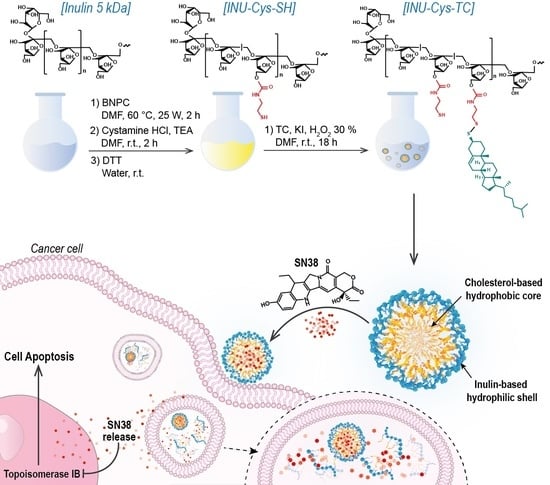
Cholesterol-Inulin Conjugates for Efficient SN38 Nuclear Delivery: Nanomedicines for Precision Cancer Therapy
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
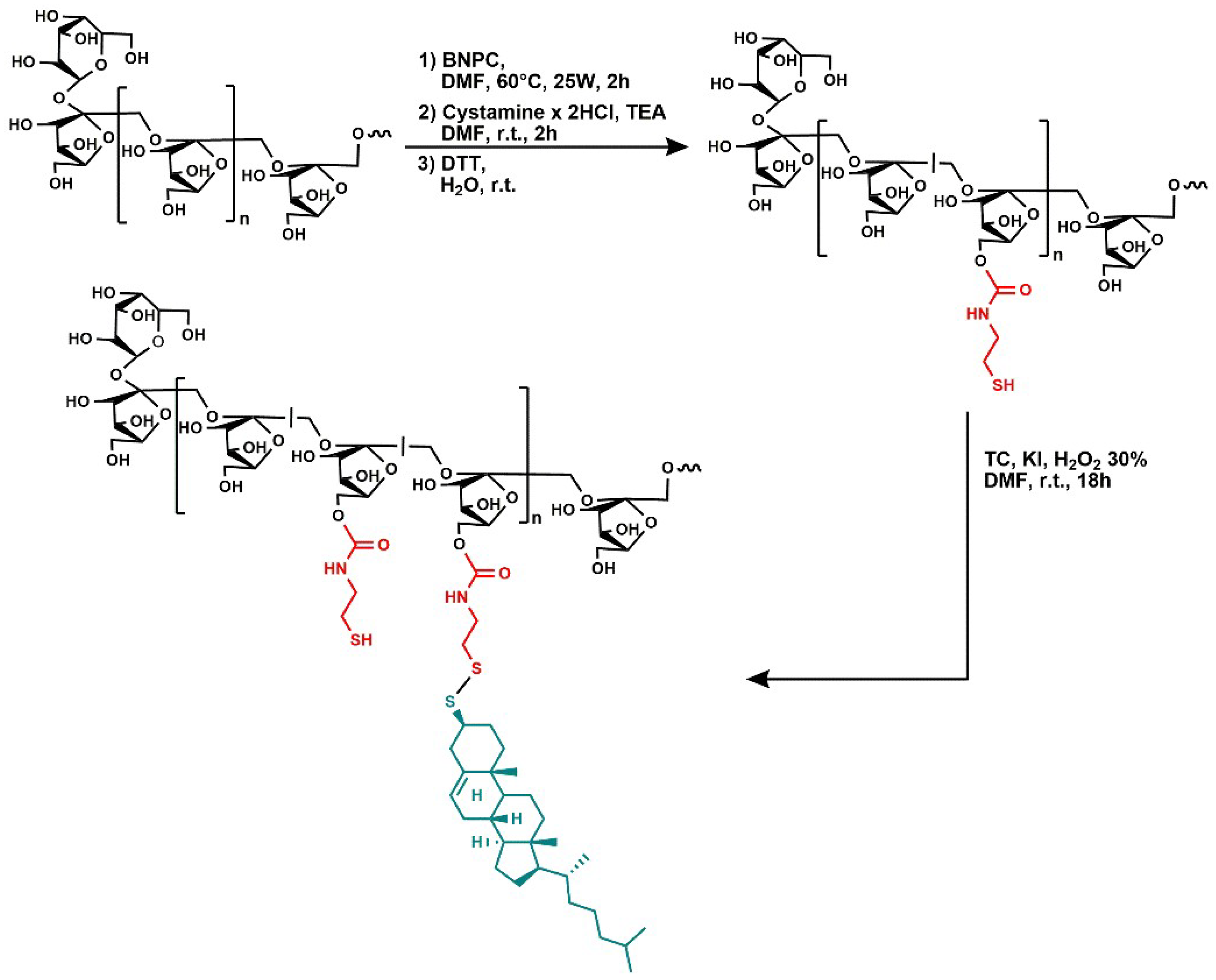
2.1. Synthesis of Cysteamine-Functionalized Inulin (INU-Cys-SH)
2.2. Synthesis of Inulin-Graft-Thiocholesterol Conjugate (INU-Cys-TC)
2.3. Determination of the Critical Aggregation Concentration (CAC) of INU-Cys-TC
2.4. Atomic Force Microscopy (AFM) of INU-Cys-TC
2.5. Scanning Electron Microscopy (SEM) of INU-Cys-TC
2.6. Preparation of SN38-Loaded INU-Cys-TC Micelles (INU-Cys-TC@SN38)
2.7. Drug Loading Determination of INU-Cys-TC@SN38
2.8. Dynamic Light Scattering (DLS) and Zeta-Potential Measurements
2.9. Cumulative Drug Release Study of INU-Cys-TC@SN38
2.10. Biological Characterization
2.10.1. Cell Internalization Study of INU-Cys-TC@SN38
2.10.2. In Vitro INU-Cys-TC and INU-Cys-TC@SN38 Cytotoxic Effects
2.11. Statistical Data Analysis
3. Results and Discussion
3.1. Synthesis of the Amphiphilic Cholesterol-Grafted-Inulin Conjugate (INU-Cys-TC)
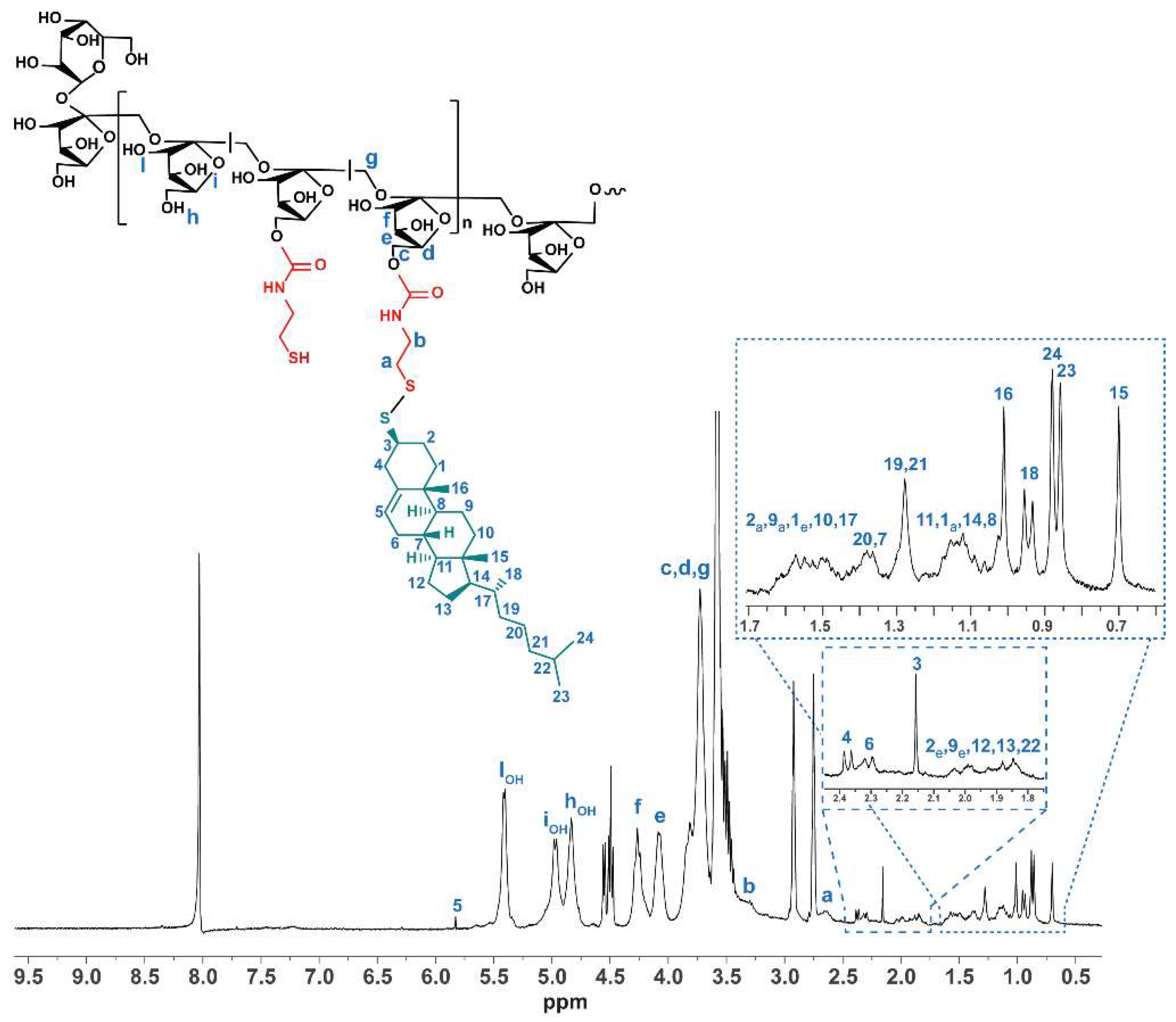
3.2. Physicochemical Characterization of INU-Cys-TC
3.3. Preparation and Characterization of Loaded INU-Cys-TC@SN38 Micelles
3.4. Dynamic Light Scattering (DLS) Measurements
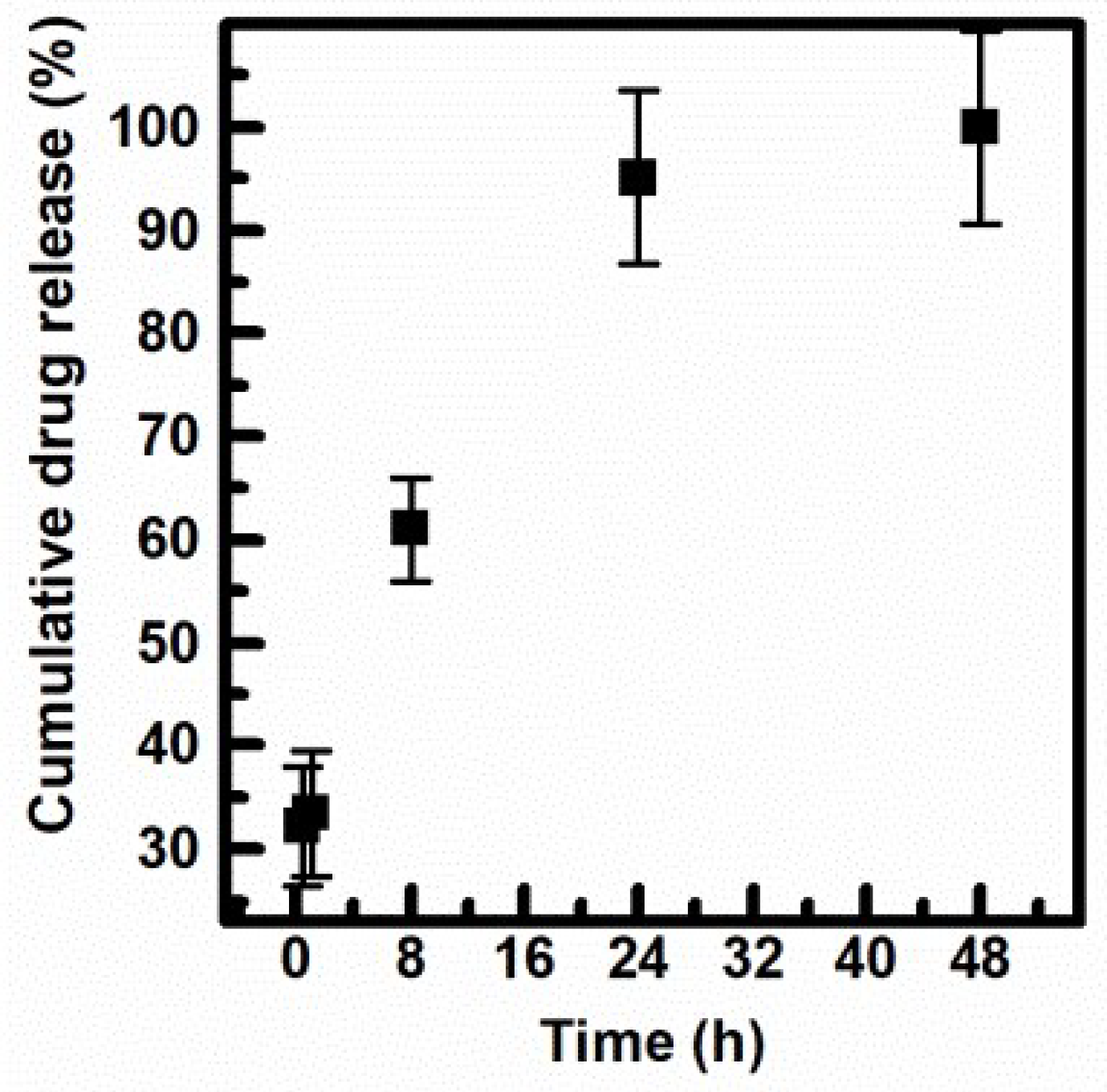
3.5. Cumulative Drug Release Study of INU-Cys-TC@SN38
3.6. Biological Characterization
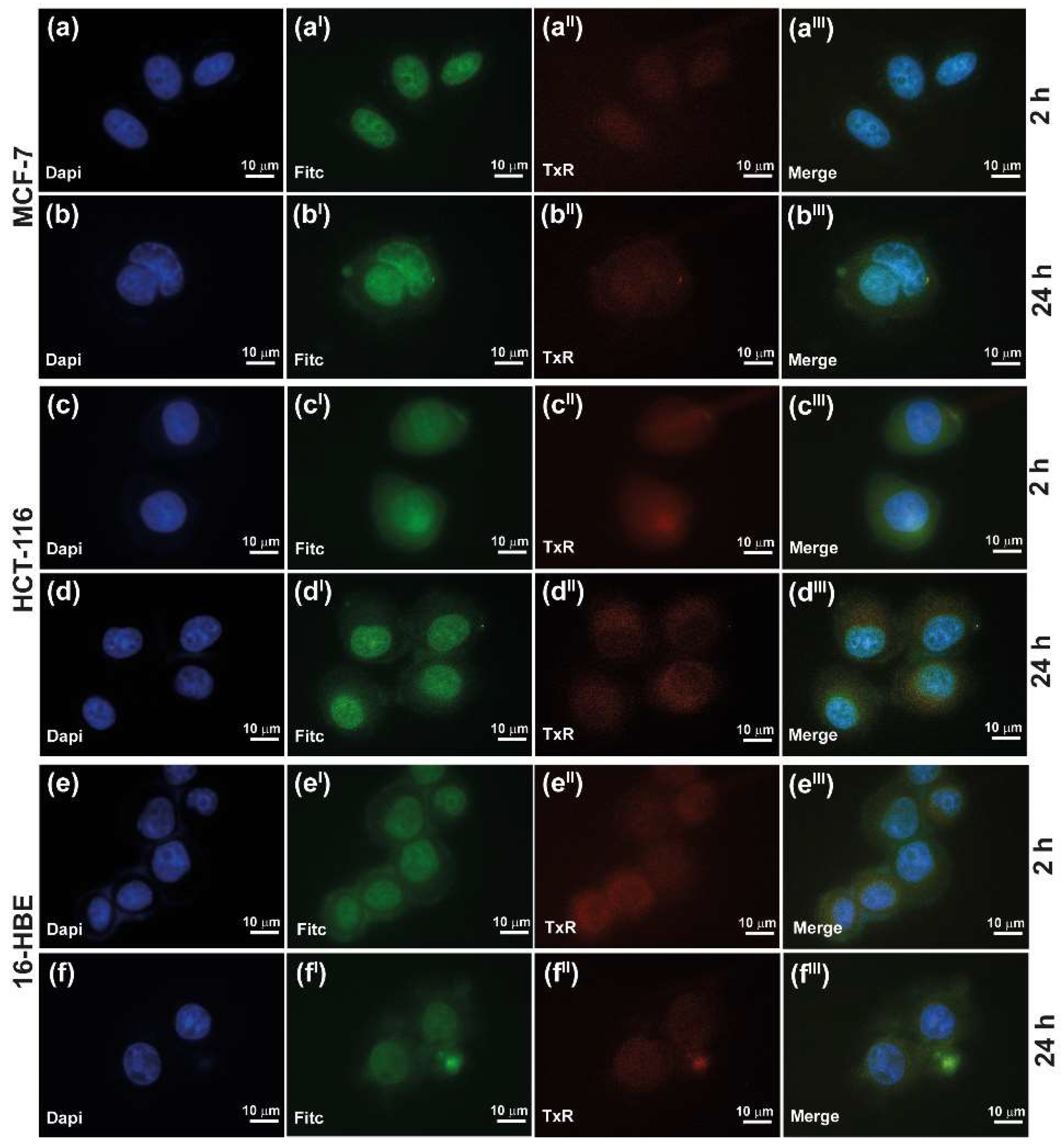
3.6.1. Cell Uptake Study of INU-Cys-TC@SN38
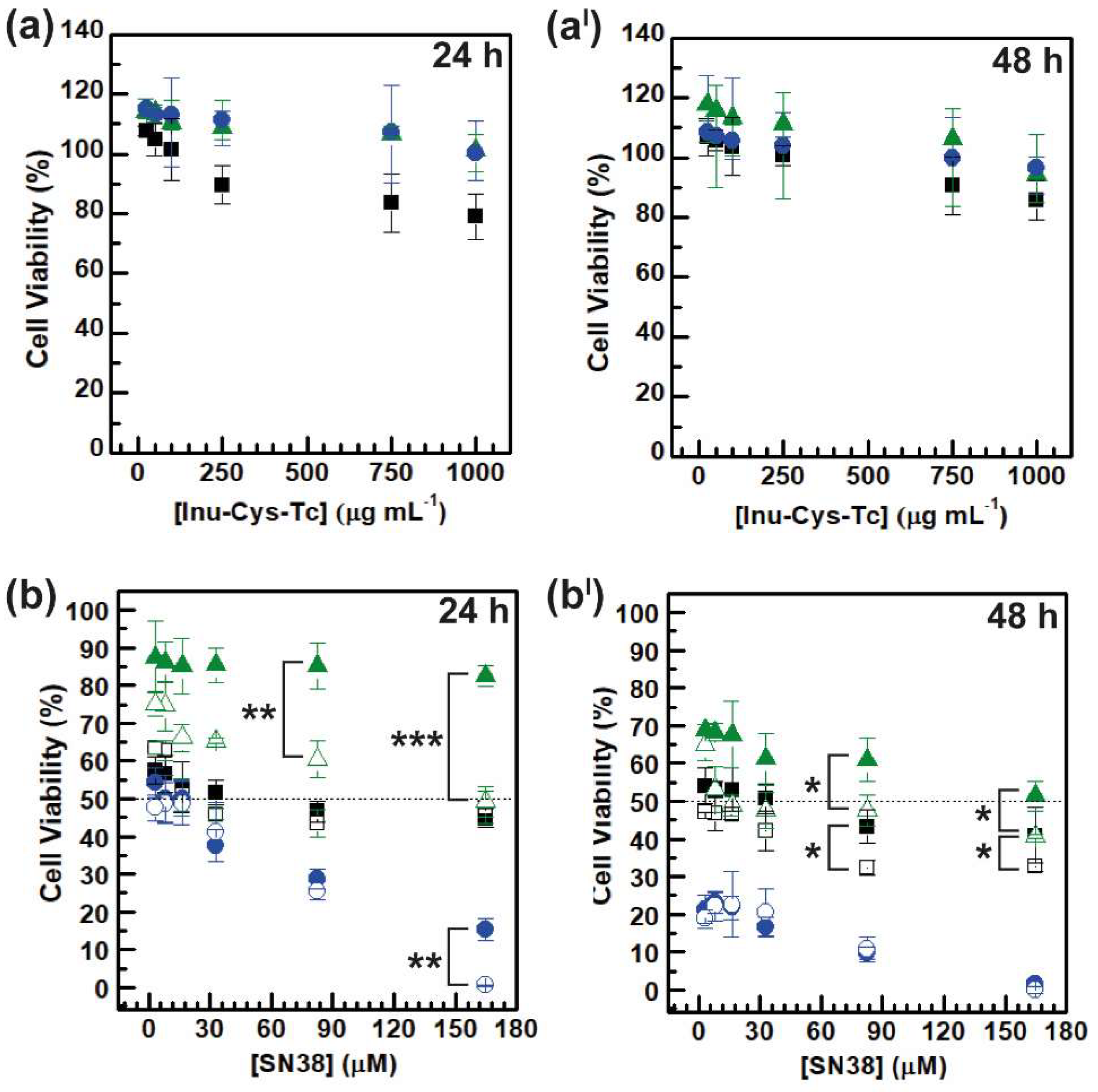
3.6.2. In Vitro Cytocompatibility and Anticancer Efficacy
