Presumed Roles of APRO Family Proteins in Cancer Invasiveness
Abstract
Simple Summary
Abstract
1. Introduction
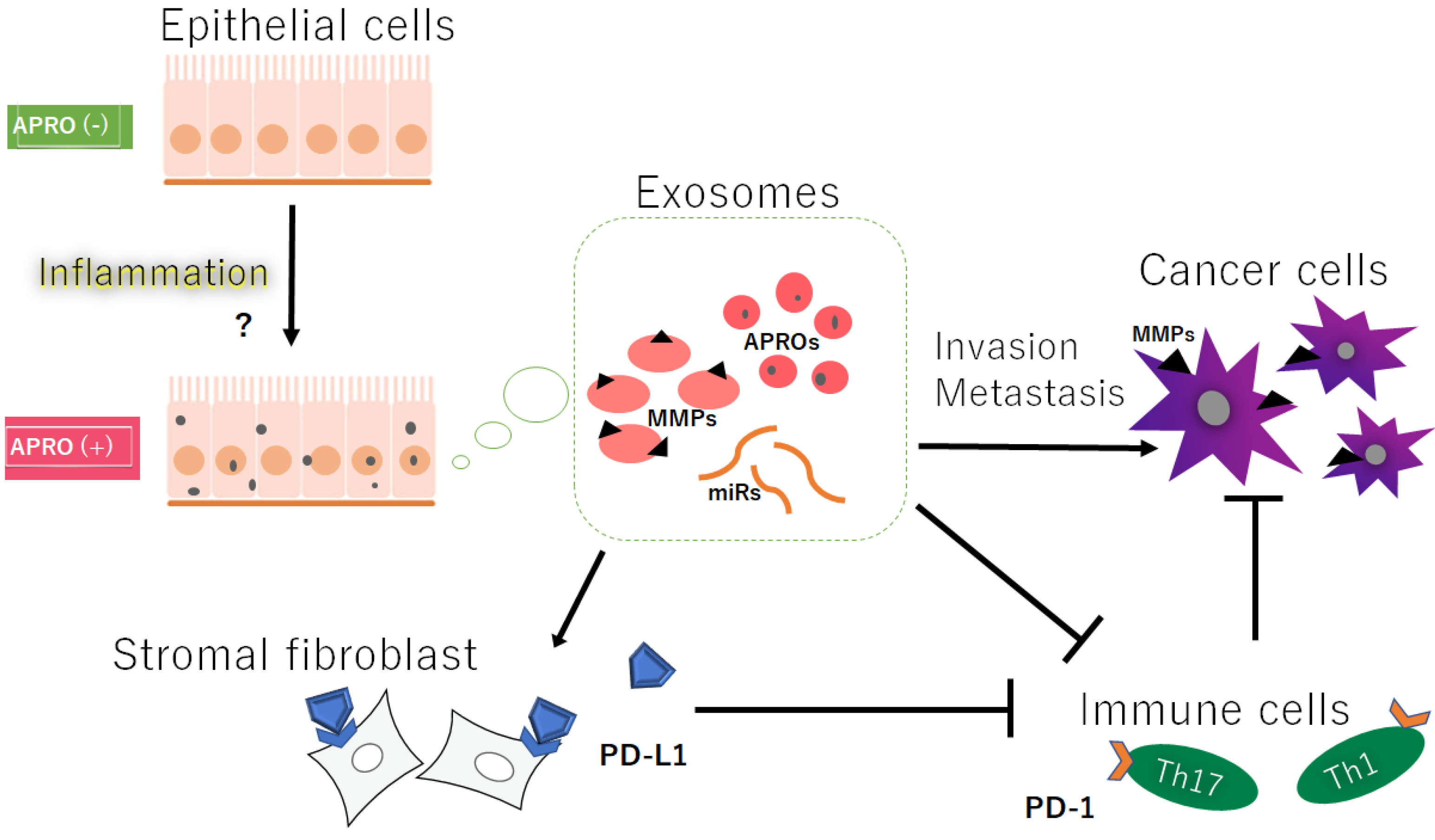
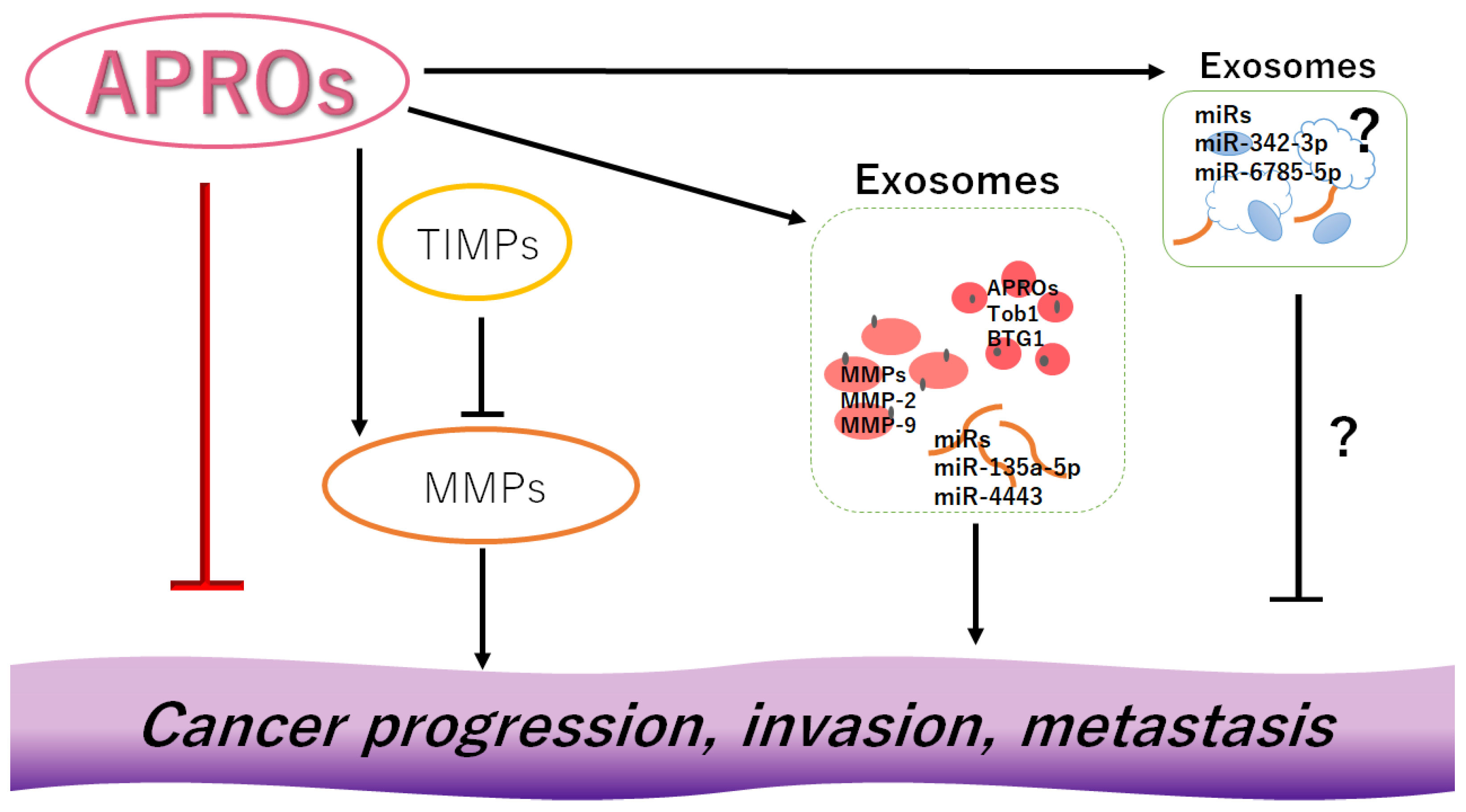
2. Exosomes with APRO Proteins and/or Certain MicroRNAs May Contribute to Cancer Invasion
3. MMPs and TIMPs Could Be Also Involved in Cancer Invasion and Metastasis
4. Activated MMPs and/or Certain MicroRNAs in Exosomes Could Contribute to the Enhanced Migration, Invasion, and/or Metastasis of Cancer Cells
5. Activated MMPs Could Also Regulate the Responses of Immune Cells against Cancers
6. Discussion and Perspectives
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| APROs | antiproliferative proteins |
| ECM | extracellular matrix |
| hCG | human chorionic gonadotropin |
| miRNAs | microRNAs |
| MMPs | matrix metalloproteinases |
| PTEN | phosphatase and tensin homolog |
| TIMP | tissue inhibitors of metalloproteinase |
| VEGF | vascular endothelial growth factor |
| VSMCs | vascular smooth muscle cells |
References
- Matsuda, S.; Rouault, J.; Magaud, J.; Berthet, C. In search of a function for the TIS21/PC3/BTG1/TOB family. FEBS Lett. 2001, 497, 67–72. [Google Scholar] [CrossRef]
- Guéhenneux, F.; Duret, L.; Callanan, M.B.; Bouhas, R.; Hayette, S.; Berthet, C.; Samarut, C.; Rimokh, R.; Birot, A.M.; Wang, Q.; et al. Cloning of the mouse BTG3 gene and definition of a new gene family (the BTG family) involved in the negative control of the cell cycle. Leukemia 1997, 11, 370–375. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, S.; Kawamura-Tsuzuku, J.; Ohsugi, M.; Yoshida, M.; Emi, M.; Nakamura, Y.; Onda, M.; Yoshida, Y.; Nishiyama, A.; Yamamoto, T. Tob, a novel protein that interacts with p185erbB2, is associated with anti-proliferative activity. Oncogene 1996, 12, 705–713. [Google Scholar] [PubMed]
- Ikematsu, N.; Yoshida, Y.; Kawamura-Tsuzuku, J.; Ohsugi, M.; Onda, M.; Hirai, M.; Fujimoto, J.; Yamamoto, T. Tob2, a novel anti-proliferative Tob/BTG1 family member, associates with a component of the CCR4 transcriptional regulatory complex capable of binding cyclin-dependent kinases. Oncogene 1999, 18, 7432–7441. [Google Scholar] [CrossRef]
- Buanne, P.; Corrente, G.; Micheli, L.; Palena, A.; Lavia, P.; Spadafora, C.; Lakshmana, M.K.; Rinaldi, A.; Banfi, S.; Quarto, M.; et al. Cloning of PC3B, a novel member of the PC3/BTG/TOB family of growth inhibitory genes, highly expressed in the olfactory epithelium. Genomics 2000, 68, 253–263. [Google Scholar] [CrossRef][Green Version]
- Zhang, S.Q.; Sun, K.K.; Wu, X.Y.; Zhong, N.; Zhao, H.; Li, D.C. Clinicopathological significance of cytoplasmic transducer of ErbB2. 1 expression in gastric cancer. Mol. Med. Rep. 2015, 12, 1177–1182. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Shen, X.; Sun, K. Prognostic value of transducer of ErbB2.1 (TOB1) expression in patients with gastric cancer: Tissue microarray analysis. Int. J. Clin. Exp. Pathol. 2018, 11, 4060–4066. [Google Scholar] [PubMed]
- Guo, H.; Zhang, R.; Afrifa, J.; Wang, Y.; Yu, J. Decreased expression levels of DAL-1 and TOB1 are associated with clinicopathological features and poor prognosis in gastric cancer. Pathol. Res. Pract. 2019, 215, 152403. [Google Scholar] [CrossRef]
