Smad7 Sustains Stat3 Expression and Signaling in Colon Cancer Cells
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Patients and Samples
2.3. Immunofluorescence
2.4. Western Blotting
2.5. Flow Cytometry
2.6. Real-Time PCR
2.7. Chromatin Immunoprecipitation Assay
2.8. Statistical Analysis
3. Results
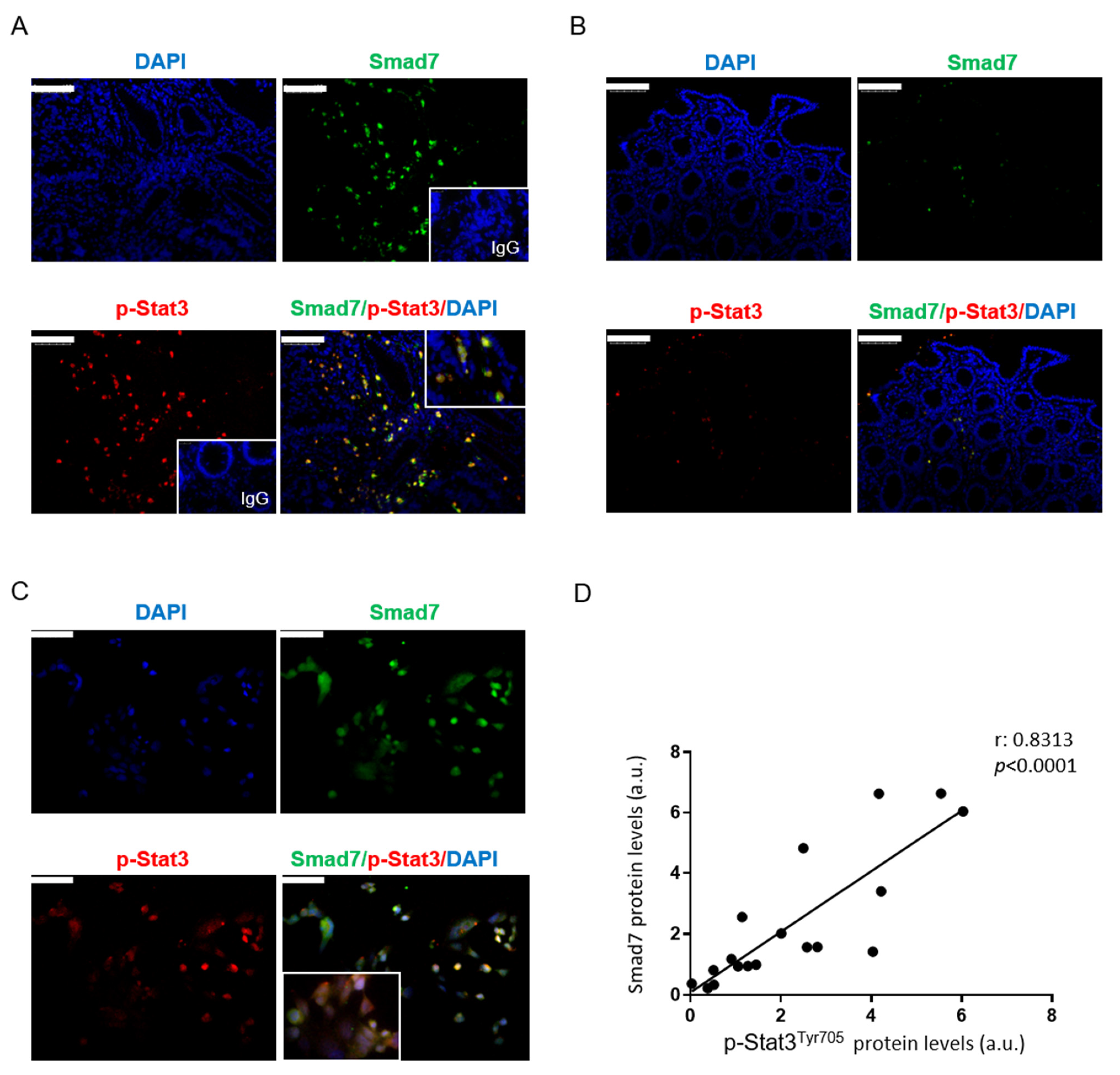
3.1. In CRC, High Smad7 Is Associated with Phosphorylated Stat3 Expression
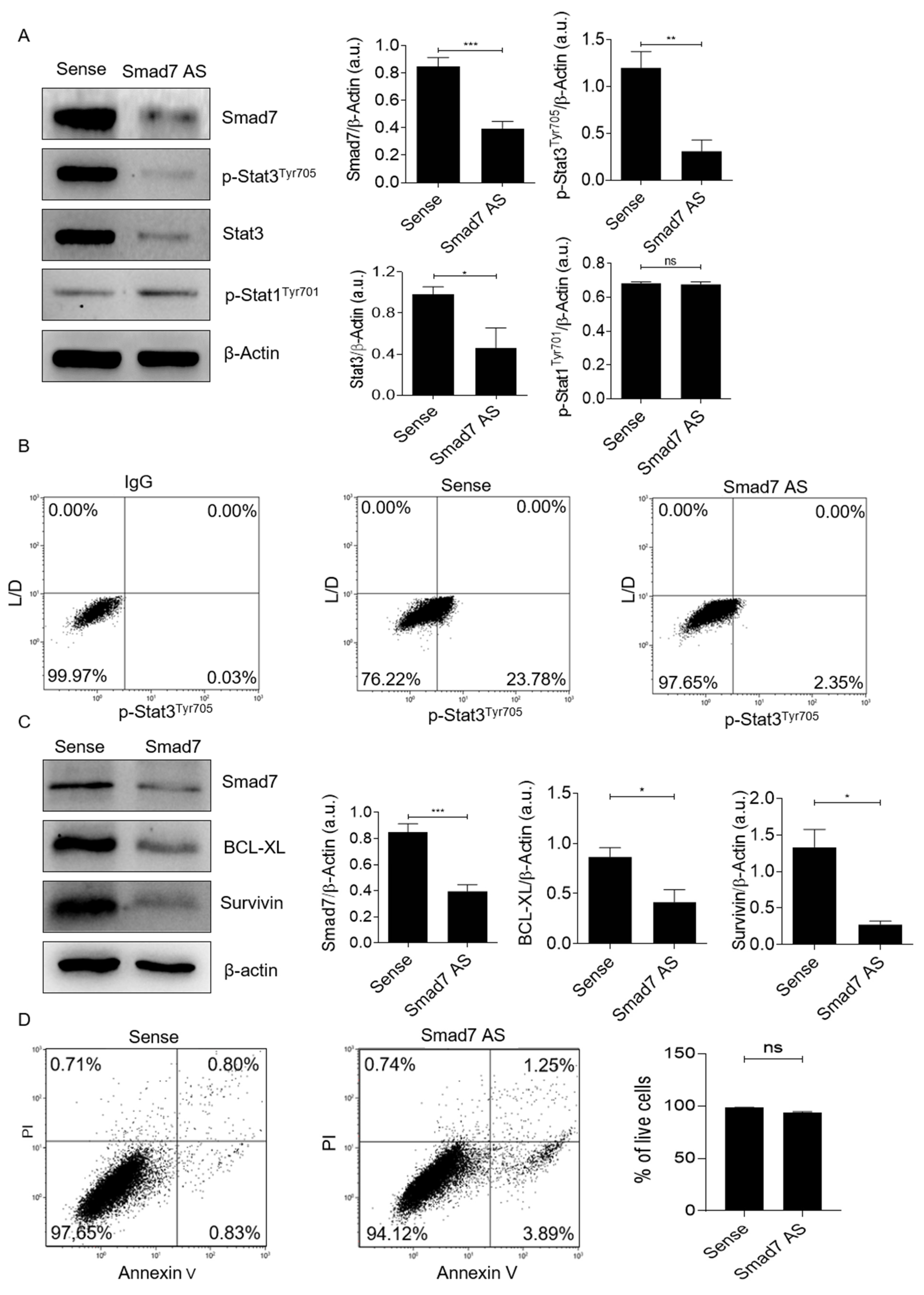
3.2. Smad7 Knockdown Reduces Stat3 Expression in CRC Cells
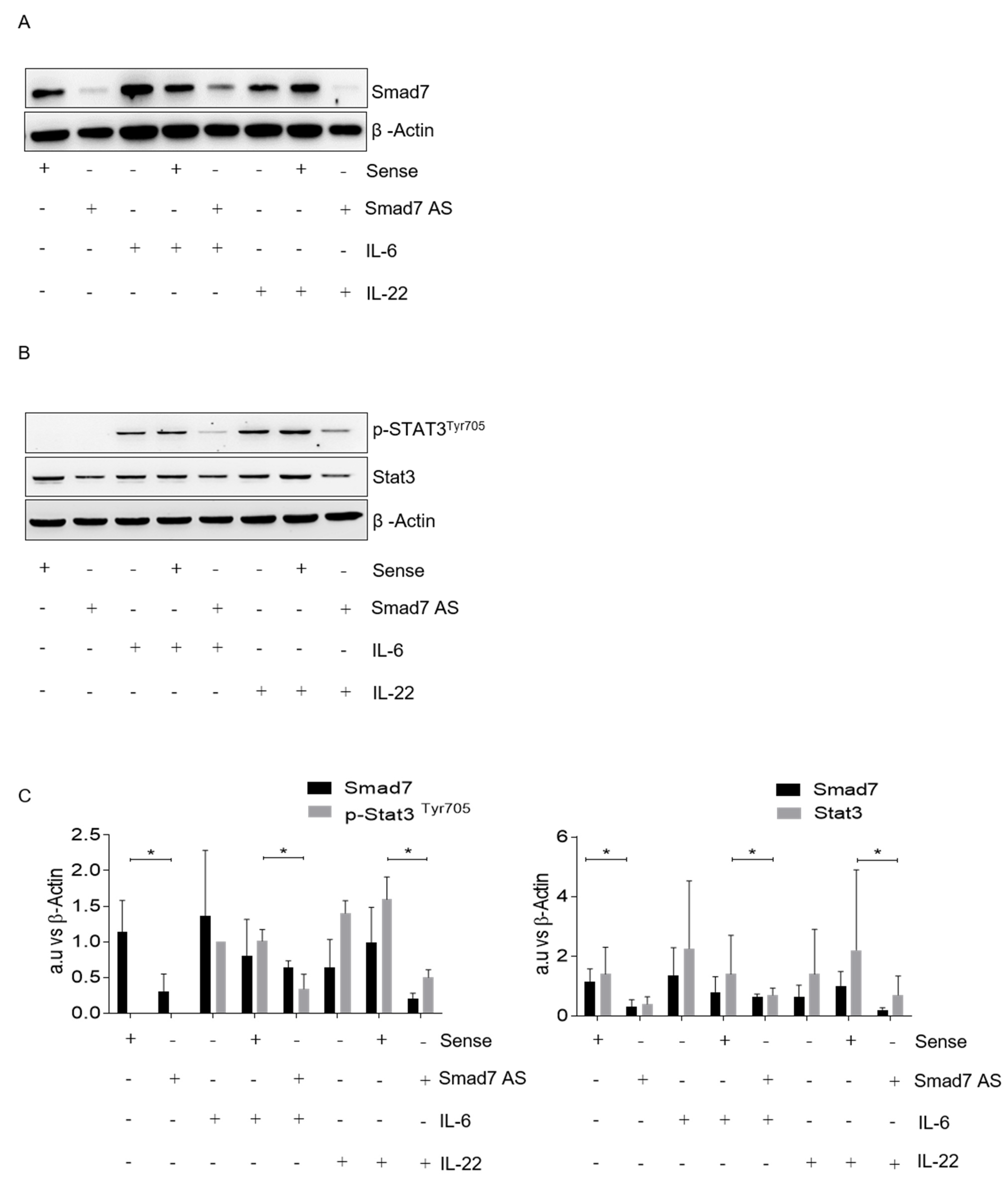
3.3. Smad7 Knockdown Reduces Cytokine-Stimulated Stat3 Expression and Signaling
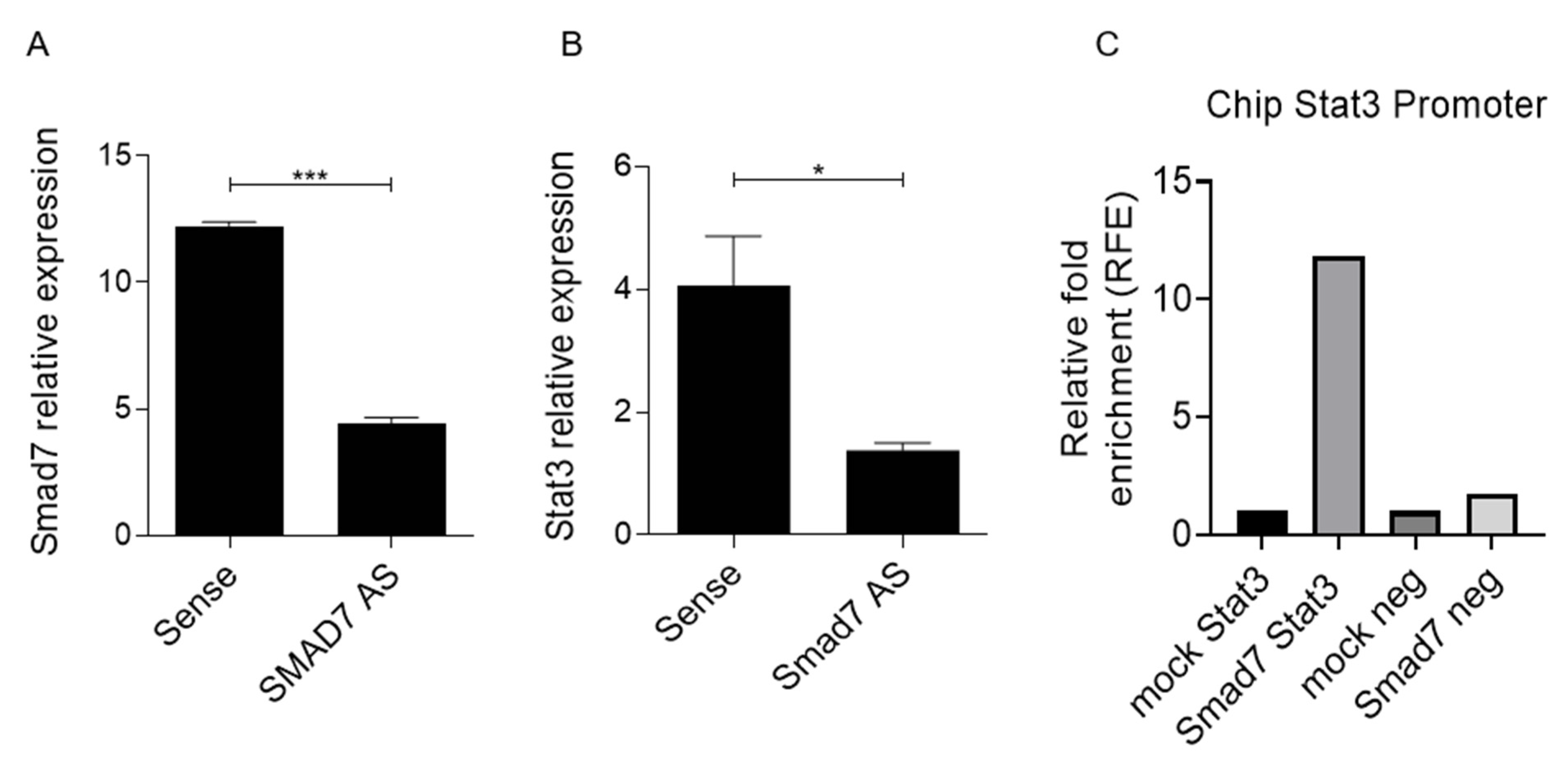
3.4. Smad7 Knockdown Is Associated with the Down-Regulation of Stat3 RNA and Protein Expression
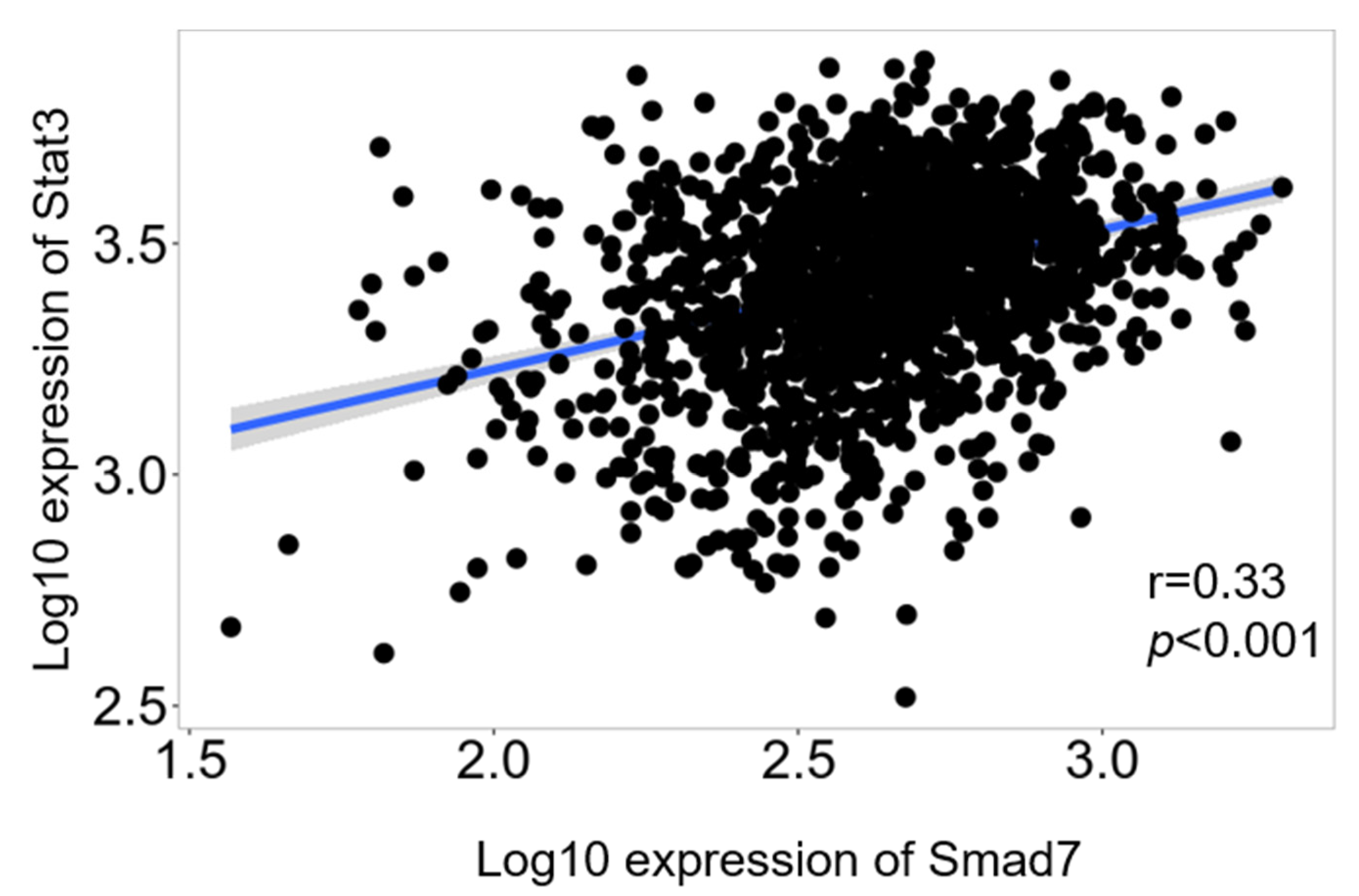
3.5. Correlation between Smad7 and Stat3 in Human CRC
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar]
- Arnold, M.; Sierra, M.S.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global patterns and trends in colorectal cancer incidence and mortality. Gut 2017, 66, 683–691. [Google Scholar] [CrossRef] [PubMed]
