Heat Modulation of Intrinsic MR Contrasts for Tumor Characterization
Abstract
:Simple Summary
Abstract
1. Introduction
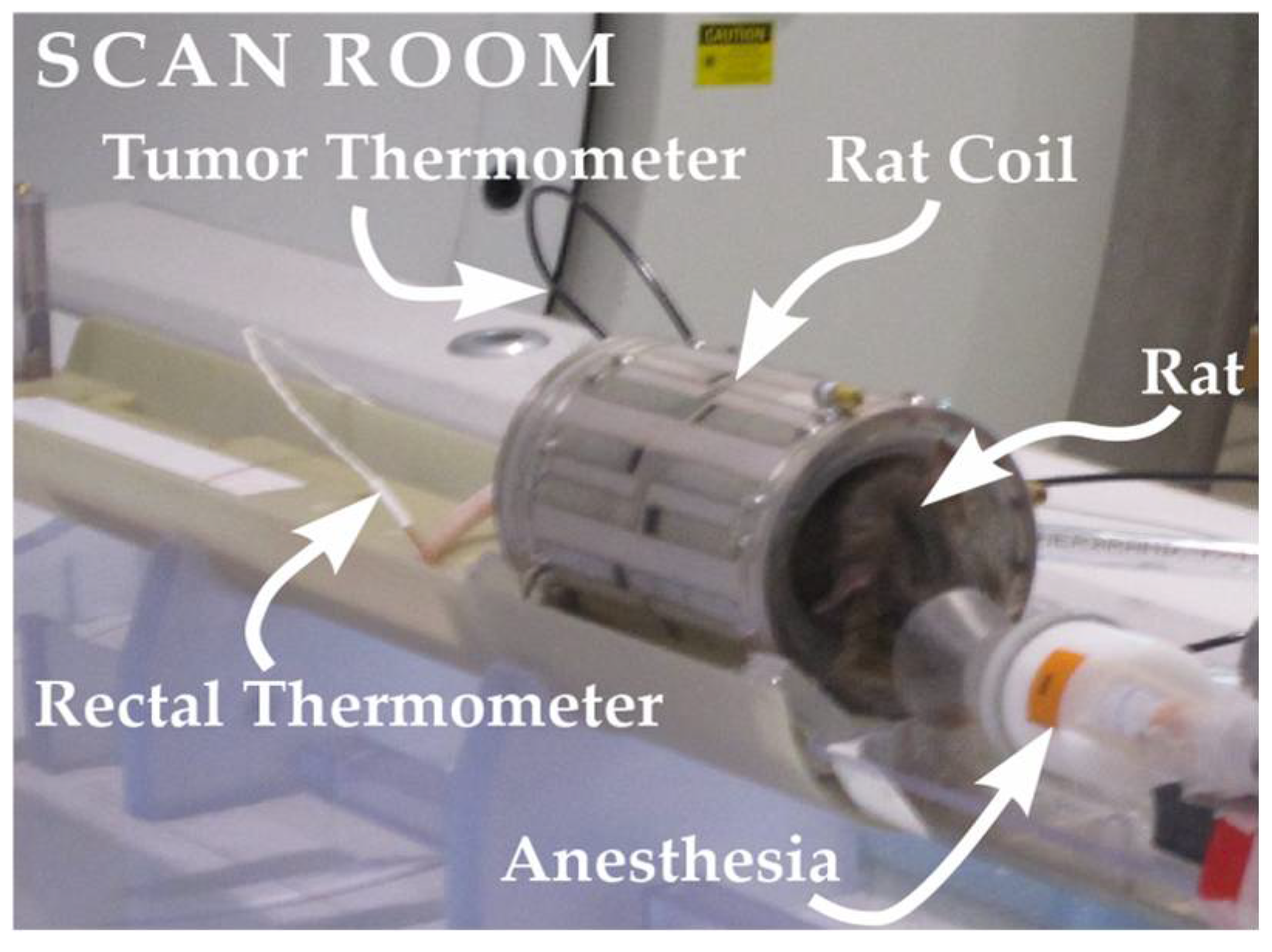
2. Materials and Methods
3. Results
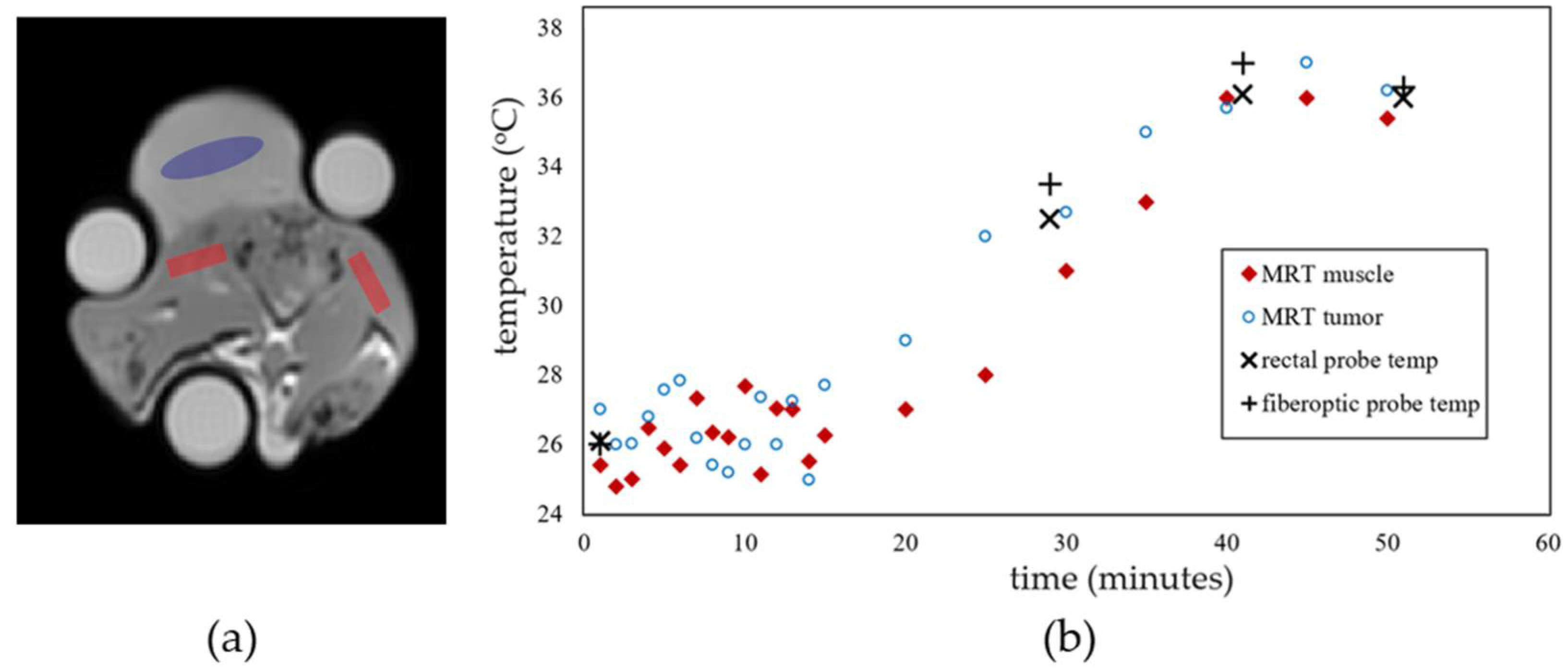
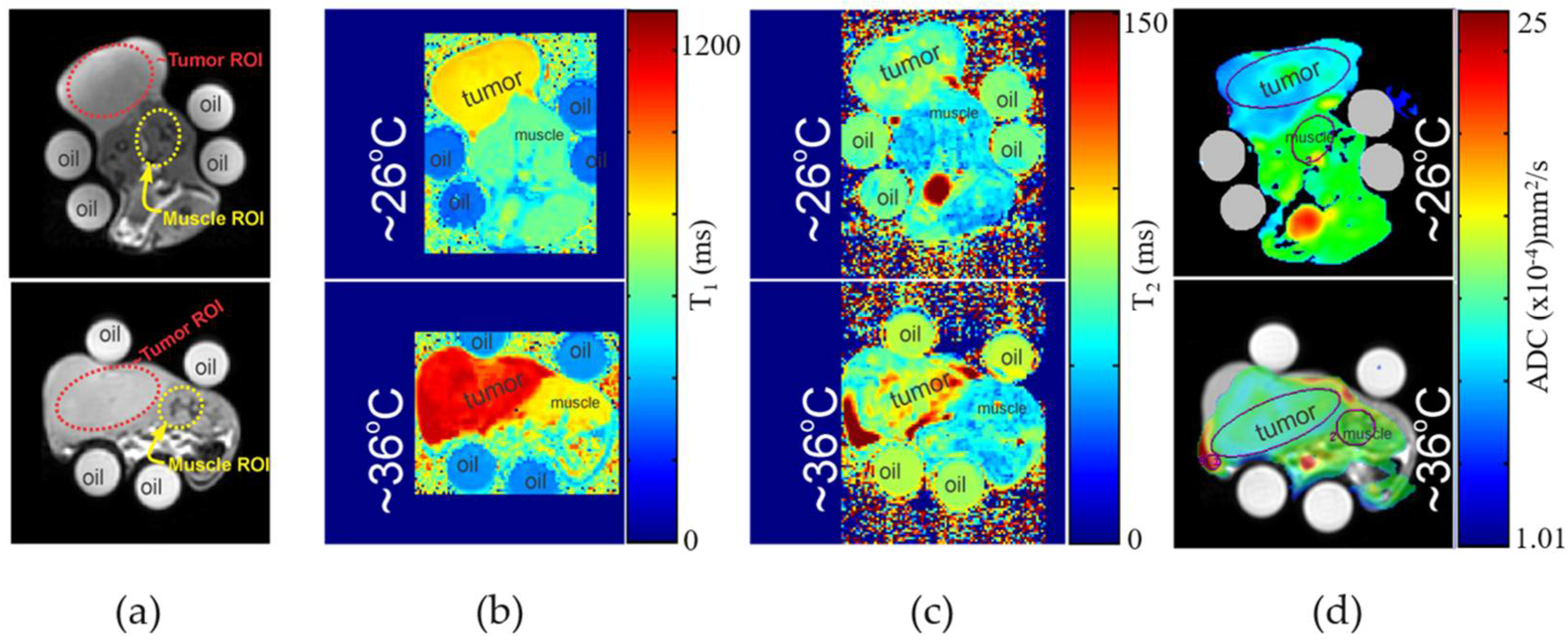
