A Clinically Significant Prostate Cancer Predictive Model Using Digital Rectal Examination Prostate Volume Category to Stratify Initial Prostate Cancer Suspicion and Reduce Magnetic Resonance Imaging Demand
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Development Cohort
2.2. Validation Cohort
2.3. MpMRI Characteristics
2.4. DRE-Prostate Volume Category Assessment
2.5. CsPCa Definition
2.6. Predictive Model Development
2.7. Endpoint Measurements
2.8. Statistical Analysis
3. Results
3.1. Characteristics of the Development and Validation Cohorts
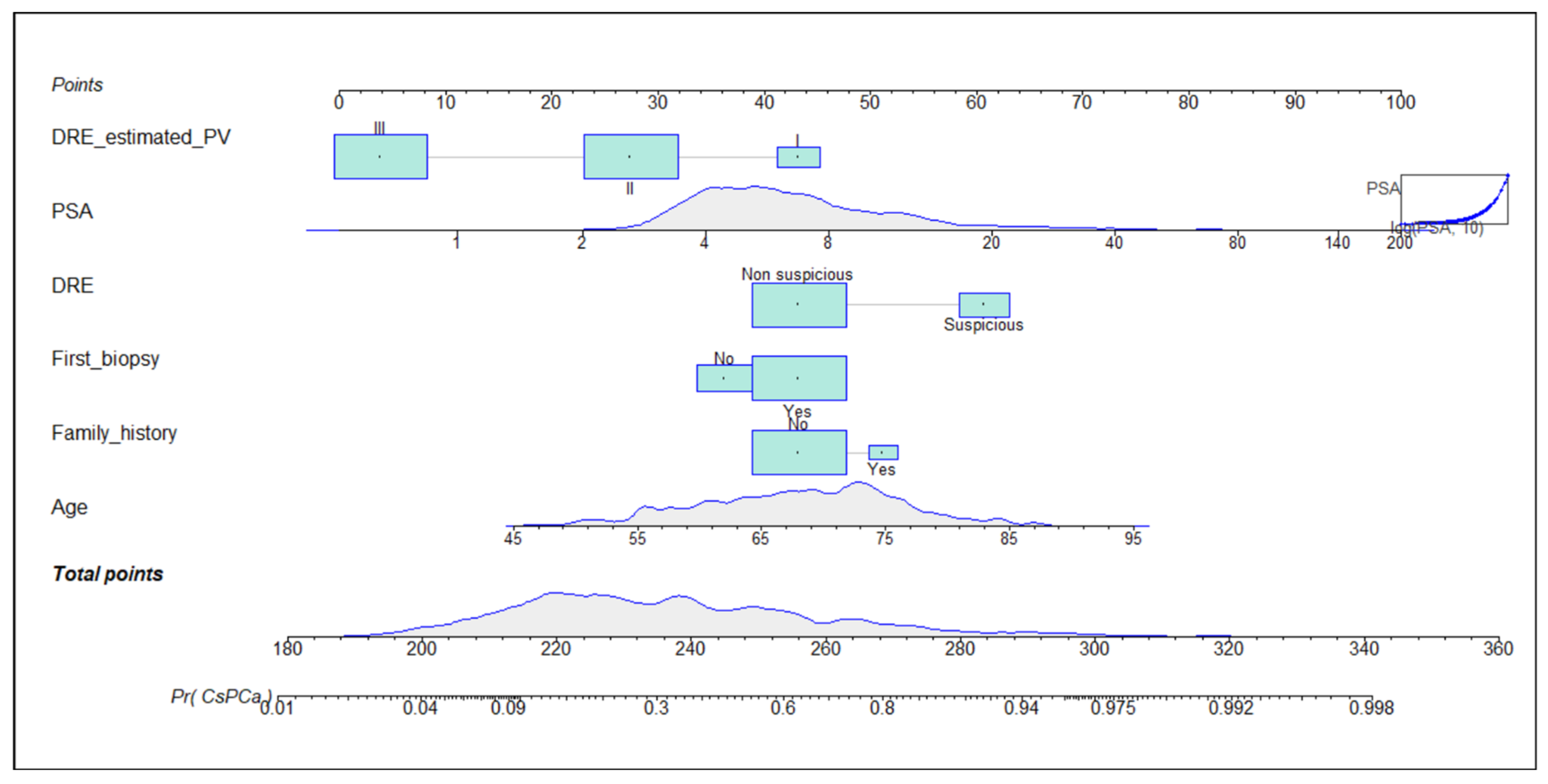
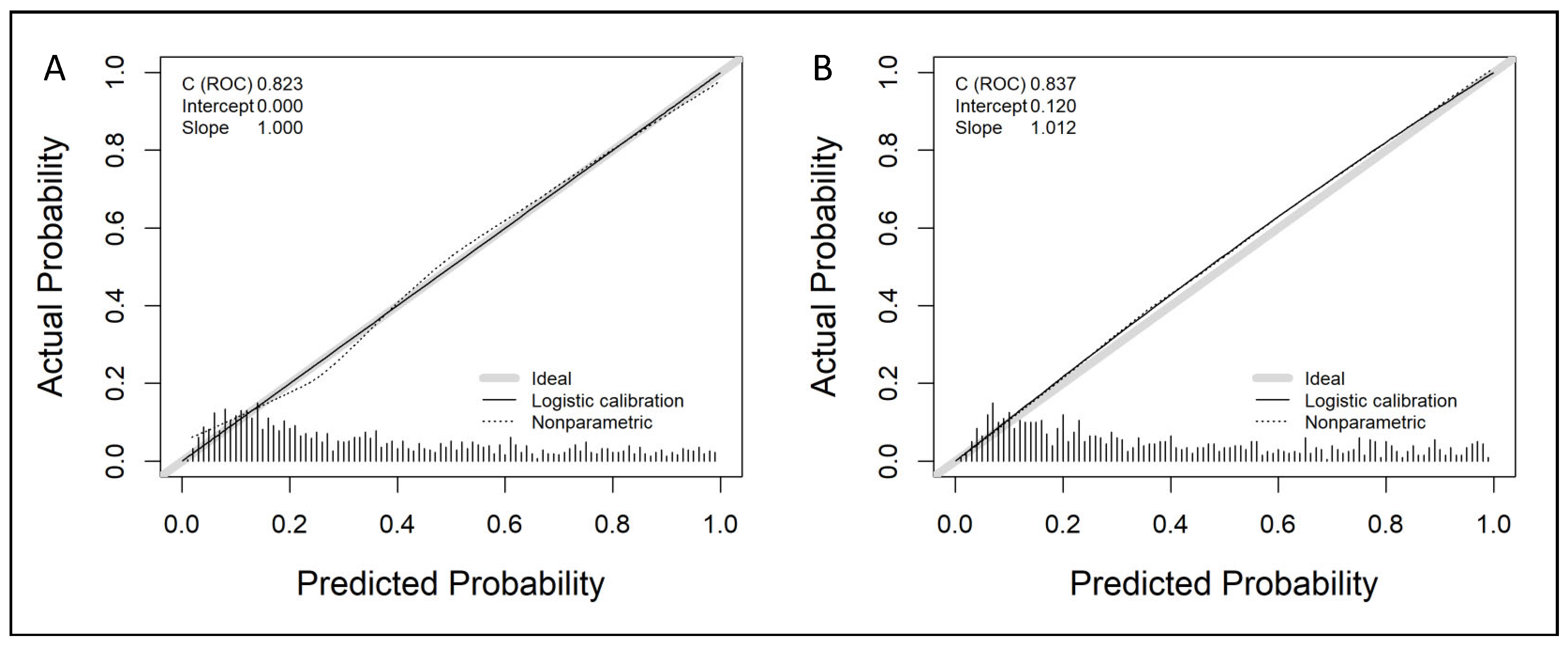
3.2. Development of the Predictive Model and Its Calibration in the Development and Validation Cohorts
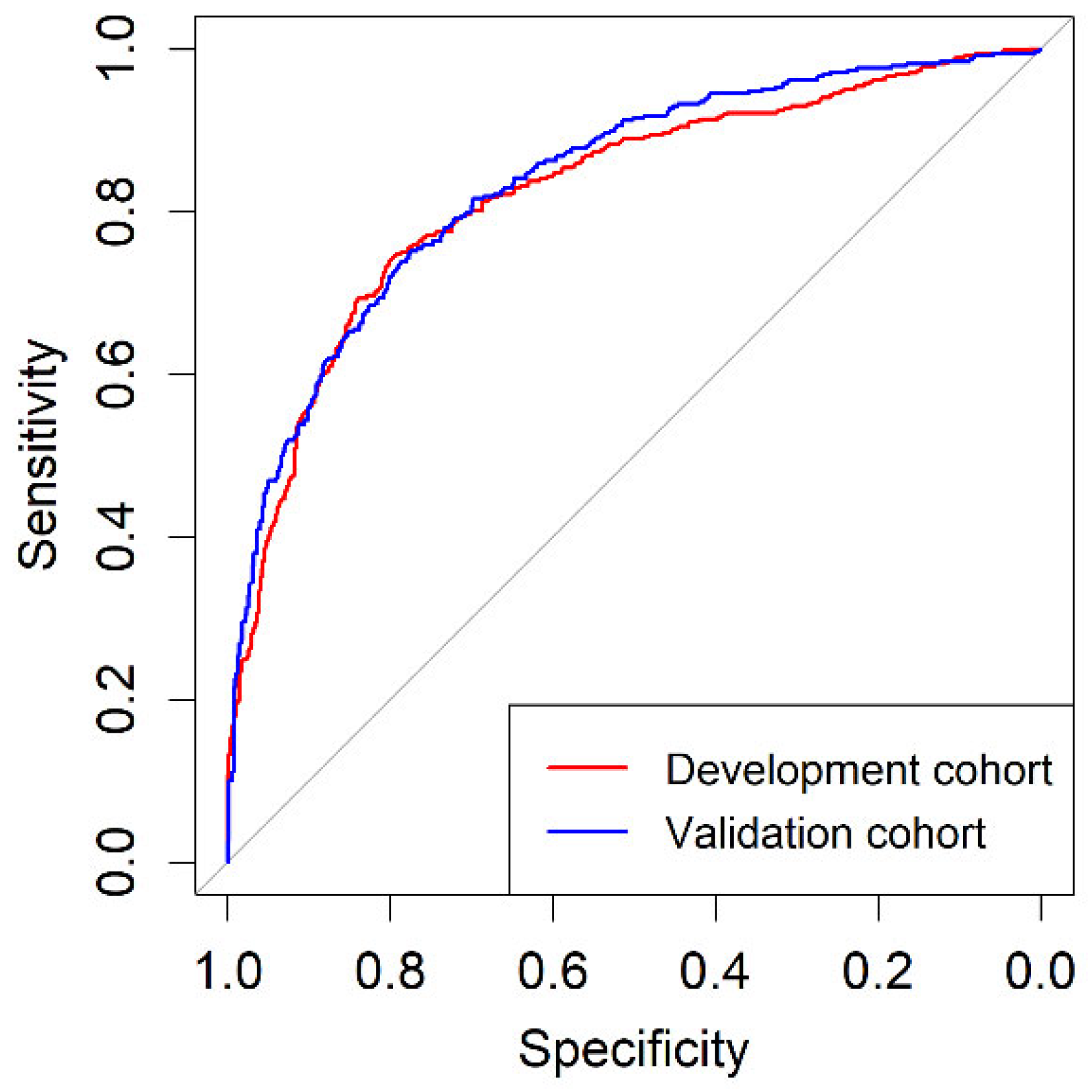
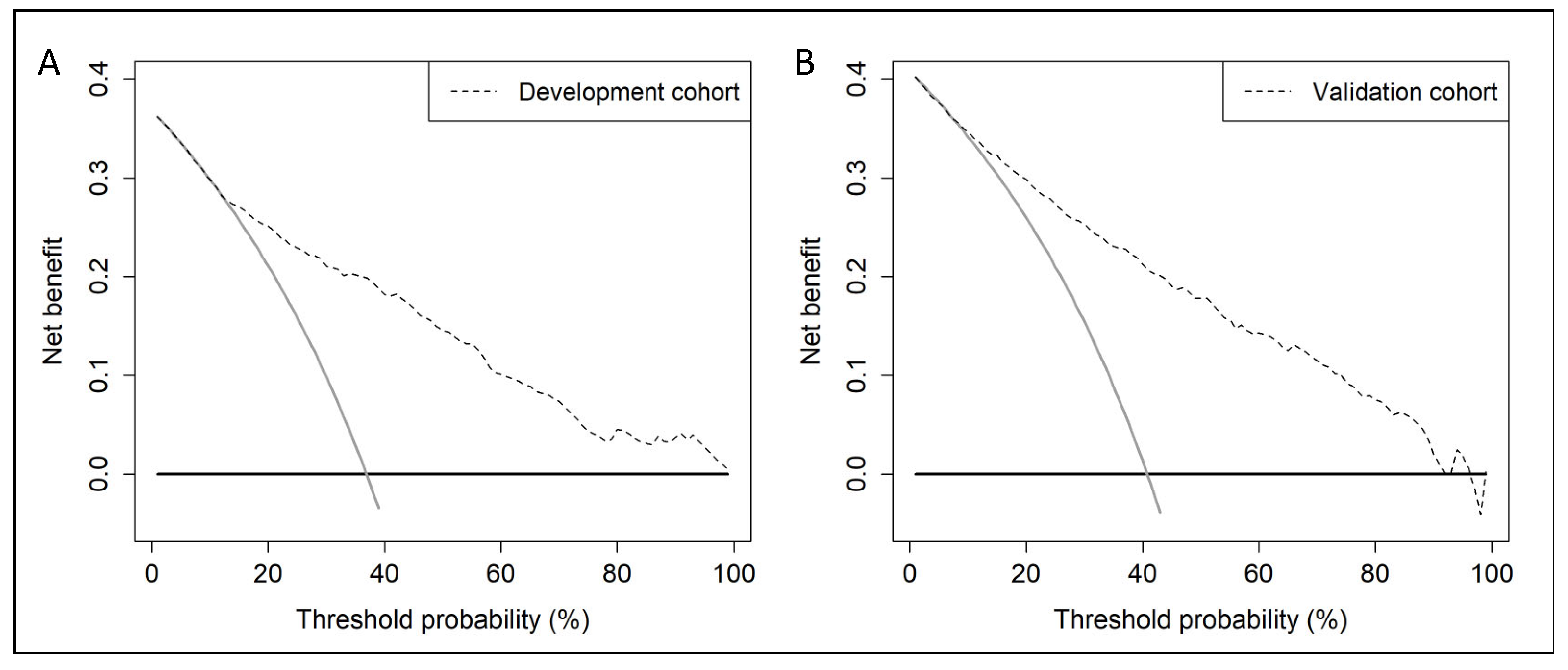
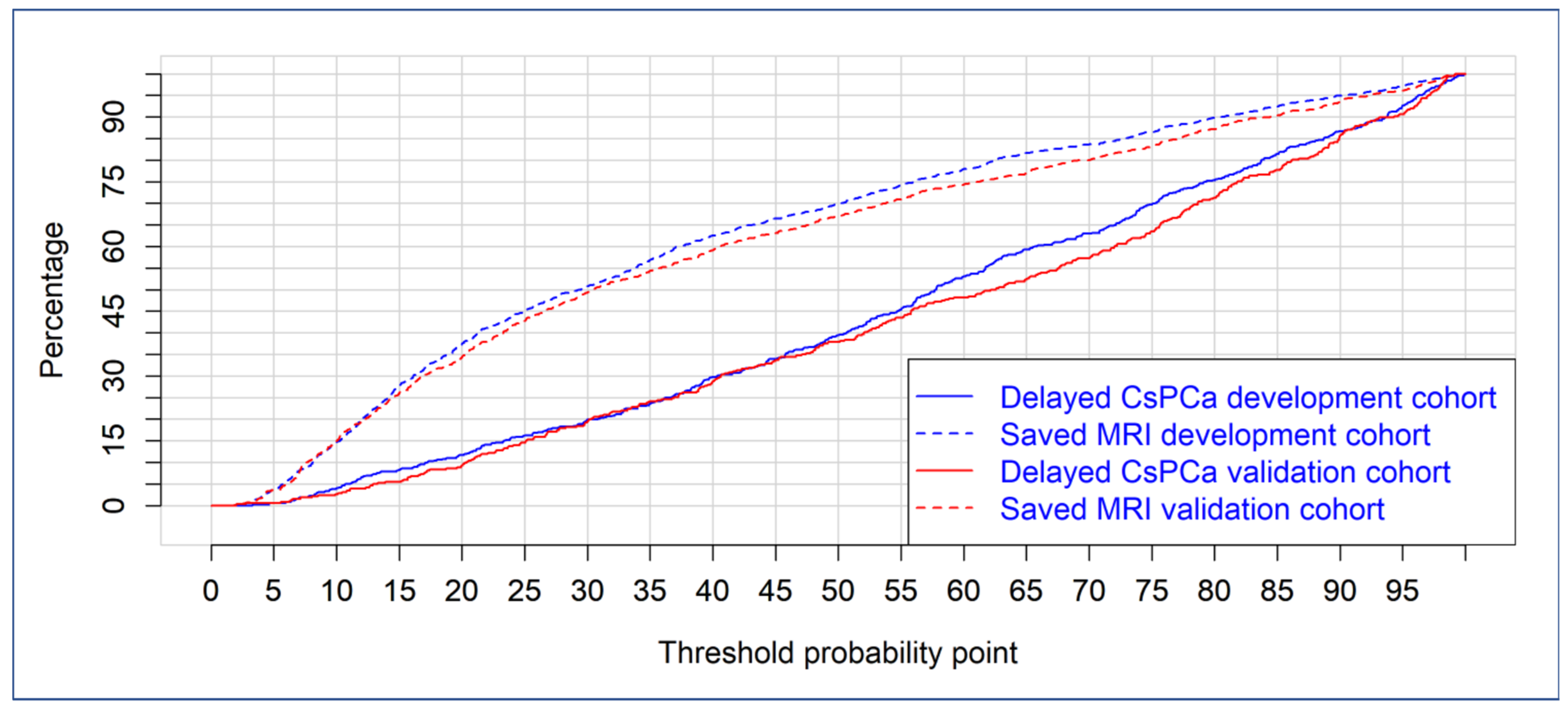
3.3. Discrimination Ability of BCN-RC 1 for csPCa, Net Benefit over Performing mpMRI in All Men, Clinical Utility and Performance in the Development and Validation Cohorts
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Correction Statement
References
- Frånlund, M.; Månsson, M.; Godtman, R.A.; Aus, G.; Holmberg, E.; Kollberg, K.S.; Lodding, P.; Pihl, C.G.; Stranne, J.; Lilja, H.; et al. Results from 22 years of Followup in the Göteborg Randomized Population-Based Prostate Cancer Screening Trial. J. Urol. 2022, 208, 292–300. [Google Scholar] [CrossRef] [PubMed]
- Chou, R.; Croswell, J.M.; Dana, T.; Bougatsos, C.; Blazina, I.; Fu, R.; Gleitsmann, K.; Koenig, H.C.; Lam, C.; Maltz, A.; et al. Screening for prostate cancer: A review of the evidence for the U.S. Preventive Services Task Force. Ann. Intern. Med. 2011, 155, 762–771. [Google Scholar] [CrossRef] [PubMed]
- Schoots, I.G.; Padhani, A.R.; Rouvière, O.; Barentsz, J.O.; Richenberg, J. Analysis of Magnetic Resonance Imaging-directed Biopsy Strategies for Changing the Paradigm of Prostate Cancer Diagnosis. Eur. Urol. Oncol. 2020, 3, 32–41. [Google Scholar] [CrossRef]
- Mottet, N.; van den Bergh, R.C.N.; Briesrs, E.; Briers, E.; Van den Broeck, T.; Cumberbatch, M.G.; De Santis, M.; Fanti, S.; Fossati, N.; Gandaglia, G.; et al. EAU-EANM-ESTRO-ESUR-SIOG Guidelines on Prostate Cancer-2020 Update. Part 1: Screening, Diagnosis, and Local Treatment with Curative Intent. Eur. Urol. 2021, 79, 243–262. [Google Scholar] [CrossRef]