- Jiao, Y.; Sun, K.K.; Zhao, L.; Xu, J.Y.; Wang, L.L.; Fan, S.J. Suppression of human lung cancer cell proliferation and metastasis in vitro by the transducer of ErbB-2.1 (TOB1). Acta Pharm. Sin. 2012, 33, 250–260. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Qiao, L.; Xie, N.; Shi, Y.; Liu, N.; Wang, J. Expression and prognosis analyses of the Tob/BTG antiproliferative (APRO) protein family in human cancers. PLoS ONE 2017, 12, e0184902. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Xiao, L.; Ge, Y.; Fu, Y.; Zhang, W.; Cao, H.; Chen, B.; Wang, H.; Zhan, Y.Y.; Hu, T. High expression of Tob1 indicates poor survival outcome and promotes tumour progression via a Wnt positive feedback loop in colon cancer. Mol. Cancer 2018, 17, 159. [Google Scholar] [CrossRef]
- Guan, R.; Peng, L.; Wang, D.; He, H.; Wang, D.; Zhang, R.; Wang, H.; Hao, H.; Zhang, J.; Song, H.; et al. Decreased TOB1 expression and increased phosphorylation of nuclear TOB1 promotes gastric cancer. Oncotarget 2017, 8, 75243–75253. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.K.; Yang, Y.; Zhao, L.; Wang, L.L.; Jiao, Y. Transducer of erbB2.1 is a potential cellular target of gefitinib in lung cancer therapy. Oncol. Lett. 2013, 5, 373–377. [Google Scholar] [CrossRef][Green Version]
- Zhou, J.; Liu, T.; Wang, W. Prognostic significance of matrix metalloproteinase 9 expression in osteosarcoma: A meta-analysis of 16 studies. Medicine 2018, 97, e13051. [Google Scholar] [CrossRef] [PubMed]
- Dofara, S.G.; Chang, S.L.; Diorio, C. Gene Polymorphisms and Circulating Levels of MMP-2 and MMP-9: A Review of Their Role in Breast Cancer Risk. Anticancer Res. 2020, 40, 3619–3631. [Google Scholar] [CrossRef]
- Chu, X.; Yang, Y.; Tian, X. Crosstalk between Pancreatic Cancer Cells and Cancer-Associated Fibroblasts in the Tumor Microenvironment Mediated by Exosomal MicroRNAs. Int. J. Mol. Sci. 2022, 23, 9512. [Google Scholar] [CrossRef] [PubMed]
- Sunami, Y.; Häußler, J.; Zourelidis, A.; Kleeff, J. Cancer-Associated Fibroblasts and Tumor Cells in Pancreatic Cancer Microenvironment and Metastasis: Paracrine Regulators, Reciprocation and Exosomes. Cancers 2022, 14, 744. [Google Scholar] [CrossRef] [PubMed]
- Thuault, S.; Ghossoub, R.; David, G.; Zimmermann, P. A Journey on Extracellular Vesicles for Matrix Metalloproteinases: A Mechanistic Perspective. Front Cell Dev. Biol. 2022, 10, 886381. [Google Scholar] [CrossRef] [PubMed]
- Conde-Vancells, J.; Rodriguez-Suarez, E.; Embade, N.; Gil, D.; Matthiesen, R.; Valle, M.; Elortza, F.; Lu, S.C.; Mato, J.M.; Falcon-Perez, J.M. Characterization and comprehensive proteome profiling of exosomes secreted by hepatocytes. J. Proteome Res. 2008, 7, 5157–5166. [Google Scholar] [CrossRef]
- Moradi-Chaleshtori, M.; Hashemi, S.M.; Soudi, S.; Bandehpour, M.; Mohammadi-Yeganeh, S. Tumor-derived exosomal microRNAs and proteins as modulators of macrophage function. J. Cell Physiol. 2019, 234, 7970–7982. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Zhang, W.S.; Guo, Y.; Peng, H.; Zhu, M.; Miao, D.; Su, G. Engineering of exosome-triggered enzyme-powered DNA motors for highly sensitive fluorescence detection of tumor-derived exosomes. Biosens. Bioelectron. 2020, 167, 112482. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Xia, B.; Wang, Y.; You, M.J.; Zhang, Y. Potential Therapeutic Roles of Exosomes in Multiple Myeloma: A Systematic Review. J. Cancer 2019, 10, 6154–6160. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Song, Y.; Zhou, L.; Wang, M.; Wang, D.; Bai, J.; Fu, S.; Yu, J. The Overexpression of TOB1 Induces Autophagy in Gastric Cancer Cells by Secreting Exosomes. Dis. Markers 2022, 2022, 7925097. [Google Scholar] [CrossRef]
