Analysis of Survival and Response to Lenvatinib in Unresectable Hepatocellular Carcinoma
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Lenvatinib Treatment Regimens
2.3. Assessment of Response to Lenvatinib
2.4. Statistical Analysis
3. Results
3.1. Clinical Characteristics of Participating Patients
3.2. Treatment Response and Survival
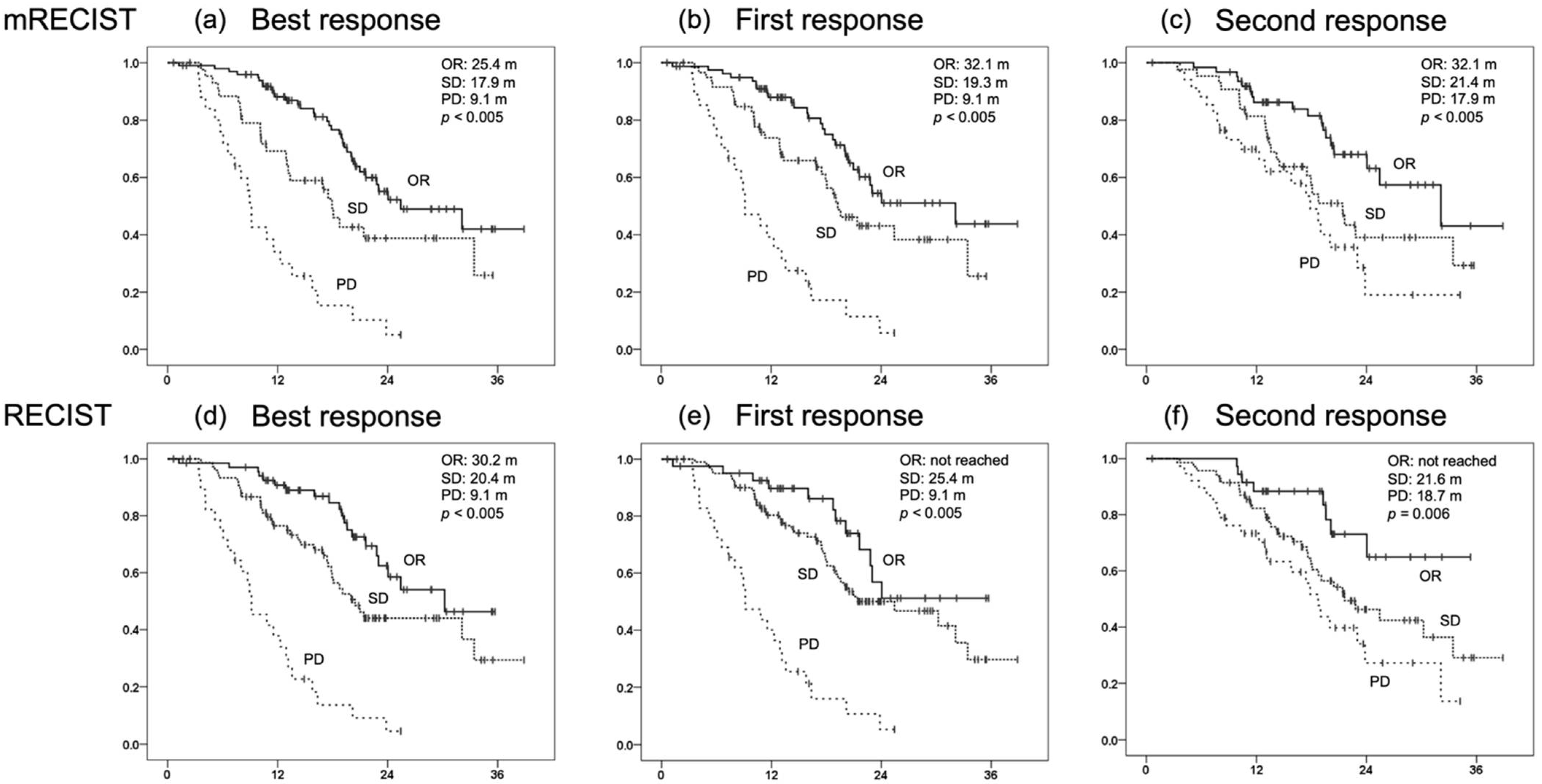
3.3. OS for Each Initial Radiological Response and Prognostic Factors for OS
3.4. Prognostic Factors for OS in Patients with SD at the Initial Radiological Response Evaluation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Forner, A.; Reig, M.; Bruix, J. Hepatocellular carcinoma. Lancet 2018, 391, 1301–1314. [Google Scholar] [CrossRef]
- Jemal, A.; Bray, F.; Center, M.M.; Ferlay, J.; Ward, E.; Forman, D. Global cancer statistics. CA Cancer J. Clin. 2011, 61, 69–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Llovet, J.M.; Real, M.I.; Montaña, X.; Planas, R.; Coll, S.; Aponte, J.; Ayuso, C.; Sala, M.; Muchart, J.; Sola, R.; et al. Arterial embolization or chemoembolization versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: A randomised controlled trial. Lancet 2002, 359, 1734–1739. [Google Scholar] [CrossRef]
- Obi, S.; Yoshida, H.; Toune, R.; Unuma, T.; Kanda, M.; Sato, S.; Tateishi, R.; Teratani, T.; Shiina, S.; Omata, M. Combination therapy of intra-arterial 5-fluorouracil and systemic interferon-alpha for advanced hepatocellular carcinoma with portal venous invasion. Cancer 2006, 106, 1990–1997. [Google Scholar] [CrossRef]
- Tateishi, R.; Shiina, S.; Teratani, T.; Obi, S.; Sato, S.; Koike, Y.; Fujishima, T.; Yoshida, H.; Kawabe, T.; Omata, M. Percutaneous radiofrequency ablation for hepatocellular carcinoma. An analysis of 1000 cases. Cancer 2005, 103, 1201–1209. [Google Scholar] [CrossRef]
- Miyagawa, S.; Makuuchi, M.; Kawasaki, S.; Kakazu, T. Criteria for safe hepatic resection. Am. J. Surg. 1995, 169, 589–594. [Google Scholar] [CrossRef]
- Uka, K.; Aikata, H.; Takaki, S.; Miki, D.; Kawaoka, T.; Jeong, S.C.; Takahashi, S.; Toyota, N.; Ito, K.; Chayama, K. Pretreatment predictor of response, time to progression, and survival to intraarterial 5-fluorouracil/interferon combination therapy in patients with advanced hepatocellular carcinoma. J. Gastroenterol. 2007, 42, 845–853. [Google Scholar] [CrossRef]
- Llovet, J.M.; Ricci, S.; Mazzaferro, V.; Hilgard, P.; Gane, E.; Blanc, J.F.; Oliveira, A.C.; Santoro, A.; Raoul, J.L.; Forner, A.; et al. Sorafenib in advanced hepatocellular carcinoma. N. Engl. J. Med. 2008, 359, 378–390. [Google Scholar] [CrossRef]