- Van Poppel, H.; Roobol, M.J.; Chapple, C.R.; Catto, J.W.F.; N’Dow, J.; Sønksen, J.; Stenzl, A.; Wirth, M. Prostate-specific Antigen Testing as Part of a Risk-Adapted Early Detection Strategy for Prostate Cancer: European Association of Urology Position and Recommendations for 2021. Eur. Urol. 2021, 80, 703–711. [Google Scholar] [CrossRef]
- Sathianathen, N.J.; Omer, A.; Harris, S.; Davies, L.; Kasivisvanathan, V.; Punwani, S.; Moore, C.M.; Kastner, C.; Barrett, T.; Van Den Bergh, R.C.; et al. Negative Predictive Value of Multiparametric Magnetic Resonance Imaging in the Detection of Clinically Significant Prostate Cancer in the Prostate Imaging Reporting and Data System Era: A Systematic Review and Meta-analysis. Eur. Urol. 2020, 78, 402–414. [Google Scholar] [CrossRef] [PubMed]
- Drost, F.H.; Osses, D.; Nieboer, D.; Bangma, C.H.; Steyerberg, E.W.; Roobol, M.J.; Schoots, I.G. Prostate Magnetic Resonance Imaging, with or Without Magnetic Resonance Imaging-targeted Biopsy, and Systematic Biopsy for Detecting Prostate Cancer: A Cochrane Systematic Review and Meta-analysis. Eur. Urol. 2020, 77, 78–94. [Google Scholar] [CrossRef] [PubMed]
- Mazzone, E.; Stabile, A.; Pellegrino, F.; Basile, G.; Cignoli, D.; Cirulli, G.O.; Sorce, G.; Barletta, F.; Scuderi, S.; Bravi, C.A.; et al. Positive Predictive Value of Prostate Imaging Reporting and DataSystem Version 2 for the Detection of Clinically Significant Prostate Cancer: A Systematic Review and Meta-analysis. Eur. Urol. Oncol. 2020, 4, 697–713. [Google Scholar] [CrossRef] [PubMed]
- Van Poppel, H.; Hogenhout, R.; Albers, P.; van den Bergh, R.C.N.; Barentsz, J.O.; Roobol, M.J. A European Model for an Organised Risk-stratified Early Detection Programme for Prostate Cancer. Eur. Urol. Oncol. 2021, 4, 731–739. [Google Scholar] [CrossRef]
- Belue, M.J.; Yilmaz, E.C.; Daryanani, A.; Turkbey, B. Current Status of Biparametric MRI in Prostate Cancer Diagnosis: Literature Analysis. Life 2022, 12, 804. [Google Scholar] [CrossRef]
- Osses, D.F.; Roobol, M.J.; Schoots, I.G. Prediction Medicine: Biomarkers, Risk Calculators and Magnetic Resonance Imaging as Risk Stratification Tools in Prostate Cancer Diagnosis. Int. J. Mol. Sci. 2020, 20, 1637. [Google Scholar] [CrossRef]
- Govers, T.M.; Hesses, D.; Vlaeminck-Guillem, V.; Schmitz-Dräger, B.J.; Stief, C.G.; Martinez-Ballesteros, C.; Ferro, M.; Borque-Fernando, A.; Rubio-Briones, J.; Sedelaar, J.P.M.; et al. Cost-effectiveness of SelectMDx for prostate cancer in four European countries: A comparative modeling study. Prostate Cancer Prostatic Dis. 2018, 22, 101–109. [Google Scholar] [CrossRef]