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wicki, A.; Witzigmann, D.; Balasubramanian, V.; Huwyler, J. Nanomedicine in cancer therapy: Challenges, opportunities, and clinical applications. J. Control. Release 2015, 200, 138–157. [Google Scholar] [CrossRef] [PubMed]
- Abbasi Kajani, A.; Haghjooy Javanmard, S.; Asadnia, M.; Razmjou, A. Recent Advances in Nanomaterials Development for Nanomedicine and Cancer. ACS Appl. Bio. Mater. 2021, 4, 5908–5925. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, B.; Biswas, S. Polymeric micelles in cancer therapy: State of the art. J. Control. Release 2021, 332, 127–147. [Google Scholar] [CrossRef] [PubMed]
- Hari, S.K.; Gauba, A.; Shrivastava, N.; Tripathi, R.M.; Jain, S.K.; Pandey, A.K. Polymeric micelles and cancer therapy: An ingenious multimodal tumor-targeted drug delivery system. Drug Deliv. Transl. Res. 2022, 1–29. [Google Scholar] [CrossRef] [PubMed]
- Marin, J.J.G.; Monte, M.J.; Blazquez, A.G.; MacIas, R.I.; Serrano, M.A.; Briz, O. The role of reduced intracellular concentrations of active drugs in the lack of response to anticancer chemotherapy. Acta Pharmacol. Sin. 2013, 35, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Veselov, V.V.; Nosyrev, A.E.; Jicsinszky, L.; Alyautdin, R.N.; Cravotto, G. Targeted Delivery Methods for Anticancer Drugs. Cancers 2022, 14, 622. [Google Scholar] [CrossRef] [PubMed]
- Ansari, M.J. An overview of techniques for multifold enhancement in solubility of poorly soluble drugs. Curr. Issues Pharm. Med. Sci. 2019, 32, 203–209. [Google Scholar] [CrossRef]
- Ghezzi, M.; Pescina, S.; Padula, C.; Santi, P.; Del Favero, E.; Cantù, L.; Nicoli, S. Polymeric micelles in drug delivery: An insight of the techniques for their characterization and assessment in biorelevant conditions. J. Control. Release 2021, 332, 312–336. [Google Scholar] [CrossRef]
