TERT Promoter and BRAF V600E Mutations in Papillary Thyroid Cancer: A Single-Institution Experience in Korea
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
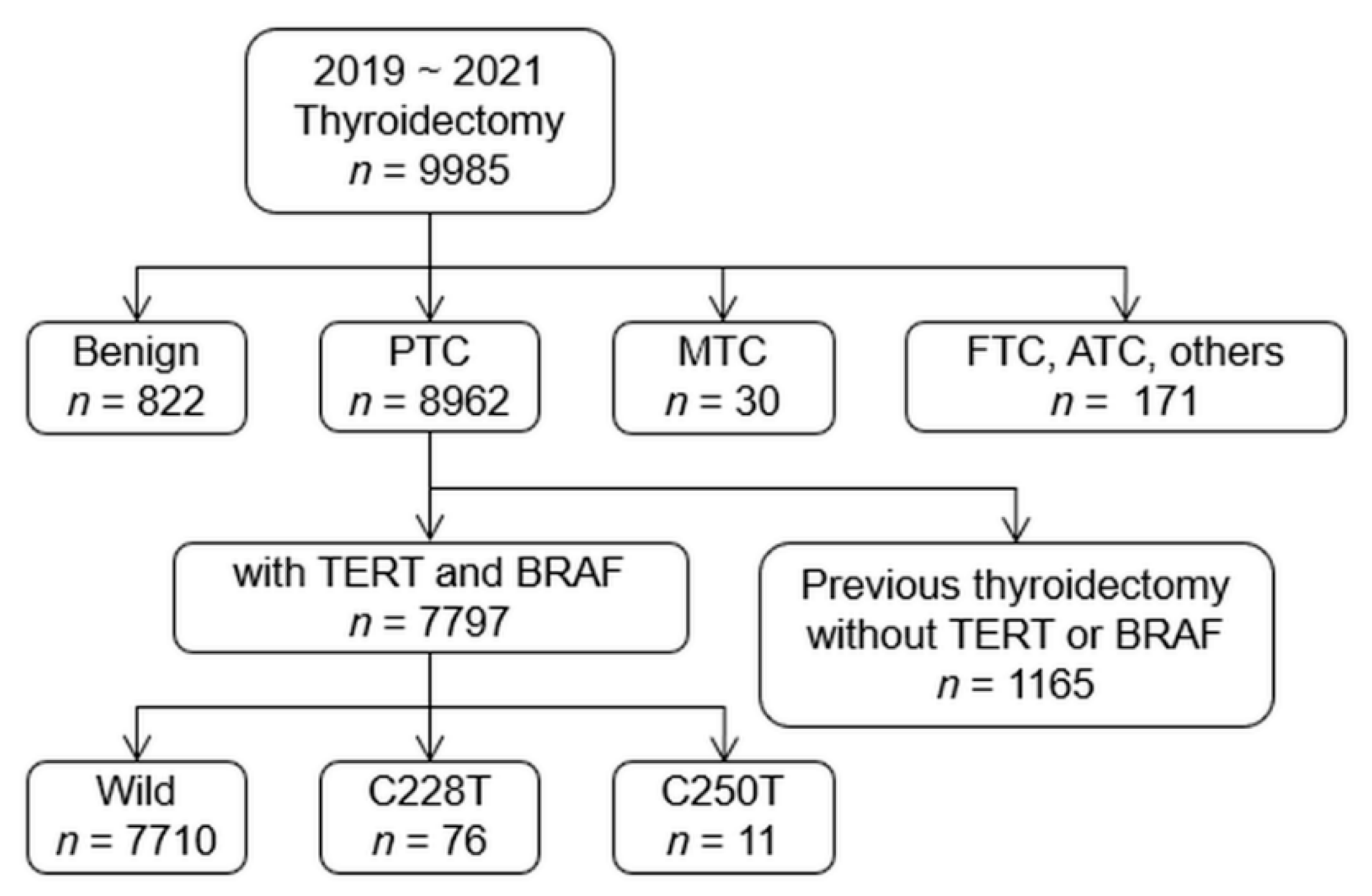
2.1. Patients
2.2. Histopathologic Diagnosis
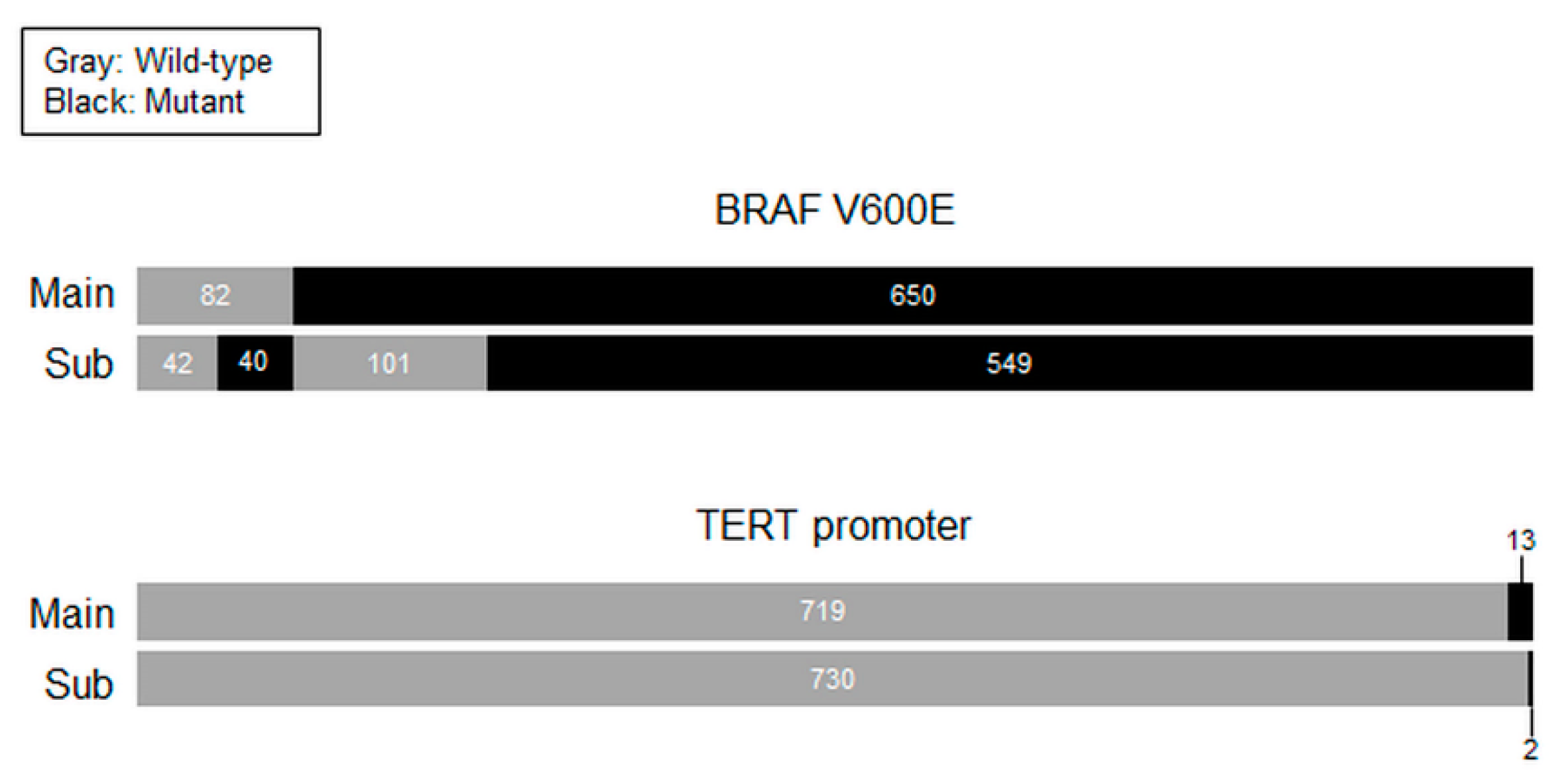
2.3. Mutational Analyses
2.4. Statistical Analyses
3. Results
3.1. Prevalence of TERT Promoter and BRAF V600E Mutations in PTC
3.2. Clinicopathologic Characteristics of PTCs with TERT Promoter Mutations
3.3. Clinicopathologic Characteristics of PTCs According to BRAF V600E and TERT Promoter Mutational Status
3.4. Clinical Significance of Coexisting BRAF V600E and TERT Promoter Mutations in PTC
3.5. Assessment of Oncologic Outcomes after Adjuvant RAI Therapy
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef]
- Ahn, H.S.; Kim, H.J.; Welch, H.G. Korea’s Thyroid-Cancer “Epidemic”—Screening and Overdiagnosis. N. Engl. J. Med. 2014, 371, 1765–1767. [Google Scholar] [CrossRef]
- Park, S.; Oh, C.-M.; Cho, H.; Lee, J.Y.; Jung, K.-W.; Jun, J.K.; Won, Y.-J.; Kong, H.-J.; Choi, K.S.; Lee, Y.J.; et al. Association between screening and the thyroid cancer “epidemic” in South Korea: Evidence from a nationwide study. BMJ 2016, 355, i5745. [Google Scholar] [CrossRef]
- Toniato, A.; Boschin, I.M.; Casara, D.; Mazzarotto, R.; Rubello, D.; Pelizzo, M. Papillary Thyroid Carcinoma: Factors Influencing Recurrence and Survival. Ann. Surg. Oncol. 2008, 15, 1518–1522. [Google Scholar] [CrossRef] [PubMed]
- Nixon, I.; Whitcher, M.M.; Palmer, F.L.; Tuttle, R.M.; Shaha, A.R.; Shah, J.; Patel, S.G.; Ganly, I. The Impact of Distant Metastases at Presentation on Prognosis in Patients with Differentiated Carcinoma of the Thyroid Gland. Thyroid 2012, 22, 884–889. [Google Scholar] [CrossRef]
- Coca-Pelaz, A.; Shah, J.P.; Hernandez-Prera, J.C.; Ghossein, R.A.; Rodrigo, J.P.; Hartl, D.M.; Olsen, K.D.; Shaha, A.R.; Zafereo, M.; Suarez, C.; et al. Papillary Thyroid Cancer—Aggressive Variants and Impact on Management: A Narrative Review. Adv. Ther. 2020, 37, 3112–3128. [Google Scholar] [CrossRef]
- Tuttle, R.M.; Alzahrani, A.S. Risk Stratification in Differentiated Thyroid Cancer: From Detection to Final Follow-Up. J. Clin. Endocrinol. Metab. 2019, 104, 4087–4100. [Google Scholar] [CrossRef]
- Tuttle, R.M.; Tala, H.; Shah, J.; Leboeuf, R.; Ghossein, R.; Gonen, M.; Brokhin, M.; Omry, G.; Fagin, J.A.; Shaha, A. Estimating Risk of Recurrence in Differentiated Thyroid Cancer After Total Thyroidectomy and Radioactive Iodine Remnant Ablation: Using Response to Therapy Variables to Modify the Initial Risk Estimates Predicted by the New American Thyroid Association Staging System. Thyroid 2010, 20, 1341–1349. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Wang, L.; Jia, X.; Hu, X.; Pang, P.; Zhao, S.; Wang, Y.; Zhang, Y.; Lyu, Z. The Coexistence of Genetic Mutations in Thyroid Carcinoma Predicts Histopathological Factors Associated With a Poor Prognosis: A Systematic Review and Network Meta-Analysis. Front. Oncol. 2020, 10, 540238. [Google Scholar] [CrossRef] [PubMed]