- Kudo, M.; Finn, R.S.; Qin, S.; Han, K.H.; Ikeda, K.; Piscaglia, F.; Baron, A.; Park, J.W.; Han, G.; Jassem, J.; et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: A randomised phase 3 non-inferiority trial. Lancet 2018, 391, 1163–1173. [Google Scholar] [CrossRef] [Green Version]
- Bruix, J.; Qin, S.; Merle, P.; Granito, A.; Huang, Y.H.; Bodoky, G.; Pracht, M.; Yokosuka, O.; Rosmorduc, O.; Breder, V.; et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017, 389, 56–66. [Google Scholar] [CrossRef] [Green Version]
- Zhu, A.X.; Finn, R.S.; Galle, P.R.; Llovet, J.M.; Kudo, M. Ramucirumab in advanced hepatocellular carcinoma in REACH-2: The true value of alpha-fetoprotein. Lancet Oncol. 2019, 20, e191. [Google Scholar] [CrossRef] [Green Version]
- Abou-Alfa, G.K.; Meyer, T.; Cheng, A.L.; El-Khoueiry, A.B.; Rimassa, L.; Ryoo, B.Y.; Cicin, I.; Merle, P.; Chen, Y.; Park, J.W.; et al. Cabozantinib in patients with advanced and progressing hepatocellular carcinoma. N. Engl. J. Med. 2018, 379, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Finn, R.S.; Qin, S.; Ikeda, M.; Galle, P.R.; Ducreux, M.; Kim, T.Y.; Kudo, M.; Breder, V.; Merle, P.; Kaseb, A.O.; et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N. Engl. J. Med. 2020, 382, 1894–1905. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, A.; Dadduzio, V.; Ricci, A.D.; Massari, F.; Di Federico, A.; Gadaleta-Caldarola, G.; Brandi, G. Lenvatinib plus pembrolizumab: The next frontier for the treatment of hepatocellular carcinoma? Expert Opin. Investig. Drugs 2021, 1–8. [Google Scholar] [CrossRef]
- Pfister, D.; Nunez, N.G.; Pinyol, R.; Govaere, O.; Pinter, M.; Szydlowska, M.; Gupta, R.; Qiu, M.; Deczkowska, A.; Weiner, A.; et al. NASH limits anti-tumour surveillance in immunotherapy-treated HCC. Nature 2021, 592, 450–456. [Google Scholar] [CrossRef]
- Ding, Z.; Dong, Z.; Chen, Z.; Hong, J.; Yan, L.; Li, H.; Yao, S.; Yan, Y.; Yang, Y.; Yang, C.; et al. Viral status and efficacy of immunotherapy in hepatocellular carcinoma: A systematic review with meta-analysis. Front. Immunol. 2021, 12, 733530. [Google Scholar] [CrossRef]
- Johnson, P.J.; Berhane, S.; Kagebayashi, C.; Satomura, S.; Teng, M.; Reeves, H.L.; O’Beirne, J.; Fox, R.; Skowronska, A.; Palmer, D.; et al. Assessment of liver function in patients with hepatocellular carcinoma: A new evidence-based approach-the ALBI grade. J. Clin. Oncol. 2015, 33, 550–558. [Google Scholar] [CrossRef]
