A Systematic Review to Evaluate Patient-Reported Outcome Measures (PROMs) for Metastatic Prostate Cancer According to the COnsensus-Based Standard for the Selection of Health Measurement INstruments (COSMIN) Methodology
Abstract
Simple Summary
Abstract
1. Introduction
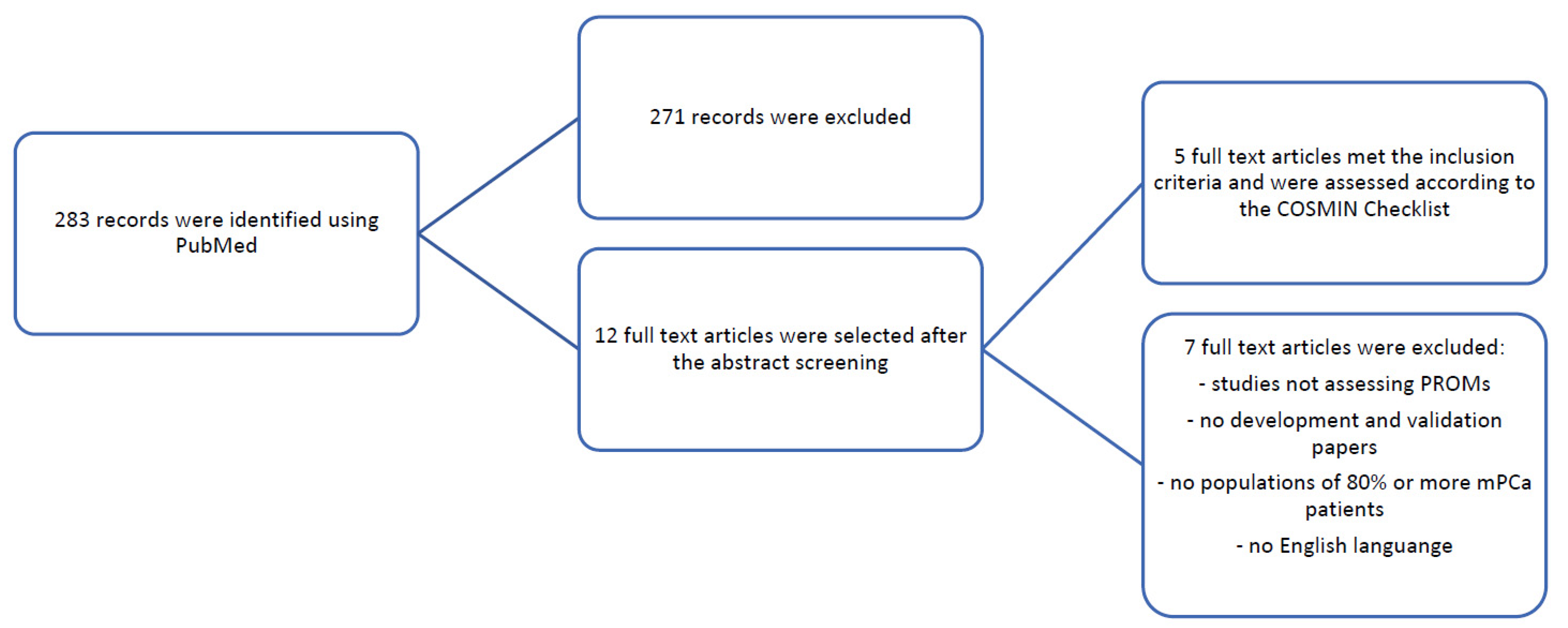
2. Evidence Acquisition
2.1. Search Strategy
2.2. Inclusion and Exclusion Criteria
2.3. Screening
2.4. Data Extraction
2.5. Appraising Methodological Quality
2.6. Reporting of Psychometric Results
2.7. Appraisal of Levels of Evidence
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Gandaglia, G.; Leni, R.; Bray, F.; Fleshner, N.; Freedland, S.J.; Kibel, A.; Stattin, P.; Van Poppel, H.; La Vecchia, C. Epidemiology and Prevention of Prostate Cancer. Eur. Urol. Oncol. 2021, 4, 877–892. [Google Scholar] [CrossRef] [PubMed]
- Cornford, P.; van den Bergh, R.; Briers, E.; Van den Broeck, T.; Cumberbatch, M.G.; De Santis, M.; Fanti, S.; Fossati, N.; Gandaglia, G.; Gillessen, S.; et al. EAU-EANM-ESTRO-ESUR-SIOG Guidelines on Prostate Cancer. Part II-2020 Update: Treatment of Relapsing and Metastatic Prostate Cancer. Eur. Urol. 2021, 79, 263–282. [Google Scholar] [CrossRef] [PubMed]
- Ratti, M.M.; Gandaglia, G.; Alleva, E.; Leardini, L.; Sisca, E.S.; Derevianko, A.; Furnari, F.; Mazzoleni Ferracini, S.; Beyer, K.; Moss, C.; et al. PIONEER Consortium. Standardising the Assessment of Patient-reported Outcome Measures in Localised Prostate Cancer. A Systematic Review. Eur. Urol. Oncol. 2022, 5, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Terwee, C.B.; Mokkink, L.B.; Knol, D.L.; Ostelo, R.W.; Bouter, L.M.; de Vet, H.C. Rating the methodological quality in systematic reviews of studies on measurement properties: A scoring system for the COSMIN checklist. Qual. Life Res. 2012, 21, 651–657. [Google Scholar] [CrossRef] [PubMed]
- Prinsen, C.A.C.; Mokkink, L.B.; Bouter, L.M.; Alonso, J.; Patrick, D.L.; De Vet, H.C.; Terwee, C.B. COSMIN guideline for systematic reviews of Patient-Reported Outcome Measures. Qual. Life Res. 2018, 27, 1147–1157. [Google Scholar] [CrossRef] [PubMed]
- Mokkink, L.B.; de Vet, H.C.W.; Prinsen, C.A.C.; Patrick, D.L.; Alonso, J.; Bouter, L.M.; Terwee, C.B. COSMIN Risk of Bias checklist for systematic reviews of Patient-Reported Outcome Measures. Qual. Life Res. 2018, 27, 1171–1179. [Google Scholar] [CrossRef] [PubMed]
- MacLennan, S.; Bekema, H.J.; Williamson, P.R.; Campbell, M.K.; Stewart, F.; MacLennan, S.J.; N’Dow, J.M.; Lam, T.B. A core outcome set for localised prostate cancer effectiveness trials: Protocol for a systematic review of the literature and stakeholder involvement through interviews and a Delphi survey. Trials 2015, 16, 76. [Google Scholar] [CrossRef] [PubMed]
- Shamseer, L.; Moher, D.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A. Preferred reporting items for systematic review and meta-analysis protocols (prisma-p) 2015: Elaboration and explanation. BMJ 2015, 349, 7647. [Google Scholar] [CrossRef] [PubMed]