- Vickers, A.J.; Russo, G.; Lilja, H.; Evans, C.; Schalken, J.A.; Klein, E.; Eggener, S. How Should Molecular Markers and Magnetic Resonance Imaging Be Used in the Early Detection of Prostate Cancer. Eur. Urol. Oncol. 2022, 5, 135–137. [Google Scholar] [CrossRef]
- Yamashiro, J.R.; de Riese, W.T.W. Any Correlation Between Prostate Volume and Incidence of Prostate Cancer: A Review of Reported Data for the Last Thirty Years. Res. Rep. Urol. 2021, 13, 749–757. [Google Scholar] [CrossRef]
- Benson, M.C.; Whang, I.S.; Pantuck, A.; Ring, K.; Kaplan, S.A.; Olsson, C.A.; Cooner, W.H. Prostate specific antigen density: A means of distinguishing benign prostatic hypertrophy and prostate cancer. J. Urol. 1992, 147, 815–816. [Google Scholar] [CrossRef]
- Dianat, S.S.; Rancier Ruiz, R.M.; Bonekamp, D.; Carter, H.B.; Macura, K.J. Prostate volumetric assessment by magnetic resonance imaging and transrectal ultrasound: Impact of variation in calculated prostate-specific antigen density on patient eligibility for active surveillance program. J. Comput. Assist. Tomogr. 2013, 37, 589–595. [Google Scholar] [CrossRef]
- Morote, J.; Celma, A.; Diaz, F.; Regis, L.; Roche, S.; Mast, R.; Semidey, M.E.; de Torres, I.M.; Planas, J.; Trilla, E. Prostatic-specific antigen density behavior according to multiparametric magnetic resonance imaging result. Urol. Oncol. 2020, 38, 410–417. [Google Scholar] [CrossRef]
- Morote, J.; Díaz, F.; Celma, A.; Planas, J.; Trilla, E. Behavior of SelectMDx and Prostate-specific Antigen Density in the Challenging Scenario of Prostate Imaging-Reporting and Data System Category 3 Lesions. Eur. Urol. 2022, 81, 124–125. [Google Scholar] [CrossRef]
- Roobol, M.J.; van Vugt, H.A.; Loeb, S.; Zhu, X.; Bul, M.; Bangma, C.H.; van Leenders, A.G.; Steyerberg, E.W.; Schröder, F.H. Prediction of prostate cancer risk: The role of prostate volume and digital rectal examination in the ERSPC risk calculators. Eur. Urol. 2012, 61, 577–583. [Google Scholar] [CrossRef]
- Roobol, M.J.; Schröder, F.H.; Hugosson, J.; Jones, J.S.; Kattan, M.W.; Klein, E.A.; Hamdy, F.; Neal, D.; Donovan, J.; Parekh, D.J.; et al. Importance of prostate volume in the European Randomised Study of Screening for Prostate Cancer (ERSPC) risk calculators: Results from the prostate biopsy collaborative group. World J. Urol. 2012, 30, 149–155. [Google Scholar] [CrossRef]
- Roehrborn, C.G.; Girman, C.J.; Rhodes, T.; Hanson, K.A.; Collins, G.N.; Sech, S.M.; Jacobsen, S.J.; Garraway, W.M.; Lieber, M.M. Correlation between prostate size estimated by digital rectal examination and measured by transrectal ultrasound. Urology 1997, 49, 548–557. [Google Scholar] [CrossRef]