- Hiraoka, A.; Kumada, T.; Tsuji, K.; Takaguchi, K.; Itobayashi, E.; Kariyama, K.; Ochi, H.; Tajiri, K.; Hirooka, M.; Shimada, N.; et al. Validation of modified ALBI grade for more detailed assessing hepatic function in hepatocellular carcinoma patients: A multicenter analysis. Liver Cancer 2019, 8, 121–129. [Google Scholar] [CrossRef]
- Kirino, S.; Tsuchiya, K.; Kurosaki, M.; Kaneko, S.; Inada, K.; Yamashita, K.; Osawa, L.; Hayakawa, Y.; Sekiguchi, S.; Okada, M.; et al. Relative dose intensity over the first four weeks of lenvatinib therapy is a factor of favorable response and overall survival in patients with unresectable hepatocellular carcinoma. PLoS ONE 2020, 15, e0231828. [Google Scholar] [CrossRef] [Green Version]
- Saeki, I.; Yamasaki, T.; Yamashita, S.; Hanazono, T.; Urata, Y.; Furutani, T.; Yokoyama, Y.; Oishi, T.; Maeda, M.; Kimura, T.; et al. Early predictors of objective response in patients with hepatocellular carcinoma undergoing lenvatinib treatment. Cancers 2020, 12, 779. [Google Scholar] [CrossRef] [Green Version]
- Fuchigami, A.; Imai, Y.; Uchida, Y.; Uchiya, H.; Fujii, Y.; Nakazawa, M.; Ando, S.; Sugawara, K.; Nakayama, N.; Tomiya, T.; et al. Therapeutic efficacy of lenvatinib for patients with unresectable hepatocellular carcinoma based on the middle-term outcome. PLoS ONE 2020, 15, e0231427. [Google Scholar] [CrossRef] [PubMed]
- Hiraoka, A.; Kumada, T.; Atsukawa, M.; Hirooka, M.; Tsuji, K.; Ishikawa, T.; Takaguchi, K.; Kariyama, K.; Itobayashi, E.; Tajiri, K.; et al. Prognostic factor of lenvatinib for unresectable hepatocellular carcinoma in real-world conditions—Multicenter analysis. Cancer Med. 2019, 8, 3719–3728. [Google Scholar] [CrossRef] [Green Version]
- Nakano, M.; Kuromatsu, R.; Niizeki, T.; Okamura, S.; Iwamoto, H.; Shimose, S.; Shirono, T.; Noda, Y.; Kamachi, N.; Koga, H.; et al. Immunological inflammatory biomarkers as prognostic predictors for advanced hepatocellular carcinoma. ESMO Open 2021, 6, 100020. [Google Scholar] [CrossRef]
- Shomura, M.; Okabe, H.; Sato, E.; Fukai, K.; Shiraishi, K.; Hirose, S.; Tsuruya, K.; Arase, Y.; Anzai, K.; Kagawa, T. Hypothyroidism is a predictive factor for better clinical outcomes in patients with advanced hepatocellular carcinoma undergoing lenvatinib therapy. Cancers 2020, 12, 3078. [Google Scholar] [CrossRef]