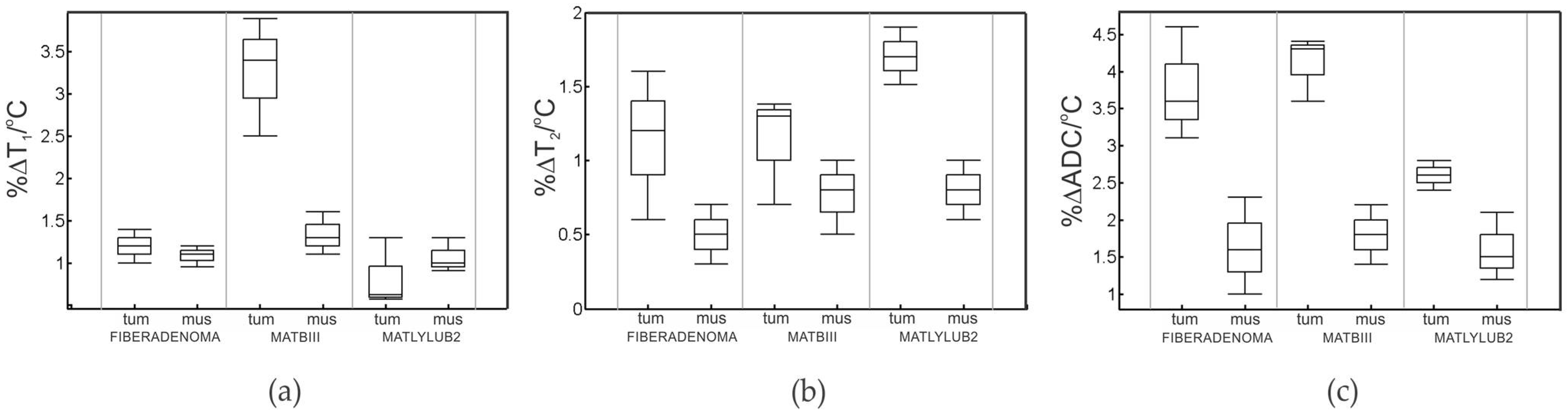
3.1. MR Imaging Experiments with Temperature Modulation
3.2. Tumor Histopathology
4. Discussion
- (i)
- measurements were obtained at temperatures less than or equal to 36 °C;
- (ii)
- accuracy of tumor temperature can be improved as temperature probe was placed just below the surface of the tumor instead of within the center of the tumor mass;
- (iii)
- the relatively small number of in vivo samples (n = 4) for each tumor type;
- (iv)
- the use of a xenograft (versus orthotopic) tumor model.
