Optimizing the Diagnosis and Biomarker Testing for Patients with Intrahepatic Cholangiocarcinoma: A Multidisciplinary Approach
Abstract
:Simple Summary
Abstract
1. Introduction
2. The Value of Biomarker Testing in iCCA
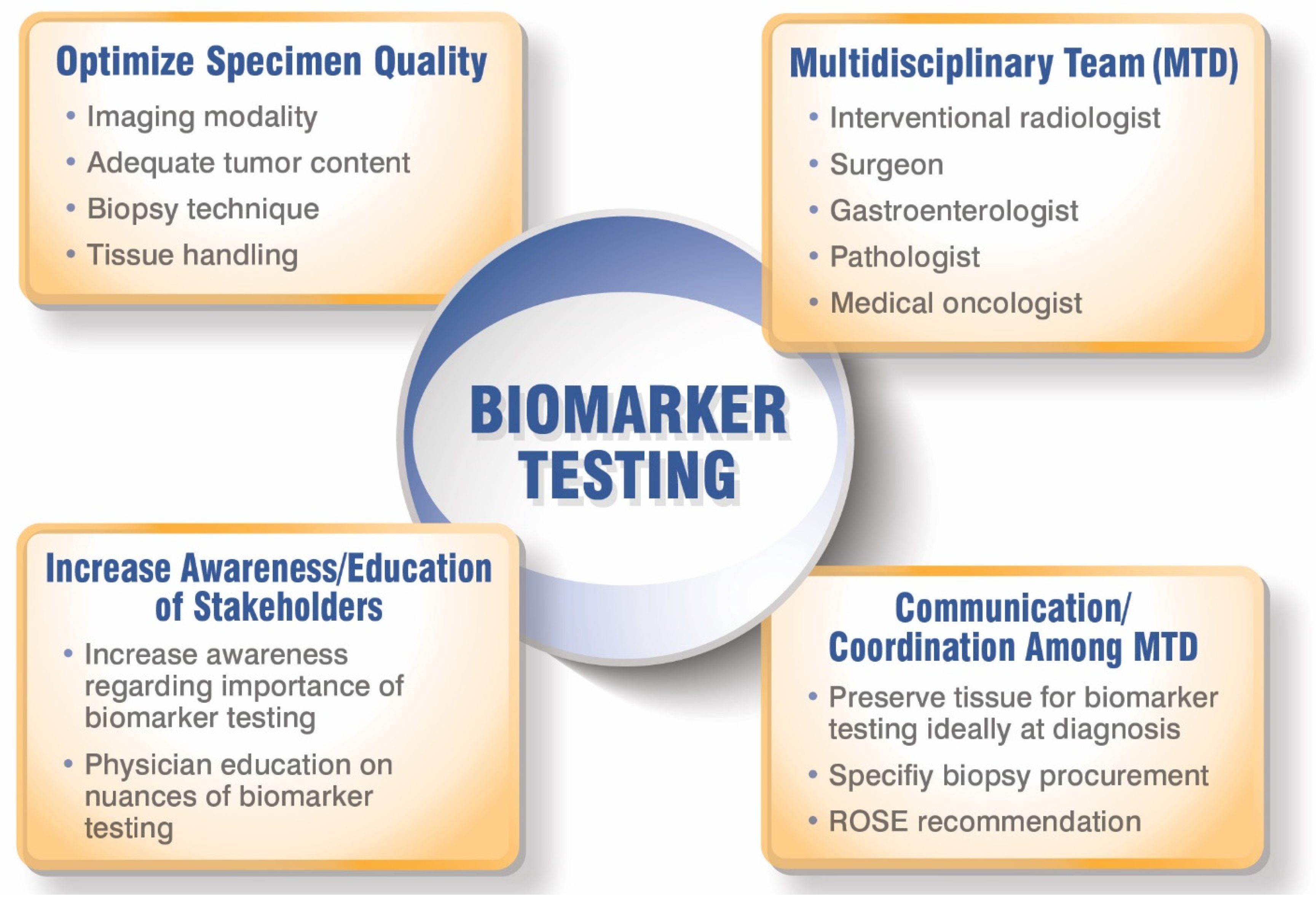
3. Identifying Potential Challenges and Solutions for Optimal Treatment of Patients with iCCA
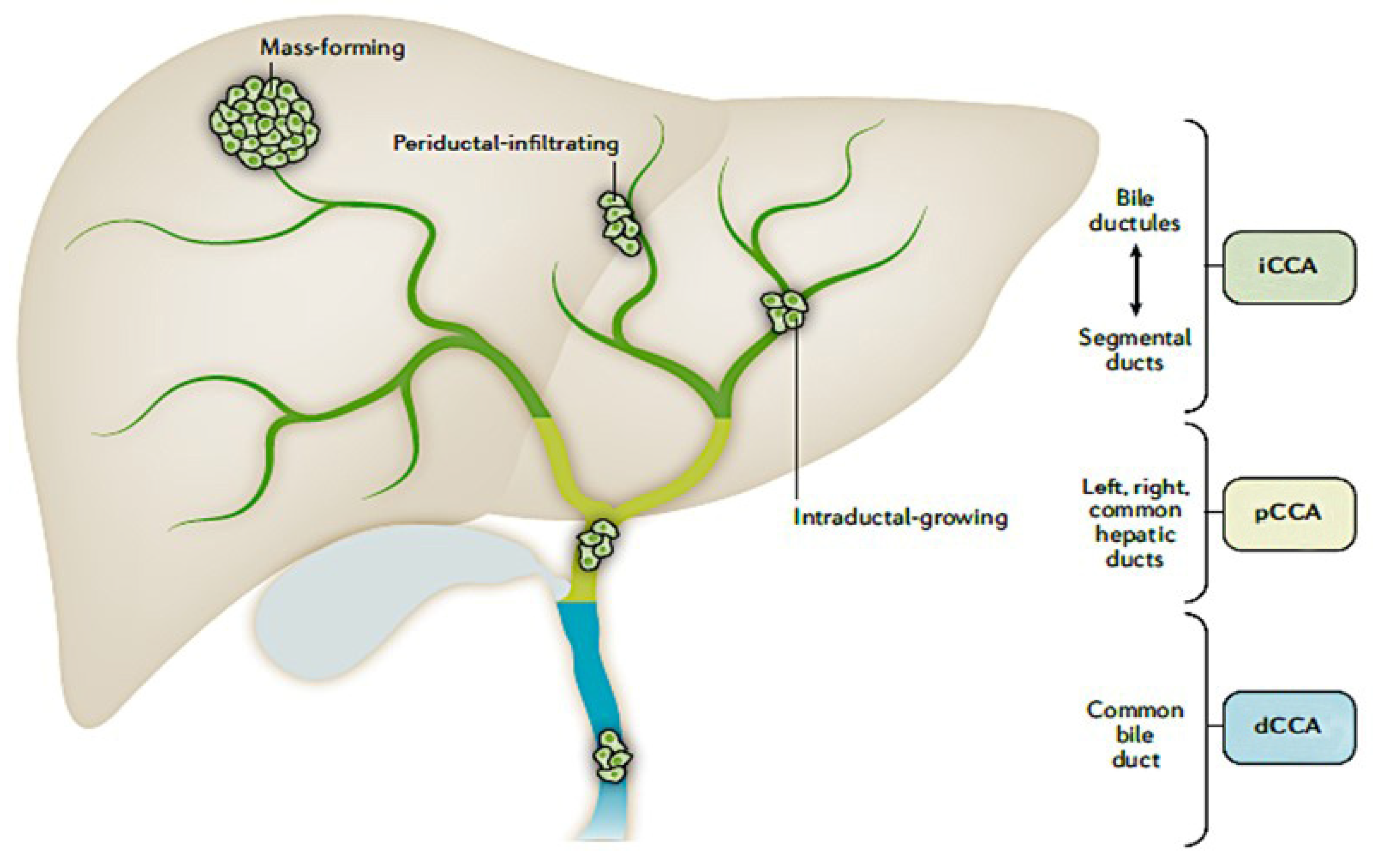
3.1. Diagnosis
3.1.1. Challenges
- Symptoms may be vague, non-specific or asymptomatic
- Absence of histologic specific markers of iCCA
- Robust diagnostic tests are unavailable and histologic features and techniques (Albumin ISH) may be underutilized
3.1.2. Best Practices
- Utilization of Albumin ISH to differentiate iCCA from liver metastases
- Incorporation of pathology techniques that identify cholangiolar pattern
- Awareness of imaging characteristics common to iCCA—for instance, capsular retraction
3.2. Biopsy Collection for Biomarker Testing
3.2.1. Challenges
- Imaging modalities are not standardized
- Identifying candidates for liver transplantation (these patients are not candidates for biopsy) and concern for adverse events associated with biopsy collection
- Tissue acquisition method (choice of needle gauge, CNB vs. FNA) is not standardized
- ROSE is not available or utilized
- Tissue is exhausted due to core samples not being separated into separate cassettes for diagnosis and biomarker testing
- Sub-optimal tumor cell content yield due to fibrotic tissue and high stromal content presence
- Location of tumor in liver can be difficult to access and image
- Feedback is not provided between pathologist and interventional radiologist (no closed-loop system)
3.2.2. Best Practices and Recommendations
- Define clear standards for biopsy collection, including specimen parameters, biopsy type and technique, and preservation parameters (consider MDA scoring system)
- Balancing the biopsy benefits and risks
- Encourage ROSE and the inclusion of a cytologist during biopsy procedures to optimize tumor cell content
- Consider collecting FNAs with core biopsies to improve tumor cell content
- Macro/microdissection of samples by pathology group to enhance tumor cell content
- When ordering NGS, provide rationale and goals of biopsy specimen for biomarker analysis
- Improve communication and feedback of tissue sample quality between the radiologist and pathologist (create a closed-loop system and consider incorporating ordering physician into discussions)
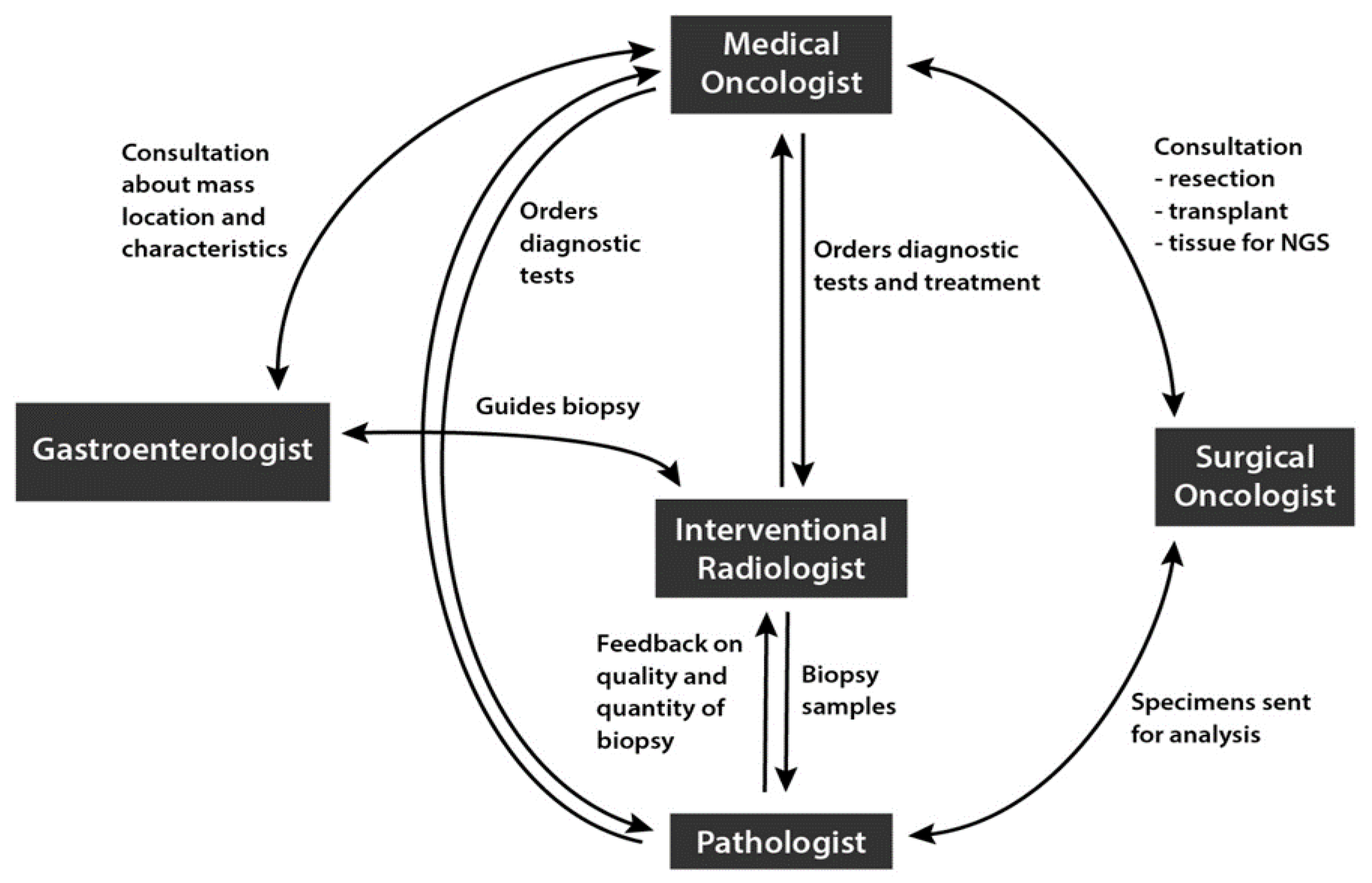
4. Best Practices among Hepatobiliary Multidisciplinary Teams (MDTs) to Optimize iCCA Diagnosis and Treatment
- Deficient communication among team regarding the complexity of iCCA diagnosis and new techniques to aid in diagnosis
- Siloed knowledge and education around biomarker testing treatment options