- Zhu, H.; Yang, Y.; Wang, L.; Xu, X.; Wang, T.; Qian, H. Leptomycin B inhibits the proliferation, migration, and invasion of cultured gastric carcinoma cells. Biosci. Biotechnol. Biochem. 2020, 84, 290–296. [Google Scholar] [CrossRef] [PubMed]
- Wan, L.; Chen, X.; Deng, J.; Zhang, S.; Tu, F.; Pei, H.; Hu, R.; Liu, J.; Yu, H. Plasma exosome-derived B-cell translation gene 1: A predictive marker for the prognosis in patients with non-small cell lung cancer. J. Cancer. 2021, 12, 1538–1547. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Zhang, W.; Zhang, C.; Wang, L.; Chen, H.; Xu, J. Exosomal non-coding RNAs have a significant effect on tumor metastasis. Mol. Nucleic Acids 2022, 29, 16–35. [Google Scholar] [CrossRef]
- Bartel, D.P. MicroRNAs: Target recognition and regulatory functions. Cell 2009, 136, 215–233. [Google Scholar] [CrossRef]
- Mohr, A.M.; Mott, J.L. Overview of microRNA biology. Semin. Liver Dis. 2015, 35, 3–11. [Google Scholar] [CrossRef]
- Dexheimer, P.J.; Cochella, L. MicroRNAs: From Mechanism to Organism. Front Cell Dev. Biol. 2020, 8, 409. [Google Scholar] [CrossRef]
- Liu, Q.; Gao, Q.; Zhang, Y.; Li, Z.; Mei, X. MicroRNA-590 promotes pathogenic Th17 cell differentiation through targeting Tob1 and is associated with multiple sclerosis. Biochem. Biophys. Res. Commun. 2017, 493, 901–908. [Google Scholar] [CrossRef]
- Tang, G.; Wang, J.; Dong, W.; Dai, K.; Du, J. Exosomal miRNA Expression Profiling and the Roles of Exosomal miR-4741, miR-32, miR-3149, and miR-6727 on Gastric Cancer Progression. Biomed. Res. Int. 2022, 2022, 1263812. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Chen, Y.; Ye, S.; Yang, H.; Yang, J.; Quan, J. Transcription factor forkhead box K1 regulates miR-32 expression and enhances cell proliferation in colorectal cancer. Oncol. Lett. 2021, 21, 407. [Google Scholar] [CrossRef]
- Sun, H.; Meng, Q.; Shi, C.; Yang, H.; Li, X.; Wu, S.; Familiari, G.; Relucenti, M.; Aschner, M.; Wang, X.; et al. Hypoxia-Inducible Exosomes Facilitate Liver-Tropic Premetastatic Niche in Colorectal Cancer. Hepatology 2021, 74, 2633–2651. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Q.; Wang, D.; Yang, S.; Zhou, S.; Xu, H.; Zhang, H.; Zhong, S.; Feng, J. Microenvironment-induced TIMP2 loss by cancer-secreted exosomal miR-4443 promotes liver metastasis of breast cancer. J. Cell Physiol. 2020, 235, 5722–5735. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Luo, L.; Bai, X.; Shen, K.; Liu, K.; Wang, J.; Hu, D. Highly-expressed micoRNA-21 in adipose derived stem cell exosomes can enhance the migration and proliferation of the HaCaT cells by increasing the MMP-9 expression through the PI3K/AKT pathway. Arch. Biochem. Biophys. 2020, 681, 108259. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Zhu, W.; Wan, D. Downregulation of microRNA-21–5p from macrophages-derived exosomes represses ventricular remodeling after myocardial infarction via inhibiting tissue inhibitors of metalloproteinase 3. Int. Immunopharmacol. 2021, 96, 107611. [Google Scholar] [CrossRef] [PubMed]
- Solé, C.; Cortés-Hernández, J.; Felip, M.L.; Vidal, M.; Ordi-Ros, J. miR-29c in urinary exosomes as predictor of early renal fibrosis in lupus nephritis. Nephrol. Dial. Transplant. 2015, 30, 1488–1496. [Google Scholar] [CrossRef] [PubMed]
- De Almeida, L.G.N.; Thode, H.; Eslambolchi, Y.; Chopra, S.; Young, D.; Gill, S.; Devel, L.; Dufour, A. Matrix Metalloproteinases: From Molecular Mechanisms to Physiology, Pathophysiology, and Pharmacology. Pharmacol. Rev. 2022, 74, 712–768. [Google Scholar] [CrossRef] [PubMed]