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Beck, E.S.; Gai, N.; Filippini, S.; Maranzano, J.; Nair, G.; Reich, D.S. Inversion recovery susceptibility weighted imaging with enhanced T2 weighting at 3 T improves visualization of subpial cortical multiple sclerosis lesions. Investig. Radiol. 2020, 55, 727–735. [Google Scholar] [CrossRef]
- Gillies, R.J.; Raghunand, N.; Karczmar, G.S.; Bhujwalla, Z.M. MRI of the tumor microenvironment. J. Magn. Reson. Imaging Off. J. Int. Soc. Magn. Reson. Med. 2002, 16, 430–450. [Google Scholar] [CrossRef]
- Joseph, C.R. Novel MRI techniques identifying vascular leak and paravascular flow reduction in early Alzheimer disease. Biomedicines 2020, 8, 228. [Google Scholar] [CrossRef]
- Wu, J.S.; Hochman, M.G. Soft-tissue tumors and tumorlike lesions: A systematic imaging approach. Radiology 2009, 253, 297–316. [Google Scholar] [CrossRef] [Green Version]
- Hakumäki, J.M.; Gröhn, O.H.; Tyynelä, K.; Valonen, P.; Ylä-Herttuala, S.; Kauppinen, R.A. Early gene therapy–induced apoptotic response in BT4C gliomas by magnetic resonance relaxation contrast T 1 in the rotating frame. Cancer Gene Ther. 2002, 9, 338–345. [Google Scholar] [CrossRef] [Green Version]
- Mountford, C.E.; Doran, S.; Lean, C.L.; Russell, P. Proton MRS can determine the pathology of human cancers with a high level of accuracy. Chem. Rev. 2004, 104, 3677–3704. [Google Scholar] [CrossRef]
- Simonetti, A.W.; Melssen, W.J.; Edelenyi, F.S.d.; van Asten, J.J.; Heerschap, A.; Buydens, L.M. Combination of feature-reduced MR spectroscopic and MR imaging data for improved brain tumor classification. NMR Biomed. Int. J. Devoted Dev. Appl. Magn. Reson. Vivo 2005, 18, 34–43. [Google Scholar] [CrossRef]
- Choyke, P.L.; Dwyer, A.J.; Knopp, M.V. Functional tumor imaging with dynamic contrast-enhanced magnetic resonance imaging. J. Magn. Reson. Imaging Off. J. Int. Soc. Magn. Reson. Med. 2003, 17, 509–520. [Google Scholar] [CrossRef]
- Knopp, M.; Hoffmann, U.; Brix, G.; Hawighorst, H.; Junkermann, H.; van Kaick, G. Fast MRI contrast medium dynamics for characterization of tumors. Experiences with functional MR-mammography. Der Radiol. 1995, 35, 964–972. [Google Scholar]
- Ma, S.; Nguyen, C.T.; Han, F.; Wang, N.; Deng, Z.; Binesh, N.; Moser, F.G.; Christodoulou, A.G.; Li, D. Three-dimensional simultaneous brain T1, T2, and ADC mapping with MR Multitasking. Magn. Reson. Med. 2020, 84, 72–88. [Google Scholar] [CrossRef]
- Méndez, C.A.; Pizzorni Ferrarese, F.; Summers, P.; Petralia, G.; Menegaz, G. DCE-MRI and DWI integration for breast lesions assessment and heterogeneity quantification. Int. J. Biomed. Imaging 2012, 2012, 2. [Google Scholar] [CrossRef]
