BHMPS Inhibits Breast Cancer Migration and Invasion by Disrupting Rab27a-Mediated EGFR and Fibronectin Secretion
Abstract
:Simple Summary
Abstract
1. Introduction
2. Results
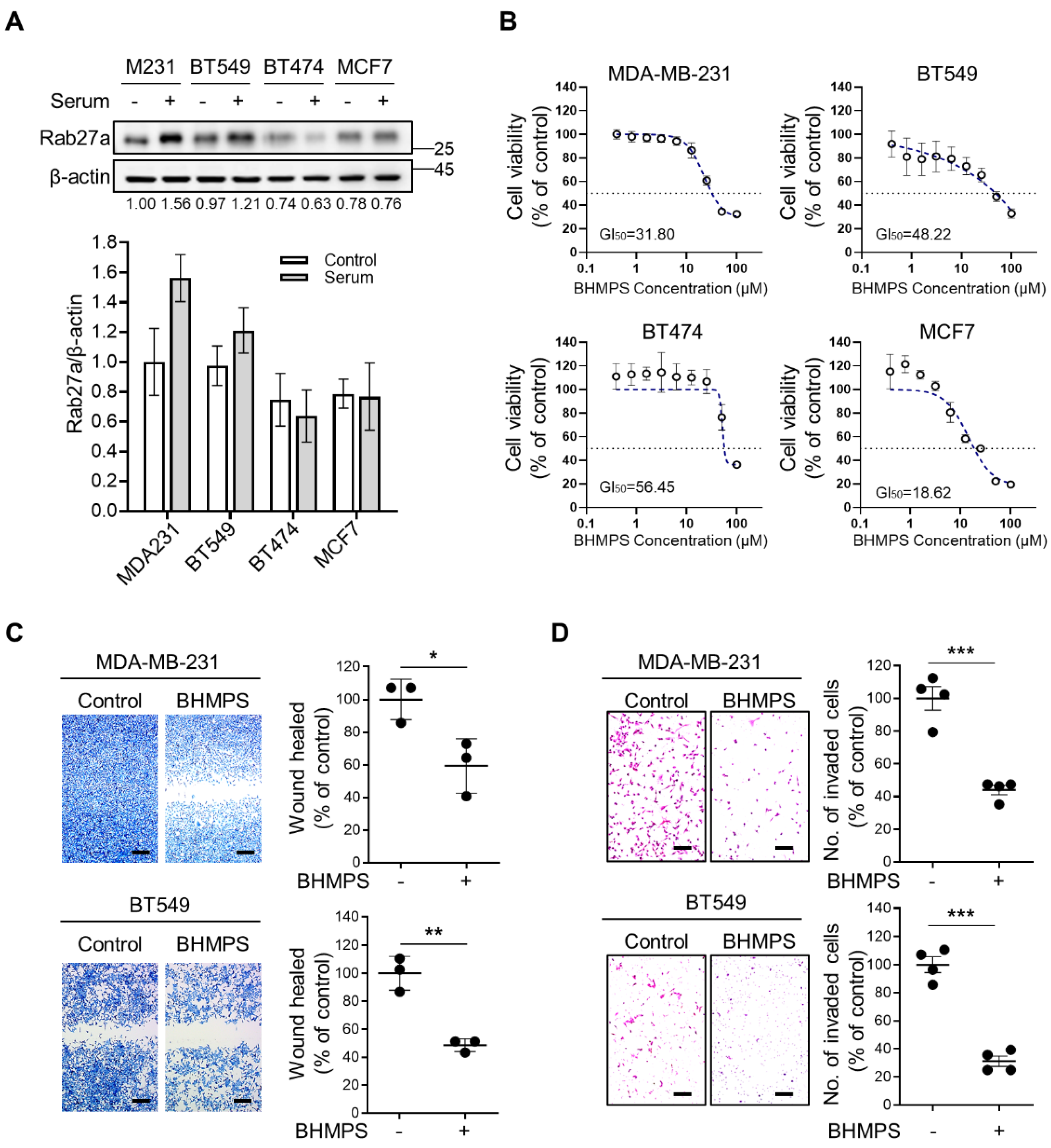
2.1. BHMPS Suppresses Migration and Invasion of Breast Cancer Cells
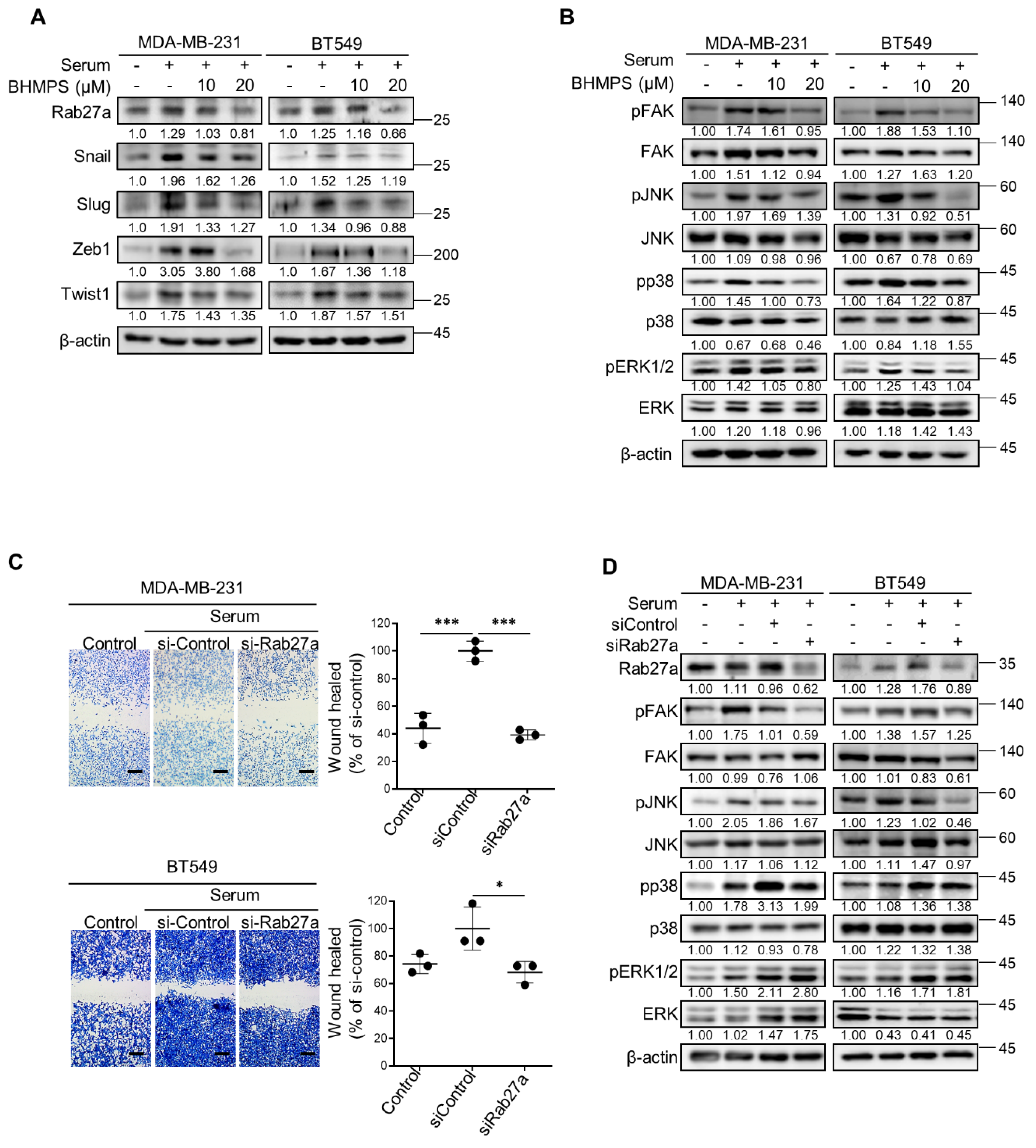
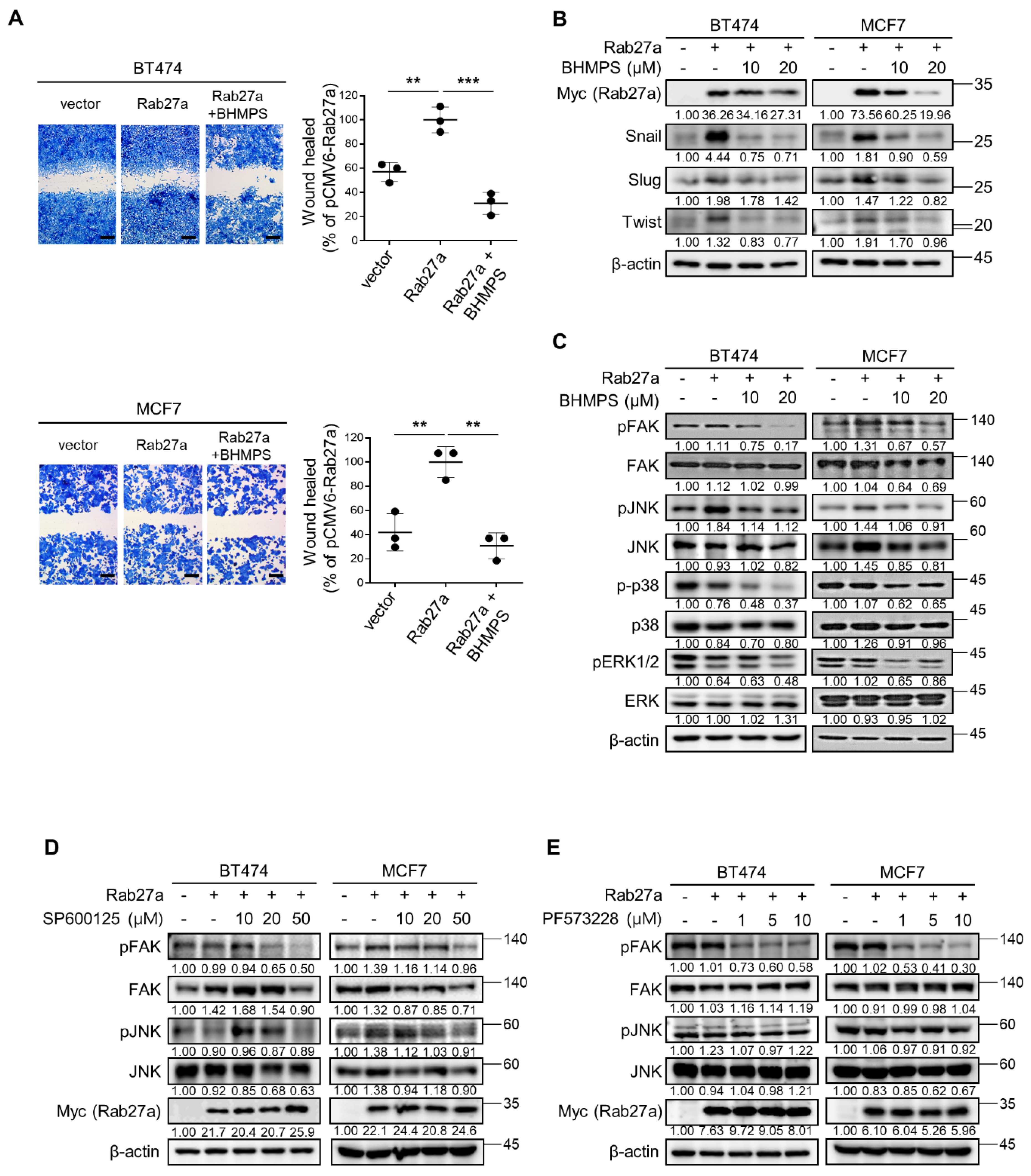
2.2. BHMPS or Rab27a Silencing Decreases Activation of FAK and JNK
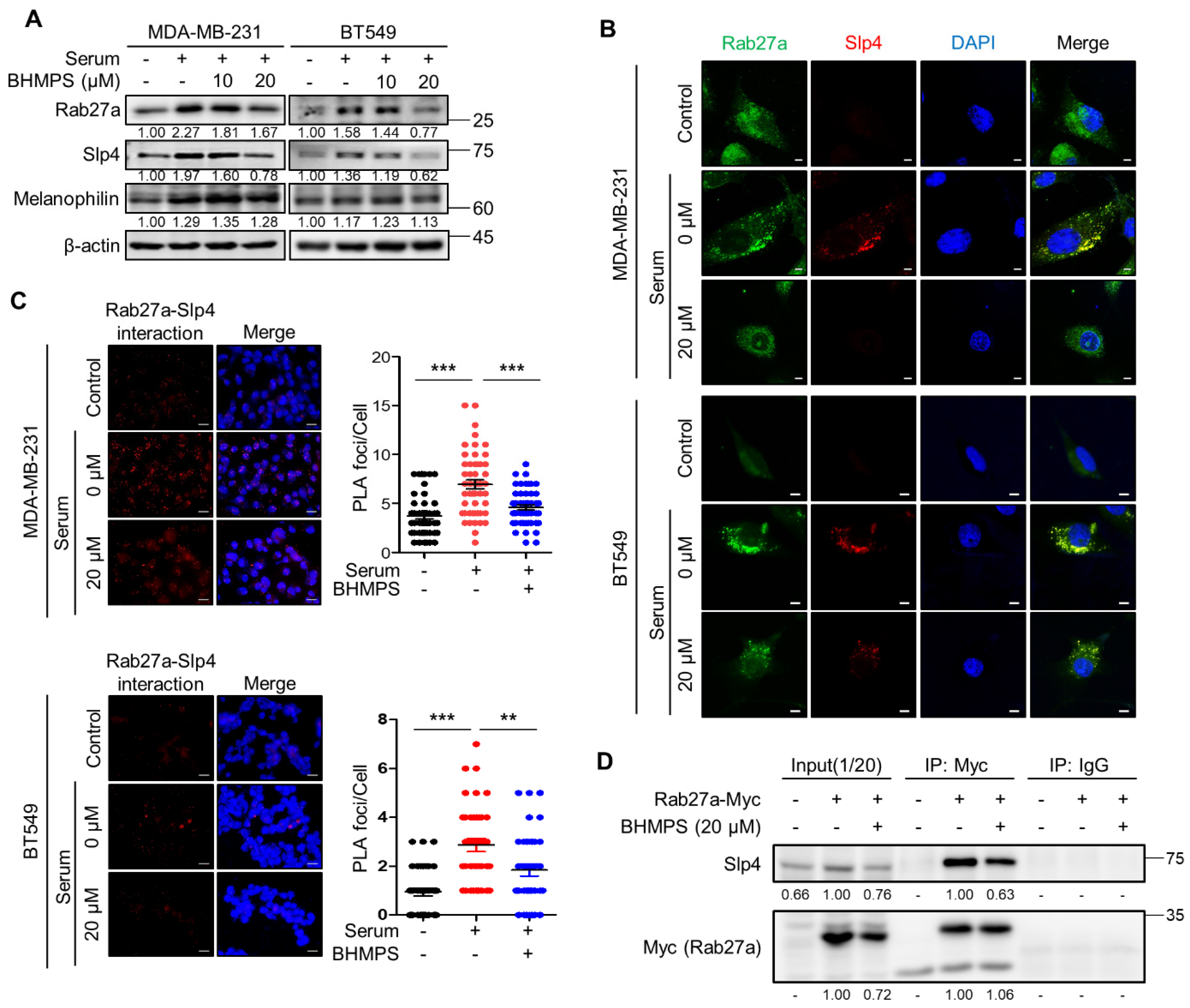
2.3. BHMPS Inhibits the Interaction of Rab27a with Slp4
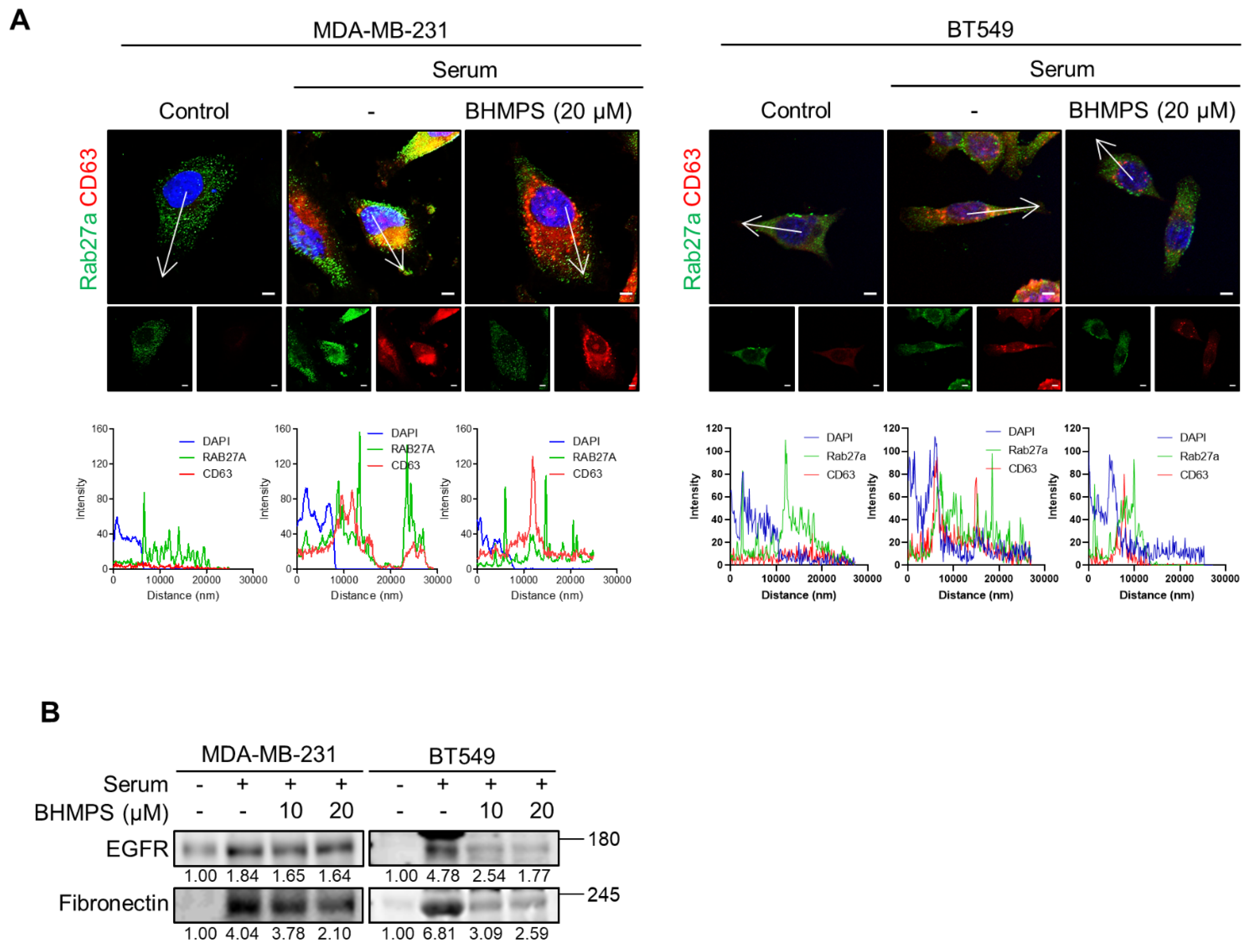
2.4. BHMPS Interferes with Vesicle Trafficking and Secretion
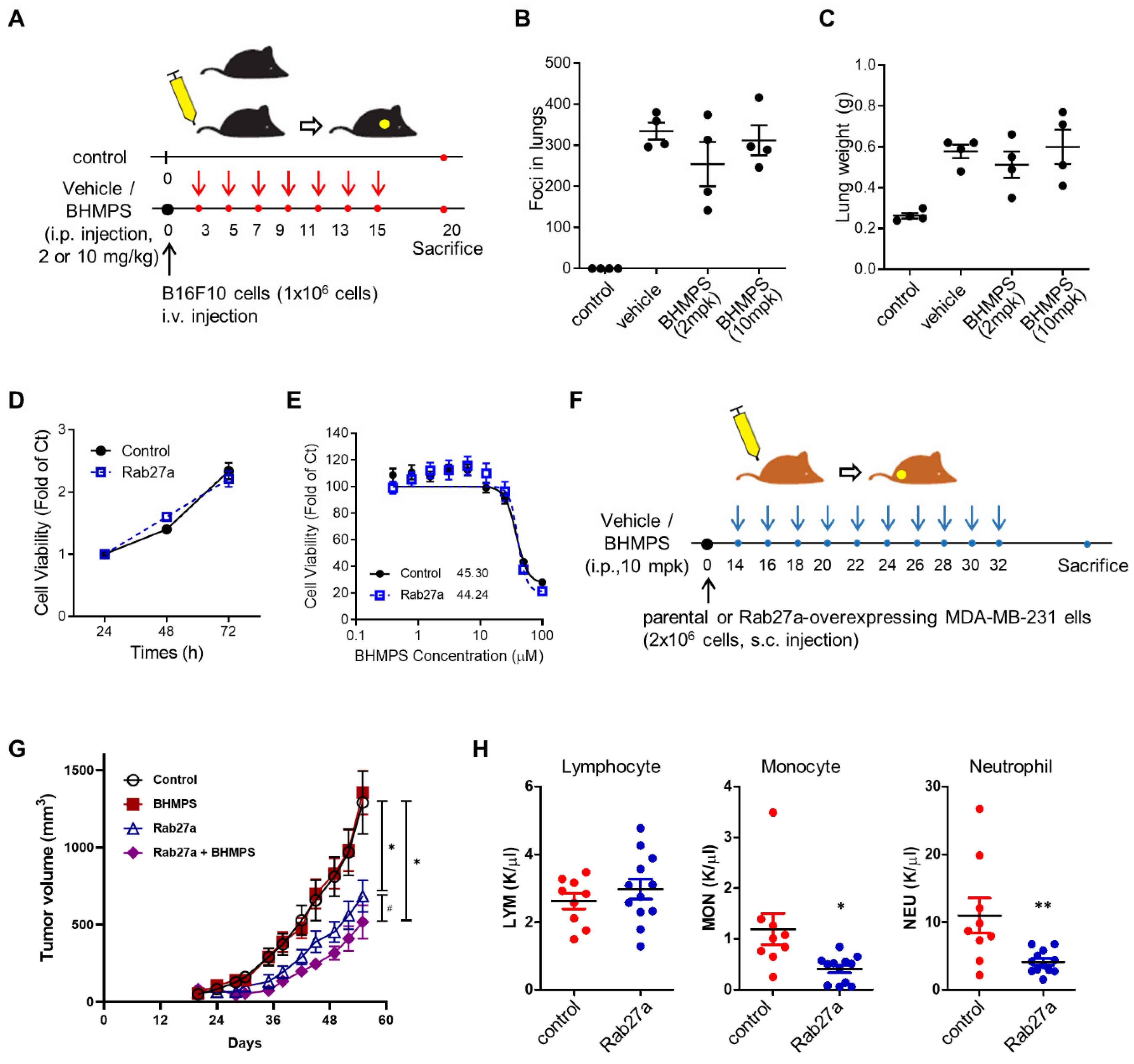
2.5. BHMPS Inhibits Tumor Growth in Rab27a-Overexpressing Tumor Xenograft Mice
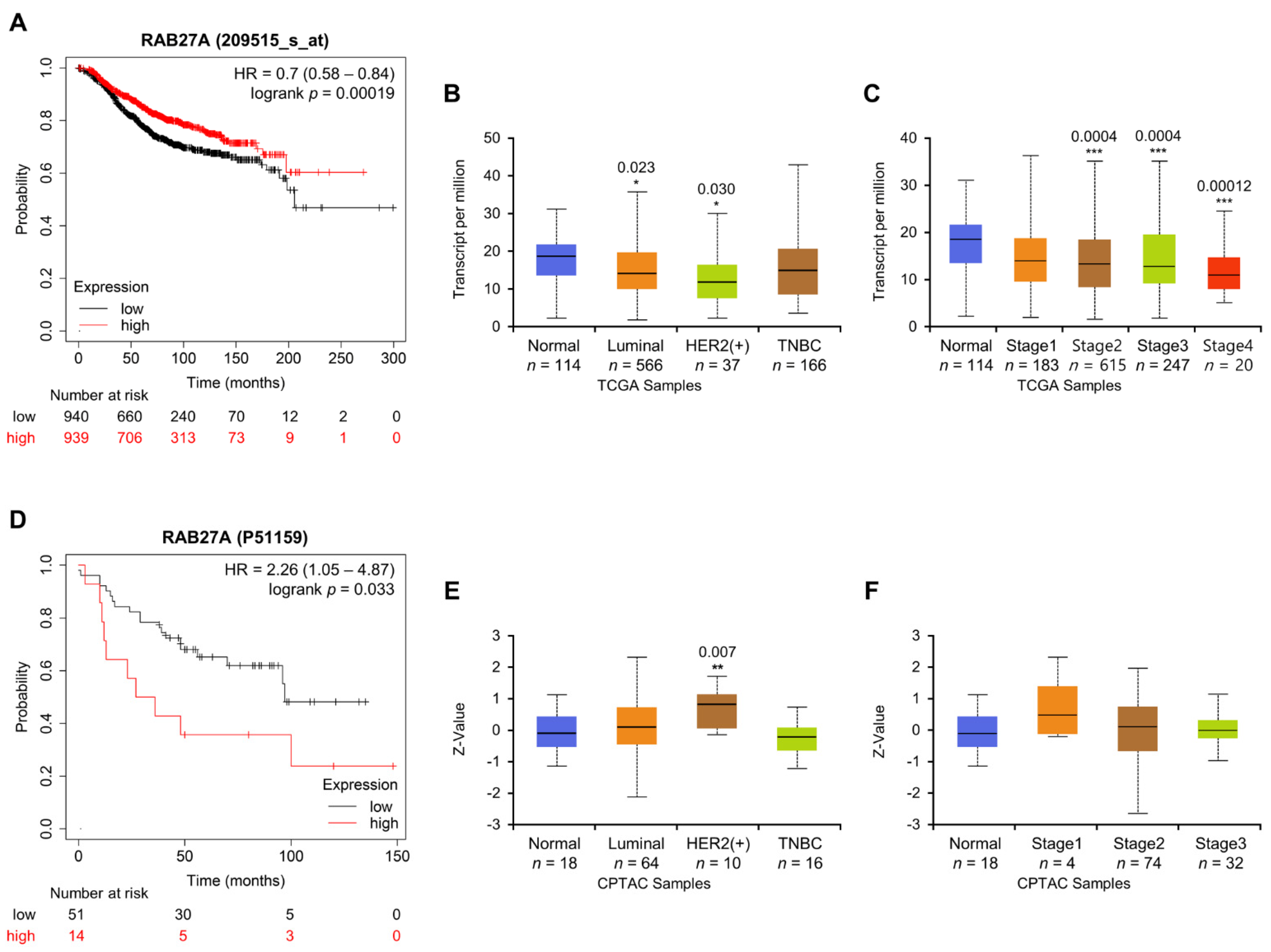
2.6. Association between Rab27a Expression and OS of Breast Cancer Patients
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Cell Cultures
4.3. Cell Viability Assay
4.4. Migration Assay
4.5. Invasion Assay
4.6. Western Blot Analysis
4.7. Transfection
4.8. Immunoprecipitation
4.9. Immunofluorescence Confocal Microscopy
4.10. Proximity Ligation Assay (PLA)
4.11. Tumor Xenograft Mouse Model
4.12. Bioinformatics Analysis
4.13. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviation
References
- Fares, J.; Fares, M.Y.; Khachfe, H.H.; Salhab, H.A.; Fares, Y. Molecular principles of metastasis: A hallmark of cancer revisited. Signal Transduct Target Ther. 2020, 5, 28. [Google Scholar] [CrossRef]
- Kalluri, R.; Weinberg, R.A. The basics of epithelial-mesenchymal transition. J. Clin. Investig. 2009, 119, 1420–1428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Recchi, C.; Seabra, M.C. Novel functions for Rab GTPases in multiple aspects of tumour progression. Biochem. Soc. Trans. 2012, 40, 1398–1403. [Google Scholar] [CrossRef] [Green Version]