- Verma, R.P.; Hansch, C. Matrix metalloproteinases (MMPs): Chemical-biological functions and (Q)SARs. Bioorg. Med. Chem. 2007, 15, 2223–2268. [Google Scholar] [CrossRef]
- Grzechocińska, B.; Dąbrowski, F.; Cyganek, A.; Panek, G.; Wielgoś, M. The role of metalloproteinases in endometrial remodelling during menstrual cycle. Ginekol. Pol. 2017, 88, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Duffy, D.M.; Stouffer, R.L. Luteinizing hormone acts directly at granulosa cells to stimulate periovulatory processes: Modulation of luteinizing hormone effects by prostaglandins. Endocrine 2003, 22, 249–256. [Google Scholar] [CrossRef]
- Kapustin, A.N.; Shanahan, C.M. Emerging roles for vascular smooth muscle cell exosomes in calcification and coagulation. J. Physiol. 2016, 594, 2905–2914. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Jia, L.; Xie, Y.; Cai, Z.; Liu, Z.; Shen, J.; Lu, Y.; Wang, Y.; Su, S.; Ma, Y.; et al. Involvement of macrophage-derived exosomes in abdominal aortic aneurysms development. Atherosclerosis 2019, 289, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, R.; Konishi, M.; Taniguchi, M.; Hatakawa, Y.; Akizawa, T. The discovery of shorter synthetic proteolytic peptides derived from Tob1 protein. Peptides 2019, 116, 71–77. [Google Scholar] [CrossRef]
- Shimoda, M. Extracellular vesicle-associated MMPs: A modulator of the tissue microenvironment. Adv. Clin. Chem. 2019, 88, 35–66. [Google Scholar] [PubMed]
- Kaur, N.; Sunil Kumar, B.V.; Mahajan, K.; Singh, S. Expression and characterization of tissue inhibitor of metalloproteinase 4 from complex canine mammary carcinomas. J. Immunoass. Immunochem. 2016, 37, 515–526. [Google Scholar] [CrossRef]
- Gomis-Rüth, F.X.; Maskos, K.; Betz, M.; Bergner, A.; Huber, R.; Suzuki, K.; Yoshida, N.; Nagase, H.; Brew, K.; Bourenkov, G.P.; et al. Mechanism of inhibition of the human matrix metalloproteinase stromelysin-1 by TIMP-1. Nature 1997, 389, 77–81. [Google Scholar] [CrossRef] [PubMed]
- Raeeszadeh-Sarmazdeh, M.; Greene, K.A.; Sankaran, B.; Downey, G.P.; Radisky, D.C.; Radisky, E.S. Directed evolution of the metalloproteinase inhibitor TIMP-1 reveals that its N- and C-terminal domains cooperate in matrix metalloproteinase recognition. J. Biol. Chem. 2019, 294, 9476–9488. [Google Scholar] [CrossRef] [PubMed]
- Thaysen-Andersen, M.; Thøgersen, I.B.; Lademann, U.; Offenberg, H.; Giessing, A.M.; Enghild, J.J.; Nielsen, H.J.; Brünner, N.; Højrup, P. Investigating the biomarker potential of glycoproteins using comparative glycoprofiling-application to tissue inhibitor of metalloproteinases-1. Biochim. Biophys. Acta. 2008, 1784, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Luparello, C.; Avanzato, G.; Carella, C.; Pucci-Minafra, I. Tissue inhibitor of metalloprotease (TIMP)-1 and proliferative behaviour of clonal breast cancer cells. Breast Cancer Res. Treat. 1999, 54, 235–244. [Google Scholar] [CrossRef]
- Guedez, L.; Stetler-Stevenson, W.G.; Wolff, L.; Wang, J.; Fukushima, P.; Mansoor, A.; Stetler-Stevenson, M. In vitro suppression of programmed cell death of B cells by tissue inhibitor of metalloproteinases-1. J. Clin. Investig. 1998, 102, 2002–2010. [Google Scholar] [CrossRef] [PubMed]
- Rao, V.S.; Gu, Q.; Tzschentke, S.; Lin, K.; Ganig, N.; Thepkaysone, M.L.; Wong, F.C.; Polster, H.; Seifert, L.; Seifert, A.M.; et al. Extravesicular TIMP-1 is a non-invasive independent prognostic marker and potential therapeutic target in colorectal liver metastases. Oncogene 2022, 41, 1809–1820. [Google Scholar] [CrossRef]