- Damadian, R.; Zaner, K.; Hor, D.; DiMaio, T. Human tumors detected by nuclear magnetic resonance. Proc. Natl. Acad. Sci. USA 1974, 71, 1471–1473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bloembergen, N.; Purcell, E.; Pound, R. Nuclear magnetic relaxation. Nature 1947, 160, 475–476. [Google Scholar] [CrossRef] [Green Version]
- Chenevert, T.L.; Galbán, C.J.; Ivancevic, M.K.; Rohrer, S.E.; Londy, F.J.; Kwee, T.C.; Meyer, C.R.; Johnson, T.D.; Rehemtulla, A.; Ross, B.D. Diffusion coefficient measurement using a temperature-controlled fluid for quality control in multicenter studies. J. Magn. Reson. Imaging 2011, 34, 983–987. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parker, D.L. Applications of NMR imaging in hyperthermia: An evaluation of the potential for localized tissue heating and noninvasive temperature monitoring. IEEE Trans. Biomed. Eng. 1984, BME-31, 161–167. [Google Scholar] [CrossRef]
- Rieke, V. MR thermometry. In Interventional Magnetic Resonance Imaging; Springer: Berlin/Heidelberg, Germany, 2011; pp. 271–288. [Google Scholar]
- Graham, S.; Stanisz, G.; Kecojevic, A.; Bronskill, M.; Henkelman, R. Analysis of changes in MR properties of tissues after heat treatment. Magn. Reson. Med. Off. J. Int. Soc. Magn. Reson. Med. 1999, 42, 1061–1071. [Google Scholar] [CrossRef]
- Graham, S.J.; Bronskill, M.J.; Henkelman, R.M. Time and temperature dependence of MR parameters during thermal coagulation of ex vivo rabbit muscle. Magn. Reson. Med. 1998, 39, 198–203. [Google Scholar] [CrossRef]
- Han, M.; Rieke, V.; Scott, S.J.; Ozhinsky, E.; Salgaonkar, V.A.; Jones, P.D.; Larson, P.E.; Diederich, C.J.; Krug, R. Quantifying temperature-dependent T 1 changes in cortical bone using ultrashort echo-time MRI. Magn. Reson. Med. 2015, 74, 1548–1555. [Google Scholar] [CrossRef] [Green Version]
- Vesanen, P.T.; Zevenhoven, K.C.; Nieminen, J.O.; Dabek, J.; Parkkonen, L.T.; Ilmoniemi, R.J. Temperature dependence of relaxation times and temperature mapping in ultra-low-field MRI. J. Magn. Reson. 2013, 235, 50–57. [Google Scholar] [CrossRef]
- Parmala, M.; Eriksson, M.; Rytioja, M.; Tanttu, J.; Köhler, M. Temperature measurement in human fat with T2 imaging. J. Magn. Reson. Imaging 2016, 43, 1171–1178. [Google Scholar] [CrossRef]
- Kuroda, K.; Oshio, K.; Chung, A.H.; Hynynen, K.; Jolesz, F.A. Temperature mapping using the water proton chemical shift: A chemical shift selective phase mapping method. Magn. Reson. Med. 1997, 38, 845–851. [Google Scholar] [CrossRef]
- Shmatukha, A.V.; Harvey, P.R.; Bakker, C.J. Correction of proton resonance frequency shift temperature maps for magnetic field disturbances using fat signal. J. Magn. Reson. Imaging Off. J. Int. Soc. Magn. Reson. Med. 2007, 25, 579–587. [Google Scholar] [CrossRef] [PubMed]
- Soher, B.J.; Wyatt, C.; Reeder, S.B.; MacFall, J.R. Noninvasive temperature mapping with MRI using chemical shift water-fat separation. Magn. Reson. Med. 2010, 63, 1238–1246. [Google Scholar] [CrossRef] [Green Version]
