Risk for Pelvic Metastasis and Role of Pelvic Lymphadenectomy in Node-Positive Vulvar Cancer-Results from the AGO-VOP.2 QS Vulva Study
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Data Collection
2.2. Statistical Analysis
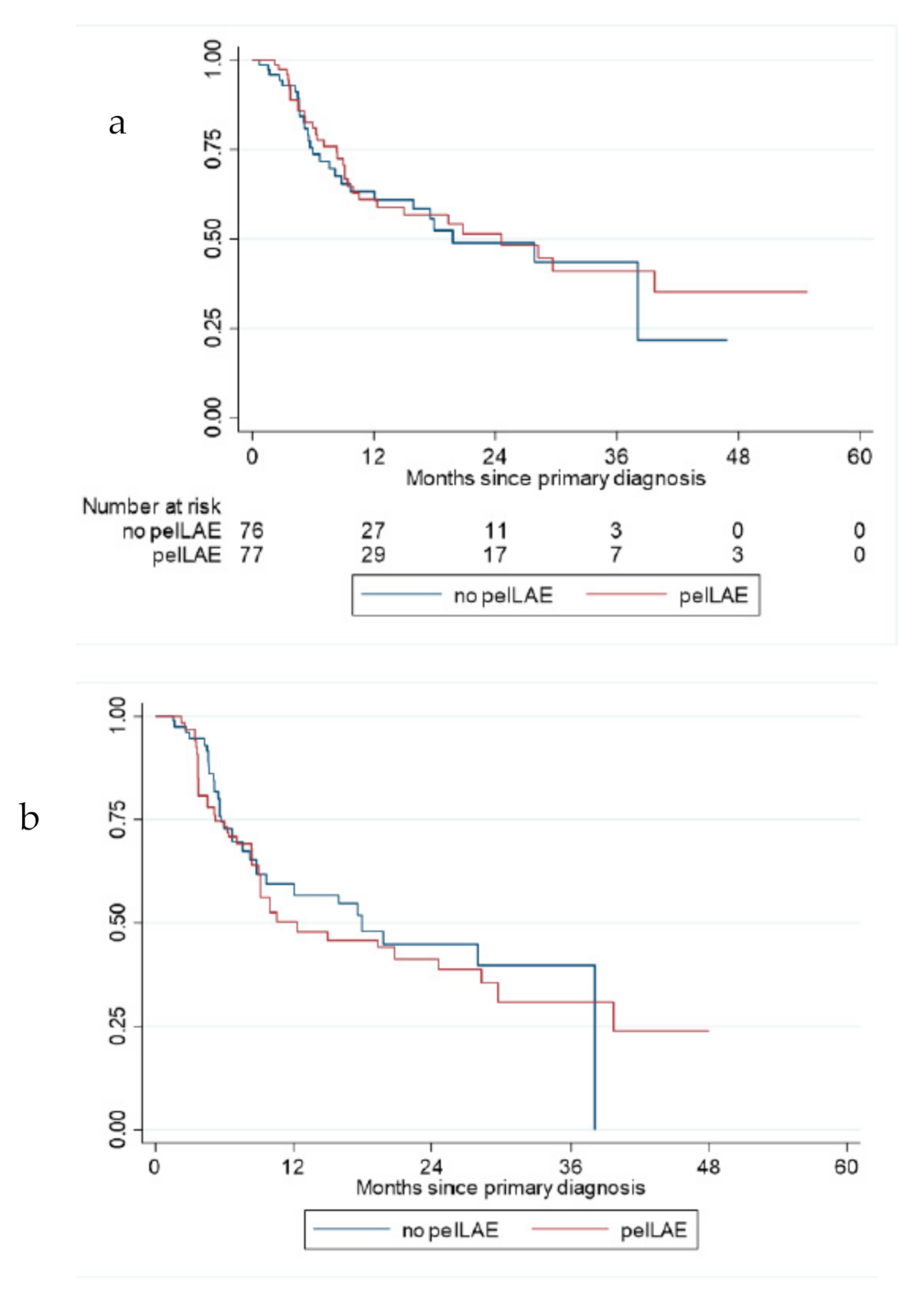
3. Results
3.1. Patients
3.2. Relation between Inguinal and Pelvic Nodal Involvement
3.3. Recurrences
3.4. Survival
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Buttmann-Schweiger, N.; Barinoff, J.; Waldmann, A.; Barnes, B.; Kraywinkel, K. Epidemiology of Vulvar and Vaginal Cancer in Germany. Springer Medizin. 2019, 2–6. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Mahner, S.; Jueckstock, J.; Hilpert, F.; Neuser, P.; Harter, P.; de Gregorio, N.; Hasenburg, A.; Sehouli, J.; Habermann, A.; Hillemanns, P.; et al. Adjuvant therapy in lymph node-positive vulvar cancer: The AGO-CaRE-1 study. J. Natl. Cancer Inst. 2015, 107, dju426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hacker, N.F.; Berek, J.S.; Lagasse, L.D.; Leuchter, R.S.; Moore, J.G. Management of regional lymph nodes and their prognostic influence in vulvar cancer. Obstet. Gynecol. 1983, 61, 408–412. [Google Scholar]
- Curry, S.L.; Wharton, J.T.; Rutledge, F. Positive lymph nodes in vulvar squamous carcinoma. Gynecol. Oncol. 1980, 9, 63–67. [Google Scholar] [CrossRef]
- Woelber, L.; Eulenburg, C.; Choschzick, M.; Kruell, A.; Petersen, C.; Gieseking, F.; Jaenicke, F.; Mahner, S. Prognostic Role of Lymph Node Metastases in Vulvar Cancer and Implications for Adjuvant Treatment. Int. J. Gynecol. Cancer 2012, 22, 503–508. [Google Scholar] [CrossRef]
- Boyce, J.; Fruchter, R.G.; Kasambilides, E.; Nicastri, A.D.; Sedlis, A.; Remy, J.C. Prognostic factors in carcinoma of the vulva. Gynecol. Oncol. 1985, 20, 364–377. [Google Scholar] [CrossRef]
- Velden, J.V.D.; Van Lindert, A.C.; Lammes, F.B.; Kate, F.J.T.; Sie-Go, D.M.; Oosting, H.; Heintz, A.P.M. Extracapsular growth of lymph node metastases in squamous cell carcinoma of the vulva. The impact on recurrence and survival. Cancer 1995, 75, 2885–2890. [Google Scholar] [CrossRef]
- Homesley, H.D.; Bundy, B.N.; Sedlis, A.; Adcock, L. Radiation therapy versus pelvic node resection for carcinoma of the vulva with positive groin nodes. Obstet. Gynecol. 1986, 68, 733–740. [Google Scholar]
- Hacker, N.F.; Eifel, P.J.; van der Velden, J. Cancer of the vulva. Int. J. Gynaecol. Obstet. 2015, 131 (Suppl. S2), S76–S83. [Google Scholar] [CrossRef] [Green Version]