- Romei, C.; Elisei, R. A Narrative Review of Genetic Alterations in Primary Thyroid Epithelial Cancer. Int. J. Mol. Sci. 2021, 22, 1726. [Google Scholar] [CrossRef]
- Xing, M.; Alzahrani, A.S.; Carson, K.A.; Viola, D.; Elisei, R.; Bendlova, B.; Yip, L.; Mian, C.; Vianello, F.; Tuttle, R.M.; et al. Association Between BRAF V600E Mutation and Mortality in Patients With Papillary Thyroid Cancer. JAMA 2013, 309, 1493–1501. [Google Scholar] [CrossRef]
- Enumah, S.; Fingeret, A.; Parangi, S.; Dias-Santagata, D.; Sadow, P.M.; Lubitz, C.C. BRAFV600E Mutation is Associated with an Increased Risk of Papillary Thyroid Cancer Recurrence. World J. Surg. 2020, 44, 2685–2691. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, S.-Z.; Guan, Y.-X.; Chen, Q.-J.; Zhu, Q.-Y. Meta-Analyses of Association Between BRAFV600E Mutation and Clinicopathological Features of Papillary Thyroid Carcinoma. Cell. Physiol. Biochem. 2016, 38, 763–776. [Google Scholar] [CrossRef]
- Hong, A.R.; Lim, J.A.; Kim, T.H.; Choi, H.S.; Yoo, W.S.; Min, H.S.; Won, J.K.; Lee, K.E.; Jung, K.C.; Park, D.J.; et al. The Frequency and Clinical Implications of the BRAFV600E Mutation in Papillary Thyroid Cancer Patients in Korea Over the Past Two Decades. Endocrinol. Metab. 2014, 29, 505–513. [Google Scholar] [CrossRef]
- Ahn, D.; Park, J.S.; Sohn, J.H.; Kim, J.H.; Park, S.-K.; Na Seo, A.; Park, J.Y. BRAFV600E mutation does not serve as a prognostic factor in Korean patients with papillary thyroid carcinoma. Auris Nasus Larynx 2012, 39, 198–203. [Google Scholar] [CrossRef]
- Kim, T.Y.; Kim, W.B.; Rhee, Y.S.; Song, J.Y.; Kim, J.M.; Gong, G.; Lee, S.; Kim, S.Y.; Kim, S.C.; Hong, S.J. The BRAF mutation is useful for prediction of clinical recurrence in low-risk patients with conventional papillary thyroid carcinoma. Clin. Endocrinol. 2006, 65, 364–368. [Google Scholar] [CrossRef]
- Günes, C.; Rudolph, K.L. The Role of Telomeres in Stem Cells and Cancer. Cell 2013, 152, 390–393. [Google Scholar] [CrossRef] [PubMed]
- Kyo, S.; Takakura, M.; Fujiwara, T.; Inoue, M. Understanding and exploiting hTERT promoter regulation for diagnosis and treatment of human cancers. Cancer Sci. 2008, 99, 1528–1538. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.-S.; Wang, Z.; He, X.-J.; Diplas, B.H.; Yang, R.; Killela, P.J.; Meng, Q.; Ye, Z.-Y.; Wang, W.; Jiang, X.-T.; et al. Recurrent TERT promoter mutations identified in a large-scale study of multiple tumour types are associated with increased TERT expression and telomerase activation. Eur. J. Cancer 2015, 51, 969–976. [Google Scholar] [CrossRef]
- Vinagre, J.; Almeida, A.; Pópulo, H.; Batista, R.; Lyra, J.; Pinto, V.; Coelho, R.; Celestino, R.; Prazeres, H.; Lima, L.; et al. Frequency of TERT promoter mutations in human cancers. Nat. Commun. 2013, 4, 2185. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Bishop, J.; Shan, Y.; Pai, S.; Liu, D.; Murugan, A.K.; Sun, H.; El-Naggar, A.K.; Xing, M. Highly prevalent TERT promoter mutations in aggressive thyroid cancers. Endocrine-Related Cancer 2013, 20, 603–610. [Google Scholar] [CrossRef]
- Alzahrani, A.S.; Alsaadi, R.; Murugan, A.K.; Bin Sadiq, B. TERT Promoter Mutations in Thyroid Cancer. Horm. Cancer 2016, 7, 165–177. [Google Scholar] [CrossRef]