- Naji, L.; Randhawa, H.; Sohani, Z.; Dennis, B.; Lautenbach, D.; Kavanagh, O.; Bawor, M.; Banfield, L.; Profetto, J. Digital Rectal Examination for Prostate Cancer Screening in Primary Care: A Systematic Review and Meta-Analysis. Ann. Fam. Med. 2018, 16, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Morote, J.; Borque-Fernando, A.; Triquell, M.; Celma, A.; Regis, L.; Abascal, J.M.; Sola, C.; Servian, P.; Escobar, M.; Mast, R.; et al. The Barcelona Predictive Model of Clinically Significant Prostate Cancer. Cancers 2022, 14, 1589. [Google Scholar] [CrossRef] [PubMed]
- Bossuyt, P.M.; Reitsma, J.B.; Bruns, D.E.; Korevaar, D.A. STARD 2015: An updated list of essential items for reporting diagnostic accuracy studies. BMJ 2015, 351, h5527. [Google Scholar] [CrossRef] [PubMed]
- Barentsz, J.O.; Richenberg, J.; Clements, R.; Choyke, P.; Verma, S.; Villeirs, G.; Rouviere, O.; Logager, V.; Futterer, J.J. ESUR prostate MR guidelines 2012. Eur. Radiol. 2012, 22, 746–757. [Google Scholar] [CrossRef] [PubMed]
- Weinreb, J.C.; Barentsz, J.O.; Choyke, P.L.; Cornud, F.; Haider, M.A.; Macura, K.J.; Margolis, D.; Schnall, M.D.; Shtern, F.; Tempany, C.M.; et al. PI-RADS Prostate Imaging—Reporting and Data System: 2015, Version 2. Eur. Urol. 2016, 69, 16–40. [Google Scholar] [CrossRef]
- Epstein, J.I.; Egevad, L.; Amin, M.B.; Delahunt, B.; Srigley, J.R.; Humphrey, P.A.; Grading, C. The 2014 International Society of Urological Pathology (ISUP) Consensus Conference on Gleason Grading of Prostatic Carcinoma: Definition of Grading Patterns and Proposal for a New Grading System. Am. J. Surg. Pathol. 2016, 40, 244–252. [Google Scholar] [CrossRef]
- McShane, L.M.; Altman, D.G.; Sauerbrei, W.; Taube, S.E.; Gion, M.; Clark, G.M. Statistics SOTNCI-EORTCWGOCD. Reporting recommendations for tumour MARKer prognostic studies (REMARK). Br. J. Cancer. 2005, 93, 387–391. [Google Scholar] [CrossRef]
- Creelman, C.D.; Donaldson, W. ROC curves for discrimination of linear extent. J. Exp. Psychol. 1968, 77, 514–516. [Google Scholar] [CrossRef]
- DeLong, E.R.; DeLong DMClarke-Pearson, D.L. Comparing the areas under two or more correlated receiver operating characteristic curves: A nonparametric approach. Biometrics 1988, 44, 837–845. [Google Scholar] [CrossRef]
- Vickers, A.J.; Elkin, E.B. Decision curve analysis: A novel method for evaluating prediction models. Med. Decis. Mak. 2006, 26, 565–574. [Google Scholar] [CrossRef] [PubMed]