- Schnürch, H.G.; Ackermann, S.; Alt, C.D.; Barinoff, J.; Böing, C.; Dannecker, C.; Gieseking, F.; Günthert, A.; Hantschmann, P.; Horn, L.C.; et al. Diagnosis, Therapy, and Follow-Up Care of Vulvar Cancer and its Precursors. National Guideline of the German Society of Gynecology and Obstetrics (S2k-Level, AWMF Registry No. 015/059, November 2015). Geburtshilfe Frauenheilkd. 2016, 76, 1035–1049. [Google Scholar]
- Klemm, P.; Marnitz, S.; Köhler, C.; Braig, U.; Schneider, A. Clinical implication of laparoscopic pelvic lymphadenectomy in patients with vulvar cancer and positive groin nodes. Gynecol. Oncol. 2005, 99, 101–105. [Google Scholar] [CrossRef]
- International Union Against Cancer (UICC). TNM Classification of Malignant Tumours, 6th ed.; Sobin, L.H., Wittekind, C., Eds.; Wiley: New York, NY, USA, 2002. [Google Scholar]
- Woelber, L.; Bommert, M.; Harter, P.; Prieske, K.; Zu Eulenburg, C.; Jueckstock, J.; Hilpert, F.; de Gregorio, N.; Iborra, S.; Sehouli, J.; et al. Role of Pelvic Lymph Node Resection in Vulvar Squamous Cell Cancer: A Subset Analysis of the AGO-CaRE-1 Study. Ann. Surg. Oncol. 2021, 28, 6696–6704. [Google Scholar] [CrossRef]
- Jaeger, A.; Prieske, K.; Mathey, S.; Fischer, I.; Vettorazzi, E.; Kuerti, S.; Reuter, S.; Dieckmann, J.; Schmalfeldt, B.; Woelber, L. Pelvic lymphadenectomy in vulvar cancer and its impact on prognosis and outcome. Arch. Gynecol. Obstet. 2021. [Google Scholar] [CrossRef]
- Woelber, L.; Bommert, M.; Prieske, K.; Fischer, I.; Zu Eulenburg, C.; Vettorazzi, E.; Harter, P.; Jueckstock, J.; Hilpert, F.; de Gregorio, N.; et al. Pelvic Lymphadenectomy in Vulvar Cancer—Does it make sense? Geburtshilfe Frauenheilkd. 2020, 80, 1221–1228. [Google Scholar] [CrossRef] [PubMed]
- Parthasarathy, A.; Cheung, M.K.; Osann, K.; Husain, A.; Teng, N.N.; Berek, J.S.; Kapp, D.S.; Chan, J.K. The benefit of adjuvant radiation therapy in single-node-positive squamous cell vulvar carcinoma. Gynecol. Oncol. 2006, 103, 1095–1099. [Google Scholar] [CrossRef]
- Woelber, L.; Kock, L.; Gieseking, F.; Petersen, C.; Trillsch, F.; Choschzick, M.; Jaenicke, F.; Mahner, S. Clinical management of primary vulvar cancer. Eur. J. Cancer 2011, 47, 2315–2321. [Google Scholar] [CrossRef] [PubMed]
- Oonk, M.H.M.; Planchamp, F.; Baldwin, P.; Bidzinski, M.; Brännström, M.; Landoni, F.; Mahner, S.; Mahantshetty, U.; Mirza, M.; Petersen, C.; et al. European Society of Gynaecological Oncology Guidelines for the Management of Patients with Vulvar Cancer. Int. J. Gynecol. Cancer 2017, 27, 832–837. [Google Scholar] [CrossRef]