- No standardization on when to order biomarker specific tests
- Lack of molecular tumor boards and team analysis of biomarker test results to determine most effective treatment options for patients
- Communication among radiologist, pathologist, and medical oncologist to collect biopsy samples for biomarker at diagnosis
- Identify which physician or physicians are responsible for ordering NGS
- Include clear leadership structure
- Initiate regular team meetings and molecular tumor boards to provide optimal analysis of biomarker test results
5. Opportunities to Improve Knowledge and Utilization of Biomarker Testing
- Restricted access to molecular tumor boards and inability to communicate with NCI-designated cancer centers to help optimize patient diagnosis, biomarker testing, and treatment options
- Reduced awareness of the importance of biomarker testing to detect targetable alterations
- Limited training on differences among biomarker tests—NGS (RNA vs. DNA, tissue vs. liquid) vs. FISH vs. RT-PCR
- Lack of knowledge of approved targeted therapies for iCCA
- Enhance physician knowledge around the advantages/disadvantages of each biomarker test (NGS-RNA vs. DNA-based, tissue vs. liquid, etc.) and interpretation of test results
- Creation of delineated guidelines and recommendations that clearly define how to integrate biomarker testing into the hepatobiliary multidisciplinary care, ideally at diagnosis
- Provide information about the importance of NGS results for precision-based treatment and patient inclusion in clinical trials through educational activities (development of iCCA multidisciplinary focus groups, CMEs, educational grants, in-person and virtual meetings)
- Improve communication between academic radiologists and community-based radiologists around standardized biopsy guidelines for biomarker testing
- Develop iCCA-specific multidisciplinary focus groups to advance knowledge around the importance of biopsies for personalized medicine/biomarker testing
- Consider implementing virtual molecular tumor boards in academic setting that incorporates community physicians (regional or statewide) to help with complex cases and analysis of biomarker test results
6. Discussion
6.1. Expert Recommendations
6.2. Future Directions
7. Conclusions by the Expert Multidisciplinary Team
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Banales, J.M.; Marin, J.J.G.; Lamarca, A.; Rodrigues, P.M.; Khan, S.A.; Roberts, L.R.; Cardinale, V.; Carpino, G.; Andersen, J.B.; Braconi, C.; et al. Cholangiocarcinoma 2020: The next horizon in mechanisms and management. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 557–588. [Google Scholar] [CrossRef]
- Lauterio, A.; De Carlis, R.; Centonze, L.; Buscemi, V.; Incarbone, N.; Vella, I.; De Carlis, L. Current surgical management of peri-hilar and intra-hepatic cholangiocarcinoma. Cancers 2021, 13, 3657. [Google Scholar] [CrossRef] [PubMed]