- Borque, A.; Rubio-Briones, J.; Esteban, L.M.; Sanz, G.; Dominguez-Escrig, J.; Ramirez-Backhaus, M.; Calatrava, A.; Solsona, E. Implementing the use of nomograms by choosing threshold points in predictive models: 2012 updated Partin Tables vs a European predictive nomogram for organ-confined disease in prostate cancer. B.J.U Int. 2014, 113, 878–886. [Google Scholar] [CrossRef] [PubMed]
- Roobol, M.J.; Steyerberg, E.W.; Kranse, R.; Wolters, T.; van den Bergh, R.C.; Bangma, C.H.; Schroder, F.H. A risk-based strategy improves prostate-specific antigen-driven detection of prostate cancer. Eur. Urol. 2010, 57, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Aberts, A.R.; Schoots, I.G.; Bokhorst, L.P.; van Leenders, G.J.; Bangma, C.H.; Roobol, M.J. Risk-based Patient Selection for Magnetic Resonance Imaging-targeted Prostate Biopsy after Negative Transrectal Ultrasound-guided Random Biopsy Avoids Unnecessary Magnetic Resonance Imaging Scans. Eur. Urol. 2016, 69, 1129–1134. [Google Scholar] [CrossRef]
- Mannaerts, C.K.; Gayet, M.; Verbeek, J.F.; Engelbrecht, M.R.W.; Savci-Heijink, C.D.; Jager, G.J.; Gielens, M.P.M.; van der Linden, H.; Beerlage, H.P.; de Reijke, T.M.; et al. Prostate Cancer Risk Assessment in Biopsy-naïve Patients: The Rotterdam Prostate Cancer Risk Calculator in Multiparametric Magnetic Resonance Imaging-Transrectal Ultrasound (TRUS) Fusion Biopsy and Systematic TRUS Biopsy. Eur. Urol. Oncol. 2018, 1, 109–117. [Google Scholar] [CrossRef]
- Remmers, S.; Kasivisvanathan, V.; Verbeek, J.F.M.; Moore, C.M.; Roobol, M.J. ERSPC RSGPRECISIONIG. Reducing Biopsies and Magnetic Resonance Imaging Scans During the Diagnostic Pathway of Prostate Cancer: Applying the Rotterdam Prostate Cancer Risk Calculator to the PRECISION Trial Data. Eur. Urol. Open. Sci. 2022, 36, 1–8. [Google Scholar] [CrossRef]
- Massanova, M.; Robertson, S.; Barone, B.; Dutto, L.; Caputo, V.F.; Bhatt, J.R.; Ahmad, I.; Bada, M.; Obeidallah, A.; Crocetto, F. The Comparison of Imaging and Clinical Methods to Estimate Prostate Volume: A Single-Centre Retrospective Study. Urol. Int. 2021, 105, 804–810. [Google Scholar] [CrossRef]
- Diniz, M.A. Statistical methods for validation of predictive models. J. Nucl. Cardiol. 2022, 1–18. [Google Scholar] [CrossRef]
- Strobl, A.N.; Vickers, A.J.; Van Calster, B.; Steyerberg, E.; Leach, R.J.; Thompson, I.M.; Ankerst, D.P. Improving patient prostate cancer risk assessment: Moving from static, globally-applied to dynamic, practice-specific risk calculators. J. Biomed. Inform. 2015, 56, 87–93. [Google Scholar] [CrossRef]