- Blanc, L.; Vidal, M. New insights into the function of Rab GTPases in the context of exosomal secretion. Small GTPases 2018, 9, 95–106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagashima, K.; Torii, S.; Yi, Z.; Igarashi, M.; Okamoto, K.; Takeuchi, T.; Izumi, T. Melanophilin directly links Rab27a and myosin Va through its distinct coiled-coil regions. FEBS Lett. 2002, 517, 233–238. [Google Scholar] [CrossRef] [Green Version]
- Yi, Z.; Yokota, H.; Torii, S.; Aoki, T.; Hosaka, M.; Zhao, S.; Takata, K.; Takeuchi, T.; Izumi, T. The Rab27a/granuphilin complex regulates the exocytosis of insulin-containing dense-core granules. Mol. Cell Biol. 2002, 22, 1858–1867. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peinado, H.; Aleckovic, M.; Lavotshkin, S.; Matei, I.; Costa-Silva, B.; Moreno-Bueno, G.; Hergueta-Redondo, M.; Williams, C.; Garcia-Santos, G.; Ghajar, C.; et al. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat. Med. 2012, 18, 883–891. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, X.; Qian, N.; Ling, S.; Li, Y.; Sun, W.; Li, J.; Du, R.; Zhong, G.; Liu, C.; Yu, G.; et al. Breast cancer exosomes contribute to pre-metastatic niche formation and promote bone metastasis of tumor cells. Theranostics 2021, 11, 1429–1445. [Google Scholar] [CrossRef]
- Dai, J.; Su, Y.; Zhong, S.; Cong, L.; Liu, B.; Yang, J.; Tao, Y.; He, Z.; Chen, C.; Jiang, Y. Exosomes: Key players in cancer and potential therapeutic strategy. Signal Transduct Target Ther. 2020, 5, 145. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Guo, N. Exosomes: Potent regulators of tumor malignancy and potential bio-tools in clinical application. Crit. Rev. Oncol. Hematol. 2015, 95, 346–358. [Google Scholar] [CrossRef] [Green Version]