- Lencioni, R.; Montal, R.; Torres, F.; Park, J.W.; Decaens, T.; Raoul, J.L.; Kudo, M.; Chang, C.; Rios, J.; Boige, V.; et al. Objective response by mRECIST as a predictor and potential surrogate end-point of overall survival in advanced HCC. J. Hepatol. 2017, 66, 1166–1172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meyer, T.; Palmer, D.H.; Cheng, A.L.; Hocke, J.; Loembe, A.B.; Yen, C.J. mRECIST to predict survival in advanced hepatocellular carcinoma: Analysis of two randomised phase II trials comparing nintedanib vs sorafenib. Liver Int. 2017, 37, 1047–1055. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vincenzi, B.; Di Maio, M.; Silletta, M.; D’Onofrio, L.; Spoto, C.; Piccirillo, M.C.; Daniele, G.; Comito, F.; Maci, E.; Bronte, G.; et al. Prognostic relevance of objective response according to EASL criteria and mRECIST criteria in hepatocellular carcinoma patients treated with loco-regional therapies: A literature-based meta-analysis. PLoS ONE 2015, 10, e0133488. [Google Scholar] [CrossRef]
- Kaneko, S.; Tsuchiya, K.; Kurosaki, M.; Kirino, S.; Inada, K.; Yamashita, K.; Osawa, L.; Hayakawa, Y.; Sekiguchi, S.; Watakabe, K.; et al. Three criteria for radiological response on survival in patients with hepatocellular carcinoma treated with lenvatinib. Hepatol. Res. 2020, 50, 137–143. [Google Scholar] [CrossRef] [Green Version]
- Kudo, M. Objective response by mRECIST is an independent prognostic factor of overall survival in systemic therapy for hepatocellular carcinoma. Liver Cancer 2019, 8, 73–77. [Google Scholar] [CrossRef]
- Hiraoka, A.; Kumada, T.; Tada, T.; Kariyama, K.; Tani, J.; Fukunishi, S.; Atsukawa, M.; Hirooka, M.; Tsuji, K.; Ishikawa, T.; et al. What can be done to solve the unmet clinical need of hepatocellular carcinoma patients following lenvatinib failure. Liver Cancer 2021, 10, 115–125. [Google Scholar] [CrossRef]
- Kuzuya, T.; Ishigami, M.; Ito, T.; Ishizu, Y.; Honda, T.; Ishikawa, T.; Fujishiro, M. Favorable radiological antitumor response at 2 weeks after starting lenvatinib for patients with advanced hepatocellular carcinoma. Hepatol. Res. 2019, 50, 374–381. [Google Scholar] [CrossRef] [PubMed]
- Yoo, C.; Kim, J.H.; Ryu, M.H.; Park, S.R.; Lee, D.; Kim, K.M.; Shim, J.H.; Lim, Y.S.; Lee, H.C.; Lee, J.; et al. Clinical outcomes with multikinase Inhibitors after progression on first-line atezolizumab plus bevacizumab in patients with advanced hepatocellular carcinoma: A multinational multicenter retrospective study. Liver Cancer 2021, 10, 107–114. [Google Scholar] [CrossRef]
- Ogasawara, S.; Ooka, Y.; Itokawa, N.; Inoue, M.; Okabe, S.; Seki, A.; Haga, Y.; Obu, M.; Atsukawa, M.; Itobayashi, E.; et al. Sequential therapy with sorafenib and regorafenib for advanced hepatocellular carcinoma: A multicenter retrospective study in Japan. Investig. New Drugs 2020, 38, 172–180. [Google Scholar] [CrossRef] [PubMed]