- Terwee, C.B.; Prinsen, C.A.; de Vet, H.C.W.; Bouter, L.M.; Alonso, J.; Westerman, M.J.; Patrick, D.L.; Mokkink, L.B. COSMIN Methodology for Assessing the Content Validity of Patient-Reported Outcome Measures (PROMs); User manual; version 1; VU University Medical Center: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Terwee, C.B.; Prinsen, C.A.C.; Chiarotto, A.; Westerman, M.J.; Patrick, D.L.; Alonso, J.; Bouter, L.M.; de Vet, H.C.W.; Mokkink, L.B. COSMIN methodology for evaluating the content validity of Patient-Reported Outcome Measures: A Delphi study. Qual. Life Res. 2018, 27, 1159–1170. [Google Scholar] [CrossRef] [PubMed]
- Esper, P.; Mo, F.; Chodak, G.; Sinner, M.; Cella, D.; Pienta, K.J. Measuring quality of life in men with prostate cancer using the functional assessment of cancer therapy-prostate instrument. Urology 1997, 50, 920–928. [Google Scholar] [CrossRef]
- Daut, R.L.; Cleeland, C.S.; Flanery, R.C. Development of the Wisconsin Brief Pain Questionnaire to Assess Pain in Cancer and other Diseases. Pain 1983, 17, 197–210. [Google Scholar] [CrossRef]
- Cleeland, C.S.; Ryan, K.M. Pain assessment: Global use of the Brief Pain Inventory. Ann. Acad. Med. Singap. 1994, 23, 129–138. [Google Scholar] [PubMed]
- Clark, M.J.; Harris, N.; Griebsch, I.; Kaschinski, D.; Copley-Merriman, C. Patient-reported outcome labeling claims and measurement approach for metastatic castration-resistant prostate cancer treatments in the United States and European Union. Health Qual. Life Outcomes 2014, 12, 104. [Google Scholar] [CrossRef] [PubMed]
- Robinson, D.W.; Zhao, N.; Dawkins, F.; Qi, M.; Revicki, D. Pain questionnaire performance in advanced prostate cancer: Comparative results from two international clinical trials. Qual. Life Res. 2013, 22, 2777–2786. [Google Scholar] [CrossRef] [PubMed]
- Gater, A.; Abetz-Webb, L.; Battersby, C.; Parasuraman, B.; McIntosh, S.; Nathan, F.; Piault, E.C. Pain in castration-resistant prostate cancer with bone metastases: A qualitative study. Health Qual. Life Outcomes 2011, 9, 88. [Google Scholar] [CrossRef] [PubMed]
- van Andel, G.; Bottomley, A.; Fosså, S.D.; Efficace, F.; Coens, C.; Guerif, S.; Kynaston, H.; Gontero, P.; Thalmann, G.; Akdas, A.; et al. An international field study of the EORTC QLQ-PR25: A questionnaire for assessing the health-related quality of life of patients with prostate cancer. Eur. J. Cancer 2008, 44, 2418–2424. [Google Scholar] [CrossRef] [PubMed]
- Piedmont, R.L. Factorial Validity. In Encyclopedia of Quality of Life and Well-Being Research; Springer: Dordrecht, The Netherlands, 2014. [Google Scholar]
- Scholar, S. Test-Retest Reliability: National Institutes of Health. Available online: https://www.semanticscholar.org/topic/Test-Retest-Reliability/697331 (accessed on 12 August 2022).
- Kirshner, B.; Guyatt, G. A methodological framework for assessing health indices. J. Chronic. Dis. 1985, 38, 27–36. [Google Scholar] [CrossRef]
| Measurement Property | Definition |
|---|---|