- Raval, N.; Maheshwari, R.; Shukla, H.; Kalia, K.; Torchilin, V.P.; Tekade, R.K. Multifunctional polymeric micellar nanomedicine in the diagnosis and treatment of cancer. Mater. Sci. Eng. C 2021, 126, 112186. [Google Scholar] [CrossRef]
- Mauro, N.; Utzeri, M.A.; Drago, S.E.; Nicosia, A.; Costa, S.; Cavallaro, G.; Giammona, G. Hyaluronic acid dressing of hydrophobic carbon nanodots: A self-assembling strategy of hybrid nanocomposites with theranostic potential. Carbohydr. Polym. 2021, 267, 118213. [Google Scholar] [CrossRef]
- Mauro, N.; Utzeri, M.A.; Drago, S.E.; Buscarino, G.; Cavallaro, G.; Giammona, G. Carbon Nanodots as Functional Excipient to Develop Highly Stable and Smart PLGA Nanoparticles Useful in Cancer Theranostics. Pharmaceutics 2020, 12, 1012. [Google Scholar] [CrossRef]
- Gong, J.; Chen, M.; Zheng, Y.; Wang, S.; Wang, Y. Polymeric micelles drug delivery system in oncology. J. Control. Release 2012, 159, 312–323. [Google Scholar] [CrossRef]
- Kedar, U.; Phutane, P.; Shidhaye, S.; Kadam, V. Advances in polymeric micelles for drug delivery and tumor targeting. Nanomed. Nanotechnol. Biol. Med. 2010, 6, 714–729. [Google Scholar] [CrossRef]
- Kang, H.; Rho, S.; Stiles, W.R.; Hu, S.; Baek, Y.; Hwang, D.W.; Kashiwagi, S.; Kim, M.S.; Choi, H.S. Size-Dependent EPR Effect of Polymeric Nanoparticles on Tumor Targeting. Adv. Healthc. Mater. 2020, 9, 1901223. [Google Scholar] [CrossRef]
- Licciardi, M.; Volsi, A.L.; Mauro, N.; Scialabba, C.; Cavallaro, G.; Giammona, G. Preparation and Characterization of Inulin Coated Gold Nanoparticles for Selective Delivery of Doxorubicin to Breast Cancer Cells. J. Nanomater. 2016, 2016, 2078315. [Google Scholar] [CrossRef]