- Ando, Y.; Kawaoka, T.; Suehiro, Y.; Yamaoka, K.; Kosaka, Y.; Uchikawa, S.; Kodama, K.; Morio, K.; Fujino, H.; Nakahara, T.; et al. Analysis of post-progression survival in patients with unresectable hepatocellular carcinoma treated with lenvatinib. Oncology 2020, 98, 787–797. [Google Scholar] [CrossRef]
- Alsina, A.; Kudo, M.; Vogel, A.; Cheng, A.L.; Tak, W.Y.; Ryoo, B.Y.; Evans, T.R.J.; Lopez, C.L.; Daniele, B.; Misir, S.; et al. Effects of subsequent systemic anticancer medication following first-line lenvatinib: A post hoc responder analysis from the phase 3 REFLECT study in unresectable hepatocellular carcinoma. Liver Cancer 2020, 9, 93–104. [Google Scholar] [CrossRef] [PubMed]
- Prospective Observational Study of Systemic Therapy for Unresectable HCC in Japan: Real World Data of Systemic Therapy for HCC; UMIN ID 000040488. Available online: https://upload.umin.ac.jp/cgi-open-bin/ctr/ctr_view.cgi?recptno=R000046213 (accessed on 21 October 2021).


| Characteristic | Median (Range) or Patients, n |
|---|---|
| Age, range, y | 74 (46–90) |
| Sex (male/female), n | 154/28 |
| Weight (<60/>60 kg), n | 84/98 |
| Performance status (0/1), n | 166/16 |
| Etiology (HBV/HCV/HBV + HCV/NBNC), n | 22/62/1/97 |
| History of non-systemic treatment (with/without), n | 146/36 |
| Total bilirubin, range, mg/dL | 0.8 (0.3–2.1) |
| Albumin, range, g/dL | 3.9 (2.9–4.9) |
| Prothrombin activity, range, % | 90 (59–131) |
| Child–Pugh score (5/6), n | 126/56 |
| mALBI grade (1/2a/2b), n | 80/51/51 |
| Size of main tumor, range, mm | 24.0 (0.0–190.0) |
| Relative tumor volume (<50/≥50%), n | 166/16 |
| Macroscopic vascular invasion (absent/present), n | 153/29 |
| Extrahepatic metastasis (absent/present), n | 130/52 |
| BCLC stage (B/C), n | 110/72 |
| Serum AFP value, range, ng/mL | 20.2 (0.5–236900.0) |
| Serum DCP value, range, mAU/mL | 174.0 (13.0–1083990.0) |
| Observation period, range, months | 14.7 (0.6–38.9) |
| Response | RECIST % (n) | mRECIST % (n) | ||||
|---|---|---|---|---|---|---|
| Best | 1st | 2nd | Best | 1st | 2nd | |
| CR | 4.0 (7) | 2.3 (4) | 2.1 (3) | 17.1 (29) | 7.7 (13) | 12.9 (18) |
| PR | 35.6 (62) | 21.4 (37) | 23.4 (34) | 42.4 (72) | 39.9 (67) | 32.1 (45) |
| SD | 44.3 (77) | 59.0 (102) | 48.3 (70) | 25.9 (44) | 35.7 (60) | 30.7 (43) |
| PD | 16.1 (28) | 17.3 (30) | 26.2 (38) | 14.7 (25) | 16.7 (28) | 24.3 (34) |
| ORR | 39.7 (69) | 23.7 (41) | 25.5 (37) | 59.4 (101) | 47.6 (80) | 45.0 (63) |