- Koh, W.-J.; Greer, B.E.; Abu-Rustum, N.R.; Campos, S.M.; Cho, K.R.; Chon, H.S.; Chu, C.; Cohn, D.; Crispens, M.A.; Dizon, D.S.; et al. Vulvar Cancer, Version 1.2017, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Canc. Netw. 2017, 15, 92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Te Grootenhuis, N.C.; van der Zee, A.G.J.; van Doorn, H.C.; van der Velden, J.; Vergote, I.; Zanagnolo, V.; Baldwin, P.J.; Gaarenstroom, K.N.; van Dorst, E.B.; Trum, J.W.; et al. Sentinel nodes in vulvar cancer: Long-term follow-up of the GROningen INternational Study on Sentinel nodes in Vulvar cancer (GROINSS-V) I. Gynecol. Oncol. 2016, 140, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Prieske, K.; Haeringer, N.; Grimm, D.; Trillsch, F.; Eulenburg, C.; Burandt, E.; Schmalfeldt, B.; Mahner, S.; Mueller, V.; Woelber, L. Patterns of distant metastases in vulvar cancer. Gynecol. Oncol. 2016, 142, 427–434. [Google Scholar] [CrossRef]
- Han, S.C.; Kim, D.H.; Higgins, S.A.; Carcangiu, M.-L.; Kacinski, B.M. Chemoradiation as primary or adjuvant treatment for locally advanced carcinoma of the vulva. Int. J. Radiat. Oncol. Biol. Phys. 2000, 47, 1235–1244. [Google Scholar] [CrossRef]
- Moore, D.H.; Ali, S.; Koh, W.J.; Michael, H.; Barnes, M.N.; McCourt, C.K.; Homesley, H.D.; Walker, J.L. A phase II trial of radiation therapy and weekly cisplatin chemotherapy for the treatment of locally-advanced squamous cell carcinoma of the vulva: A gynecologic oncology group study. Gynecol. Oncol. 2012, 124, 529–533. [Google Scholar] [CrossRef] [PubMed]
- Pigge, D.C.; Gaudenz, R. Invasive carcinoma of the vulva. Am. J. Obstet. Gynecol. 1974, 119, 382–395. [Google Scholar] [CrossRef]
- Morris, L.; Do, V.; Chard, J.; Brand, A.H. Radiation-induced vaginal stenosis: Current perspectives. Int. J. Women’s Health 2017, 9, 273–279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Characteristics | Total n = 306 | Pelvic LAE No n = 197 (64.4%) | Pelvic LAE Yes n = 109 (35.6%) | p-Value * |
|---|---|---|---|---|
| Tumor stage | 0.768 b | |||
| pT1b | 259 (84.6%) | 168 (85.3%) | 91 (83.5%) | |
| pT2 | 33 (10.8%) | 22 (11.2%) | 11 (10.1%) | |
| pT3 | 6 (1.9%) | 3 (1.5%) | 3 (2.8%) | |
| T4 | 2 (0.7%) | 1 (0.5%) | 1 (0.8%) | |
| unknown | 6 (2%) | 3 (1.5%) | 3 (2.8%) | |
| BMI median (range) | 26 (15–45) | 25.5 (15–45) | 27.1 (18–45) | 0.195 a |
| ECOG Status | 0.388 c | |||
| ECOG 0 | 89 (29.1%) | 56 (28.4%) | 33 (30.3%) | |
| ECOG 1 | 87 (28.4%) | 62 (31.5%) | 25 (22.9%) | |
| ECOG 2 | 26 (8.5%) | 20 (10.2%) | 6 (5.5%) | |