- Sharon, E.; Streicher, H.; Goncalves, P.; Chen, H.X. Immune checkpoints in cancer clinical trials. Chin. J. Cancer 2014, 33, 434–444. [Google Scholar] [CrossRef]
- Hirano, H.; Takashima, A.; Hamaguchi, T.; Shida, D.; Kanemitsu, Y. Current status and perspectives of immune checkpoint inhibitors for colorectal cancer. Jpn. J. Clin. Oncol. 2021, 51, 10–19. [Google Scholar] [CrossRef]
- Overman, M.J.; Ernstoff, M.S.; Morse, M.A. Where We Stand With Immunotherapy in Colorectal Cancer: Deficient Mismatch Repair, Proficient Mismatch Repair, and Toxicity Management. Am. Soc. Clin. Oncol. Educ. B 2018, 38, 239–347. [Google Scholar] [CrossRef] [PubMed]
- Corvinus, F.M.; Orth, C.; Moriggl, R.; Tsareva, S.A.; Wagner, S.; Pfitzner, E.B.; Baus, D.; Kaufmann, R.; Huber, L.A.; Zatloukal, K.; et al. Persistent STAT3 activation in colon cancer is associated with enhanced cell proliferation and tumor growth. Neoplasia 2005, 7, 545–555. [Google Scholar] [CrossRef] [PubMed]