- Checcucci, E.; De Cillis, S.; Granato, S.; Chang, P.; Afyouni, A.S.; Okhunov, Z.; Uro-technology and SoMe Working Group of the Young Academic Urologists Working Party of the European Association of Urology. Applications of neural networks in urology: A systematic review. Curr. Opin. Urol. 2020, 30, 788–807. [Google Scholar] [CrossRef]
- Checcucci, E.; Rosati, S.; De Cillis, S.; Vagni, M.; Giordano, N.; Piana, A.; Granato, S.; Amparore, D.; De Luca, S.; Fiori, C.; et al. Artificial intelligence for target prostate biopsy outcomes prediction the potential application of fuzzy logic. Prostate Cancer Prostatic Dis. 2022, 25, 359–362. [Google Scholar] [CrossRef] [PubMed]
- Nandi, A.; Xhafa, F. A federated learning method for real-time emotion state classification from multi modal streaming. Methods 2022, 204, 340–344. [Google Scholar] [CrossRef] [PubMed]
- Morote, J.; Celma, A.; Roche, S.; de Torres, I.M.; Mast, R.; Semedey, M.E.; Regis, L.; Planas, J. Who Benefits from Multiparametric Magnetic Resonance Imaging After Suspicion of Prostate Cancer. Eur. Urol. Oncol. 2019, 2, 664–669. [Google Scholar] [CrossRef]
| Variable | Development Cohort | Validation Cohort | p-Value |
|---|---|---|---|
| Number of men | 1486 | 946 | - |
| Caucasian ethnicity, n (%) | 1.465 (98.6) | 931 (98.4) | 0.738 |
| Median age at biopsy (IQR), years | 69 (62–74) | 67 (61–72) | <0.001 |
| Median serum PSA (IQR), ng/mL | 6.0 (4.4–9.2) | 7.4 (5.5–10.9) | <0.001 |
| Abnormal DRE, n (%) | 329 (22.1) | 283 (29.9) | <0.001 |
| PCa family history, n (%) | 127 (8.5) | 34 (3.6) | <0.001 |
| Prior negative prostate biopsy, n (%) | 388 (26.1) | 293 (31.0) | =0.010 |
| Median prostate volume (IQR), mL | 55 (40–76) | 55 (40–78) | =0.559 |
| DRE-prostate volume category, n (%) | =0.675 | ||
| I | 140 (9.4) | 96 (10.2) | |
| II | 681 (45.8) | 417 (44.2) | |
| III | 665 (44.8) | 431 (45.7) | |
| PI-RADS v.2.0, n (%) | <0.001 | ||
| 1 | 242 (16.3) | 185 (19.6) | |
| 2 | 73 (4.9) | 50 (5.3) | |
| 3 | 444 (29.9) | 201 (21.2) | |
| 4 | 450 (30.3) | 391 (41.3) | |
| 5 | 277 (18.6) | 119 (12.6) | |
| PCa detection, n (%) | 693 (46.6) | 521 (55.1) | <0.001 |
| csPCa detection, n (%) | 548 (36.9) | 386 (40.8) | =0.058 |
| iPCa detection, n (%) | 145 (9.8) | 135 (14.3) | <0.001 |
| csPCa detection according to PI-RADS | <0.001 | ||
| <3 | 13 (4.1) | 42 (17.9) | |
| 3 | 68 (15.3) | 41 (20.4) | |
| 4 | 236 (52.4) | 203 (51.9) | |
| 5 | 231 (83.4) | 100 (84.0) |