- Rizvi, S.; Khan, S.A.; Hallemeier, C.L.; Kelley, R.K.; Gores, G.J. Cholangiocarcinoma-evolving concepts and therapeutic strategies. Nat. Rev. Clin. Oncol. 2018, 15, 95–111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsilimigras, D.I.; Sahara, K.; Wu, L.; Moris, D.; Bagante, F.; Guglielmi, A.; Aldrighetti, L.; Weiss, M.; Bauer, T.W.; Alexandrescu, S.; et al. Very early recurrence after liver resection for intrahepatic cholangiocarcinoma: Considering alternative treatment approaches. JAMA Surg. 2020, 155, 823–831. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-F.; Beal, E.W.; Bagante, F.; Chakedis, J.; Weiss, M.; Popescu, I.; Marques, H.P.; Aldrighetti, L.; Maithel, S.K.; Pulitano, C.; et al. Early versus late recurrence of intrahepatic cholangiocarcinoma after resection with curative intent. Br. J. Surg. 2018, 105, 848–856. [Google Scholar] [CrossRef] [PubMed]
- Doussot, A.; Gonen, M.; Wiggers, J.K.; Groot-Koerkamp, B.; DeMatteo, R.P.; Fuks, D.; Allen, P.J.; Farges, O.; Kingham, T.P.; Regimbeau, J.M.; et al. Recurrence patterns and disease-free survival after resection of intrahepatic cholangiocarcinoma: Preoperative and postoperative prognostic models. J. Am. Coll. Surg. 2016, 223, 493–505.e2. [Google Scholar] [CrossRef] [Green Version]
- Valle, J.; Wasan, H.; Palmer, D.H.; Cunningham, D.; Anthoney, A.; Maraveyas, A.; Madhusudan, S.; Iveson, T.; Hughes, S.; Pereira, S.P.; et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N. Engl. J. Med. 2010, 362, 1273–1281. [Google Scholar] [CrossRef] [Green Version]
- Shroff, R.T.; Javle, M.M.; Xiao, L.; Kaseb, A.O.; Varadhachary, G.R.; Wolff, R.A.; Raghav, K.P.S.; Iwasaki, M.; Masci, P.; Ramanathan, R.K.; et al. Gemcitabine, cisplatin, and nab-paclitaxel for the treatment of advanced biliary tract cancers: A phase 2 clinical trial. JAMA Oncol. 2019, 5, 824–830. [Google Scholar] [CrossRef]
- Lamarca, A.; Palmer, D.H.; Wasan, H.S.; Ross, P.J.; Ma, Y.T.; Arora, A.; Falk, S.; Gillmore, R.; Wadsley, J.; Patel, K.; et al. Second-line FOLFOX chemotherapy versus active symptom control for advanced biliary tract cancer (ABC-06): A phase 3, open-label, randomised, controlled trial. Lancet Oncol. 2021, 22, 690–701. [Google Scholar] [CrossRef]
- Bekaii-Saab, T.S.; Briedgewater, J.; Normanno, N. Practical considerations in screening for genetic alterations in cholangiocarcinoma. Ann. Oncol. 2021, 32, 1111–1126. [Google Scholar] [CrossRef]
- US Food and Drug Administration. FDA Grants Accelerated Approval to Pemigatinib for Cholangiocarcinoma with an FGFR2 Rearrangement or Fusion. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-pemigatinib-cholangiocarcinoma-fgfr2-rearrangement-or-fusion (accessed on 24 June 2021).