- Kim, T.H.; Kim, Y.-E.; Ahn, S.; Kim, J.-Y.; Ki, C.-S.; Oh, Y.L.; Kim, K.; Yun, J.W.; Park, W.-Y.; Choe, J.-H.; et al. TERT promoter mutations and long-term survival in patients with thyroid cancer. Endocr. Relat. Cancer 2016, 23, 813–823. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Liu, Z.; Chen, T.; Zeng, W.; Guo, Y.; Huang, T. TERT promoter Mutation and Its Association with Clinicopathological Features and Prognosis of Papillary Thyroid Cancer: A Meta-analysis. Sci. Rep. 2016, 6, 36990. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.-P.; Lai, H.-F.; Kuo, C.-Y. Significance of telomerase reverse-transcriptase promoter mutations in differentiated thyroid cancer. Formos. J. Surg. 2021, 54, 171. [Google Scholar] [CrossRef]
- Liu, R.; Xing, M. TERT promoter mutations in thyroid cancer. Endocr. Relat. Cancer 2016, 23, R143–R155. [Google Scholar] [CrossRef]
- Jin, L.; Chen, E.; Dong, S.; Cai, Y.; Zhang, X.; Zhou, Y.; Zeng, R.; Yang, F.; Pan, C.; Liu, Y.; et al. BRAF and TERT promoter mutations in the aggressiveness of papillary thyroid carcinoma: A study of 653 patients. Oncotarget 2016, 7, 18346–18355. [Google Scholar] [CrossRef]
- Liu, R.; Bishop, J.; Zhu, G.; Zhang, T.; Ladenson, P.W.; Xing, M. Mortality Risk Stratification by Combining BRAF V600E and TERT Promoter Mutations in Papillary Thyroid Cancer: Genetic Duet of BRAF and TERT Promoter Mutations in Thyroid Cancer Mortality. JAMA Oncol. 2017, 3, 202–208. [Google Scholar] [CrossRef]
- Song, Y.S.; Lim, J.A.; Choi, H.; Won, J.K.; Moon, J.H.; Cho, S.W.; Lee, K.E.; Park, Y.J.; Yi, K.H.; Park, D.J.; et al. Prognostic effects of TERT promoter mutations are enhanced by coexistence with BRAF or RAS mutations and strengthen the risk prediction by the ATA or TNM staging system in differentiated thyroid cancer patients. Cancer 2016, 122, 1370–1379. [Google Scholar] [CrossRef]
- Xing, M.; Liu, R.; Liu, X.; Murugan, A.K.; Zhu, G.; Zeiger, M.A.; Pai, S.; Bishop, J. BRAF V600E and TERT Promoter Mutations Cooperatively Identify the Most Aggressive Papillary Thyroid Cancer With Highest Recurrence. J. Clin. Oncol. 2014, 32, 2718–2726. [Google Scholar] [CrossRef]
- Kim, M.J.; Lee, S.G.; Kim, K.; Lee, C.R.; Kang, S.W.; Lee, J.; Nam, K.H.; Chung, W.Y.; Jeong, J.J. Current trends in the features of male thyroid cancer: Retrospective evaluation of their prognostic value. Medicine 2019, 98, e15559. [Google Scholar] [CrossRef]
- Lloyd, R.; Osamura, R.; Klöppel, G.; Rosai, J. WHO Classification of Tumours of Endocrine Organs, 4th ed.; IARC Press: Lyon, France, 2017. [Google Scholar]
- Amin, M.B.; Greene, F.L.; Edge, S.B.; Compton, C.C.; Gershenwald, J.E.; Brookland, R.K.; Meyer, L.; Gress, D.M.; Byrd, D.R.; Winchester, D.P. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J. Clin. 2017, 67, 93–99. [Google Scholar] [CrossRef]
- Tuttle, R.M.; Haugen, B.; Perrier, N.D. Updated American Joint Committee on Cancer/Tumor-Node-Metastasis Staging System for Differentiated and Anaplastic Thyroid Cancer (Eighth Edition): What Changed and Why? Thyroid 2017, 27, 751–756. [Google Scholar] [CrossRef]