| Predictive Variable | Univariate OR (95% CI) | p-Value | Multivariate OR (95% CI) | p-Value |
|---|---|---|---|---|
| Age at biopsy, years | 1.08 (1.06–1.09) | <0.001 | 1.08 (1.06–1.10) | <0.001 |
| Median log serum PSA, ng/mL | 8.03 (5.31–12.14) | <0.001 | 12.96 (7.69–21.84) | <0.001 |
| Abnormal DRE, yes vs. no | 4.51 (3.48–5.84) | <0.001 | 3.19 (2.34–4.34) | <0.001 |
| PCa family history, yes vs. no | 1.77 (1.23–2.56) | =0.002 | 1.69 (1.06–2.68) | =0.026 |
| Prior negative prostate biopsy, yes vs. no | 0.68 (0.53–0.87) | =0.002 | 0.63 (046–0.85) | =0.003 |
| DRE-prostate volume category, II vs. I | 0.37 (0.25–0.55) | <0.001 | 0.35 (0.22–0.55) | <0.001 |
| DRE-prostate volume category, III vs. I | 0.11 (0.07–0.16) | <0.001 | 0.07 (0.04–0.12) | <0.001 |
| Sensitivity | Development Cohort | Validation Cohort | p Value | ||
|---|---|---|---|---|---|
| Specificy (95% CI) | Threshold (%) | Specificy (95% CI) | Threshold (%) | ||
| 0.80 | 0.70 (0.68–0.72) | 30.8 | 0.70 (0.67–0.73) | 30.3 | 0.927 |
| 0.85 | 0.59 (0.56–0.61) | 23.4 | 0.63 (0.59–0.66) | 25.1 | 0.187 |
| 0.90 | 0.45 (0.43–0.48) | 17.2 | 0.53 (0.49–0.56) | 30.3 | <0.001 |
| 0.95 | 0.24 (0.22–0.26) | 11.1 | 0.34 (0.31–0.37) | 13.3 | <0.001 |
| Parameter | Development Cohort | Validation Cohort |
|---|---|---|
| Sensitivity, number (%) | 520/548 (95.0) | 367/386 (95.0) |
| Specificity, number (%) | 228/938 (24.3) | 192/560 34.3) |
| Positive predictive value, number (%) | 520/1230 (42.3) | 367/737 (49.8) |
| Negative predictive value, number (%) | 228/256 (89.1) | 192/209 (91.9) |
| Accuracy, number (%) | 748/1486 (50.3) | 559/946 (59.1) |
| Avoided mpMRI exams, number (%) | 256/1486 (17.2) | 211/946 (22.3) |
| Missed csPCa, number (%) | 28/548 (5.0) | 19/386 (5.0) |
| Odds ratio (95% confidence interval) | 6.19 (4.06–9.43) | 9.92 (6.06–16.24) |
| Threshold Probability | Development Cohort | Validation Cohort | ||
|---|---|---|---|---|
| Missed csPCa | Saved mpMRI | Missed csPCa | Saved mpMRI | |
| 1 | 0 | 0 | 0 | 0 |
| 2 | 0 | 1 | 1 | 2 |
| 3 | 0 | 7 | 2 | 6 |
| 4 | 1 | 20 | 2 | 17 |
| 5 | 2 | 38 | 2 | 35 |
| 6 | 3 | 55 | 3 | 49 |
| 7 | 7 | 81 | 6 | 74 |
| 8 | 8 | 97 | 7 | 106 |
| 9 | 11 | 124 | 10 | 127 |
| 10 | 15 | 146 | 11 | 150 |
| 11 | 19 | 170 | 14 | 177 |
| 12 | 24 | 197 | 16 | 195 |
| 13 | 27 | 224 | 20 | 217 |
| 14 | 29 | 247 | 22 | 238 |
| 15 | 30 | 278 | 22 | 259 |
| 16 | 32 | 295 | 27 | 280 |
| 17 | 36 | 318 | 31 | 302 |
| 18 | 39 | 336 | 34 | 317 |
| 19 | 40 | 353 | 35 | 326 |
| 20 | 43 | 374 | 38 | 344 |