- US Food and Drug Administration. FDA Grants Accelerated Approval to Infigratinib for Metastatic Cholangiocarcinoma. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-infigratinib-metastatic-cholangiocarcinoma (accessed on 24 June 2021).
- US Food and Drug Administration. FDA Approves Ivosidenib for Advanced or Metastatic Cholangiocarcinoma. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-ivosidenib-advanced-or-metastatic-cholangiocarcinoma (accessed on 24 June 2021).
- Bibeau, K.; Bachini, M.; Lindley, A.; Barkey, N.M.; Lindsey, S. Exploring the diagnostic journey and life impact of patients with cholangiocarcinoma (CCA): Results from a large patient survey in the United States. J. Clin. Oncol. 2021, 39, 277. [Google Scholar] [CrossRef]
- Lamarca, A.; Kapacee, A.; Breeze, M.; Bell, C.; Belcher, D.; Staiger, H.; Taylor, C.; McNamara, M.G.; Hubner, R.A.; Valle, J.W. Molecular profiling in daily clinical practice: Practicalities in advanced cholangiocarcinoma and other biliary trat cancers. J. Clin. Med. 2020, 9, 2854. [Google Scholar] [CrossRef]
- Nakamura, Y.; Taniguchi, H.; Ikeda, M.; Bando, H.; Kato, K.; Morizane, C.; Esaki, T.; Komatsu, Y.; Kawamoto, Y.; Takahashi, N.; et al. Clinical utility of circulating tumor DNA sequencing in advanced gastrointestinal cancer: SCRUM-Japan GI- SCREEN and GOZILA studies. Nat. Med. 2020, 26, 1859–1864. [Google Scholar] [CrossRef]
- Tomlins, S.A.; Hovelson, D.H.; Suga, J.M.; Anderson, D.M.; Koh, H.A.; Dees, E.C.; McNulty, B.; Burkard, M.E.; Guarino, M.; Khatri, J.; et al. Real-world performance of a comprehensive genomic profiling test optimized for small tumor simples. JCO Precis. Oncol. 2021, 5, 1312–1324. [Google Scholar] [CrossRef] [PubMed]
- Parikh, K.; Ragoonath Cameron, D.; Abair, T.; Kugel, P.; Vogel, A. Targeted therapies in cholangiocarcinoma: Assessment of US oncologist practice patterns. J. Clin. Oncol. 2021, 39, 347. [Google Scholar] [CrossRef]
- Ersek, J.L.; Black, L.J.; Thompson, M.A.; Kim, E.S. Implementing precision medicine programs and clinical trials in community-based oncology practice: Barriers and best practices. Am. Soc. Clin. Oncol. Educ. Book 2018, 38, 188–196. [Google Scholar] [CrossRef]
- Jia, A.Y.; Popovic, A.; Mohan, A.A.; Zorzi, J.; Griffith, P.; Kim, A.K.; Anders, R.A.; Burkhart, R.A.; Lafaro, K.; Georgiades, C.; et al. Development, practice patterns, and early clinical outcomes of a multidisciplinary liver cancer clinic. Cancer Control 2021, 28, 10732748211009945:1–10732748211009945:11. [Google Scholar] [CrossRef]
- Levit, L.A.; Byatt, L.; Lyss, A.P.; Paskett, E.D.; Levit, K.; Kirkwood, K.; Schenkel, C.; Schilsky, R.L. Closing the rural cancer care gap: Three institutional approaches. JCO Oncol. Pract. 2020, 16, 422–430. [Google Scholar] [CrossRef] [PubMed]
- Kato, S.; Kim, K.H.; Lim, H.J.; Boichard, A.; Nikanjam, M.; Weihe, E.; Kuo, D.J.; Eskander, R.N.; Goodman, A.; Galanina, N.; et al. Real-world data from a molecular tumor board demonstrates improved outcomes with a precision N-of-One strategy. Nat. Commun. 2020, 11, 4965:1–4965:9. [Google Scholar] [CrossRef]
- Sutton, T.L.; Walker, B.S.; Nabavizadeh, N.; Grossberg, A.; Thomas, C.R., Jr.; Lopez, C.D.; Kardosh, A.; Chen, E.Y.; Sheppard, B.C.; Mayo, S.C. Geographic disparities in referral and oncologic outcomes in intrahepatic cholangiocarcinoma: A population-based study. Ann. Surg. Oncol. 2021, 28, 8152–8159. [Google Scholar] [CrossRef]
- Martin, N.A.; Tepper, J.E.; Giri, V.N.; Stinchcombe, T.E.; Cheng, H.H.; Javle, M.M.; Konnick, E.Q. Adopting consensus terms for testing in precision medicine. JCO Precis. Oncol. 2021, 5, 1563–1567. [Google Scholar] [CrossRef]
- Pellino, A.; Loupakis, F.; Cadamuro, M.; Dadduzio, V.; Fassan, M.; Guido, M.; Cillo, U.; Indraccolo, S.; Fabris, L. Precision medicine in cholangiocarcinoma. Transl. Gastroenterol. Hepatol. 2018, 3, 40:1–40:15. [Google Scholar] [CrossRef] [PubMed]
- Avogadri-Connors, F.; Wei, G.; Dambkowski, C.L.; Li, G.; Soifer, H.S. Abstract 2940: Molecular tumor profiling identifies actionable targets in patients with cholangiocarcinoma. Cancer Res. 2020, 80, 2940. [Google Scholar] [CrossRef]
- Lamarca, A.; Barriuso, J.; McNamara, M.G.; Valle, J.W. Molecular targeted therapies: Ready for “prime time” in biliary tract cancer. J. Hepatol. 2020, 73, 170–185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kelley, R.K.; Bridgewater, J.; Gores, G.J.; Zhu, A.X. Systemic therapies for intrahepatic cholangiocarcinoma. J. Hepatol. 2020, 72, 353–363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abou-Alfa, G.K.; Macarulla, T.; Javle, M.M.; Kelley, R.K.; Lubner, S.J.; Adeva, J.; Cleary, J.M.; Catenacci, D.V.; Borad, M.J.; Bridgewater, J.; et al. Ivosidenib in IDHa-mutant, chemotherapy-refractory cholangiocarcinoma (ClarIDHy): A multicentre, randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol. 2020, 21, 796–807. [Google Scholar] [CrossRef]