- Licciardi, M.; Li Volsi, A.; Sardo, C.; Mauro, N.; Cavallaro, G.; Giammona, G. Inulin-Ethylenediamine Coated SPIONs Magnetoplexes: A Promising Tool for Improving siRNA Delivery. Pharm. Res. 2015, 32, 3674–3687. [Google Scholar] [CrossRef]
- Ahmed, W.; Rashid, S. Functional and therapeutic potential of inulin: A comprehensive review. Crit. Rev. Food Sci. Nutr. 2019, 59, 1–13. [Google Scholar] [CrossRef]
- Mauro, N.; Scialabba, C.; Giammona, G.; Mauro, N.; Scialabba, C. Inulin for Cancer Therapy: Present and Perspectives Plasma-induced graphene oxide deposition onto polymeric scaffolds for cancer cells recruitment view project Inulin for Cancer Therapy: Present and Perspectives. Int. J. Pharma. Res. Rev. 2016, 5, 63–69. [Google Scholar]
- Afinjuomo, F.; Abdella, S.; Youssef, S.H.; Song, Y.; Garg, S. Inulin and Its Application in Drug Delivery. Pharmaceuticals 2021, 14, 855. [Google Scholar] [CrossRef]
- Kesharwani, S.S.; Dachineni, R.; Bhat, G.J.; Tummala, H. Hydrophobically modified inulin-based micelles: Transport mechanisms and drug delivery applications for breast cancer. J. Drug Deliv. Sci. Technol. 2019, 54, 101254. [Google Scholar] [CrossRef]
- Matsumura, Y. Preclinical and clinical studies of NK012, an SN-38-incorporating polymeric micelles, which is designed based on EPR effect. Adv. Drug Deliv. Rev. 2011, 63, 184–192. [Google Scholar] [CrossRef]
- Gu, Q.; Xing, J.Z.; Huang, M.; He, C.; Chen, J. SN-38 loaded polymeric micelles to enhance cancer therapy. Nanotechnology 2012, 23, 205101. [Google Scholar] [CrossRef]