- Lin, C.; Liang, S.; Li, Y.; Peng, Y.; Huang, Z.; Li, Z.; Yang, Y.; Luo, X. Localized plasmonic sensor for direct identifying lung and colon cancer from the blood. Biosens. Bioelectron. 2022, 211, 114372. [Google Scholar] [CrossRef]
- Liao, W.T.; Ye, Y.P.; Deng, Y.J.; Bian, X.W.; Ding, Y.Q. Metastatic cancer stem cells: From the concept to therapeutics. Am. J. Stem. Cells 2014, 3, 46–62. [Google Scholar] [PubMed]
- Huang, Q.; Lan, F.; Wang, X.; Yu, Y.; Ouyang, X.; Zheng, F.; Han, J.; Lin, Y.; Xie, Y.; Xie, F.; et al. IL-1β-induced activation of p38 promotes metastasis in gastric adenocarcinoma via upregulation of AP-1/c-fos, MMP2 and MMP9. Mol. Cancer 2014, 13, 18. [Google Scholar] [CrossRef] [PubMed]
- Roy, R.; Yang, J.; Moses, M.A. Matrix metalloproteinases as novel biomarkers and potential therapeutic targets in human cancer. J. Clin. Oncol. 2009, 27, 5287–5297. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.M.; Deng, S.H.; Liu, T.; Han, R.; Zhang, T.; Xu, Y. TGF-β-mediated exosomal lnc-MMP2–2 regulates migration and invasion of lung cancer cells to the vasculature by promoting MMP2 expression. Cancer Med. 2018, 7, 5118–5129. [Google Scholar] [CrossRef]
- McCready, J.; Sims, J.D.; Chan, D.; Jay, D.G. Secretion of extracellular hsp90alpha via exosomes increases cancer cell motility: A role for plasminogen activation. BMC Cancer 2010, 10, 294. [Google Scholar] [CrossRef]
- Chen, G.; Zhang, Y.; Wu, X. 786–0 Renal cancer cell line-derived exosomes promote 786–0 cell migration and invasion in vitro. Oncol. Lett. 2014, 7, 1576–1580. [Google Scholar] [CrossRef]
- Shan, Y.; You, B.; Shi, S.; Shi, W.; Zhang, Z.; Zhang, Q.; Gu, M.; Chen, J.; Bao, L.; Liu, D.; et al. Hypoxia-Induced Matrix Metalloproteinase-13 Expression in Exosomes from Nasopharyngeal Carcinoma Enhances Metastases. Cell Death Dis. 2018, 9, 382. [Google Scholar] [CrossRef]
- Zhu, Y.; Tao, Z.; Chen, Y.; Lin, S.; Zhu, M.; Ji, W.; Liu, X.; Li, T.; Hu, X. Exosomal MMP-1 transfers metastasis potential in triple-negative breast cancer through PAR1-mediated EMT. Breast Cancer Res. Treat. 2022, 193, 65–81. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Li, D.; Shen, L.; Hu, D.; Tang, B.; Guo, W.; Wang, Z.; Zhang, Z.; Wei, G.; He, D. Exosomes derived from Piwil2-induced cancer stem cells transform fibroblasts into cancer-associated fibroblasts. Oncol. Rep. 2020, 43, 1125–1132. [Google Scholar] [CrossRef] [PubMed]
- Xue, X.; Huang, J.; Yu, K.; Chen, X.; He, Y.; Qi, D.; Wu, Y. YB-1 transferred by gastric cancer exosomes promotes angiogenesis via enhancing the expression of angiogenic factors in vascular endothelial cells. BMC Cancer 2020, 20, 996. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wang, Y.; Chang, X.; Ba, X.; Hu, N.; Liu, Q.; Fang, L.; Wang, Z. Melanoma-Derived Exosomes Endow Fibroblasts with an Invasive Potential via miR-21 Target Signaling Pathway. Cancer Manag. Res. 2020, 12, 12965–12974. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Tan, S.; Yang, L.; Chen, X.; Yang, R.; Oyang, L.; Lin, J.; Xia, L.; Wu, N.; Han, Y.; et al. Exosomal miR-205–5p enhances angiogenesis and nasopharyngeal carcinoma metastasis by targeting desmocollin-2. Mol. Ther. Oncolytics. 2022, 24, 612–623. [Google Scholar] [CrossRef]
- Sun, S.; Chen, H.; Xu, C.; Zhang, Y.; Zhang, Q.; Chen, L.; Ding, Q.; Deng, Z. Exosomal miR-106b serves as a novel marker for lung cancer and promotes cancer metastasis via targeting PTEN. Life Sci. 2020, 244, 117297. [Google Scholar] [CrossRef]