- Javle, M.M.; Roychowdhury, S.; Kelley, R.K.; Sadeghi, S.; Macarulla, T.; Waldschmidt, D.T.; Goyal, L.; Borbath, I.; El-Khoueiry, A.B.; Yong, W.-P.; et al. Final results from a phase II study of infigratinib (BGJ398), an FGFR-selective tyrosine kinase inhibitor, in patients with previously treated advanced cholangiocarcinoma harboring an FGFR2 gene fusion or rearrangement. J. Clin. Oncol. 2021, 39, 265. [Google Scholar] [CrossRef]
- Goyal, L. Primary results of phase 2 FOENIX-CCA2: The irreversible FGFR1-4 inhibitor futibatinib in intrahepatic cholangiocarcinoma with FGFR2 fusions/rearrangements. Cancer Res. 2021, 81, CT010. [Google Scholar] [CrossRef]
- Busset, M.D.D.; Shaib, W.L.; Kody, M.; Personeni, N.; Damjanov, N.; Harris, W.P.; Bergamo, F.; Brandi, G.; Masi, G.; Halfdanarson, T.R.; et al. Derazantinib for patients with intrahepatic cholangiocarcinoma harboring FGFR2 fusions/rearrangements: Primary results from the phase II study FIDES-01. Ann. Oncol. 2021, 32, S376. [Google Scholar] [CrossRef]
- Lee, P.C.; Hendifar, A.; Osipov, A.; Cho, M.; Li, D.; Gong, J. Targeting the fibroblast growth factor receptor (FGFR) in advanced cholangiocarcinoma: Clinical trial progress and future considerations. Cancers 2021, 13, 1706. [Google Scholar] [CrossRef]
- Abou-Alfa, G.K.; Sahai, V.; Hollebecque, A.; Vaccaro, G.; Melisi, D.; Al-Rajabi, R.; Paulson, A.S.; Borad, M.J.; Gallinson, D.; Murphy, A.G.; et al. Pemigatinib for previously treated, locally advanced or metastatic cholangiocarcinoma: A multicentre, open-label, phase 2 study. Lancet Oncol. 2020, 21, 671–684. [Google Scholar] [CrossRef]
- Keenan, B.P.; Kelley, R.K.K. Key challenges for drugs in clinical development for cholangiocarcinoma. Expert Opin. Investig. Drugs 2021, 30, 285–290. [Google Scholar] [CrossRef] [PubMed]
- Lamarca, A.; Hubner, R.A.; Ryder, W.D.; Valle, J.W. Second-line chemotherapy in advanced biliary cancer: A systematic review. Ann. Oncol. 2014, 25, 2328–2338. [Google Scholar] [CrossRef] [PubMed]
- Yoo, C.; Kim, K.-P.; Kim, I.; Kang, M.J.; Cheon, J.; Kang, B.W.; Ryu, H.; Jeong, J.H.; Lee, J.S.; Kim, K.W.; et al. Liposomal irinotecan (nal-IRI) in combination with fluorouracil (5-FU) and leucovorin (LV) for patients with metastatic biliary tract cancer (BTC) after progression on gemcitabine plus cisplatin (GemCis): Multicenter comparative randomized phase 2b study (NIFTY). J. Clin. Oncol. 2021, 39, 4006. [Google Scholar] [CrossRef]
- Tella, S.H.; Kommalapati, A.; Borad, M.J.; Mahipal, A. Second-line therapies in advanced biliary tract cancers. Lancet Oncol. 2020, 21, e29–e41. [Google Scholar] [CrossRef]
- Walter, T.; Horgan, A.M.; McNamara, M.; McKeever, L.; Min, T.; Hedley, D.; Serra, S.; Krzyzanowska, M.K.; Chen, E.; Mackay, H.; et al. Feasibility and benefits of second-line chemotherapy in advanced biliary tract cancer: A large retrospective study. Eur. J. Cancer 2013, 49, 329–335. [Google Scholar] [CrossRef]
- Benson, A.B.; D’Angelica, M.I.; Abbott, D.E.; Anaya, D.A.; Anders, R.; Are, C.; Bachini, M.; Borad, M.; Brown, D.; Burgoyne, A.; et al. Hepatobiliary Cancers, Version 2.2021, NCCN Clinical Practice Guidelies in Oncology. J. Natl. Compr. Canc. Netw. 2021, 19, 541–565. [Google Scholar] [CrossRef]
- Mosele, F.; Remon, J.; Mateo, J.; Westphalen, C.B.; Barlesi, F.; Lolkema, M.P.; Normanno, N.; Scarpa, A.; Robson, M.; Meric-Bernstam, F.; et al. Recommendations for the use of next-generation sequencing (NGS) for patients with metastatic cancers: A report from the ESMO Precision Medicine Working Group. Ann. Oncol. 2020, 31, 1491–1505. [Google Scholar] [CrossRef]
- Brindley, P.J.; Bachini, M.; Ilyas, S.I.; Kahn, S.A.; Loukas, A.; Sirica, A.E.; Teh, B.T.; Wongkam, S.; Gores, G.J. Cholangiocarcinoma. Nat. Rev. Dis. Primers 2021, 7, 65. [Google Scholar] [CrossRef] [PubMed]
- Bourien, H.; Lamarca, A.; McNamara, M.G.; Hubner, R.A.; Valle, J.W.; Edeline, J. Druggable molecular alterations in bile duct cancer: Potential and current therapeutic applications in clinical trials. Expert Opin. Investig. Drugs 2021, 30, 975–983. [Google Scholar] [CrossRef] [PubMed]
- Malaguarnera, G.; Paladina, I.; Giordano, M.; Malaguarnera, M.; Bertino, G.; Berretta, M. Serum markers of intrahepatic cholangiocarcinoma. Dis. Markers 2013, 34, 219–228. [Google Scholar] [CrossRef]
- Mar, W.A.; Chan, H.K.; Trivedi, S.B.; Berggruen, S.M. Imaging of intrahepatic cholangiocarcinoma. Semin. Ultrasound CT MR 2021, 42, 366–380. [Google Scholar] [CrossRef] [PubMed]
- Joo, I.; Lee, J.M.; Yoon, J.H. Imaging diagnosis of intrahepatic and perihilar cholangiocarcinoma: Recent advances and challenges. Radiology 2018, 288, 7–13. [Google Scholar] [CrossRef] [Green Version]
- Razumilava, N.; Gores, G.J. Cholangiocarcinoma. Lancet 2014, 383, 2168–2179. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.H.; Yoon, J.H.; Joo, I.; Lee, J.M. Evaluation of primary liver cancers using hepatocyte-specific contrast-enhanced MRI: Pitfalls and potential tips. J. Magn. Reson. Imaging 2021, 53, 655–675. [Google Scholar] [CrossRef] [PubMed]