| Reliability | The proportion of the total variance in the measurements that is due to “true” † differences between patients. Usually expressed as an intraclass correlation coefficient (ICC). |
| Internal Consistency | The degree of the interrelatedness among the items. Usually expressed as Cronbach’s α, which measures the extent to which items in a PROM (sub)scale are correlated. |
| Measurement Error | The systematic and random error of a patient’s score that is not attributed to true changes in the construct to be measured. The difference between the true or actual value and the measured value. |
| Content Validity | The degree to which the content of a PROM is an adequate reflection of the construct to be measured. Reflects whether all items of a PROM are relevant, comprehensive, and comprehensible for the population of interest and requires an element of subjectivity when assessing. |
| Structural Validity | The degree to which the scores of a PROM are an adequate reflection of the dimensionality of the construct to be measured. Examines the extent to which the underlying putative structure of a scale is recoverable in a set of test scores. |
| Cross-cultural Validity | The degree to which the performance of the items on a translated or culturally adapted PROM are an adequate reflection of the performance of the items of the original version of the PROM. |
| Hypotheses Testing | Item construct validity. Concerns the degree to which the scores of an instrument are consistent with hypotheses based on the assumption that the instrument validly measures the construct to be measured. |
| Criterion Validity | The degree to which the scores of a PROM are an adequate reflection of a “gold standard”. Criterion validity is an estimate of the extent to which a measure agrees with a gold standard. |
| Responsiveness | The ability of a PROM to detect change over time in the construct to be measured. Refers to the ability of an instrument to distinguish clinically important changes as the result of an intervention. |
| Quality Level | Definition |
|---|---|
| High | We are very confident that the true measurement property lies close to that of the estimate * of the measurement property |
| Moderate | We are moderately confident in the measurement property estimate: the true measurement property is likely to be close to the estimate of the measurement property, but there is a possibility that it is substantially different |
| Low | Our confidence in the measurement property estimate is limited: the true measurement property may be substantially different from the estimate of the measurement property |
| Very low | We have very little confidence in the measurement property estimate: the true measurement property is likely to be substantially different from the estimate of the measurement property |
| Prom | Full Name | Abstract Screening | Full-Text Screening | Full-Text Extracted |
|---|---|---|---|---|
| FACT-P | Functional Assessment of Cancer Therapy—Prostate Cancer | 65 | 3 | 2 |
| EORTC QLQ-C30 | EORTC QLQ Quality of Life Questionnaire | 67 | 4 | 0 |
| BPI | Brief Pain Inventory | 62 | 3 | 1 |