- Xiong, A.; Yang, Z.; Shen, Y.; Zhou, J.; Shen, Q. Transcription factor STAT3 as a novel molecular target for cancer prevention. Cancers 2014, 6, 927–957. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Lee, H.; Herrmann, A.; Buettner, R.; Jove, R. Revisiting STAT3 signalling in cancer: New and unexpected biological functions. Nat. Rev. Cancer 2014, 14, 736–746. [Google Scholar] [CrossRef] [PubMed]
- de Simone, V.; Ronchetti, G.; Franzè, E.; Colantoni, A.; Ortenzi, A.; Fantini, M.C.; Rizzo, A.; Sica, G.S.; Sileri, P.; Rossi, P.; et al. Interleukin-21 sustains inflammatory signals that contribute to sporadic colon tumorigenesis. Oncotarget 2015, 6, 9908–9923. [Google Scholar] [CrossRef]
- De Simone, V.; Franzè, E.; Ronchetti, G.; Colantoni, A.; Fantini, M.C.; Di Fusco, D.; Sica, G.S.; Sileri, P.; MacDonald, T.T.; Pallone, F.; et al. Th17-type cytokines, IL-6 and TNF-α synergistically activate STAT3 and NF-kB to promote colorectal cancer cell growth. Oncogene 2015, 34, 3493–3503. [Google Scholar] [CrossRef]
- Tolomeo, M.; Cascio, A. The multifaced role of stat3 in cancer and its implication for anticancer therapy. Int. J. Mol. Sci. 2021, 22, 603. [Google Scholar] [CrossRef]