| ECOG 3+4 | 7 (2.3%) | 6 (3%) | 1 (0.9%) | |
| unknown | 97 (31.7%) | 53 (26.9%) | 44 (40.4%) | |
| Median tumor diameter (mm) (range) | 32 (2–110) | 32.5 (2–106) | 32 (4–110) | 0.863 a |
| Median depth of invasion (mm) (range) | 7 (1–120) | 7 (1–120) | 7 (1–65) | 0.280 a |
| Resection status of vulvar primary | 0.225 b | |||
| R0 | 273 (89.2%) | 172 (87.3%) | 101 (92.6%) | |
| R1 | 21 (6.9%) | 16 (8.1%) | 5 (4.6%) | |
| unknown | 12 (3.9%) | 9 (4.6%) | 3 (2.8%) | |
| Median minimal resection margin (mm) (range) | 3.0 (0–22) | 3.0 (0–22) | 3.25 (0–15) | 0.970 a |
| Nodal status groin | <0.001 b | |||
| pN1a | 73 (23.9%) | 66 (33.5%) | 7 (6.4%) | |
| with one metastasis <5 mm | 53 (17.3%) | 49 (24.9%) | 4 (3.6%) | |
| with two metastasis <5 mm | 20 (6.6%) | 17 (8.6%) | 3 (2.8%) | |
| pN1b | 72 (23.5%) | 51 (25.9%) | 21 (19.3%) | |
| pN2a/b | 62 (20.2%) | 34 (17.3%) | 28 (25.7%) | |
| pN2c/pN3 | 97 (31.7%) | 44 (22.3%) | 53 (48.6%) | |
| unknown | 2 (0.7%) | 2 (1.0%) | 0 | |
| Median number of removed LN groin (range) | 7 (1–23) | 6 (1–21) | 7 (1–23) | 0.003 a |
| Median number of affected LN groin (range) | 1 (1–11) | 1 (1–7) | 1 (1–11) | 0.065 a |
| Median number of removed SN LN groin (range) | 2 (1–11) | 2 (1–11) | 2 (1–10) | 0.990 a |
| Median number of affected SN LN groin (range) | 1 (1–6) | 1 (1–6) | 1 (1–4) | 0.141 a |
| Median number of removed LN pelvis (range) | 9 (1–34) | n.a. | 9 (1–34) | n.a. |
| Median number of affected LN pelvis (range) | 2 (1–5) | n.a. | 2 (1–5) | n.a. |
| Distant metastasis | <0.001 b | |||
| M0 | 256 (83.6%) | 178 (90.4%) | 78 (71.6%) | |
| M1 | 32 (10.5%) | 8 (4%) | 24 (22%) | |
| unknown | 18 (5.9%) | 11 (5.6%) | 7 (6.4%) | |
| Treatment of the primary | 0.346 b | |||
| surgery | 289 (94.4%) | 189 (95.9%) | 100 (91.7%) | |
| no surgical treatment (radiotherapy or chemoradiation) | 14 (4.6%) | 6 (3%) | 8 (7.3%) | |
| (radiotherapy or chemoradiation) | ||||
| unknown | 3 (1%) | 2 (0.1%) | 1 (0.9%) | |
| Surgical therapy vulva | 0.832 b | |||
| complete vulvectomy | 75 (24.5%) | 48 (24.4%) | 27 (24.7%) | |
| partial vulvectomy | 183 (59.8%) | 119 (60.4%) | 64 (58.7%) | |
| wide excision | 35 (11.4%) | 22 (11.2%) | 13 (12%) | |
| exenteration | 3 (1%) | 1 (0.5%) | 2 (1.8%) | |
| unknown | 10 (3.3%) | 7 (3.5%) | 3 (2.8%) | |
| SNL—LAE groin | 0.012 b | |||
| unilateral | 18 (5.9%) | 14 (7.1%) | 4 (3.6%) | |
| bilateral | 137 (44.8%) | 98 (49.7%) | 39 (35.8%) | |
| no SNL-LAE groin | 151 (49.3%) | 85 (43.2%) | 66 (60.6%) | |
| Inguino-femoral LAE | 0.001 b | |||