| BPI-SF (Short Form) | Brief Pain Inventory Short Form | 23 | 2 | 2 |
| EORTC QLQ-PR25 | EORTC Prostate Cancer Module | 39 | 0 | 0 |
| EQ-5D-5L | EuroQol five dimensions, five levels questionnaire | 12 | 0 | 0 |
| BFI | Brief Fatigue Inventory | 15 | 0 | 0 |
| Authors | Content Validity * | Structural Validity * | Internal Consistency * | Reliability * | Measurement Error * | Hypothesis Testing * | Responsiveness * | Cross-Cultural Validity * | Criterion Validity * | |
|---|---|---|---|---|---|---|---|---|---|---|
| FACT-P | Clark et al., 2014 [14] | Very high | Very low | Very high | High | / | Very high | Very high | / | / |
| Robinson et al., 2013 [15] | High | Moderate | Very high | Very high | / | High | / | / | Moderate | |
| BPI-SF | Clark et al., 2014 [14] | Very high | Very low | / | Moderate | / | Very low | Very high | / | / |
| Gater et al., 2011 [16] | Very high | Very low | / | / | / | / | / | / | / | |
| BPI | Robinson et al., 2013 [15] | High | Moderate | Very high | Very high | / | Very high | / | / | Moderate |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ratti, M.M.; Gandaglia, G.; Sisca, E.S.; Derevianko, A.; Alleva, E.; Beyer, K.; Moss, C.; Barletta, F.; Scuderi, S.; Omar, M.I.; et al. A Systematic Review to Evaluate Patient-Reported Outcome Measures (PROMs) for Metastatic Prostate Cancer According to the COnsensus-Based Standard for the Selection of Health Measurement INstruments (COSMIN) Methodology. Cancers 2022, 14, 5120. https://doi.org/10.3390/cancers14205120
Ratti MM, Gandaglia G, Sisca ES, Derevianko A, Alleva E, Beyer K, Moss C, Barletta F, Scuderi S, Omar MI, et al. A Systematic Review to Evaluate Patient-Reported Outcome Measures (PROMs) for Metastatic Prostate Cancer According to the COnsensus-Based Standard for the Selection of Health Measurement INstruments (COSMIN) Methodology. Cancers. 2022; 14(20):5120. https://doi.org/10.3390/cancers14205120
Chicago/Turabian StyleRatti, Maria Monica, Giorgio Gandaglia, Elena Silvia Sisca, Alexandra Derevianko, Eugenia Alleva, Katharina Beyer, Charlotte Moss, Francesco Barletta, Simone Scuderi, Muhammad Imran Omar, and et al. 2022. "A Systematic Review to Evaluate Patient-Reported Outcome Measures (PROMs) for Metastatic Prostate Cancer According to the COnsensus-Based Standard for the Selection of Health Measurement INstruments (COSMIN) Methodology" Cancers 14, no. 20: 5120. https://doi.org/10.3390/cancers14205120
APA StyleRatti, M. M., Gandaglia, G., Sisca, E. S., Derevianko, A., Alleva, E., Beyer, K., Moss, C., Barletta, F., Scuderi, S., Omar, M. I., MacLennan, S., Williamson, P. R., Zong, J., MacLennan, S. J., Mottet, N., Cornford, P., Aiyegbusi, O. L., Van Hemelrijck, M., N’Dow, J., & Briganti, A. (2022). A Systematic Review to Evaluate Patient-Reported Outcome Measures (PROMs) for Metastatic Prostate Cancer According to the COnsensus-Based Standard for the Selection of Health Measurement INstruments (COSMIN) Methodology. Cancers, 14(20), 5120. https://doi.org/10.3390/cancers14205120