- Yoon, J.; Lee, E.; Koo, J.S.; Yoon, J.H.; Nam, K.H.; Lee, J.; Jo, Y.S.; Moon, H.J.; Park, V.Y.; Kwak, J.Y. Artificial intelligence to predict the BRAFV600E mutation in patients with thyroid cancer. PLoS ONE 2020, 15, e0242806. [Google Scholar] [CrossRef]
- Lee, S.E.; Hwang, T.S.; Choi, Y.L.; Kim, W.Y.; Han, H.S.; Lim, S.D.; Kim, W.S.; Yoo, Y.B.; Kim, S.K. Molecular Profiling of Papillary Thyroid Carcinoma in Korea with a High Prevalence of BRAF(V600E) Mutation. Thyroid 2017, 27, 802–810. [Google Scholar] [CrossRef]
- Huang, M.; Yan, C.; Xiao, J.; Wang, T.; Ling, R. Relevance and clinicopathologic relationship of BRAF V600E, TERT and NRAS mutations for papillary thyroid carcinoma patients in Northwest China. Diagn. Pathol. 2019, 14, 74. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Kim, T.; Kim, K.; Bae, J.S.; Kim, J.S.; Jung, C.K. Highly prevalent BRAF V600E and low-frequency TERT promoter mutations underlie papillary thyroid carcinoma in Koreans. J. Pathol. Transl. Med. 2020, 54, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Gong, Y.; Yan, S.; Chen, H.; Qin, S.; Gong, R. Association between TERT promoter mutations and clinical behaviors in differentiated thyroid carcinoma: A systematic review and meta-analysis. Endocrine 2020, 67, 44–57. [Google Scholar] [CrossRef]
- Song, Y.S.; Yoo, S.K.; Kim, H.H.; Jung, G.; Oh, A.R.; Cha, J.Y.; Kim, S.J.; Cho, S.W.; Lee, K.E.; Seo, J.S.; et al. Interaction of BRAF-induced ETS factors with mutant TERT promoter in papillary thyroid cancer. Endocr. Relat. Cancer 2019, 26, 629–641. [Google Scholar] [CrossRef]
- Yang, X.; Li, J.; Li, X.; Liang, Z.; Gao, W.; Liang, J.; Cheng, S.; Lin, Y. TERT Promoter Mutation Predicts Radioiodine-Refractory Character in Distant Metastatic Differentiated Thyroid Cancer. J. Nucl. Med. 2017, 58, 258–265. [Google Scholar] [CrossRef]
- Yang, H.; Park, H.; Ryu, H.J.; Heo, J.; Kim, J.-S.; Oh, Y.L.; Choe, J.-H.; Kim, J.H.; Kim, J.S.; Jang, H.W.; et al. Frequency of TERT Promoter Mutations in Real-World Analysis of 2,092 Thyroid Carcinoma Patients. Endocrinol. Metab. 2022, 37, 652–663. [Google Scholar] [CrossRef]
| Variables | Wild-Type TERT | TERT Promoter Mutation C228T/C250T | Total |
|---|---|---|---|
| Wild-type BRAF | 1234 (15.8) | 16/1 (0.2) | 1251 (16.0) |
| BRAF V600E | 6476 (83.1) | 60/10 (0.9) | 6546 (84.0) |
| Total | 7710 (89.9) | 76/11 (1.1) | 7797 (100.0) |
| Total PTC | PTC > 1 cm | |||||
|---|---|---|---|---|---|---|
| Variables | Wild-Type TERT | pTERT Mutation | p-Value | Wild-Type TERT | pTERT Mutation | p-Value |
| Number of cases (%) | 7710 (98.9) | 87 (1.1) | 2134 (96.8) | 71 (3.2) | ||
| Age (year) | 43.04 ± 12.03 | 62.18 ± 12.17 | <0.001 | 41.72 ± 12.96 | 63.04 ± 12.55 | <0.001 |
| ≥55 | 1481 (19.2) | 66 (75.9) | <0.001 | 383 (17.9) | 54 (76.1) | <0.001 |
| Sex, Male | 1765 (22.9) | 39 (44.8) | <0.001 | 591 (27.7) | 34 (47.9) | <0.001 |
| Tumor size (cm) | 0.90 ± 0.67 | 1.95 ± 1.28 | <0.001 | 1.69 ± 0.79 | 2.25 ± 1.24 | <0.001 |
| >1.0 | 2134 (27.7) | 71 (81.6) | <0.001 | - | - | - |
| Multifocality | 2537 (32.9) | 45 (51.7) | <0.001 | 877 (41.1) | 39 (54.9) | 0.020 |
| Extrathyroidal extension | 692 (9.0) | 43 (49.4) | <0.001 | 515 (24.1) | 40 (56.3) | <0.001 |