- Blechacz, B.; Komuta, M.; Roskams, T.; Gores, G.J. Clinical diagnosis and staging of cholangiocarcinoma. Nat. Rev. Gastroenterol. Hepatol. 2011, 8, 512–522. [Google Scholar] [CrossRef] [Green Version]
- Vijgen, S.; Terris, B.; Rubbia-Brandt, L. Pathology of intrahepatic cholangiocarcinoma. Hepatobiliary Surg. Nutr. 2017, 6, 22–34. [Google Scholar] [CrossRef] [Green Version]
- Goyal, L.; Chen, C.T.; Pierce, T.T.; Deshpande, V. Case 8-2021: A 34-year-old woman with cholangiocarcinoma. N. Engl. J. Med. 2021, 384, 1054–1064. [Google Scholar] [CrossRef] [PubMed]
- Brackett, D.G.; Neyaz, A.; Arora, K.; Masia, R.; Mattia, A.; Zukerberg, L.; Misdraji, J.; Goyal, L.; Zhu, A.X.; Ferrone, C.R.; et al. Cholangiolar pattern and albumin in situ hybridisation enable a diagnosis of intrahepatic cholangiocarcinoma. J. Clin. Pathol. 2020, 73, 23–29. [Google Scholar] [CrossRef]
- Ferrone, C.R.; Ting, D.T.; Shahid, M.; Konstantinidis, I.T.; Sabbatino, F.; Goyal, L.; Rice-Stitt, T.; Mubeen, A.; Arora, K.; Bardeesey, N.; et al. The ability to diagnose intrahepatic cholangiocarcinoma definitively using novel branched DNA-enhanced albumin RNA in situ hybridization technology. Ann. Surg. Oncol. 2016, 23, 290–296. [Google Scholar] [CrossRef] [PubMed]
- Bledsoe, J.R.; Shinagare, S.A.; Deshpande, V. Difficult diagnostic problems in pancreatobiliary neoplasia. Arch. Pathol. Lab. Med. 2015, 139, 848–857. [Google Scholar] [CrossRef] [Green Version]
- Lowery, M.A.; Ptashkin, R.; Jordan, E.; Berger, M.F.; Zehir, A.; Capanu, M.; Kemeny, N.E.; O’Reilly, E.M.; El-Dika, I.; Jarnagin, W.R.; et al. Comprehensive molecular profiling of intrahepatic and extrahepatic cholangiocarcinomas: Potential targets for intervention. Clin. Cancer Res. 2018, 24, 4154–4161. [Google Scholar] [CrossRef] [Green Version]
- Verlingue, L.; Malka, D.; Allorant, A.; Massard, C.; Ferté, C.; Lacroix, L.; Rouleau, E.; Auger, N.; Ngo, M.; Nicotra, C.; et al. Precision medicine for patients with advanced biliary tract cancers: An effective strategy within the prospective MOSCATO-01 trial. Eur. J. Cancer 2017, 87, 122–130. [Google Scholar] [CrossRef]
- Roberts, M.C.; Spees, L.P.; Freedman, A.N.; Klein, W.M.P.; Das, I.P.; Butler, E.N.; de Moor, J.S. Oncologist-reported reasons for not ordering multimarker tumor panels: Results from a nationally representative survey. JCO Precis. Oncol. 2021, 5, 701–709. [Google Scholar] [CrossRef] [PubMed]
- Levy, E.B.; Fiel, M.I.; Hamilton, S.R.; Kleiner, D.E.; McCall, S.J.; Schirmacher, P.; Travis, W.; Kuo, M.D.; Suh, R.D.; Tam, A.L.; et al. State of the art: Toward improving outcomes of lung and liver tumor biopsies in clinical trials-a multidisciplinary approach. J. Clin. Oncol. 2020, 38, 1633–1640. [Google Scholar] [CrossRef] [PubMed]
- Goldhoff, P.E.; Vohra, P.; Kolli, K.P.; Ljung, B.-M. Fine-needle aspiration biopsy of liver lesions yields higher tumor fraction for molecular studies: A direct comparison with concurrent core needle biopsy. J. Natl. Compr. Canc. Netw. 2019, 17, 1075–1081. [Google Scholar] [CrossRef] [PubMed]
- Gan, Q.; Roy-Chowdury, S. Small but powerful: The promising role of small specimens for biomarker testing. J. Am. Soc. Cytopathol. 2020, 9, 450–460. [Google Scholar] [CrossRef]
- Roy-Chowdhuri, S.; Aisner, D.L.; Allen, T.C.; Beasley, M.B.; Borczuk, A.; Cagle, P.T.; Capelozzi, V.; Dacic, S.; da Cunha Santos, G.; Hariri, L.P.; et al. Biomarker testig in lung carcinoma cytology specimens: A perspective from members of the Pulmonary Pathology Society. Arch. Pathol. Lab. Med. 2016, 140, 1267–1272. [Google Scholar] [CrossRef] [Green Version]
- Tam, A.L.; Papadimitrakopoulou, V.; Wistuba, I.I.; Lee, J.J.; Ensor, J.E.; Kim, E.S.; Kalhor, N.; Blumenschein, G.R., Jr.; Tsao, A.S.; Heymach, J.V.; et al. The value of interventional radiology in clinical trial teams: Experience from the BATTLE lung cancer trials. Clin. Radiol. 2021, 76, 155.e25–155.e35. [Google Scholar] [CrossRef]
- Sirica, A.E.; Gores, G.J. Desmoplastic stroma and cholangiocarcinoma: Clinical implications and therapeutic targeting. Hepatology 2014, 59, 2397–2402. [Google Scholar] [CrossRef] [Green Version]
- Choo, M.; Ahn, S.; Hong, M.; Bang, H.; Van Vrancken, M.; Kim, S.; Lee, J.; Park, S.H.; Park, J.O.; Park, Y.S.; et al. Tissue recommendations for precision cancer therapy using next generation sequencing: A comprehensive single cancer center’s experiences. Oncotarget 2017, 8, 42478–42486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ascierto, P.A.; Bifulco, C.; Palmieri, G.; Peters, S.; Sidiropoulos, N. Preanalytic variables and tissue stewardship for reliable next-generation sequencing (NGS) clinical analysis. J. Mol. Diagn. 2019, 21, 756–767. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheth, R.A.; Baerlocher, M.O.; Connolly, B.L.; Dariushnia, S.R.; Shyn, P.B.; Vatsky, S.; Tam, A.L.; Gupta, S. Society of Intervnetional Radiology quality improvement standards on percutaneous needle biopsy in adult and pediatric patients. J. Vasc. Interv. Radiol. 2020, 31, 1840–1848. [Google Scholar] [CrossRef]
