Study) Investigators. Comparison between different D-Dimer cutoff values to assess the individual risk of recurrent venous thromboembolism: Analysis of results obtained in the DULCIS study. Int. J. Lab. Hematol. 2016, 38, 42–49. [Google Scholar] [CrossRef]
- Chłopik, A.; Selim, M.A.; Peng, Y.; Wu, C.-L.; Tell-Marti, G.; Paral, K.M.; Shalin, S.C.; Kraft, S.; Hsu, C.-K.; Shea, C.R.; et al. Prognostic role of tumoral PDL1 expression and peritumoral FoxP3+ lymphocytes in vulvar melanomas. Hum. Pathol. 2018, 73, 176–183. [Google Scholar] [CrossRef]
- Indini, A.; Di Guardo, L.; Cimminiello, C.; Lorusso, D.; Raspagliesi, F.; Del Vecchio, M. Investigating the role of immunotherapy in advanced/recurrent female genital tract melanoma: A preliminary experience. J. Gynecol. Oncol. 2019, 30, e94. [Google Scholar] [CrossRef] [PubMed]
- Sezen, D.; Patel, R.R.; Tang, C.; Onstad, M.; Nagarajan, P.; Patel, S.P.; Welsh, J.W.; Lin, L.L. Immunotherapy combined with high- and low-dose radiation to all sites leads to complete clearance of disease in a patient with metastatic vaginal melanoma. Gynecol. Oncol. 2021, 161, 645–652. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Song, J.; Sun, Y.; Cui, Z. Multiple metastases after surgery for a rare vulvar malignant melanoma combined with immunotherapy: A case report. J. Int. Med. Res. 2020, 48, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Chanal, J.; Kramkimel, N.; Guegan, S.; Moguelet, P.; Fourchotte, V.; Avril, M.-F. Locally Advanced Unresectable Vaginal Melanoma: Response With Anti–Programmed Death Receptor 1. J. Low. Genit. Tract Dis. 2016, 20, e4–e5. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).