- Wu, Q.; Zhou, L.; Lv, D.; Zhu, X.; Tang, H. Exosome-mediated communication in the tumor microenvironment contributes to hepatocellular carcinoma development and progression. J. Hematol. Oncol. 2019, 12, 53. [Google Scholar] [CrossRef]
- Li, Z.; Fang, R.; Fang, J.; He, S.; Liu, T. Functional implications of Rab27 GTPases in Cancer. Cell. Commun. Signal 2018, 16, 44. [Google Scholar] [CrossRef] [Green Version]
- Joung, J.Y.; Lee, H.Y.; Park, J.; Lee, J.Y.; Chang, B.H.; No, K.T.; Nam, K.Y.; Hwang, J.S. Identification of novel rab27a/melanophilin blockers by pharmacophore-based virtual screening. Appl. Biochem. Biotechnol. 2014, 172, 1882–1897. [Google Scholar] [CrossRef]
- Kang, S.M.; Nam, K.Y.; Jung, S.Y.; Song, K.H.; Kho, S.; No, K.T.; Choi, H.K.; Song, J.Y. Inhibition of cancer cell invasion by new ((3,4-dihydroxy benzylidene)hydrazinyl)pyridine-3-sulfonamide analogs. Bioorg. Med. Chem. Lett. 2016, 26, 1322–1328. [Google Scholar] [CrossRef]
- De Craene, B.; Berx, G. Regulatory networks defining EMT during cancer initiation and progression. Nat. Rev. Cancer 2013, 13, 97–110. [Google Scholar] [CrossRef]
- Braicu, C.; Buse, M.; Busuioc, C.; Drula, R.; Gulei, D.; Raduly, L.; Rusu, A.; Irimie, A.; Atanasov, A.G.; Slaby, O.; et al. A Comprehensive Review on MAPK: A Promising Therapeutic Target in Cancer. Cancers 2019, 11, 1618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McLean, G.W.; Carragher, N.O.; Avizienyte, E.; Evans, J.; Brunton, V.G.; Frame, M.C. The role of focal-adhesion kinase in cancer—A new therapeutic opportunity. Nat. Rev. Cancer 2005, 5, 505–515. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, M. Rab27 effectors, pleiotropic regulators in secretory pathways. Traffic 2013, 14, 949–963. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.S.; Wang, F.B.; Zhang, Q.G.; Shen, Z.Z.; Shao, Z.M. Enhanced expression of Rab27A gene by breast cancer cells promoting invasiveness and the metastasis potential by secretion of insulin-like growth factor-II. Mol. Cancer Res. 2008, 6, 372–382. [Google Scholar] [CrossRef] [Green Version]
- Hessvik, N.P.; Llorente, A. Current knowledge on exosome biogenesis and release. Cell Mol. Life Sci. 2018, 75, 193–208. [Google Scholar] [CrossRef] [Green Version]
- Huang, T.; Deng, C.X. Current Progresses of Exosomes as Cancer Diagnostic and Prognostic Biomarkers. Int. J. Biol. Sci. 2019, 15, 1–11. [Google Scholar] [CrossRef]
- Schlaepfer, D.D.; Mitra, S.K. Multiple connections link FAK to cell motility and invasion. Curr. Opin. Genet. Dev. 2004, 14, 92–101. [Google Scholar] [CrossRef]
- Zhao, J.; Guan, J.L. Signal transduction by focal adhesion kinase in cancer. Cancer Metastasis Rev. 2009, 28, 35–49. [Google Scholar] [CrossRef]
- De Franceschi, N.; Hamidi, H.; Alanko, J.; Sahgal, P.; Ivaska, J. Integrin traffic—The update. J. Cell Sci. 2015, 128, 839–852. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alanko, J.; Ivaska, J. Endosomes: Emerging Platforms for Integrin-Mediated FAK Signalling. Trends Cell Biol 2016, 26, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Harburger, D.S.; Calderwood, D.A. Integrin signalling at a glance. J. Cell Sci. 2009, 122 Pt 2, 159–163. [Google Scholar] [CrossRef] [Green Version]
- Luo, M.; Fan, H.; Nagy, T.; Wei, H.; Wang, C.; Liu, S.; Wicha, M.S.; Guan, J.L. Mammary epithelial-specific ablation of the focal adhesion kinase suppresses mammary tumorigenesis by affecting mammary cancer stem/progenitor cells. Cancer Res. 2009, 69, 