| DCR | 83.9 (146) | 82.7 (143) | 73.8 (107) | 85.3 (145) | 83.3 (140) | 75.7 (106) |
| Factors | Univariate p-Value | Multivariate | ||
|---|---|---|---|---|
| HR | 95% CI | p-Value | ||
| Age (<74 vs. ≥74 years) | 0.302 | |||
| Sex (male vs. female) | 0.279 | |||
| Etiology (NBNC vs. viral) | 0.010 | 0.605 | 0.380–0.962 | 0.034 |
| History of non-systemic treatment (with vs. without) | 0.981 | |||
| mALBI grade (1/2a vs. 2b) | <0.005 | 0.409 | 0.249–0.674 | <0.005 |
| Macroscopic vascular invasion (absent vs. present) | <0.005 | 0.838 | 0.320–1.129 | 0.113 |
| Extrahepatic metastasis (absent vs. present) | 0.010 | 0.601 | 0.456–1.199 | 0.221 |
| Relative tumor volume (<50% vs. ≥50%) | <0.005 | 0.740 | 0.377–1.866 | 0.666 |
| Serum AFP value (<400 vs. ≥400), ng/mL | <0.005 | 0.409 | 0.251–0.667 | <0.005 |
| Serum DCP value (<174 vs. ≥174), ng/mL | 0.133 | |||
| Initial objective response by RECIST (OR vs. non-OR) | 0.007 | 0.369 | 0.197–0.691 | <0.005 |
| Age (<74 vs. ≥74 years) | 0.302 | |||
| Sex (male vs. female) | 0.279 | |||
| Etiology (NBNC vs. viral) | 0.010 | 0.662 | 0.416–1.055 | 0.083 |
| History of non-systemic treatment (with vs. without) | 0.981 | |||
| mALBI grade (1/2a vs. 2b) | <0.005 | 0.451 | 0.277–0.734 | <0.005 |
| Macroscopic vascular invasion (absent vs. present) | <0.005 | 0.861 | 0.437–1.697 | 0.666 |
| Extrahepatic metastasis (absent vs. present) | 0.010 | 0.786 | 0.484–1.278 | 0.332 |
| Relative tumor volume (<50% vs. ≥50%) | <0.005 | 0.488 | 0.215–1.111 | 0.087 |
| Serum AFP value (<400 vs. ≥400), ng/mL | <0.005 | 0.359 | 0.221–0.583 | <0.005 |
| Serum DCP value (<174 vs. ≥174), ng/mL | 0.133 | |||
| Initial objective response by mRECIST (OR vs. non-OR) | <0.005 | 0.378 | 0.234–0.611 | <0.005 |
| Factors | Univariate p-Value | Multivariate | ||
|---|---|---|---|---|
| HR | 95% CI | p-Value | ||
| Age (<74 vs. ≥74 years) | 0.444 | |||
| Sex (female vs. male) | 0.072 | |||
| Etiology (NBNC vs. viral) | 0.016 | 0.584 | 0.303–1.124 | 0.107 |
| History of non-systemic treatment (with vs. without) | 0.555 | |||
| mALBI grade at initial objective evaluation (1/2a vs. 2b) | 0.031 | 0.743 | 0.352–1.567 | 0.435 |
| Decrease in AFP value up to initial objective evaluation (yes vs. no) | 0.482 | |||
| Decrease in DCP value up to initial objective evaluation (yes vs. no) | 0.574 | |||
| Relative dose intensity up to initial objective evaluation (<0.8 vs. ≥0.8) | 0.540 | |||
| Macroscopic vascular invasion at initial objective evaluation (absent vs. present) | <0.005 | 0.347 | 0.143–0.843 | 0.019 |