- Hong, J.W.; Kim, J.M.; Kim, J.E.; Cho, H.; Kim, D.; Kim, W.; Oh, J.W.; Kwon, H.J. MiR-4435 is an UQCRB-related circulating miRNA in human colorectal cancer. Sci. Rep. 2020, 10, 2833. [Google Scholar] [CrossRef]
- Solga, R.; Behrens, J.; Ziemann, A.; Riou, A.; Berwanger, C.; Becker, L.; Garrett, L.; de Angelis, M.H.; Fischer, L.; Coras, R.; et al. CRN2 binds to TIMP4 and MMP14 and promotes perivascular invasion of glioblastoma cells. Eur. J. Cell Biol. 2019, 98, 151046. [Google Scholar] [CrossRef]
- Li, M.; Lu, Y.; Xu, Y.; Wang, J.; Zhang, C.; Du, Y.; Wang, L.; Li, L.; Wang, B.; Shen, J.; et al. Horizontal transfer of exosomal CXCR4 promotes murine hepatocarcinoma cell migration, invasion and lymphangiogenesis. Gene 2018, 676, 101–109. [Google Scholar] [CrossRef]
- Srivastava, M.K.; Zhu, L.; Harris-White, M.; Huang, M.; St John, M.; Lee, J.M.; Salgia, R.; Cameron, R.B.; Strieter, R.; Dubinett, S.; et al. Targeting myeloid-derived suppressor cells augments antitumor activity against lung cancer. Immunotargets Ther. 2012, 2012, 7–12. [Google Scholar]
- Wang, B.Q.; Zhang, C.M.; Gao, W.; Wang, X.F.; Zhang, H.L.; Yang, P.C. Cancer-derived matrix metalloproteinase-9 contributes to tumor tolerance. J. Cancer Res. Clin. Oncol. 2011, 137, 1525–1533. [Google Scholar] [CrossRef]
- Lee, S.; Yamamoto, S.; Srinivas, B.; Shimizu, Y.; Sada, N.; Yoshitome, K.; Ito, T.; Kumagai-Takei, N.; Nishimura, Y.; Otsuki, T. Increased production of matrix metalloproteinase-7 (MMP-7) by asbestos exposure enhances tissue migration of human regulatory T-like cells. Toxicology 2021, 452, 152717. [Google Scholar] [CrossRef]
- Zeng, Y.; Gao, M.; Lin, D.; Du, G.; Cai, Y. Prognostic and Immunological Roles of MMP-9 in Pan-Cancer. Biomed Res. Int. 2022, 2022, 2592962. [Google Scholar] [CrossRef]
- Liu, D.; Zhang, R.; Wu, J.; Pu, Y.; Yin, X.; Cheng, Y.; Wu, J.; Feng, C.; Luo, Y.; Zhang, J. Interleukin-17A promotes esophageal adenocarcinoma cell invasiveness through ROS-dependent, NF-κB-mediated MMP-2/9 activation. Oncol. Rep. 2017, 37, 1779–1785. [Google Scholar] [CrossRef]
- Krstic, J.; Santibanez, J.F. Transforming growth factor-beta and matrix metalloproteinases: Functional interactions in tumor stroma-infiltrating myeloid cells. Sci. World J. 2014, 2014, 521754. [Google Scholar] [CrossRef]
- Wilson, K.T.; Crabtree, J.E. Immunology of Helicobacter pylori: Insights into the failure of the immune response and perspectives on vaccine studies. Gastroenterology 2007, 133, 288–308. [Google Scholar] [CrossRef]
- Ogden, S.R.; Noto, J.M.; Allen, S.S.; Patel, D.A.; Romero-Gallo, J.; Washington, M.K.; Fingleton, B.; Israel, D.A.; Lewis, N.D.; Wilson, K.T.; et al. Matrix metalloproteinase-7 and premalignant host responses in Helicobacter pylori-infected mice. Cancer Res. 2010, 70, 30–35. [Google Scholar] [CrossRef]
- Khare, P.; Bose, A.; Singh, P.; Singh, S.; Javed, S.; Jain, S.K.; Singh, O.; Pal, R. Gonadotropin and tumorigenesis: Direct and indirect effects on inflammatory and immunosuppressive mediators and invasion. Mol. Carcinog. 2017, 56, 359–370. [Google Scholar] [CrossRef]
- Ye, Y.; Kuang, X.; Xie, Z.; Liang, L.; Zhang, Z.; Zhang, Y.; Ma, F.; Gao, Q.; Chang, R.; Lee, H.-H.; et al. Small-molecule MMP2/MMP9 inhibitor SB-3CT modulates tumor immune surveillance by regulating PD-L1. Genome Med. 2020, 12, 83. [Google Scholar] [CrossRef]
- Chen, Z.; Zhuo, S.; He, G.; Tang, J.; Hao, W.; Gao, W.Q.; Yang, K.; Xu, H. Prognosis and Immunotherapy Significances of a Cancer-Associated Fibroblasts-Related Gene Signature in Gliomas. Front Cell Dev. Biol. 2021, 9, 721897. [Google Scholar] [CrossRef]