466–474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Provenzano, P.P.; Inman, D.R.; Eliceiri, K.W.; Beggs, H.E.; Keely, P.J. Mammary epithelial-specific disruption of focal adhesion kinase retards tumor formation and metastasis in a transgenic mouse model of human breast cancer. Am. J. Pathol. 2008, 173, 1551–1565. [Google Scholar] [CrossRef] [Green Version]
- Pylayeva, Y.; Gillen, K.M.; Gerald, W.; Beggs, H.E.; Reichardt, L.F.; Giancotti, F.G. Ras- and PI3K-dependent breast tumorigenesis in mice and humans requires focal adhesion kinase signaling. J. Clin. Investig. 2009, 119, 252–266. [Google Scholar] [CrossRef] [Green Version]
- Ulu, A.; Frost, J.A. Regulation of RhoA activation and cell motility by c-Jun N-terminal kinases and Net1. Small GTPases 2020, 11, 385–391. [Google Scholar] [CrossRef]
- Cheng, H.L.; Lin, C.W.; Yang, J.S.; Hsieh, M.J.; Yang, S.F.; Lu, K.H. Zoledronate blocks geranylgeranylation not farnesylation to suppress human osteosarcoma U2OS cells metastasis by EMT via Rho A activation and FAK-inhibited JNK and p38 pathways. Oncotarget 2016, 7, 9742–9758. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.F.; Zhao, K.; Chen, Y.Y.; Qiu, Y.; Zhu, J.H.; Li, B.P.; Wang, Z.; Chen, J.Q. NKCC1 promotes proliferation, invasion and migration in human gastric cancer cells via activation of the MAPK-JNK/EMT signaling pathway. J. Cancer 2021, 12, 253–263. [Google Scholar] [CrossRef]
- Miller, I.V.; Grunewald, T.G.P. Tumour-derived exosomes: Tiny envelopes for big stories. Biol. Cell 2015, 107, 287–305. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.K.; Mizuno, K.; Wasmeier, C.; Wavre-Shapton, S.T.; Recchi, C.; Catz, S.D.; Futter, C.; Tolmachova, T.; Hume, A.N.; Seabra, M.C. Distinct and opposing roles for Rab27a/Mlph/MyoVa and Rab27b/Munc13–4 in mast cell secretion. FEBS J. 2013, 280, 892–903. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.L.; Brzezinska, A.A.; Tolmachova, T.; Munafo, D.B.; Ellis, B.A.; Seabra, M.C.; Hong, H.; Catz, S.D. Rab27a and Rab27b regulate neutrophil azurophilic granule exocytosis and NADPH oxidase activity by independent mechanisms. Traffic 2010, 11, 533–547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ostrowski, M.; Carmo, N.B.; Krumeich, S.; Fanget, I.; Raposo, G.; Savina, A.; Moita, C.F.; Schauer, K.; Hume, A.N.; Freitas, R.P.; et al. Rab27a and Rab27b control different steps of the exosome secretion pathway. Nat. Cell Biol. 2010, 12, 19–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bobrie, A.; Krumeich, S.; Reyal, F.; Recchi, C.; Moita, L.F.; Seabra, M.C.; Ostrowski, M.; Thery, C. Rab27a supports exosome-dependent and -independent mechanisms that modify the tumor microenvironment and can promote tumor progression. Cancer Res. 2012, 72, 4920–4930. [Google Scholar] [CrossRef] [Green Version]
- Guo, D.; Lui, G.Y.L.; Lai, S.L.; Wilmott, J.S.; Tikoo, S.; Jackett, L.A.; Quek, C.; Brown, D.L.; Sharp, D.M.; Kwan, R.Y.Q.; et al. RAB27A promotes melanoma cell invasion and metastasis via regulation of pro-invasive exosomes. Int. J. Cancer 2019, 144, 3070–3085. [Google Scholar] [CrossRef] [Green Version]
- Song, X.; Liu, Z.; Yu, Z. EGFR Promotes the Development of Triple Negative Breast Cancer Through JAK/STAT3 Signaling. Cancer Manag. Res. 2020, 12, 703–717. [Google Scholar] [CrossRef] [Green Version]
- Corkery, B.; Crown, J.; Clynes, M.; O’Donovan, N. Epidermal growth factor receptor as a potential therapeutic target in triple-negative breast cancer. Ann. Oncol. 2009, 20, 862–867. [Google Scholar] [CrossRef]