- Wyatt, C.R.; Soher, B.J.; MacFall, J.R. Correction of breathing-induced errors in magnetic resonance thermometry of hyperthermia using multiecho field fitting techniques. Med. Phys. 2010, 37, 6300–6309. [Google Scholar] [CrossRef] [Green Version]
- Hofstetter, L.W.; Yeo, D.T.; Dixon, W.T.; Kempf, J.G.; Davis, C.E.; Foo, T.K. Fat-referenced MR thermometry in the breast and prostate using IDEAL. J. Magn. Reson. Imaging 2012, 36, 722–732. [Google Scholar] [CrossRef]
- Tarasek, M.R.; Pellicer, R.; Hofstetter, L.W.; Numan, W.C.; Bakker, J.F.; Kotek, G.; Togni, P.; Verhaart, R.F.; Fiveland, E.W.; Houston, G.C.; et al. Validation of MR thermometry: Method for temperature probe sensor registration accuracy in head and neck phantoms. Int. J. Hyperth. 2014, 30, 142–149. [Google Scholar] [CrossRef]
- MacFall, J.R.; Wehrli, F.W.; Breger, R.K.; Johnson, G.A. Methodology for the measurement and analysis of relaxation times in proton imaging. Magn. Reson. Imaging 1987, 5, 209–220. [Google Scholar] [CrossRef]
- de Marini, P.; Cazzato, R.L.; Garnon, J.; Shaygi, B.; Koch, G.; Auloge, P.; Tricard, T.; Lang, H.; Gangi, A. Percutaneous MR-guided prostate cancer cryoablation technical updates and literature review. BJR Open 2019, 1, 20180043. [Google Scholar] [CrossRef]
- Gangi, A.; Tsoumakidou, G.; Abdelli, O.; Buy, X.; de Mathelin, M.; Jacqmin, D.; Lang, H. Percutaneous MR-guided cryoablation of prostate cancer: Initial experience. Eur. Radiol. 2012, 22, 1829–1835. [Google Scholar] [CrossRef]
- Hua, Y.; Ma, S.; Fu, Z.; Hu, Q.; Wang, L.; Piao, Y. Intracavity hyperthermia in nasopharyngeal cancer: A phase III clinical study. Int. J. Hyperth. 2011, 27, 180–186. [Google Scholar] [CrossRef]
- Huilgol, N.G.; Gupta, S.; Sridhar, C. Hyperthermia with radiation in the treatment of locally advanced head and neck cancer: A report of randomized trial. J. Cancer Res. Ther. 2010, 6, 492. [Google Scholar] [CrossRef] [PubMed]
- Hynynen, K.; Pomeroy, O.; Smith, D.N.; Huber, P.E.; McDannold, N.J.; Kettenbach, J.; Baum, J.; Singer, S.; Jolesz, F.A. MR imaging-guided focused ultrasound surgery of fibroadenomas in the breast: A feasibility study. Radiology 2001, 219, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Mahnken, A.H.; Günther, R.W.; Tacke, J. Radiofrequency ablation of renal tumors. Eur. Radiol. 2004, 14, 1449–1455. [Google Scholar] [CrossRef]
- Caravan, P.; Ellison, J.J.; McMurry, T.J.; Lauffer, R.B. Gadolinium (III) chelates as MRI contrast agents: Structure, dynamics, and applications. Chem. Rev. 1999, 99, 2293–2352. [Google Scholar] [CrossRef] [PubMed]
- Golman, K.; Olsson, L.E.; Axelsson, O.; Mansson, S.; Karlsson, M.; Petersson, J. Molecular imaging using hyperpolarized 13C. Br. J. Radiol. 2003, 76, S118–S127. [Google Scholar] [CrossRef]