- Garbers, C.; Hermanns, H.M.; Schaper, F.; Müller-Newen, G.; Grötzinger, J.; Rose-John, S.; Scheller, J. Plasticity and cross-talk of Interleukin 6-type cytokines. Cytokine Growth Factor Rev. 2012, 23, 85–97. [Google Scholar] [CrossRef]
- Jones, S.A.; Jenkins, B.J. Recent insights into targeting the IL-6 cytokine family in inflammatory diseases and cancer. Nat. Rev. Immunol. 2018, 18, 773–789. [Google Scholar] [CrossRef]
- Stolfi, C.; De Simone, V.; Colantoni, A.; Franzè, E.; Ribichini, E.; Fantini, M.C.; Caruso, R.; Monteleone, I.; Sica, G.S.; Sileri, P.; et al. A functional role for Smad7 in sustaining colon cancer cell growth and survival. Cell Death Dis. 2014, 5, 1073. [Google Scholar] [CrossRef]
- Nakao, A.; Afrakhte, M.; Moren, A.; Nakayama, T.; Christian, J.L.; Heuchel, R.; Itoh, S.; Kawabata, M.; Heldin, N.E.; Heldin, C.H.; et al. Identification of Smad7, a TGFbeta-inducible antagonist of TGF-beta signaling. Nature 1997, 389, 631–635. [Google Scholar] [CrossRef]
- De Simone, V.; Bevivino, G.; Sedda, S.; Izzo, R.; Laudisi, F.; Dinallo, V.; Franzè, E.; Colantoni, A.; Ortenzi, A.; Salvatori, S.; et al. Smad7 knockdown activates protein kinase RNA-associated eIF2α pathway leading to colon cancer cell death. Cell Death Dis. 2017, 8, 2681. [Google Scholar] [CrossRef]