| unilateral | 49 (16%) | 38 (19.3%) | 11 (1.1%) | |
| bilateral | 237 (77.4%) | 140 (71.1%) | 97 (89%) | |
| no inguino-femoral LAE | 20 (6.6%) | 19 (9.6%) | 1 (0.9%) | |
| Pelvic LAE | n.a. | |||
| unilateral | 43 (14.1%) | 0 | 43 (39.4%) | |
| bilateral | 66 (21.5%) | 0 | 66 (60.6%) | |
| no pelvic LAE | 197 (64.4%) | 197 (100%) | 0 | |
| Surgical approach pelvic LAE | n.a. | |||
| laparascopy | 67 (21.9%) | 0 | 67 (61.4%) | |
| extraperitoneal via groin | 35 (11.4%) | 0 | 35 (32.1%) | |
| open | 6 (2%) | 0 | 6 (5.6%) | |
| unknown | 198 (64.7%) | 197 | 1 (0.9%) | |
| Adjuvant radiotherapy | 0.001b | |||
| yes | 197 (64.4%) | 115 (58.3%) | 82 (75.2%) | |
| no | 103 (33.7%) | 80 (40.6%) | 23 (21.1%) | |
| unknown | 6 (1.9%) | 2 (0.1%) | 4 (3.7%) | |
| Adjuvant chemoradiation | ||||
| yes | 47 (15.4%) | 20 (10.2%) | 27 (24.8%) | |
| no | 256 (83.7%) | 176 (89.3%) | 80 (73.4%) | |
| unknown | 3 (0.9%) | 1 (0.5%) | 2 (1.8%) | |
| Primary chemoradiation | 0.085 b | |||
| yes | 14 (4.6%) | 6 (3%) | 8 (7.3%) | |
| no | 289 (94.4%) | 189 (95.9%) | 100 (91.7%) | |
| unknown | 3 (1%) | 2 (0.1%) | 1 (0.9%) | |
| Median total dose applied (Gy) (range) | 50.4 (2–70) | 50.4 (2–66) | 50.4 (26–70) | 0.332 a |
| Recurrent disease | 0.079 d | |||
| yes | 102 (33.3%) | 54 (27.4%) | 48 (44%) | |
| no | 181 (59.2%) | 125 (63.4%) | 56 (51.4%) | |
| unknown | 11 (3.6%) | 8 (4.1%) | 3 (2.8%) | |
| death without relapse | 12 (3.9%) | 10 (5.1%) | 2 (1.8%) |
| Nodal Status Groin | Total n = 108 | Nodal Status Pelvis N0 (Pelvic N−) n = 88 | Nodal Status Pelvis N+ (Pelvic N+) n = 20 | p-Value * |
|---|---|---|---|---|
| 0.001 | ||||
| pN1a | 7 (6.5%) | 7 (8.0%) | 0 | |
| pN1b | 21 (19.4%) | 21 (23.9%) | 0 | |
| pN2a/b | 28 (25.9%) | 26 (29.5%) | 2 (10.0%) | |
| pN2c/pN3 | 52 (48.2%) | 34 (38.6%) | 18 (90.0%) |
| Radiation Fields | Total n = 305 | No Pelvic LAE n = 197 | Pelvic LAE Nodal Status Pelvis N0 (Pelvic N−) n = 88 | Pelvic LAE Nodal Status Pelvis N1 (Pelvic N+) n = 20 |
|---|---|---|---|---|
| No groin RT (including vulva only) | 99 (32.4%) | 81 (41.1%) | 12 (13.6%) | 6 (30.0%) |
| Groins+/− vulva | 125 (41.0%) | 70 (35.6%) | 51 (58.0%) | 4 (20.0%) |
| Groins + pelvis +− vulva | 50 (16.4%) | 31 (15.7%) | 10 (11.3%) | 9 (45.0%) |
| pelvis only | 3 (1%) | 3 (1.5%) | 0 | 0 |
| Missing | 28 (9.2%) | 12 (6.1%) | 15 (17.1%) | 1 (5.0%) |
| Complications | Total n = 306 | Pelvic LAE No n = 197 (64.4%) | Pelvic LAE Yes n = 109 (35.6%) | p-Value * |
|---|---|---|---|---|