- Bottomley, P.A.; Hardy, C.; Argersinger, R.; Allen-Moore, G. A review of 1H nuclear magnetic resonance relaxation in pathology: Are T1 and T2 diagnostic? Med. Phys. 1987, 14, 1–37. [Google Scholar] [CrossRef] [PubMed]
- Just, M.; Higer, H.; Pfannenstiel, P. Errors in T1-determination using multislice technique and Gaussian slice profiles. Magn. Reson. Imaging 1988, 6, 53–56. [Google Scholar] [CrossRef]
- Katz, J.; Boxt, L.M.; Sciacca, R.R.; Cannon, P.J. Motion dependence of myocardial transverse relaxation time in magnetic resonance imaging. Magn. Reson. Imaging 1990, 8, 449–458. [Google Scholar] [CrossRef]
- Majumdar, S.; Sostman, H.D.; MacFall, J. Contrast and accuracy of relaxation time measurements in acquired and synthesized multislice magnetic resonance images. Investig. Radiol. 1989, 24, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Bottomley, P.A.; Andrew, E.R. RF magnetic field penetration, phase shift and power dissipation in biological tissue: Implications for NMR imaging. Phys. Med. Biol. 1978, 23, 630. [Google Scholar] [CrossRef]
- Dixon, W.T.; Engels, H.; Castillo, M.; Sardashti, M. Incidental magnetization transfer contrast in standard multislice imaging. Magn. Reson. Imaging 1990, 8, 417–422. [Google Scholar] [CrossRef]

| Fibroadenoma (Benign) | Breast Tumor/MAT B III | Prostate Tumor/ MatLyLuB2 | ||||
|---|---|---|---|---|---|---|
| Muscle c | Benign Tumor | Muscle c | Breast Malignant | Muscle c | Prostate Malignant | |
| T1 (ms) a | 769 ± 12 | 651 ± 20 | 767 ± 10 | 1201 ± 26 | 770 ± 13 | 1145 ± 22 |
| %ΔT1/°C b | 1.16 ± 0.20 | 1.20 ± 0.91 | 1.40 ± 0.42 | 3.41 ± 0.64 | 1.05 ± 0.3 | 0.60 ± 0.51 |
| T2 (ms) a | 26.0 ± 0.75 | 23.8 ± 1.2 | 25.8 ± 0.8 | 63.7 ± 2.1 | 26.6 ± 0.6 | 64.6 ± 1.7 |
| %ΔT2/°C b | 0.50 ± 0.3 | 1.17 ± 0.8 | 0.72 ± 0.5 | 1.11 ± 0.7 | 0.70 ± 0.3 | 1.7 ± 0.9 |
| ADC (×10−4 mm2/s) a | 14.88 ± 1.1 | 11.22 ± 1.6 | 14.91 ± 1.0 | 8.9 ± 1.7 | 13.52 ± 1.2 | 10.3 ± 1.4 |
| %ΔADC/°C b | 1.6 ± 0.7 | 4.00 ± 0.78 | 1.8 ± 0.6 | 4.20 ± 0.81 | 1.5 ± 0.6 | 2.60 ± 0.56 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tarasek, M.; Akin, O.; Roberts, J.; Foo, T.; Yeo, D. Heat Modulation of Intrinsic MR Contrasts for Tumor Characterization. Cancers 2022, 14, 405. https://doi.org/10.3390/cancers14020405
Tarasek M, Akin O, Roberts J, Foo T, Yeo D. Heat Modulation of Intrinsic MR Contrasts for Tumor Characterization. Cancers. 2022; 14(2):405. https://doi.org/10.3390/cancers14020405
Chicago/Turabian StyleTarasek, Matthew, Oguz Akin, Jeannette Roberts, Thomas Foo, and Desmond Yeo. 2022. "Heat Modulation of Intrinsic MR Contrasts for Tumor Characterization" Cancers 14, no. 2: 405. https://doi.org/10.3390/cancers14020405
APA StyleTarasek, M., Akin, O., Roberts, J., Foo, T., & Yeo, D. (2022). Heat Modulation of Intrinsic MR Contrasts for Tumor Characterization. Cancers, 14(2), 405. https://doi.org/10.3390/cancers14020405