- Ferry-Galow, K.V.; Datta, V.; Makhlouf, H.R.; Wright, J.; Wood, B.J.; Levy, E.; Pisano, E.D.; Tam, A.L.; Lee, S.I.; Mahmood, U.; et al. What can be done to improve research biopsy quality in oncology clinical trials? J. Oncol. Pract. 2018, 14, e722–e728. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.A.; Hegab, B.; Hyde, C.; Guo, B.; Buckels, J.A.C.; Mirza, D.F. Needle track seeding following biopsy of liver lesions in the diagnosis of hepatocellular cancer: A systematic review and meta-analysis. Gut 2008, 57, 1592–1596. [Google Scholar] [CrossRef] [PubMed]
- Javle, M.; Bekaii-Saab, T.; Jain, A.; Wang, Y.; Kelley, R.K.; Wang, K.; Kang, H.C.; Catenacci, D.; Ali, S.; Krishnan, S.; et al. Biliary cancer: Utility of next-generation sequencing for clinical management. Cancer 2016, 122, 3838–3847. [Google Scholar] [CrossRef] [Green Version]
- Schwartzberg, L.; Kim, E.S.; Liu, D.; Schrag, D. Precision oncology: Who, how, what, when, and when not? Am. Soc. Clin. Oncol. Educ. Book 2017, 37, 160–169. [Google Scholar] [CrossRef]
- McKenzie, A.J.; Dilks, H.H.; Jones, S.F.; Burris, H., 3rd. Should next-generation sequencing tests be performed on all cancer patients? Expert Rev. Mol. Diagn. 2019, 19, 89–93. [Google Scholar] [CrossRef]
- O’Shea, A.; Tam, A.L.; Kilcoyne, A.; Flaherty, K.T.; Lee, S.I. Image-guided biopsy in the age of personalised medicine: Strategies for success and safety. Clin. Radiol. 2021, 76, 154.e1–154.e9. [Google Scholar] [CrossRef]
- Walia, S.; Aron, M.; Hu, E.; Chopra, S. Utility of rapid on-site evaluation for needle core biopsies and fine-needle aspiration cytology done for diagnosis of mass lesions of the liver. J. Am. Soc. Cytopathol. 2019, 8, 69–77. [Google Scholar] [CrossRef]
- Okamura, R.; Kurzrock, R.; Mallory, R.J.; Fanta, P.T.; Burgoyne, A.M.; Clary, B.M.; Kato, S.; Sicklick, J.K. Comprehensive genomic landscape and precision therapeutic approach in biliary tract cancers. Int. J. Cancer 2021, 148, 701–712. [Google Scholar] [CrossRef]
- Mody, K.; Kasi, P.M.; Yang, J.D.; Surapeneni, P.K.; Ritter, A.; Roberts, A.; Nagy, R.; Borad, M.J. Feasibility of circulating tumor DNA testig in hepatocellular carcinoma. J. Gastrointest. Oncol. 2019, 10, 745–750. [Google Scholar] [CrossRef] [PubMed]
- Ettrich, T.J.; Schwerdel, D.; Dolnik, A.; Beuter, F.; Blätte, T.J.; Schmidt, S.A.; Stanescu-Siegmund, N.; Steinacker, J.; Marienfeld, R.; Kleger, A.; et al. Genotyping of circulating tumor DNA in cholangiocarcinoma reveals diagnostic and prognostic information. Sci. Rep. 2019, 9, 13261:1–13261:11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ross, J.S.; Sokol, E.; Vergilio, J.-A.; Killian, K.; Lin, D.I.; Williams, E.; Danziger, N.; Ramkissoon, S.H.; Severson, E.A.; Hemmerich, A.; et al. Primary versus metastatic intrahepatic cholangiocarcinoma: A comparative comprehensive genomic profiling (CGP) study. J. Clin. Oncol. 2020, 38, 578. [Google Scholar] [CrossRef]
- Israel, M.A.; Danziger, N.; McGregor, K.A.; Murugesan, K.; Gjoerup, O.; Sokol, E.S.; Tukachinsky, H.; Kurzrock, R.; Kato, S.; Sicklick, J.K.; et al. Comparative genomic analysis of intrahepatic cholangiocarcinoma: Biopsy type, ancestry, and testing patterns. Oncologist 2021, 26, 787–796. [Google Scholar] [CrossRef] [PubMed]
- Selby, P.; Popescu, R.; Lawler, M.; Butcher, H.; Costa, A. The value and future developments of multidisciplinary team cancer care. Am. Soc. Clin. Oncol. Educ. Book 2019, 39, 332–340. [Google Scholar] [CrossRef]
- Kommalapati, A.; Tella, S.H.; Goyal, G.; Borad, M.; Alberts, S.R.; Roberts, L.; Hubbard, J.M.; Durgin, L.; Cleary, S.; Mahipal, A. Association between treatment facility volume, therapy types and overall survival in patients with intrahepatic cholangiocarcinoma. HPB 2019, 21, 379–386. [Google Scholar] [CrossRef] [Green Version]
- Lee, G.C.; Gamblin, T.C.; Fong, Z.V.; Ferrone, C.R.; Goyal, L.; Lillemoe, K.D.; Blaszkowsky, L.S.; Tanabe, K.K.; Qadan, M. Facility type is associated with margin status and overall survival of patients with resected intrahepatic cholangiocarcinoma. Ann. Surg. Oncol. 2019, 26, 4091–4099. [Google Scholar] [CrossRef]
- Levit, L.A.; Kim, E.S.; McAneny, B.L.; Nadauld, L.D.; Levit, K.; Schenkel, C.; Schilsky, R.L. Implementing precision medicine in community-based oncology programs: Three models. J. Oncol. Pract. 2020, 15, 325–329. [Google Scholar] [CrossRef]