- Yu, Y.; Gu, S.; Li, W.; Sun, C.; Chen, F.; Xiao, M.; Wang, L.; Xu, D.; Li, Y.; Ding, C.; et al. Smad7 enables STAT3 activation and promotes pluripotency independent of TGF-β signaling. Proc. Natl. Acad. Sci. USA 2017, 114, 10113–10118. [Google Scholar] [CrossRef]
- Thakur, N.; Hamidi, A.; Song, J.; Itoh, S.; Bergh, A.; Heldin, C.H.; Landström, M. Smad7 Enhances TGF-β-Induced Transcription of c-Jun and HDAC6 Promoting Invasion of Prostate Cancer Cells. iScience 2020, 23, 101470. [Google Scholar] [CrossRef]
- Carpenter, R.L.; Lo, H.W. STAT3 target genes relevant to human cancers. Cancers 2014, 6, 897–925. [Google Scholar] [CrossRef]
- Xi, X.; Hu, R.; Wang, Q.; Xu, K.; Yang, H.; Cui, Z.; Zhang, Y.; Teng, M.; Xia, L.; Chen, J.; et al. Interleukin-22 promotes PD-L1 expression via STAT3 in colon cancer cells. Oncol. Lett. 2021, 22, 716. [Google Scholar] [CrossRef]
- Xie, M.H.; Aggarwal, S.; Ho, W.H.; Foster, J.; Zhang, Z.; Stinson, J.; Wood, W.I.; Goddard, A.D.; Gurney, A.L. Interleukin (IL)-22, a novel human cytokine that signals through the interferon receptor-related proteins CRF2-4 and IL-22R. J. Biol. Chem. 2000, 275, 31335–31339. [Google Scholar] [CrossRef] [PubMed]