- Jabbari, N.; Nawaz, M.; Rezaie, J. Ionizing Radiation Increases the Activity of Exosomal Secretory Pathway in MCF-7 Human Breast Cancer Cells: A Possible Way to Communicate Resistance against Radiotherapy. Int. J. Mol. Sci. 2019, 20, 3649. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Zhao, J.; Zhang, L.; Wei, F.; Lian, Y.; Wu, Y.; Gong, Z.; Zhang, S.; Zhou, J.; Cao, K.; et al. Role of tumor microenvironment in tumorigenesis. J. Cancer 2017, 8, 761–773. [Google Scholar] [CrossRef] [PubMed]
- Soysal, S.D.; Tzankov, A.; Muenst, S.E. Role of the Tumor Microenvironment in Breast Cancer. Pathobiology 2015, 82, 142–152. [Google Scholar] [CrossRef] [PubMed]
- Fridlender, Z.G.; Albelda, S.M. Tumor-associated neutrophils: Friend or foe? Carcinogenesis 2012, 33, 949–955. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akavia, U.D.; Litvin, O.; Kim, J.; Sanchez-Garcia, F.; Kotliar, D.; Causton, H.C.; Pochanard, P.; Mozes, E.; Garraway, L.A.; Pe’er, D. An integrated approach to uncover drivers of cancer. Cell 2010, 143, 1005–1017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, W.W.; Mou, Q.; Chen, J.; Cui, J.T.; Li, W.M.; Xiao, W.H. Differential expression of Rab27A/B correlates with clinical outcome in hepatocellular carcinoma. World J. Gastroenterol. 2012, 18, 1806–1813. [Google Scholar] [CrossRef] [PubMed]
- Moritz, C.P.; Muhlhaus, T.; Tenzer, S.; Schulenborg, T.; Friauf, E. Poor transcript-protein correlation in the brain: Negatively correlating gene products reveal neuronal polarity as a potential cause. J. Neurochem. 2019, 149, 582–604. [Google Scholar] [CrossRef] [Green Version]
- Koh, H.M.; Jang, B.G.; Kim, D.C. Prognostic significance of Rab27 expression in solid cancer: A systematic review and meta-analysis. Sci. Rep. 2020, 10, 14136. [Google Scholar] [CrossRef]