| Histology | ||||||
| Classic | 7109 (92.2) | 73 (83.9) | 0.020 | 1875 (87.9) | 58 (81.7) | 0.184 |
| Follicular variant | 396 (5.1) | 9 (10.3) | 179 (8.4) | 8 (11.3) | ||
| Tall-cell variant | 76 (1.0) | 3 (3.4) | 19 (0.9) | 3 (4.2) | ||
| Others | 129 (1.7) | 2 (2.3) | 61 (2.9) | 2 (2.8) | ||
| Histology, Variants | 601 (7.8) | 14 (16.1) | 0.004 | 259 (12.1) | 13 (18.3) | 0.120 |
| BRAF V600E mutation | 6476 (84.0) | 70 (80.5) | 0.372 | 1669 (78.2) | 55 (77.5) | 0.881 |
| Perinodal infiltration | 701 (9.1) | 30 (34.5) | <0.001 | 412 (19.3) | 26 (36.6) | <0.001 |
| T stage | ||||||
| T1 | 6760 (87.7) | 34 (39.1) | <0.001 | 1361 (63.8) | 21 (29.6) | <0.001 |
| T2 | 234 (3.0) | 8 (9.2) | 234 (11.0) | 8 (11.3) | ||
| T3 | 501 (6.5) | 27 (31.0) | 390 (18.3) | 27 (38.0) | ||
| T4 | 215 (2.8) | 18 (20.7) | 149 (7.0) | 15 (21.1) | ||
| T stage, T3–T4 | 716 (9.3) | 45 (51.7) | <0.001 | 539 (25.3) | 42 (59.2) | <0.001 |
| N stage | ||||||
| N0 | 4275 (55.4) | 36 (41.4) | <0.001 | 747 (35.0) | 28 (39.4) | 0.749 |
| N1a | 2687 (34.9) | 29 (33.3) | 912 (42.7) | 22 (31.0) | ||
| N1b | 748 (9.7) | 22 (25.3) | 475 (22.3) | 21 (29.6) | ||
| N stage, N1 | 3435 (44.6) | 51 (58.6) | 0.009 | 1387 (65.0) | 43 (60.6) | 0.442 |
| M stage, M1 | 15 (0.2) | 8 (9.2) | <0.001 | 12 (0.6) | 7 (9.9) | <0.001 |
| TNM stage | ||||||
| I | 6535 (84.8) | 26 (29.9) | <0.001 | 1661 (77.8) | 19 (26.8) | <0.001 |
| II | 1124 (14.6) | 40 (46.0) | 439 (20.6) | 34 (47.9) | ||
| III | 46 (0.6) | 13 (14.9) | 30 (1.4) | 11 (15.5) | ||
| IV | 5 (0.1) | 8 (9.2) | 4 (0.2) | 7 (9.9) | ||
| TNM stage, III–IV | 51 (0.7) | 21 (24.1) | <0.001 | 34 (1.6) | 18 (25.4) | <0.001 |
| RAI therapy | 1432 (18.6) | 54 (62.1) | <0.001 | 824 (38.6) | 49 (69.0) | <0.001 |
| Total PTC | PTC > 1 cm | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variables | No Mutation | BRAF V600E Mutation Only | p- Value | pTERT Mutation Only | p- Value | BRAF+ pTERT Mutations | p- Value | No Mutation | BRAF V600E Mutation Only | p- Value | pTERT Mutation Only | p- Value | BRAF+ pTERT Mutations | p- Value |
| No. of cases | 1234 (15.8) | 6476 (83.1) | 17 (0.2) | 70 (0.9) | 465 (21.1) | 1669 (75.7) | 16 (0.7) | 55 (2.5) | ||||||
| Age (year) | 42.52 ± 12.56 | 43.14 ± 11.93 | 0.113 | 58.18 ± 14.22 | <0.001 | 63.16 ± 11.52 | <0.001 | 39.67 ± 12.71 | 42.29 ± 12.97 | <0.001 | 58.75 ± 14.48 | <0.001 | 64.29 ± 11.79 | <0.001 |
| ≥55 | 240 (19.4) | 1241 (19.2) | 0.815 | 9 (52.9) | 0.001 | 57 (81.4) | <0.001 | 64 (13.8) | 319 (19.1) | 0.008 | 9 (56.3) | <0.001 | 45 (81.8) | <0.001 |
| Sex, Male | 228 (18.5) | 1537 (23.7) | <0.001 | 4 (23.5) | 0.573 | 35 (50.0) | <0.001 | 108 (23.2) | 483 (28.9) | 0.015 | 4 (25.0) | 0.772 | 30 (54.5) | <0.001 |
| Tumor size (cm) | 1.09 ± 0.95 | 0.86 ± 0.59 | <0.001 | 2.92 ± 1.98 | 0.002 | 1.72 ± 0.93 | <0.001 | 1.95 ± 1.05 | 1.62 ± 0.68 | <0.001 | 3.06 ± 1.95 | 0.039 | 2.01 ± 0.83 | 0.666 |
| >1.0 | 465 (37.7) | 1669 (25.8) | <0.001 | 16 (94.1) | <0.001 | 55 (78.6) | <0.001 | - | - | - | - | - | ||
| Multiplicity | 384 (31.1) | 2153 (33.2) | 0.145 | 13 (76.5) | <0.001 | 32 (45.7) | 0.011 | 179 (38.5) | 698 (41.8) | 0.197 | 12 (75.0) | 0.007 | 27 (49.1) | 0.129 |