- Kotenko, S.V.; Izotova, L.S.; Mirochnitchenko, O.V.; Esterova, E.; Dickensheets, H.; Donnelly, R.P.; Pestka, S. Identification of the functional interleukin-22 (IL-22) receptor complex. The IL-10R2 chain (IL-10Rβ) is a common chain of both the IL-10 and IL-22 (IL-10-related T cell-derived inducible factor, IL-TIF) receptor complexes. J. Biol. Chem. 2001, 276, 2725–2732. [Google Scholar] [CrossRef] [PubMed]
- Meng, G.; Lauria, A.; Maldotti, M.; Anselmi, F.; Polignano, I.L.; Rapelli, S.; Donna, D.; Oliviero, S. Genome-wide analysis of smad7-mediated transcription in mouse embryonic stem cells. Int. J. Mol. Sci. 2021, 22, 13598. [Google Scholar] [CrossRef]
- Burstein, H.J.; Schwartz, R.S. Molecular Origins of Cancer. N. Engl. J. Med. 2008, 358, 527. [Google Scholar] [CrossRef] [PubMed]
- Loh, C.Y.; Arya, A.; Naema, A.F.; Wong, W.F.; Sethi, G.; Looi, C.Y. Signal transducer and activator of transcription (STATs) proteins in cancer and inflammation: Functions and therapeutic implication. Front. Oncol. 2019, 9, 48. [Google Scholar] [CrossRef] [PubMed]