- Jones, S.F.; McKenzie, A.J. Molecular profiling in drug development: Paving a way forward. Am. Soc. Clin. Oncol. Educ. Book 2020, 40, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Burkard, M.E.; Deming, D.A.; Parsons, B.M.; Kenny, P.A.; Schuh, M.R.; Leal, T.; Uboha, N.; Lang, J.M.; Thompson, M.A.; Warren, R.; et al. Implementation and clinical utility of an integrated academic-community regional molecular tumor board. JCO Precis. Oncol. 2017, 1, PO.16.00022:1–PO.16.00022:10. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Mavros, M.N.; Cosgrove, D.; Hirose, K.; Herman, J.M.; Smallwood-Massey, S.; Kamel, I.; Gurakar, A.; Anders, R.; Cameron, A.; et al. Impact of a single-day multidisciplinary clinic on the management of patients with liver tumours. Curr. Oncol. 2013, 20, e123–e131. [Google Scholar] [CrossRef] [Green Version]
- El-Deiry, W.S.; Goldberg, R.M.; Lenz, H.-J.; Shields, A.F.; Gibney, G.T.; Tan, A.R.; Brown, J.; Eisenberg, B.; Heath, E.I.; Phuphanich, S.; et al. The current state of molecular testing in the treatment of patients with solid tumors, 2019. CA Cancer J. Clin. 2019, 69, 305–343. [Google Scholar] [CrossRef]
- Colomer, R.; Mondejar, R.; Romero-Laorden, N.; Alfranca, A.; Sanchez-Madrid, F.; Quintela-Fandino, M. When should we order a next generation sequencing test in a patient with cancer? EClinicalMedicine 2020, 25, 100487:1–100487:9. [Google Scholar] [CrossRef]
- Davies, K.D.; Aisner, D.L. Wake up and smell the fusions: Single-modality molecular testing misses drivers. Clin. Cancer Res. 2019, 25, 1–3. [Google Scholar] [CrossRef]
- De Luca, A.; Esposito Abate, R.; Rachiglio, A.M.; Rosaria Maiello, M.; Esposito, C.; Schettino, C.; Izzo, F.; Nasti, G.; Normanno, N. FGFR fusions in cancer: From diagnostic approaches to therapeutic intervention. Int. J. Mol. Sci. 2020, 21, 6856. [Google Scholar] [CrossRef]
- Saborowski, A.; Lehmann, U.; Vogel, A. FGFR inhibitors in cholangiocarcinoma: What’s now and what’s next? Ther. Adv. Med. Oncol. 2020, 12, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Pennell, N.A.; Arcila, M.E.; Gandara, D.R.; West, H. Biomarker testing for patients with non-small cell lung cancer: Real-world issues and tough choices. Am. Soc. Clin. Oncol. Educ. Book 2019, 39, 531–542. [Google Scholar] [CrossRef]
- Schwaederle, M.; Parker, B.A.; Schwab, R.B.; Fanta, P.T.; Boles, S.G.; Daniels, G.A.; Bazhenova, L.A.; Subramanian, R.; Coutinho, A.C.; Ojeda-Fournier, H.; et al. Molecular tumor board: The University of California-San Diego Moores Cancer Center experience. Oncologist 2014, 19, 631–636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sicklick, J.K.; Kato, S.; Okamura, R.; Schwaederle, M.; Hahn, M.E.; Williams, C.B.; De, P.; Krie, A.; Piccioni, D.E.; Miller, V.A. Molecular profiling of cancer patients enables personalized combination therapy: The I-PREDICT study. Nat. Med. 2019, 25, 744–750. [Google Scholar] [CrossRef]
- Haslem, D.S.; Chakravarty, I.; Fulde, G.; Gilbert, H.; Tudor, B.P.; Lin, K.; Ford, J.M.; Nadauld, L.D. Precision oncology in advanced cancer patients improves overall survival with lower weekly healthcare costs. Oncotarget 2018, 9, 12316–12322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lewis, M.A. Disunited state of cancer care in America. JCO Oncol. Pract. 2021, 17, 3–6. [Google Scholar] [CrossRef] [PubMed]
| Gene | Prevalence * |
|---|---|
| IDH1 mutations | 10–20% [27] |
| FGFR2 fusions/rearrangements | 10–16% [25] |
| BRAF mutations | <5% [27] |
| NTRK fusions | <5% [27] |
| MSI-High/dMMR, TMB > 10 mutations/megabase | <5% [27] |
| Test | Advantage | Disadvantage |
|---|---|---|
| Next-Generation Sequencing (NGS): Tissue-Based |
|
|
| NGS—Liquid-Based |
|
|
| FISH Testing |
|
|
| Single-Analyte Tests (PCR/RT-PCR, Sanger sequencing) |
|
|
| Immunohisto-chemistry (IHC) |
|
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, M.T.; Gholami, S.; Gui, D.; Tejaswi, S.L.; Fananapazir, G.; Abi-Jaoudeh, N.; Jutric, Z.; Samarasena, J.B.; Li, X.; Valerin, J.B.; et al. Optimizing the Diagnosis and Biomarker Testing for Patients with Intrahepatic Cholangiocarcinoma: A Multidisciplinary Approach. Cancers 2022, 14, 392. https://doi.org/10.3390/cancers14020392
Cho MT, Gholami S, Gui D, Tejaswi SL, Fananapazir G, Abi-Jaoudeh N, Jutric Z, Samarasena JB, Li X, Valerin JB, et al. Optimizing the Diagnosis and Biomarker Testing for Patients with Intrahepatic Cholangiocarcinoma: A Multidisciplinary Approach. Cancers. 2022; 14(2):392. https://doi.org/10.3390/cancers14020392
Chicago/Turabian StyleCho, May T., Sepideh Gholami, Dorina Gui, Sooraj L. Tejaswi, Ghaneh Fananapazir, Nadine Abi-Jaoudeh, Zeljka Jutric, Jason B. Samarasena, Xiaodong Li, Jennifer B. Valerin, and et al. 2022. "Optimizing the Diagnosis and Biomarker Testing for Patients with Intrahepatic Cholangiocarcinoma: A Multidisciplinary Approach" Cancers 14, no. 2: 392. https://doi.org/10.3390/cancers14020392
APA StyleCho, M. T., Gholami, S., Gui, D., Tejaswi, S. L., Fananapazir, G., Abi-Jaoudeh, N., Jutric, Z., Samarasena, J. B., Li, X., Valerin, J. B., Mercer, J., & Dayyani, F. (2022). Optimizing the Diagnosis and Biomarker Testing for Patients with Intrahepatic Cholangiocarcinoma: A Multidisciplinary Approach. Cancers, 14(2), 392. https://doi.org/10.3390/cancers14020392