| Extrathyroidal extension | 95 (7.7) | 597 (9.2) | 0.087 | 6 (35.3) | <0.001 | 37 (52.9) | <0.001 | 80 (17.2) | 435 (26.1) | <0.001 | 6 (37.5) | 0.037 | 34 (61.8) | <0.001 |
| Histology | ||||||||||||||
| Classic | 945 (76.6) | 6164 (95.2) | <0.001 | 7 (41.2) | 0.043 | 66 (94.3) | 0.018 | 300 (64.5) | 1575 (94.4) | <0.001 | 7 (43.8) | 0.485 | 51 (92.7) | 0.003 |
| Follicular variant | 223 (18.1) | 173 (2.7) | 9 (52.9) | 0 (0.0) | 124 (26.7) | 55 (3.3) | 8 (50.0) | 0 (0.0) | ||||||
| Tall-cell variant | 1 (0.1) | 75 (1.2) | 0 (0.0) | 3 (4.3) | 0 (0.0) | 19 (1.1) | 0 (0.0) | 3 (5.5) | ||||||
| Others | 65 (5.3) | 64 (1.0) | <0.001 | 1 (5.9) | 0.001 | 1 (1.4) | <0.001 | 41 (8.8) | 20 (1.2) | 1 (6.3) | 1 (1.8) | |||
| Histology, Variants | 289 (23.4) | 312 (4.8) | 10 (58.8) | 4 (5.7) | 165 (35.5) | 94 (5.6) | <0.001 | 9 (56.3) | 0.089 | 4 (7.3) | <0.001 | |||
| Perinodal infiltration | 138 (11.2) | 563 (8.7) | 0.005 | 2 (11.8) | 1.000 | 28 (40.0) | <0.001 | 100 (21.5) | 312 (18.7) | 0.174 | 2 (12.5) | 0.541 | 24 (43.6) | <0.001 |
| T stage | ||||||||||||||
| T1 | 1028 (83.3) | 5732 (88.5) | <0.001 | 6 (35.3) | <0.001 | 28 (40.0) | <0.001 | 274 (58.9) | 1087 (65.1) | 0.860 | 5 (31.3) | 0.008 | 16 (29.1) | <0.001 |
| T2 | 93 (7.5) | 141 (2.2) | 3 (17.6) | 5 (7.1) | 93 (20.0) | 141 (8.4) | 3 (18.8) | 5 (9.1) | ||||||
| T3 | 82 (6.6) | 419 (6.5) | 6 (35.3) | 21 (30.0) | 72 (15.5) | 318 (19.1) | 6 (37.5) | 21 (38.2) | ||||||
| T4 | 31 (2.5) | 184 (2.8) | 2 (11.8) | 16 (22.9) | 26 (5.6) | 123 (7.4) | 2 (12.5) | 13 (23.6) | ||||||
| T stage, T3–T4 | 113 (9.2) | 603 (9.3) | 0.864 | 8 (47.1) | <0.001 | 37 (52.9) | <0.001 | 98 (21.1) | 441 (26.4) | 0.019 | 8 (50.0) | 0.006 | 34 (61.8) | <0.001 |
| N stage | ||||||||||||||
| N0 | 726 (58.8) | 3549 (54.8) | <0.001 | 10 (58.8) | 0.416 | 26 (37.1) | 0.001 | 201 (43.2) | 546 (32.7) | 0.687 | 10 (62.5) | 0.421 | 18 (32.7) | 0.385 |
| N1a | 326 (26.4) | 2361 (36.5) | 2 (11.8) | 27 (38.6) | 128 (27.5) | 784 (47.0) | 1 (6.3) | 21 (38.2) | ||||||
| N1b | 182 (14.7) | 566 (8.7) | 5 (29.4) | 17 (24.3) | 136 (29.2) | 339 (20.3) | 5 (31.3) | 16 (29.1) | ||||||
| N stage, N1 | 508 (41.2) | 2927 (45.2) | 0.009 | 7 (41.2) | 0.999 | 44 (62.9) | <0.001 | 264 (56.8) | 1123 (67.3) | <0.001 | 6 (37.5) | 0.127 | 37 (67.3) | 0.136 |
| M stage | ||||||||||||||
| M0 | 1225 (99.3) | 6470 (99.9) | <0.001 | 13 (76.5) | <0.001 | 66 (94.3) | 0.004 | 456 (98.1) | 1666 (99.8) | <0.001 | 12 (75.0) | <0.001 | 52 (94.5) | 0.124 |
| M1 | 9 (0.7) | 6 (0.1) | 4 (23.5) | 4 (5.7) | 9 (1.9) | 3 (0.2) | 4 (25.0) | 3 (5.5) | ||||||
| TNM stage | ||||||||||||||
| I | 1059 (85.8) | 5476 (84.6) | 0.435 | 6 (35.3) | <0.001 | 20 (28.6) | <0.001 | 383 (82.4) | 1278 (76.6) | 0.005 | 6 (37.5) | <0.001 | 13 (23.6) | <0.001 |
| II | 170 (13.8) | 954 (14.7) | 5 (29.4) | 35 (50.0) | 79 (17.0) | 360 (21.6) | 4 (25.0) | 30 (54.5) | ||||||
| III | 4 (0.3) | 42 (0.6) | 2 (11.8) | 11 (15.7) | 2 (0.4) | 28 (1.7) | 2 (12.5) | 9 (16.4) | ||||||
| IV | 1 (0.1) | 4 (0.1) | 4 (23.5) | 4 (5.7) | 1 (0.2) | 3 (0.2) | 4 (25.0) | 3 (5.5) | ||||||