- Sadat, S.M.A.; Vakili, M.R.; Paiva, I.M.; Weinfeld, M.; Lavasanifar, A. Development of self-associating sn-38-conjugated poly(Ethylene oxide)-poly(ester) micelles for colorectal cancer therapy. Pharmaceutics 2020, 12, 1033. [Google Scholar] [CrossRef]
- Palakurthi, S. Challenges in SN38 drug delivery: Current success and future directions. Expert Opin. Drug Deliv. 2015, 12, 1911–1921. [Google Scholar] [CrossRef]
- Wu, D.; Li, Y.; Zhu, L.; Zhang, W.; Xu, S.; Yang, Y.; Yan, Q.; Yang, G. A biocompatible superparamagnetic chitosan-based nanoplatform enabling targeted SN-38 delivery for colorectal cancer therapy. Carbohydr. Polym. 2021, 274, 118641. [Google Scholar] [CrossRef]
- Ranucci, E.; Suardi, M.A.; Annunziata, R.; Ferruti, P.; Chiellini, F.; Bartoli, C. Poly(amidoamine) conjugates with disulfide-linked cholesterol pendants self-assembling into redox-sensitive nanoparticles. Biomacromolecules 2008, 9, 2693–2704. [Google Scholar] [CrossRef]
- Chen, Q.; Han, F.; Lin, C.; Wen, X.; Zhao, P. Synthesis of bioreducible core crosslinked star polymers with N,N′-bis(acryloyl)cystamine crosslinker via aqueous ethanol dispersion RAFT polymerization. Polymer 2018, 146, 378–385. [Google Scholar] [CrossRef]
- Ling, X.; Tu, J.; Wang, J.; Shajii, A.; Kong, N.; Feng, C.; Zhang, Y.; Yu, M.; Xie, T.; Bharwani, Z.; et al. Glutathione-Responsive Prodrug Nanoparticles for Effective Drug Delivery and Cancer Therapy. ACS Nano 2019, 13, 357–370. [Google Scholar] [CrossRef]
- Gamcsik, M.P.; Kasibhatla, M.S.; Teeter, S.D.; Colvin, O.M. Glutathione Levels in Human Tumors. Biomarkers 2012, 17, 671. [Google Scholar] [CrossRef]
- Huang, Z.; Li, W.; MacKay, J.A.; Szoka, F.C. Thiocholesterol-based lipids for ordered assembly of bioresponsive gene carriers. Mol. Ther. 2005, 11, 409–417. [Google Scholar] [CrossRef]