| 21 | 46 | 392 | 44 | 369 |
| 22 | 52 | 410 | 49 | 379 |
| 23 | 54 | 424 | 52 | 395 |
| 24 | 58 | 439 | 56 | 418 |
| 25 | 60 | 451 | 59 | 428 |
| 26 | 63 | 466 | 64 | 442 |
| 27 | 65 | 476 | 70 | 456 |
| 28 | 67 | 491 | 73 | 468 |
| 29 | 68 | 497 | 74 | 478 |
| 30 | 73 | 507 | 79 | 494 |
| 35 | 87 | 569 | 98 | 543 |
| 40 | 110 | 625 | 117 | 592 |
| 45 | 125 | 664 | 137 | 631 |
| 50 | 145 | 699 | 154 | 670 |
| 55 | 168 | 742 | 178 | 709 |
| 60 | 196 | 779 | 197 | 743 |
| 65 | 219 | 817 | 214 | 768 |
| 70 | 232 | 836 | 234 | 800 |
| 75 | 257 | 865 | 259 | 832 |
| 80 | 278 | 898 | 291 | 872 |
| 85 | 300 | 925 | 317 | 904 |
| 90 | 320 | 950 | 350 | 938 |
| 95 | 342 | 973 | 370 | 961 |
| 100 | 369 | 1000 | 408 | 1000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morote, J.; Borque-Fernando, Á.; Triquell, M.; Campistol, M.; Celma, A.; Regis, L.; Abascal, J.M.; Servian, P.; Planas, J.; Mendez, O.; et al. A Clinically Significant Prostate Cancer Predictive Model Using Digital Rectal Examination Prostate Volume Category to Stratify Initial Prostate Cancer Suspicion and Reduce Magnetic Resonance Imaging Demand. Cancers 2022, 14, 5100. https://doi.org/10.3390/cancers14205100
Morote J, Borque-Fernando Á, Triquell M, Campistol M, Celma A, Regis L, Abascal JM, Servian P, Planas J, Mendez O, et al. A Clinically Significant Prostate Cancer Predictive Model Using Digital Rectal Examination Prostate Volume Category to Stratify Initial Prostate Cancer Suspicion and Reduce Magnetic Resonance Imaging Demand. Cancers. 2022; 14(20):5100. https://doi.org/10.3390/cancers14205100
Chicago/Turabian StyleMorote, Juan, Ángel Borque-Fernando, Marina Triquell, Miriam Campistol, Anna Celma, Lucas Regis, José M. Abascal, Pol Servian, Jacques Planas, Olga Mendez, and et al. 2022. "A Clinically Significant Prostate Cancer Predictive Model Using Digital Rectal Examination Prostate Volume Category to Stratify Initial Prostate Cancer Suspicion and Reduce Magnetic Resonance Imaging Demand" Cancers 14, no. 20: 5100. https://doi.org/10.3390/cancers14205100
APA StyleMorote, J., Borque-Fernando, Á., Triquell, M., Campistol, M., Celma, A., Regis, L., Abascal, J. M., Servian, P., Planas, J., Mendez, O., Esteban, L. M., & Trilla, E. (2022). A Clinically Significant Prostate Cancer Predictive Model Using Digital Rectal Examination Prostate Volume Category to Stratify Initial Prostate Cancer Suspicion and Reduce Magnetic Resonance Imaging Demand. Cancers, 14(20), 5100. https://doi.org/10.3390/cancers14205100