- Aguirre, J.E.; Beswick, E.J.; Grim, C.; Uribe, G.; Tafoya, M.; Chacon Palma, G.; Samedi, V.; McKee, R.; Villeger, R.; Fofanov, Y.; et al. Matrix metalloproteinases cleave membrane-bound PD-L1 on CD90+ (myo-)fibroblasts in Crohn’s disease and regulate Th1/Th17 cell responses. Int. Immunol. 2020, 32, 57–68. [Google Scholar] [CrossRef]
- Dezutter-Dambuyant, C.; Durand, I.; Alberti, L.; Bendriss-Vermare, N.; Valladeau-Guilemond, J.; Duc, A.; Magron, A.; Morel, A.P.; Sisirak, V.; Rodriguez, C.; et al. A novel regulation of PD-1 ligands on mesenchymal stromal cells through MMP-mediated proteolytic cleavage. Oncoimmunology 2015, 5, e1091146. [Google Scholar] [CrossRef]
- Li, M.; Xing, S.; Zhang, H.; Shang, S.; Li, X.; Ren, B.; Li, G.; Chang, X.; Li, Y.; Li, W. A matrix metalloproteinase inhibitor enhances anti-cytotoxic T lymphocyte antigen-4 antibody immunotherapy in breast cancer by reprogramming the tumor microenvironment. Oncol. Rep. 2016, 35, 1329–1339. [Google Scholar] [CrossRef]
- Juric, V.; O’Sullivan, C.; Stefanutti, E.; Kovalenko, M.; Greenstein, A.; Barry-Hamilton, V.; Mikaelian, I.; Degenhardt, J.; Yue, P.; Smith, V.; et al. MMP-9 inhibition promotes anti-tumor immunity through disruption of biochemical and physical barriers to T-cell trafficking to tumors. PLoS ONE. 2018, 13, e0207255. [Google Scholar] [CrossRef]
- Huang, Y.; Zheng, J.; Tan, T.; Song, L.; Huang, S.; Zhang, Y.; Lin, L.; Liu, J.; Zheng, P.; Chen, X.; et al. BTG1 low expression in pancreatic ductal adenocarcinoma is associated with a poorer prognosis. Int. J. Biol. Markers. 2018, 33, 189–194. [Google Scholar] [CrossRef]
- Jung, Y.Y.; Sung, J.Y.; Kim, J.Y.; Kim, H.S. Down-regulation of B-Cell Translocation Gene 1 by Promoter Methylation in Colorectal Carcinoma. Anticancer Res. 2018, 38, 691–697. [Google Scholar]
- Lu, Y.F.; Sun, G.G.; Liu, Q.; Yang, C.R.; Cheng, Y.J. BTG1 expression in thyroid carcinoma: Diagnostic indicator and prognostic marker. Int. J. Oncol. 2014, 45, 1574–1582. [Google Scholar] [CrossRef][Green Version]
- Sun, G.G.; Wang, Y.D.; Cheng, Y.J.; Hu, W.N. BTG1 underexpression is an independent prognostic marker in esophageal squamous cell carcinoma. Tumour. Biol. 2014, 35, 9707–9716. [Google Scholar] [CrossRef]
- Li, G.; Yue, Y.; Li, W.; Chen, Y.; Fan, H.; Yuan, J.; Chen, Z.; Liu, J. Prognostic significance of low expression of B-cell translocation gene 1 (BTG 1) in skin squamous cell carcinoma. J. Biosci. 2020, 45, 70. [Google Scholar] [CrossRef]
- Zhang, J.; Dong, W. Expression of B Cell Translocation Gene 1 Protein in Colon Carcinoma and its Clinical Significance. Recent Pat. Anticancer Drug Discov. 2020, 15, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.C.; Li, J.; Shen, D.F.; Yang, X.F.; Zhao, S.; Wu, Y.Z.; Takano, Y.; Sun, H.Z.; Su, R.J.; Luo, J.S.; et al. BTG1 expression correlates with pathogenesis, aggressive behaviors and prognosis of gastric cancer: A potential target for gene therapy. Oncotarget 2015, 6, 19685–19705. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhao, S.; Xue, H.; Hao, C.L.; Jiang, H.M.; Zheng, H.C. BTG1 Overexpression Might Promote Invasion and Metastasis of Colorectal Cancer via Decreasing Adhesion and Inducing Epithelial-Mesenchymal Transition. Front. Oncol. 2020, 10, 598192. [Google Scholar] [CrossRef] [PubMed]