- Guo, X.; Cheng, Y.; Zhao, X.; Luo, Y.; Chen, J.; Yuan, W.E. Advances in redox-responsive drug delivery systems of tumor microenvironment. J. Nanobiotechnol. 2018, 16, 74. [Google Scholar] [CrossRef] [PubMed]
- Sharma, D.; Rajarathnam, K. 13C NMR chemical shifts can predict disulfide bond formation. J. Biomol. NMR 2000, 18, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2020, 20, 101–124. [Google Scholar] [CrossRef] [PubMed]
- Alibolandi, M.; Abnous, K.; Anvari, S.; Mohammadi, M.; Ramezani, M.; Taghdisi, S.M. CD133-targeted delivery of self-assembled PEGylated carboxymethylcellulose-SN38 nanoparticles to colorectal cancer. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1159–1169. [Google Scholar] [CrossRef]
- Scialabba, C.; Licciardi, M.; Mauro, N.; Rocco, F.; Ceruti, M.; Giammona, G. Inulin-based polymer coated SPIONs as potential drug delivery systems for targeted cancer therapy. Eur. J. Pharm. Biopharm. 2014, 88, 695–705. [Google Scholar] [CrossRef]
- Mauro, N.; Campora, S.; Scialabba, C.; Adamo, G.; Licciardi, M.; Ghersi, G.; Giammona, G. Self-organized environment-sensitive inulin-doxorubicin conjugate with a selective cytotoxic effect towards cancer cells. RSC Adv. 2015, 5, 32421–32430. [Google Scholar] [CrossRef]
- Lamers, R.J.A.N.; Wessels, E.C.H.H.; Van de Sandt, J.J.M.; Venema, K.; Schaafsma, G.; Van der Greef, J.; Van Nesselrooij, J.H.J. A Pilot Study to Investigate Effects of Inulin on Caco-2 Cells through In Vitro Metabolic Fingerprinting. J. Nutr. 2003, 133, 3080–3084. [Google Scholar] [CrossRef]
- Liu, D.; Mori, A.; Huang, L. Role of liposome size and RES blockade in controlling biodistribution and tumor uptake of GM1-containing liposomes. Biochim. Biophys. Acta—Biomembr. 1992, 1104, 95–101. [Google Scholar] [CrossRef]