| Woundhealing problems postoperatively | 0.930 | |||
| yes | 89 (29.0%) | 57 (29.0%) | 32 (29.4%) | |
| no | 192 (62.8%) | 124 (62.9%) | 68 (62.3%) | |
| unknown | 25 (8.2%) | 16 (8.1%) | 9 (8.3%) | |
| Infection of the groin | 0.025 | |||
| yes | 77 (25.1%) | 41 (20.8%) | 36 (33%) | |
| no | 204 (66.7%) | 138 (70%) | 66 (60.6%) | |
| unknown | 25 (8.2%) | 18 (9.2%) | 7 (6.4%) | |
| Secondary surgery needed | 0.341 | |||
| yes | 80 (26.1%) | 48 (24.4%) | 32 (29.3%) | |
| no | 226 (73.9%) | 149 (75.6%) | 77 (70.7%) | |
| Persistent lymphocele | 0.223 | |||
| yes | 61 (19.9%) | 36 (18.3%) | 25 (22.9%) | |
| no | 197 (64.4%) | 133 (67.5%) | 64 (58.7%) | |
| missing | 48 (15.7%) | 28 (14.2%) | 20 (18.4%) |
| Site of Recurrence | Total n = 291 * | No Pelvic LAE n = 186 | Pelvic LAE Nodal Status Pelvis N0 (Pelvic N−) n = 85 | Pelvic LAE Nodal Status Pelvis N1 (Pelvic N+) n = 20 |
|---|---|---|---|---|
| No recurrence | 169 (58.1%) | 117 (62.9%) | 45 (52.9%) | 7 (35.0%) |
| Local (Vulva only) | 34 (11.7%) | 20 (10.7%) | 12 (15.3%) | 1 (5.0%) |
| Groins only | 14 (4.8%) | 10 (5.3%) | 3 (3.5%) | 1 (5.0%) |
| Vulva + groins | 4 (1.4%) | 4 (2.1%) | 0 | 0 |
| Pelvis only | 9 (3.1%) | 6 (3.2%) | 2 (2.4%) | 1 (5.0%) |
| Including pelvis | 5 (1.7%) | 1 (0.5%) | 2 (2.4%) | 2 (10.0%) |
| Including distant | 35 (12.0%) | 12 (6.5%) | 16 (18.8%) | 7 (35.0%) |
| Unknown location | 10 (2.4%) | 7 (3.7%) | 3 (3.5%) | 0 |
| Death without relapse | 11 (3.8%) | 10 (5.1%) | 1 (1.2%) | 1 (5.0%) |
| Site of Recurrence | Total n = 265 * | No Pelvic LAE RT Including Pelvis (Yes) n = 142 | No Pelvic LAE RT Including Pelvis (No) n = 33 | Pelvic LAE (Pelvic N+) RT Including Pelvis (Yes) n = 9 | Pelvic LAE (Pelvic N+) RT Including Pelvis (No) n = 10 | Pelvic LAE (Pelvic N−) RT Including Pelvis (Yes) n = 10 | Pelvic LAE (Pelvic N−) RT Including Pelvis (No) n = 61 |
|---|---|---|---|---|---|---|---|
| Including pelvis | 14 (5.3%) | 5 (3.5%) | 2 (6.1%) | 0 | 3 (30.0%) | 1 (10.0%) | 3 (4.9%) |
| Including distant | 33 (12.4%) | 11 (7.7%) | 1 (3.0%) | 5 (55.5%) | 2 (20.0%) | 2 (20.0%) | 12 (19.7%) |
| Unknown | 7 (2.6%) | 6 (4.2%) | 0 | 0 | 0 | 0 | 1 (1.6%) |
| Total | 54 (20.4%) | 22 (15.4%) | 3 (9.1%) | 5 (55.5%) | 5 (50.0%) | 3 (30.0%) | 16 (26.2%) |
| Total # n = 291 | Pelvic LAE No n = 86 (64.4%) | Pelvic LAE Yes n = 105 (35.6%) | p-Value * | |
|---|---|---|---|---|
| 1year DFS in % (95% CI) | 65.9 (59.3–71.6) | 70.1 (61.9–76.9) | 58.9 (47.7–68.5) | 0.079 |
| 1year OS in % (95% CI) | 86.6 (81.3–90.6) | 90.2 (83.9–94.1) | 80.7 (69.8–87.9) | 0.100 |