- Stolfi, C.; Marafini, I.; De Simone, V.; Pallone, F.; Monteleone, G. The dual role of Smad7 in the control of cancer growth and metastasis. Int. J. Mol. Sci. 2013, 14, 23774–23790. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; McBride, K.M.; Reich, N.C. STAT3 nuclear import is independent of tyrosine phosphorylation and mediated by importin-α3. Proc. Natl. Acad. Sci. USA 2005, 102, 8150–8155. [Google Scholar] [CrossRef]
- Yang, J.; Chatterjee-Kishore, M.; Staugaitis, S.M.; Nguyen, H.; Schlessinger, K.; Levy, D.E.; Stark, G.R. Novel roles of unphosphorylated STAT3 in oncogenesis and transcriptional regulation. Cancer Res. 2005, 65, 939–947. [Google Scholar] [CrossRef]
- Luwor, R.B.; Baradaran, B.; Taylor, L.E.; Iaria, J.; Nheu, T.V.; Amiry, N.; Hovens, C.M.; Wang, B.; Kaye, A.H.; Zhu, H.J. Targeting Stat3 and Smad7 to restore TGF-β cytostatic regulation of tumor cells in vitro and in vivo. Oncogene 2013, 32, 2433–2441. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maresca, C.; Di Maggio, G.; Stolfi, C.; Laudisi, F.; Colella, M.; Pacifico, T.; Di Grazia, A.; Di Fusco, D.; Congiu, D.; Guida, A.M.; et al. Smad7 Sustains Stat3 Expression and Signaling in Colon Cancer Cells. Cancers 2022, 14, 4993. https://doi.org/10.3390/cancers14204993
Maresca C, Di Maggio G, Stolfi C, Laudisi F, Colella M, Pacifico T, Di Grazia A, Di Fusco D, Congiu D, Guida AM, et al. Smad7 Sustains Stat3 Expression and Signaling in Colon Cancer Cells. Cancers. 2022; 14(20):4993. https://doi.org/10.3390/cancers14204993
Chicago/Turabian StyleMaresca, Claudia, Giulia Di Maggio, Carmine Stolfi, Federica Laudisi, Marco Colella, Teresa Pacifico, Antonio Di Grazia, Davide Di Fusco, Daniele Congiu, Andrea Martina Guida, and et al. 2022. "Smad7 Sustains Stat3 Expression and Signaling in Colon Cancer Cells" Cancers 14, no. 20: 4993. https://doi.org/10.3390/cancers14204993
APA StyleMaresca, C., Di Maggio, G., Stolfi, C., Laudisi, F., Colella, M., Pacifico, T., Di Grazia, A., Di Fusco, D., Congiu, D., Guida, A. M., Sica, G., Monteleone, I., & Monteleone, G. (2022). Smad7 Sustains Stat3 Expression and Signaling in Colon Cancer Cells. Cancers, 14(20), 4993. https://doi.org/10.3390/cancers14204993