- Iwai, K.; Hirata, K.; Ishida, T.; Takeuchi, S.; Hirase, T.; Rikitake, Y.; Kojima, Y.; Inoue, N.; Kawashima, S.; Yokoyama, M. An anti-proliferative gene BTG1 regulates angiogenesis in vitro. Biochem. Biophys. Res. Commun. 2004, 316, 628–635. [Google Scholar] [CrossRef] [PubMed]
- Pickart, C.M. Mechanisms underlying ubiquitination. Annu. Rev. Biochem. 2001, 70, 503–533. [Google Scholar] [CrossRef]
- Ficazzola, M.A.; Fraiman, M.; Gitlin, J.; Woo, K.; Melamed, J.; Rubin, M.A.; Walden, P.D. Antiproliferative B cell translocation gene 2 protein is down-regulated post-transcriptionally as an early event in prostate carcinogenesis. Carcinogenesis 2001, 22, 1271–1279. [Google Scholar] [CrossRef]
- Yadav, D.; Lee, J.Y.; Puranik, N.; Chauhan, P.S.; Chavda, V.; Jin, J.O.; Lee, P.C.W. Modulating the Ubiquitin-Proteasome System: A Therapeutic Strategy for Autoimmune Diseases. Cells 2022, 11, 1093. [Google Scholar] [CrossRef]
- Yu, S.; Zhou, Y.; Niu, L.; Qiao, Y.; Yan, Y. Mesenchymal stem cell-derived exosome mir-342–3p inhibits metastasis and chemo-resistance of breast cancer through regulating ID4. Genes Genom. 2022, 44, 539–550. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Xie, Y.; Chen, W.; Li, T.; Chen, X.; Liu, B. microRNA-6785–5p-loaded human umbilical cord mesenchymal stem cells-derived exosomes suppress angiogenesis and metastasis in gastric cancer via INHBA. Life Sci. 2021, 284, 119222. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, G.; Yu, T.; He, J.; Liu, J.; Chai, X.; Zhao, G.; Yin, D.; Zhang, C. Exosomes deliver lncRNA DARS-AS1 siRNA to inhibit chronic unpredictable mild stress-induced TNBC metastasis. Cancer Lett. 2022, 543, 215781. [Google Scholar] [CrossRef]
- Ikeda, Y.; Taniguchi, K.; Nagase, N.; Tsuji, A.; Kitagishi, Y.; Matsuda, S. Reactive oxygen species may influence on the crossroads of stemness, senescence, and carcinogenesis in a cell via the roles of APRO family proteins. Explor. Med. 2021, 2, 443–454. [Google Scholar] [CrossRef]
- Weng, W.; Liu, N.; Toiyama, Y.; Kusunoki, M.; Nagasaka, T.; Fujiwara, T.; Wei, Q.; Qin, H.; Lin, H.; Ma, Y.; et al. Novel evidence for a PIWI-interacting RNA (piRNA) as an oncogenic mediator of disease progression, and a potential prognostic biomarker in colorectal cancer. Mol. Cancer. 2018, 17, 16. [Google Scholar] [CrossRef]
- Moyano, M.; Stefani, G. piRNA involvement in genome stability and human cancer. J. Hematol. Oncol. 2015, 8, 38. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Chen, G.Q.; Cao, G.M. Abnormal expression and mechanism of miR-330–3p/BTG1 axis in hepatocellular carcinoma. Eur. Rev. Med. Pharm. Sci. 2019, 23, 6888–6898. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ikeda, Y.; Taniguchi, K.; Sawamura, H.; Yoshikawa, S.; Tsuji, A.; Matsuda, S. Presumed Roles of APRO Family Proteins in Cancer Invasiveness. Cancers 2022, 14, 4931. https://doi.org/10.3390/cancers14194931
Ikeda Y, Taniguchi K, Sawamura H, Yoshikawa S, Tsuji A, Matsuda S. Presumed Roles of APRO Family Proteins in Cancer Invasiveness. Cancers. 2022; 14(19):4931. https://doi.org/10.3390/cancers14194931
Chicago/Turabian StyleIkeda, Yuka, Kurumi Taniguchi, Haruka Sawamura, Sayuri Yoshikawa, Ai Tsuji, and Satoru Matsuda. 2022. "Presumed Roles of APRO Family Proteins in Cancer Invasiveness" Cancers 14, no. 19: 4931. https://doi.org/10.3390/cancers14194931
APA StyleIkeda, Y., Taniguchi, K., Sawamura, H., Yoshikawa, S., Tsuji, A., & Matsuda, S. (2022). Presumed Roles of APRO Family Proteins in Cancer Invasiveness. Cancers, 14(19), 4931. https://doi.org/10.3390/cancers14194931