- Revia, R.A.; Zhang, M. Magnetite nanoparticles for cancer diagnosis, treatment, and treatment monitoring: Recent advances. Mater. Today 2016, 19, 157–168. [Google Scholar] [CrossRef]
- Rodríguez-Cáceres, M.I.; Gil, D.B.; Durán-Merás, I.; Sánchez, M.C.H. Spectrofluorimetric determination of SN-38, a promising new anti-tumor agent, in the presence and absence of organized media. Appl. Spectrosc. 2011, 65, 298–306. [Google Scholar] [CrossRef]
- Van der Zee, M.; Stoutjesdijk, J.H.; van der Heijden, P.A.A.W.; de Wit, D. Structure-biodegradation relationships of polymeric materials. 1. Effect of degree of oxidation on Biodegradability of carbohydrate polymers. J. Environ. Polym. Degrad. 1995, 3, 235–242. [Google Scholar] [CrossRef]
- Niness, K.R. Inulin and oligofructose: What are they? J. Nutr. 1999, 129, 1402S–1406S. [Google Scholar] [CrossRef]
- Mirgayazova, R.; Khadiullina, R.; Mingaleeva, R.; Chasov, V.; Gomzikova, M.; Garanina, E.; Rizvanov, A.; Bulatov, E. Novel Isatin-based activator of p53 transcriptional functions in tumor cells. Mol. Biol. Res. Commun. 2019, 8, 119–128. [Google Scholar] [CrossRef]
- Mauro, N.; Li Volsi, A.; Scialabba, C.; Licciardi, M.; Cavallaro, G.; Giammona, G. Photothermal Ablation of Cancer Cells Using Folate-Coated Gold/Graphene Oxide Composite. Curr. Drug Deliv. 2017, 14, 433–443. [Google Scholar] [CrossRef]



| Sample | DDCys (mol%) | DDTC (mol%) | Mw | PD |
|---|---|---|---|---|
| INU-Cys-SH | 14 | - | 3391 | 1.39 |
| INU-Cys-TC | 14 | 8 | 4413 | 1.53 |
| Sample | Z-Average (nm) | PDI | Zeta-Potential (mV) |
|---|---|---|---|