| TNM stage, III–IV | 5 (0.4) | 46 (0.7) | 0.226 | 6 (35.3) | <0.001 | 15 (21.4) | <0.001 | 3 (0.6) | 31 (1.9) | 0.091 | 6 (37.5) | <0.001 | 12 (21.8) | <0.001 |
| RAI therapy | 250 (20.3) | 1182 (18.3) | 0.097 | 12 (70.6) | <0.001 | 42 (60.0) | <0.001 | 167 (35.9) | 657 (39.4) | 0.176 | 12 (75.0) | 0.003 | 37 (67.3) | <0.001 |
| Total PTC | PTC >1 cm | |||||||
|---|---|---|---|---|---|---|---|---|
| Variables | No Mutation | BRAF Mutation Only | BRAF + pTERT Mutations | p-Value | No Mutation | BRAF Mutation Only | BRAF + pTERT Mutations | p-Value |
| No. of cases | 1234 (15.9) | 6476 (83.2) | 70 (0.9) | 465 (21.2) | 1669 (76.2) | 55 (2.5) | ||
| Age (year) | 42.52 ± 12.56 | 43.14 ± 11.93 | 63.16 ± 11.52 *, # | <0.001 | 39.67 ± 12.71 | 42.29 ± 12.97 * | 64.29 ± 11.79 *, # | <0.001 |
| ≥55 | 240 (19.4) | 1241 (19.2) | 57 (81.4) *, # | <0.001 | 64 (13.8) | 319 (19.1) * | 45 (81.8) *, # | <0.001 |
| Sex, Male | 228 (18.5) | 1537 (18.5) * | 35 (50.0) *, # | <0.001 | 108 (23.2) | 483 (28.9) * | 30 (54.5) *, # | <0.001 |
| Tumor size (cm) | 1.09 ± 0.95 | 0.86 ± 0.59 * | 1.72 ± 0.93 *, # | <0.001 | 1.95 ± 1.05 | 1.62 ± 0.68 * | 2.01 ± 0.83 # | <0.001 |
| >1.0 | 465 (37.7) | 1669 (25.8) * | 55 (78.6) *, # | 0.004 | - | - | - | - |
| Multifocality | 384 (31.1) | 2153 (33.2) | 32 (45.7) *, # | 0.017 | 179 (38.5) | 698 (41.8) | 27 (49.1) | 0.080 |
| Extrathyroidal extension | 80 (17.2) | 435 (26.1) | 37 (52.9) *, # | <0.001 | 80 (17.2) | 435 (26.1) | 34 (61.8) *, # | <0.001 |
| Histology | ||||||||
| Classic | 945 (76.6) | 6164 (95.2) * | 66 (94.3) * | <0.001 | 300 (64.5) | 1575 (94.4) * | 51 (92.7) * | <0.001 |
| Follicular variant | 223 (18.1) | 173 (2.7) | 0 (0.0) | 124 (26.7) | 55 (3.3) | 0 (0.0) | ||
| Tall-cell variant | 1 (0.1) | 75 (1.2) | 3 (4.3) | 0 (0.0) | 19 (1.1) | 3 (5.5) | ||
| Others | 65 (5.3) | 64 (1.0) | 1 (1.4) | 41 (8.8) | 20 (1.2) | 1 (1.8) | ||
| Histology, Variants | 289 (23.4) | 312 (4.8) * | 4 (5.7) * | <0.001 | 165 (35.5) | 94 (5.6) * | 4 (7.3) * | <0.001 |
| Perinodal infiltration | 138 (11.2) | 563 (8.7) * | 28 (40.0) *, # | 0.056 | 100 (21.5) | 312 (18.7) | 24 (43.6) *, # | 0.062 |
| T stage | ||||||||
| T1 | 1028 (83.3) | 5732 (88.5) * | 28 (40.0) *, # | <0.001 | 274 (58.9) | 1087 (65.1) * | 16 (29.1) *, # | <0.001 |
| T2 | 93 (7.5) | 141 (2.2) | 5 (7.1) | 93 (20.0) | 141 (8.4) | 5 (9.1) | ||
| T3 | 82 (6.6) | 419 (6.5) | 21 (30.0) | 72 (15.5) | 318 (19.1) | 21 (38.2) | ||
| T4 | 31 (2.5) | 184 (2.8) | 16 (22.9) | 26 (5.6) | 123 (7.4) | 13 (23.6) | ||
| T stage, T3-T4 | 113 (9.2) | 603 (9.3) | 37 (52.9) *, # | <0.001 | 98 (21.1) | 441 (26.4) * | 34 (61.8) *, # | <0.001 |
| N stage | ||||||||
| N0 | 726 (58.8) | 3549 (54.8) * | 26 (37.1) *, # | 0.242 | 201 (43.2) | 546 (32.7) * | 18 (32.7) | 0.376 |
| N1a | 326 (26.4) | 2361 (36.5) | 27 (38.6) | 128 (27.5) | 784 (47.0) | 21 (38.2) | ||
| N1b | 182 (14.7) | 566 (8.7) | 17 (24.3) | 136 (29.2) | 339 (20.3) | 16 (29.1) | ||
| N stage, N1 | 508 (41.2) | 2927 (45.2) * | 44 (62.9) *, # | <0.001 | 264 (56.8) | 1123 (67.3) * | 37 (67.3) * | 0.001 |
| M stage | ||||||||
| M0 | 1225 (99.3) | 6470 (99.9) * | 66 (94.3) *, # | 0.358 | 456 (98.1) | 1666 (99.8) * | 52 (94.5) # | 0.784 |