| Total # n = 265 | No Pelvic LAE Rt Including Pelvis (Yes) n = 142 | No Pelvic LAE Rt Including Pelvis (No) n = 33 | Pelvic LAE (Pelvic N+) Rt Including Pelvis (Yes) n = 9 | Pelvic LAE (Pelvic N+) Rt Including Pelvis (No) n = 10 | Pelvic LAE (Pelvic N−) Rt Including Pelvis (Yes and No) n = 19 | Pelvic LAE (Pelvic N−) Rt Including Pelvis (Yes) n = 10 | Pelvic LAE (Pelvic n-) Rt Including Pelvis (No) n = 61 | Pelvic LAE (Pelvic N−) Rt Including Pelvis (Yes and No) n = 71 | |
|---|---|---|---|---|---|---|---|---|---|
| 1year DFS in % (95% CI) | 67.7 (58.2–75.6) | 78.8 (58.3–90.0) | 43.8 (10.1–74.2) | 11.1 (0.6–38.8) | 19.8 (3.7–45.2) | 45.0 (13.8–72.4) | 69.2 (47.1–72.1) | 65.4 (51.8–76.0) | |
| Incidence of a DFS event in 10 person-years | 2.06 | 3.04 | 8.88 | 13.19 | 9.1 | 3.81 | 3.19 | 3.29 | |
| Incidence of an OS event in 10 person-years | 1.27 | 0.21 | 3.81 | 7.26 | 4.5 | 2.21 | 0.99 | 1.17 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Woelber, L.; Hampl, M.; Eulenburg, C.z.; Prieske, K.; Hambrecht, J.; Fuerst, S.; Klapdor, R.; Heublein, S.; Gass, P.; Rohner, A.; et al. Risk for Pelvic Metastasis and Role of Pelvic Lymphadenectomy in Node-Positive Vulvar Cancer-Results from the AGO-VOP.2 QS Vulva Study. Cancers 2022, 14, 418. https://doi.org/10.3390/cancers14020418
Woelber L, Hampl M, Eulenburg Cz, Prieske K, Hambrecht J, Fuerst S, Klapdor R, Heublein S, Gass P, Rohner A, et al. Risk for Pelvic Metastasis and Role of Pelvic Lymphadenectomy in Node-Positive Vulvar Cancer-Results from the AGO-VOP.2 QS Vulva Study. Cancers. 2022; 14(2):418. https://doi.org/10.3390/cancers14020418
Chicago/Turabian StyleWoelber, Linn, Monika Hampl, Christine zu Eulenburg, Katharina Prieske, Johanna Hambrecht, Sophie Fuerst, Ruediger Klapdor, Sabine Heublein, Paul Gass, Annika Rohner, and et al. 2022. "Risk for Pelvic Metastasis and Role of Pelvic Lymphadenectomy in Node-Positive Vulvar Cancer-Results from the AGO-VOP.2 QS Vulva Study" Cancers 14, no. 2: 418. https://doi.org/10.3390/cancers14020418
APA StyleWoelber, L., Hampl, M., Eulenburg, C. z., Prieske, K., Hambrecht, J., Fuerst, S., Klapdor, R., Heublein, S., Gass, P., Rohner, A., Canzler, U., Becker, S., Bommert, M., Bauerschlag, D., Denecke, A., Hanker, L., Runnebaumn, I., Forner, D. M., Schochter, F., ... Jaeger, A. (2022). Risk for Pelvic Metastasis and Role of Pelvic Lymphadenectomy in Node-Positive Vulvar Cancer-Results from the AGO-VOP.2 QS Vulva Study. Cancers, 14(2), 418. https://doi.org/10.3390/cancers14020418