| INU-Cys-TC | 177.7 ± 0.9 | 0.135 | −25.3 ± 5.4 |
| INU-Cys-TC@SN38 | 225.4 ± 0.7 | 0.136 | −18.2 ± 3.9 |
| Sample | Cell Line | IC5024h (mM) | IC5048h (mM) | Imax24h (%) | Imax48h (%) |
|---|---|---|---|---|---|
| INU-Cys-TC@SN38 | MCF-7 | 47 ± 3.1 | 33 ± 1.4 | 55 ± 0.5 | 59 ± 0.7 |
| HCT-116 | 3.3 ± 0.3 | <3.3 | 85 ± 0.6 | 99 ± 1.1 | |
| 16-HBE | >165 | 165 ± 9.0 | 18 ± 0.2 | 48 ± 0.8 | |
| Free SN38 | MCF-7 | 17 ± 0.9 | <3.3 | 55 ± 0.9 | 67 ± 0.9 |
| HCT-116 | <3.3 | <3.3 | 100 ± 0.3 | 100 ± 0.2 | |
| 16-HBE | 130 ± 3.7 | 20 ± 0.6 | 50 ± 0.5 | 59 ± 0.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mauro, N.; Utzeri, M.A.; Cillari, R.; Scialabba, C.; Giammona, G.; Cavallaro, G. Cholesterol-Inulin Conjugates for Efficient SN38 Nuclear Delivery: Nanomedicines for Precision Cancer Therapy. Cancers 2022, 14, 4857. https://doi.org/10.3390/cancers14194857
Mauro N, Utzeri MA, Cillari R, Scialabba C, Giammona G, Cavallaro G. Cholesterol-Inulin Conjugates for Efficient SN38 Nuclear Delivery: Nanomedicines for Precision Cancer Therapy. Cancers. 2022; 14(19):4857. https://doi.org/10.3390/cancers14194857
Chicago/Turabian StyleMauro, Nicolò, Mara Andrea Utzeri, Roberta Cillari, Cinzia Scialabba, Gaetano Giammona, and Gennara Cavallaro. 2022. "Cholesterol-Inulin Conjugates for Efficient SN38 Nuclear Delivery: Nanomedicines for Precision Cancer Therapy" Cancers 14, no. 19: 4857. https://doi.org/10.3390/cancers14194857
APA StyleMauro, N., Utzeri, M. A., Cillari, R., Scialabba, C., Giammona, G., & Cavallaro, G. (2022). Cholesterol-Inulin Conjugates for Efficient SN38 Nuclear Delivery: Nanomedicines for Precision Cancer Therapy. Cancers, 14(19), 4857. https://doi.org/10.3390/cancers14194857