| Extrahepatic metastasis at initial objective evaluation (absent vs. present) | 0.169 | |||
| Relative tumor volume at initial objective evaluation (<50% vs. ≥50%) | 0.005 | 0.464 | 0.158–1.361 | 0.162 |
| Second objective response by RECIST (OR vs. non-OR) | 0.225 | |||
| Age (<74 vs. ≥74 years) | 0.946 | |||
| Sex (female vs. male) | 0.542 | |||
| Etiology (NBNC vs. viral) | 0.052 | |||
| History of non-systemic treatment (with vs. without) | 0.911 | |||
| mALBI grade at initial objective evaluation (1/2a vs. 2b) | 0.009 | 0.381 | 0.156–0.932 | 0.035 |
| Decrease in AFP value up to initial objective evaluation (yes vs. no) | 0.323 | |||
| Decrease in DCP value up to initial objective evaluation (yes vs. no) | 0.848 | |||
| Relative dose intensity up to initial objective evaluation (<0.8 vs. ≥0.8) | 0.302 | |||
| Macroscopic vascular invasion at initial objective evaluation (absent vs. present) | 0.013 | 0.671 | 0.247–1.824 | 0.435 |
| Extrahepatic metastasis at initial objective evaluation (absent vs. present) | 0.212 | |||
| Relative tumor volume at initial objective evaluation (<50% vs. ≥50%) | <0.005 | 0.216 | 0.042–1.114 | 0.067 |
| Second objective response by mRECIST (OR vs. non-OR) | 0.443 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amioka, K.; Kawaoka, T.; Kosaka, M.; Johira, Y.; Shirane, Y.; Miura, R.; Murakami, S.; Yano, S.; Naruto, K.; Ando, Y.; et al. Analysis of Survival and Response to Lenvatinib in Unresectable Hepatocellular Carcinoma. Cancers 2022, 14, 320. https://doi.org/10.3390/cancers14020320
Amioka K, Kawaoka T, Kosaka M, Johira Y, Shirane Y, Miura R, Murakami S, Yano S, Naruto K, Ando Y, et al. Analysis of Survival and Response to Lenvatinib in Unresectable Hepatocellular Carcinoma. Cancers. 2022; 14(2):320. https://doi.org/10.3390/cancers14020320
Chicago/Turabian StyleAmioka, Kei, Tomokazu Kawaoka, Masanari Kosaka, Yusuke Johira, Yuki Shirane, Ryoichi Miura, Serami Murakami, Shigeki Yano, Kensuke Naruto, Yuwa Ando, and et al. 2022. "Analysis of Survival and Response to Lenvatinib in Unresectable Hepatocellular Carcinoma" Cancers 14, no. 2: 320. https://doi.org/10.3390/cancers14020320
APA StyleAmioka, K., Kawaoka, T., Kosaka, M., Johira, Y., Shirane, Y., Miura, R., Murakami, S., Yano, S., Naruto, K., Ando, Y., Kosaka, Y., Fujii, Y., Kodama, K., Uchikawa, S., Fujino, H., Ono, A., Nakahara, T., Murakami, E., Okamoto, W., ... Aikata, H. (2022). Analysis of Survival and Response to Lenvatinib in Unresectable Hepatocellular Carcinoma. Cancers, 14(2), 320. https://doi.org/10.3390/cancers14020320