| M1 | 9 (0.7) | 6 (0.1) | 4 (5.7) | 9 (1.9) | 3 (0.2) | 3 (5.5) | ||
| TNM stage | ||||||||
| I | 1059 (85.8) | 5476 (84.6) | 20 (28.6) *, # | <0.001 | 383 (82.4) | 1278 (76.6) * | 13 (23.6) *, # | <0.001 |
| II | 170 (13.8) | 954 (14.7) | 35 (50.0) | 79 (17.0) | 360 (21.6) | 30 (54.5) | ||
| III | 4 (0.3) | 42 (0.6) | 11 (15.7) | 2 (0.4) | 28 (1.7) | 9 (16.4) | ||
| IV | 1 (0.1) | 4 (0.1) | 4 (5.7) | 1 (0.2) | 3 (0.2) | 3 (5.5) | ||
| TNM stage, III–IV | 5 (0.4) | 46 (0.7) | 15 (21.4) *, # | <0.001 | 3 (0.6) | 31 (1.9) | 12 (21.8) *, # | <0.001 |
| RAI therapy | 250 (20.3) | 1182 (18.3) | 42 (60.0) *, # | 0.004 | 167 (35.9) | 657 (39.4) | 37 (67.3) *, # | <0.001 |
| Total PTC | PTC > 1 cm | |||||
|---|---|---|---|---|---|---|
| Variables | Wild-Type TERT | pTERT Mutation | p-Value | Wild-Type TERT | pTERT Mutation | p-Value |
| Number of adjuvant RAI cases | 1432 (96.4) | 54 (3.6) | 824 (94.4) | 49 (5.6) | ||
| TSH level before RAI (uIU/mL) | 75.50 ± 42.51 | 77.47 ± 66.65 | 0.830 | 74.07 ± 43.85 | 79.49 ± 69.34 | 0.591 |
| Stimulated Tg before RAI (mg/mL) | 15.75 ± 87.19 | 422.74 ± 1247.07 | 0.020 | 23.06 ± 110.95 | 446.77 ± 1302.63 | 0.027 |
| Tg Ab before RAI (IU/mL) | 60.78 ± 256.97 | 88.21 ± 287.04 | 0.443 | 63.12 ± 279.15 | 89.28 ± 297.97 | 0.526 |
| Results of post-therapeutic WBS | ||||||
| Thyroid bed only uptake | 1318 (92.0) | 43 (79.6) | 0.001 | 740 (89.8) | 39 (79.6) | 0.025 |
| Cervical LN or extracervical uptake | 114 (8.0) | 11 (20.4) | 84 (10.2) | 10 (20.4) | ||
| Number of cases at 6 months after RAI | 1076 (72.4) | 40 (2.7) | 605 (69.3) | 35 (4.0) | ||
| TSH (uIU/mL) | 1.02 ± 6.96 | 0.32 ± 0.79 | 0.522 | 1.12 ± 7.60 | 0.35 ± 0.84 | 0.552 |
| Unstimulated Tg (mg/mL) | 1.10 ± 11.912 | 80.14 ± 291.90 | 0.095 | 1.70 ± 15.77 | 86.12 ± 310.80 | 0.117 |
| Tg Ab (IU/mL) | 42.11 ± 200.81 | 18.79 ± 42.55 | 0.463 | 52.97 ± 261.33 | 17.42 ± 42.92 | 0.422 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, M.J.; Kim, J.K.; Kim, G.J.; Kang, S.-W.; Lee, J.; Jeong, J.J.; Chung, W.Y.; Kim, D.; Nam, K.-H. TERT Promoter and BRAF V600E Mutations in Papillary Thyroid Cancer: A Single-Institution Experience in Korea. Cancers 2022, 14, 4928. https://doi.org/10.3390/cancers14194928
Kim MJ, Kim JK, Kim GJ, Kang S-W, Lee J, Jeong JJ, Chung WY, Kim D, Nam K-H. TERT Promoter and BRAF V600E Mutations in Papillary Thyroid Cancer: A Single-Institution Experience in Korea. Cancers. 2022; 14(19):4928. https://doi.org/10.3390/cancers14194928
Chicago/Turabian StyleKim, Min Jhi, Jin Kyong Kim, Gi Jeong Kim, Sang-Wook Kang, Jandee Lee, Jong Ju Jeong, Woong Youn Chung, Daham Kim, and Kee-Hyun Nam. 2022. "TERT Promoter and BRAF V600E Mutations in Papillary Thyroid Cancer: A Single-Institution Experience in Korea" Cancers 14, no. 19: 4928. https://doi.org/10.3390/cancers14194928
APA StyleKim, M. J., Kim, J. K., Kim, G. J., Kang, S.-W., Lee, J., Jeong, J. J., Chung, W. Y., Kim, D., & Nam, K.-H. (2022). TERT Promoter and BRAF V600E Mutations in Papillary Thyroid Cancer: A Single-Institution Experience in Korea. Cancers, 14(19), 4928. https://doi.org/10.3390/cancers14194928