- Shi, C.; Yang, X.; Ni, Y.; Hou, N.; Xu, L.; Zhan, F.; Zhu, H.; Xiong, L.; Chen, P. High Rab27A expression indicates favorable prognosis in CRC. Diagn. Pathol. 2015, 10, 68. [Google Scholar] [CrossRef] [Green Version]
- Koos, B.; Andersson, L.; Clausson, C.M.; Grannas, K.; Klaesson, A.; Cane, G.; Soderberg, O. Analysis of protein interactions in situ by proximity ligation assays. Curr. Top. Microbiol. Immunol. 2014, 377, 111–126. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, J.-I.; Song, K.-H.; Kang, S.-M.; Lee, J.; Cho, S.-J.; Choi, H.K.; Ahn, J.; Park, J.-K.; Kim, J.; Hwang, S.-G.; et al. BHMPS Inhibits Breast Cancer Migration and Invasion by Disrupting Rab27a-Mediated EGFR and Fibronectin Secretion. Cancers 2022, 14, 373. https://doi.org/10.3390/cancers14020373
Park J-I, Song K-H, Kang S-M, Lee J, Cho S-J, Choi HK, Ahn J, Park J-K, Kim J, Hwang S-G, et al. BHMPS Inhibits Breast Cancer Migration and Invasion by Disrupting Rab27a-Mediated EGFR and Fibronectin Secretion. Cancers. 2022; 14(2):373. https://doi.org/10.3390/cancers14020373
Chicago/Turabian StylePark, Jeong-In, Kyung-Hee Song, Seong-Mook Kang, Jeeyong Lee, Seong-Jun Cho, Hyun Kyung Choi, Jiyeon Ahn, Jong-Kuk Park, Jaesung Kim, Sang-Gu Hwang, and et al. 2022. "BHMPS Inhibits Breast Cancer Migration and Invasion by Disrupting Rab27a-Mediated EGFR and Fibronectin Secretion" Cancers 14, no. 2: 373. https://doi.org/10.3390/cancers14020373
APA StylePark, J.-I., Song, K.-H., Kang, S.-M., Lee, J., Cho, S.-J., Choi, H. K., Ahn, J., Park, J.-K., Kim, J., Hwang, S.-G., Lim, D.-S., Kim, J., Jung, S.-Y., & Song, J.-Y. (2022). BHMPS Inhibits Breast Cancer Migration and Invasion by Disrupting Rab27a-Mediated EGFR and Fibronectin Secretion. Cancers, 14(2), 373. https://doi.org/10.3390/cancers14020373





